Abstract
Naturally derived regulatory T cells (nTregs) may prevent graft-versus-host disease (GVHD) while preserving graft-versus-leukemia (GVL) activity. However, clinical application of nTregs has been severely hampered by their scarce availability and non-selectivity. To overcome these limitations, we took alternative approaches to generate Ag-specific induced Tregs (iTregs) and tested their efficacy and selectivity in the prevention of GVHD in pre-clinical models of bone marrow transplantation (BMT). We selected HY as a target antigen because it is a naturally processed, ubiquitously expressed minor histocompatibility antigen (miHAg) with a proven role in GVHD and GVL effect. We generated HY-specific iTregs (HY-iTregs) from resting CD4 T cells derived from TCR transgenic mice, in which CD4 cells specifically recognize HY peptide. We found that HY-iTregs were highly effective in preventing GVHD in male (HY+) but not female (HY−) recipients using MHC II-mismatched, parent → F1 and miHAg-mismatched murine BMT models. Interestingly, the expression of target Ag (HY) on the hematopoietic or non- hematopoietic compartment alone was sufficient for iTregs to prevent GVHD. Furthermore, treatment with HY-iTregs still preserved the GVL effect even against pre-established leukemia. We found that HY-iTregs were more stable in male than in female recipients. Furthermore, HY-iTregs expanded extensively in male but not female recipients, which in turn significantly reduced donor effector T-cell (Teff) expansion, activation, and migration into GVHD target organs resulting in effective prevention of GVHD. This study demonstrates that iTregs specific for HY miHAgs are highly effective in controlling GVHD in an Ag-dependent manner while sparing the GVL effect.
Introduction
Allogeneic bone marrow transplantation (BMT), as a treatment for leukemias, lymphomas, and myelomas, has historically been hampered by the detrimental effects of graft-versus-host disease (GVHD). Allogeneic T cells within the graft inoculum recognize both major and minor mismatch antigens on leukemic and host tissues, resulting in either beneficial graft versus leukemic (GVL) or deleterious graft-versus host (GVH) effect. Clinicians and scientists still struggle to separate the GVL and GVH responses; among other strategies, the use of naturally derived regulatory T cells (nTregs) has been shown to be a promising approach to effectively control GVHD in animal studies and initial clinical trials. However, isolation and expansion of nTregs still remains a significant obstacle to establishing nTreg therapy as a standard for GVHD treatment. This is due to the low frequency and high number of nTregs needed to effectively control GVHD. Another concern regarding nTreg therapy centers on the loss of the GVL effect. Given that nTregs are non-selective suppressors, this therapy could result in suppression of allogeneic T cells responding to leukemic cells and therefore increased relapse in patients. Establishing Ag-specific inducible T regulatory (iTreg) cell therapy for the treatment of GVHD may solve the previously stated disadvantages of nTreg therapy. First, iTregs can be generated from naïve T cells, under specific polarizing conditions, offering a greater number of primary cells for initial expansion. Secondly, we propose, by conferring antigen specificity or antigen education during iTreg generation, we can overcome the high number needed for efficiency as compared to non-specific nTreg cell therapy. Finally, we propose drawing the fine line between GVL and GVH responses can be obtained by conferring Ag-specificity.
In experimental autoimmune disease models, Ag-specific Tregs are highly effective in controlling autoimmune diabetes, gastritis, and encephalomyelitis (1–3). We and others have initiated studies to evaluate the effects of Ag-specific iTregs in the prevention of GVHD and in the maintenance of GVL activity. We previously generated OVA-specific iTregs by foxp3 transduction or TGFβ-induction, and demonstrated that they persist long-term in vivo and suppress GVHD in non-myeloablative and myeloablative BMT models when activated by the cognate Ag; either constitutively expressed or introduced via immunization (4, 5). However, we used a nominal Ag to activate Ag-specific iTregs in our preliminary studies, which may not represent clinical settings. Therefore, it is crucial to extend these studies by testing iTregs specific for naturally processed alloantigens, in this case, HY Ag. HY is a minor histocompatibility Ag (miHAg) expressed solely by male recipients. Clinical data shows that MHC-matched BMT between female donors and male recipients increased the risk for acute GVHD development (6) and HY-specific alloresponses (7–10). Therefore, due to its clinical relevance, we generated HY specific iTregs and tested their efficiency, stability, and selectivity in suppressing acute murine GVHD.
Materials and Methods
Mice
C57BL/6 (B6, H-2b, CD45.2+, BALB/c (H-2d) and (B6 x DBA2) F1 (BDF1, H-2b/d) mice were purchased from the National Cancer Institute. B6 Ly5.1 (H-2b, CD45.1+), B6 bm12 (H-2b), BALB.b (H-2b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Foxp3gfp knock-in (KI) strain was obtained from A. Rudensky’s laboratory (11, 12). Luciferase-transgenic (Luc-Tg) strain on B6 background was kindly provided by R. Negrin (Stanford Univ., CA)(13). Anti-HY TCR Tg Marilyn mice (CD4+Tg, H-2b, I-Ab restricted) was kindly provided by C.R. Mainhart (NIAID, Bethesda, MD). Marilynn Foxp3gfp knock-in (KI) and (B6 x bm12) F1 strains were produced by cross-breeding. All the mice were housed in a pathogen-free condition at H. Lee Moffitt Cancer Center and Drug Discovery Building at MUSC. All experimental procedures were approved by the IACUC.
T-cell purification and iTreg generation
Total T cells or CD4+CD25− T cells were purified through negative selection using magnetic beads as described in our previous work (5, 14). The purity of CD4+CD25− cells ranged from 85 to 95%, but CD4+CD25+ cells was always less than 1% among total CD4+ cells. To generate HY-specific iTregs, CD4+CD25− T cells from TCR Tg (Marilynn) Foxp3gfp KI mice were seeded at 2.5 x 105/ml and stimulated with 0.5 μg/ml HY peptide in the presence of 1.25 x 106/ml irradiated syngeneic T-cell depleted (TCD)-splenoctyes as APCs with 5 ng/ml TGF-β1, 5 ng/ml IL-2 and 10 nM retinoic acid for 6 days.
Immuno-fluorescence analysis
Multiple-color flow cytometry was performed to measure the expression of surface molecules according to standard techniques. Intracellular Foxp3 expression was measured with a Foxp3 detection kit from eBioscience (San Diego, CA), according to manufacturer’s instruction. Intracellular cytokines were measured according to standard techniques, as described in our previous work (15).
BMT and bioluminescent imaging (BLI)
The procedures for induction of acute GVHD were described in previous publications (5, 15). BALB.b mice were exposed to total body irradiation (TBI) at 850–900 cGy (2 split doses) at day −1. (B6 x bm12)F1 or BDF1 mice were exposed to 1200 – 1300 cGy TBI (2 split doses). TCD-BM cells alone or in combination with purified T cells from B6 donors were injected via the tail vein into recipients within 24 hrs after irradiation. Recipient mice were monitored every other day for clinical signs of GVHD, such as ruffled fur, hunched back, lethargy or diarrhea, and mortality. Animals judged to be moribund were euthanized and counted as GVHD lethality. To generate BM chimeras, female or male recipients were lethally irradiated and transplanted with TCD-BM from male or female syngeneic donors, and thus HY antigens were expressed only on epithelial tissues (F → M) or hematopoietic cells (M → F). In vivo BLI of the recipients transplanted with allogeneic T cells from Luc-Tg B6 donors and BM from non-Tg B6 donors was performed using an IVIS200 charge-coupled device imaging system (Xenogen). For GVL induction, p815-luc mastocytoma cells were injected either on day of transplant with iTregs or three days prior to irradiation (pre-established tumor model).
Results
HY-specific iTregs suppress polyclonal T-cell response to alloantigens in vitro
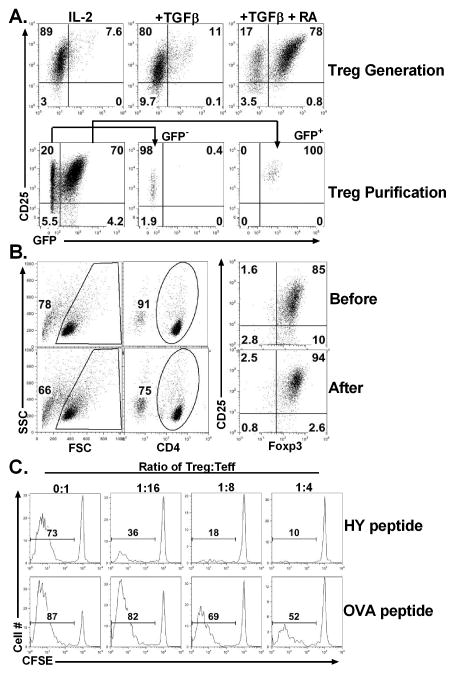
iTregs can be generated from conventional CD4 T cells upon TCR stimulation in the presence of TGFβ, and addition of retinoic acid (RA) further increases the generation of iTregs (14, 16, 17). In this study we selected HY as target antigen, because it is a naturally processed and ubiquitously expressed miHAg with a proven role in GVHD and GVL responses (6–10). HY-specific iTregs were generated from CD4+CD25− T cells from Marilyn Foxp3gfp KI mice by stimulating with HYAbDby, in the presence of IL-2, TGFβ, and RA (Fig. 1A, upper panels) and purified (purity 94%± 3%) by FACS sorting (Fig. 1A, lower panels).
Fig. 1. Generation and isolation of HY-specific iTregs.
(A) CD4+CD25− cells were purified from spleen and lymph nodes of TCR Marilynn Foxp3gfp KI mice, and stimulated with HY-peptide (0.5 μM) in the presence of irradiated TCD-splenocytes plus IL-2 (5 ng/ml). To generate iTregs, media was supplied with either TGFβ (5 ng/ml) alone or TGFβ and RA (10 nM). Five to six days after culture, cells were harvested and tested for expression of CD4, CD25, and GFP by flow cytometry. The phenotype of cultured cells under the different condition is shown on gated live CD4+ cells (upper panels). CD4+ CD25+GFP+ (iTregs) and CD25+GFP− cells (controls) were purified by FACS sorting (lower panels). (B) CD4+CD25− cells were purified from spleen and lymph node of TCR Marilyn mice, and iTregs were generated in the presence of TGFβ and RA as described in A. Six days after culture, CD4+CD25hi cells (iTregs) were isolated by enriching CD4+ cells through negative selection and then purifying CD25hi cells through positive selection using magnetic beads. The phenotype of cultured cells is shown on gated live CD4+ cells before (upper panels) and after (lower panels) iTreg isolation. These results represent accumulative data obtained from more than 10 experiments. (C) CD4+CD25− purified T cells from B6 Ly5.1+ mice were labeled with CFSE and stimulated at 2 x 105/well with irradiated TCD-splenocytes from female (B6 x bm12) F1 mice at 6 x 105/well in 96-well plates. Various numbers of HY-specific iTregs were added into culture to achieve indicated Treg: Teff ratios in the presence of HY (upper panels) or control OVA (lower panels) peptide at 0.5 μg/ml. Six days after cell stimulation, cultured cells were harvested and stained for the expression of Ly5.1 and CD4. CSFE profiles were shown on gated Ly5.1+CD4+ Teffs. These data represents 1 of 3 replicate experiments. ND: not done.
Foxp3gfp reporter gene allows us to obtain purified Foxp3+ iTregs, but this strategy cannot be applied in humans and GFP KI may affect the function of Tregs (18, 19). Therefore, to exclude any confounding effect, we generated HY-specific iTregs from CD4+CD25− T cells of Marilyn mice (Fig. 1B, upper panels). CD4+CD25hi cells (purity 92% ± 3%) were purified through positive selection for CD25 using magnetic beads (Fig. 1B, lower panels). Thus, iTregs were routinely generated from non-Foxp3gfp CD4+CD25− T cells and isolated for CD4+CD25hi using magnetic beads.
We then tested the suppressive function of HY-specific iTregs in vitro, and found that these iTregs suppressed ~50% proliferation of polyclonal T cells in response to allogeneic APCs at 1:16 ratio of Treg: Teff in the presence of HY-peptide, but the same iTregs had little suppressive activity in the presence of nominal OVA peptide (Fig. 1C), confirming that the activation of iTregs is required for their suppressive function in vitro.
HY-specific iTregs prevent GVHD in activation-dependent manner
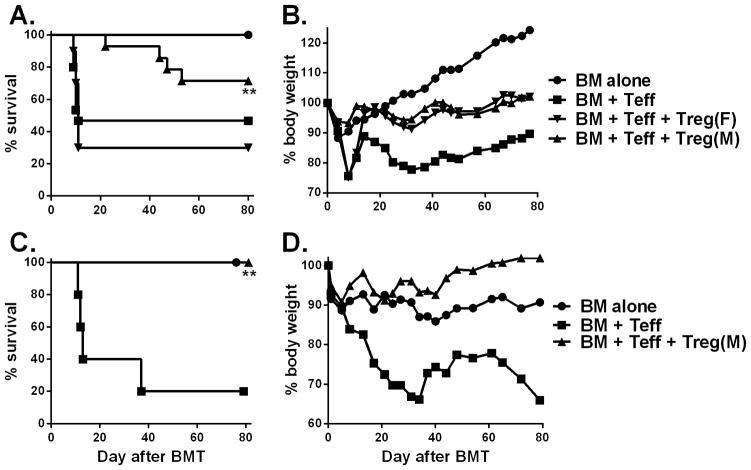
Next, we examined whether HY-specific iTregs were able to prevent GVHD induced by polyclonal T cells in a B6 → (B6 x bm12)F1 BMT model, in which donor effector CD4+ T cells (Teff) recognize mismatched recipient MHC II alloantigen (H2bm12). In this model, Teffs at indicated dose induced ~60% GVHD lethality while addition of iTregs at the same time of BMT significantly reduced GVHD lethality in male (p < 0.01) but not in female recipients (p = 0.7) (Fig. 2A and B), indicating that recognition of HY antigen by HY-specific iTregs was indispensable for their suppressive function of allogeneic responses in vivo. To assess whether donor reconstitution was impaired by iTreg therapy, 80 days post BMT, we observed male recipients that received Teffs plus HY-specific iTregs had comparable numbers of total spleen, B and T cells to those of BM alone (controls without GVHD), whereas the recipients of BM + Teff (GVHD controls) had significantly reduced numbers of spleen, B and T cells (vs BM alone, p<0.05, Fig. S1). These results indicate that HY-specific iTregs promoted long-term immune reconstitution and did not cause chronic GVHD in male recipients. We next determined whether infusion of HY-specific iTregs prior to Teffs promotes Treg expansion and increases therapeutic potential of Tregs (20). To this end, we utilized the same model and infused HY-specific iTregs 3 days prior to Teffs and found that these iTregs completely prevented GVHD lethality in male recipients (p<0.01) (Fig. 2C and D).
Fig. 2. HY-specific iTreg attenuation of GVHD is antigen dependent.
Male or female (B6 x bm12) F1 mice were lethally irradiated and transferred with 5 x 106 TCD-BM alone or plus 1–1.5 x 106/mouse CD25-depleted CD4+ T cells (Teffs) from B6 donors. HY-specific iTregs were generated and isolated as described in figure 1, were added at 0.5–0.75 x 106/mouse into donor graft at the same time of BMT. Overall survival (A) and body weight changes (B) are shown. The data are pooled from 3 replicate experiments with 10–15 mice in each group. In a separate experiment (n = 5–6), male (B6 x bm12) F1 mice were lethally irradiated and transferred with TCD-BM alone or plus 1 x 106/mouse HY-specific iTregs on day 0. CD25-depleted CD4+ T cells (Teffs) from B6 donors were injected at 2 x 106/mouse on day 3 after BMT. Recipient survival (C) and body weight changes (D) are shown. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001
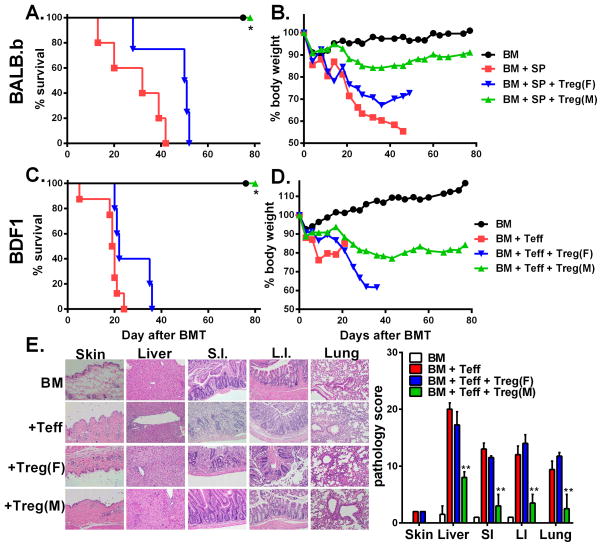
In clinical BMT, most patients receive grafts from MHC-matched and multiple mHAg-mismatched donors. In an effort to mimic a clinical scenario, we used the B6 → BALB.b (both H2b) model, in which donor and recipient mice differ by at least 29 different miHA loci (21). HY-specific iTregs were highly effective in preventing GVHD in male (p<0.05) but not female BALB.B recipients (Fig. 3A and B). Likewise, haploidentical transplantation is extensively used in clinic. Utilizing B6 → BDF1 model, we further confirmed that HY-specific iTregs were highly effective in preventing GVHD in male (p<0.05) but not female BDF1 recipients (Fig. 3C and D). To further support our long-term data in the B6 → BDF1 model, we analyzed pathology scores and found male recipients that received iTregs had significantly reduced pathologic damage within the liver, small intestine, large intestine, and lung compared to all other groups (Fig. 3E; p < 0.01). In agreement with survival data, female recipients receiving iTregs had comparable pathologic injury in target organs to Teff alone groups, further supporting necessity for iTregs to recognize specific Ag to exert their suppressive function. Taken together, these findings support the use of HY-specific iTregs in clinically relevant BMT models.
Fig. 3. Effect of HY-specific iTregs in the prevention of GVHD in mHAg- or halpo-mismatched BMT model.
Male or female BALB.b mice were lethally irradiated and transferred with TCD-BM alone or plus 4 x 106/mouse HY-specific iTregs. On day 3, 25 x 106/mouse total splenocytes from normal B6 donors were injected into the recipients previously transferred with BM alone or BM plus iTregs. Recipient survival (A) and body weight changes (B) are shown. The data are pooled from 2 replicate experiments with 9–10 mice in each group. Male or female BDF1 mice were lethally irradiated and transferred with TCD-BM alone or plus at 4 x 106/mouse CD25-depleted total T cells from normal B6 donors. HY-specific iTregs were also included at 2 x 106/mouse into donor graft 3 days after BMT for some recipients. Recipient survival (C) and body weight changes (D) are shown. The experiments were done 2–3 times for each BMT model, but the data presented are from one experiment with 5–8 mice in each group using cell doses indicated. Seven days post Teff injection, BDF1 recipients as described in the legend were sacrificed and pathology samples of the skin, lung, liver, small and large intestine were collected (E) represents the pathology score of the various organs with 4 mice per group. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001
HY-specific iTregs suppress the expansion, activation, and migration of donor T cells
We next assessed the cellular mechanism by which HY-specific iTregs suppress alloreactive Teffs in vivo. Taking advantage of Luc-Tg mice, the expansion and infiltration of Luc-Tg Teffs can be measured in vivo over time using BLI assay (22). To use this method, we first titrated the dose of T cells that are required for mediating GVHD and found that at least 4-fold lower numbers of Luc-Tg T cells were required to cause GVHD lethality comparable to normal B6 T cells (Fig. S2). This data suggested that Luc-Tg T cells might be significantly more pathogenic in the induction of acute GVHD. Using 0.25 x 106 Luc-Tg CD4+, although there were relatively low signal intensities and no significant difference among groups on day 6 following Teff injection (Fig. S3A), throughout later observation periods, the BLI intensity was significantly reduced in male recipients that received HY-specific iTregs compared to recipients with Teff alone (p< 0.001) or in female recipients transplanted HY-specific iTregs (p = 0.02) (Fig. S3A, B). Furthermore, male recipients that received HY-specific iTreg showed less dispersed BLI signal, mainly confined to the spleen, compared to other recipients (Fig. S3B). Similar results were observed in miHAg- or haplo-mismatched BMT models (Fig. S3C–F). These data suggest that HY specific iTregs regulate allogeneic Teff expansion and infiltration into GVHD target organs, such as the gut and liver.
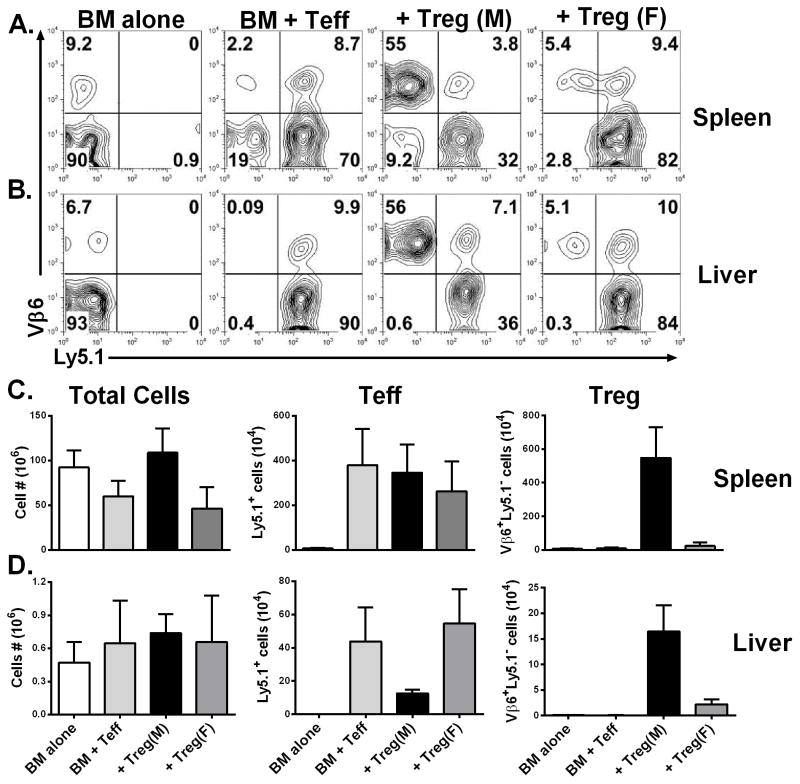
To further evaluate the effect of iTregs on the expansion and migration of Teffs, we transferred Teffs isolated from B6 Ly5.1+ mice and HY-specific iTregs along with TCD-BM isolated from normal B6 donors into (B6 x bm12) F1 recipients. Seven days after BMT, we measured Teffs (CD4+Ly5.1+) and iTregs (CD4+TCRVβ6+Ly5.1−) in recipient spleen (Fig. 4A and C) and liver (Fig. 4B and D). We found that iTregs expanded extensively in the spleen and migrated substantially to the liver of male but not female recipients, (p < 0.01, in spleen and liver), and the number of Teffs in the liver of male recipients was dramatically reduced (Fig. 4D).
Fig. 4. Stability and Efficiency of HY-specific iTregs.
Lethally irradiated male or female (B6 x bm12) F1 mice were transplanted with B6 TCD-BM plus 1.5 x 106/mouse Teffs (CD4+CD25−) isolated from B6 Ly5.1+ mice. At the same time, 0.75 x 106/mouse HY-specific iTregs were also included into the donor graft to some recipients. Seven days after BMT, recipient spleen and liver were harvested and measured for expansion and infiltration of iTregs and Teffs. Mononuclear cells were isolated from recipient spleen (A) and liver (B), and expression of TCRVβ6 and Ly5.1 was shown in gated CD4+ live cells. The average numbers of total cells, Teffs (CD4+Ly5.1+) and iTregs (CD4+Vβ6+Ly5.1−) per mouse were shown in recipient spleen (C) and liver (D). Each group includes 3–4 mice, and the data represents 1 of 3 replicate experiments. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001
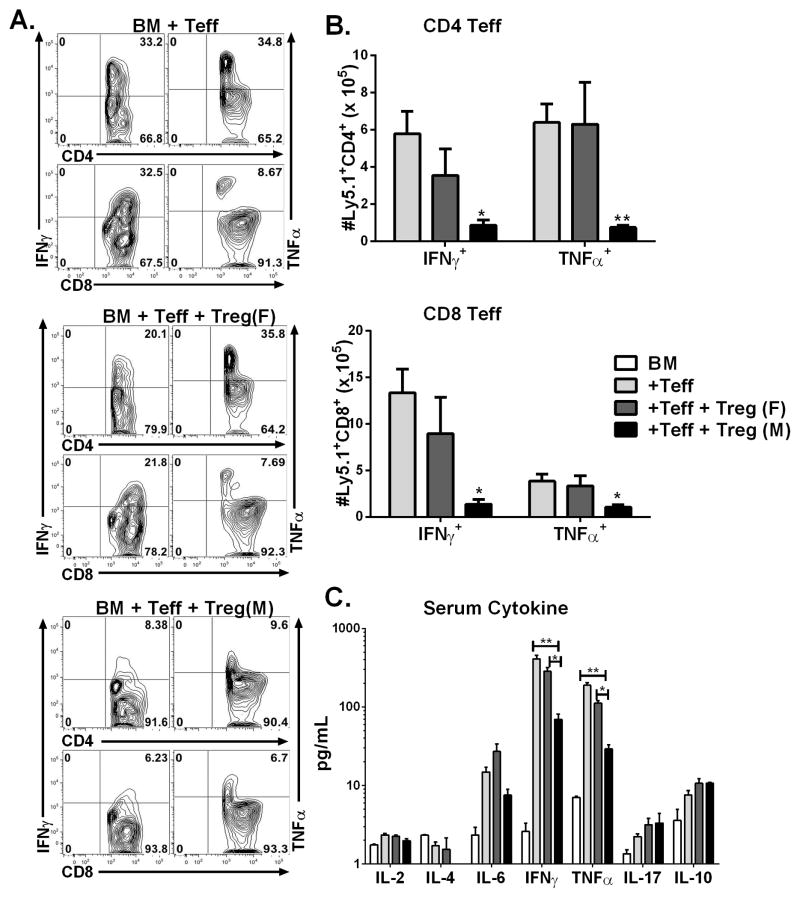
To extend these findings to the haploidentical BMT model, we transferred BM and Teffs from B6 Ly5.1+ mice and HY-specific iTregs (Ly5.1−) into irradiated B6D2F1 recipients. Fourteen days after BMT, analysis of IFNγ and TNFα secretion within the spleen of effector CD4 and CD8 T cells, showed male recipients had significantly decreased secretion of pro-inflammatory cytokines ( p<0.01) whereas there was no significant difference between Teff alone and female recipients (Fig 5A,B). In correlation with our flow data, analysis of the serum cytokine levels, 14 days post BMT, revealed male recipients had significantly reduced levels of IFNγ and TNFα (p<0.01) whereas there was no differences between female recipients and Teff alone groups (Fig. 5C). These cellular findings are consistent with our long-term survival data, proving the recognition of cognate antigen is necessary for iTregs to control Teffs in order to attenuate GVHD.
Fig 5. HY-specific iTregs suppress activation and expansion of Teffs.
Male or Female BDF1 mice were lethally irradiated and transferred with TCD-BM alone or plus 4 x 106/mouse CD25-depleted total T cells from normal B6 Ly5.1+ donors. HY-specific iTregs were also transplanted at 2 x 106/mouse on day 0 of BMT. Fourteen days after Teff injection, recipient spleen and liver were harvested and total T cells isolated, 4 mice per group. (A) Representative flow analysis of IFNγ and TNFα with effector CD4 and CD8 T cells. B) Represents the absolute number of CD4 and CD8 Teffs secreting IFNγ and TNFα within the recipient spleen. (C) Serum was collected on day 14 and cytokine levels were assessed by cytometric bead analysis, one representative analysis of two independent experiments. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001.
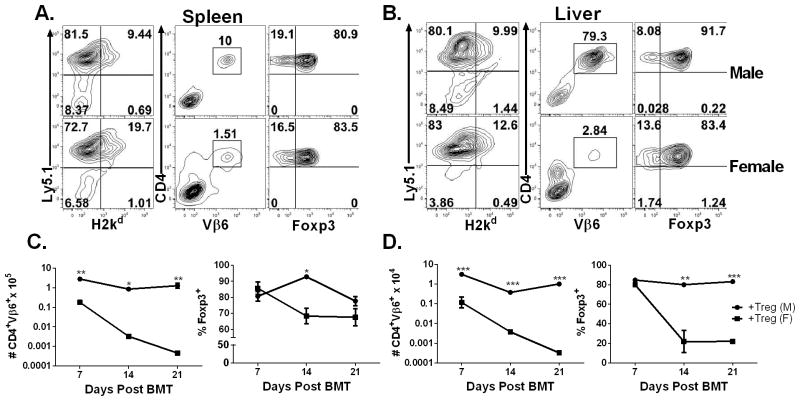
To address the stability of Ag-specific iTregs after injection, we performed a time course analysis of HY-specific iTregs within male and female recipient’s spleen and liver 7, 14 and 21 days after our previously described B6→BDF1 model. The male recipients retained the high number of CD4+Vβ6+Foxp3+ iTregs in their spleens on day 7, 14, and 21 post BMT (p< 0.001), whereas the number of HY-specific iTregs declined over time in the female recipients (Fig 6A, B). The male recipients also had higher numbers of CD4+Vβ6+ iTregs than female recipients on day 7 in their livers, although Foxp3 expression was retained similarly at this time point (Fig 6C, D). In the liver, the numbers of iTregs were more strikingly different on days 14 or 21 between male and female recipients. Furthermore, iTregs were highly stable in the male recipients reflected by their Foxp3 expression, whereas iTregs rapidly lost their Foxp3 expression in female recipients (p<0.001) (Fig. 6D). Taken together, iTregs were highly stable and expanded extensively after Ag-stimulation, and in turn effectively suppress Teff expansion and activation and migration into GVHD target organs regardless of BMT models
Fig. 6. HY-specific iTregs are highly stable under inflammatory conditions.
Male or Female BDF1 mice were lethally irradiated and transferred with TCD-BM alone or plus 4 x 106/mouse CD25-depleted total T cells from normal B6 Ly5.1+ donors. HY-specific iTregs were also transplanted at 2 x 106/mouse on day 0 of BMT. On days 7, 14, and 21 post BMT spleen (A, C) and liver (B, D) were collected from recipient mice for analysis. Phenotypes of cells isolated from recipient spleen (A) or liver (B) are displayed. Absolute numbers of originally infused iTregs (H2Kb+CD4+Vβ6+) in spleen (C) or liver (D) are depicted on the left panels. Percentages of Foxp3 expression on gated iTregs in spleen (C) or liver (D) were shown on the right panels. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001.
Expression of target antigen on epithelial tissues is not required for iTregs to prevent GVHD
It has been widely accepted that donor T cells have to recognize alloantigens expressed on epithelial tissues in order to cause GVHD in myeloablative BMT models (23). However, it is not clear whether Tregs require expression of target antigen on epithelial tissues in order to suppress GVHD. To address this question, we created 2 types of BM chimeras by transplanting donor (male or female) BM into lethally irradiated syngeneic recipients (female or male), so that the HY antigen was only expressed either on hematopoietic cells (M → F chimeras) or on epithelial tissues (F → M chimeras). We then transplanted TCD-BM plus Teffs from B6 donors with or without additional HY-specific iTregs into these lethally irradiated chimeric recipients. In B6 → BALB.b (miHAg-mismatched) and B6 → BDF1 (haplo-mismatched) BMT models, we found that HY-specific iTregs were highly capable in preventing GVHD, and the efficacy was comparable in either type of chimeric recipients (Fig. 7A,B), indicating target antigen expressed on either compartment is sufficient for iTregs to exert their suppression in GVHD.
Fig. 7. Effect of HY-antigen distribution on HY-specific iTreg-medicated protection.
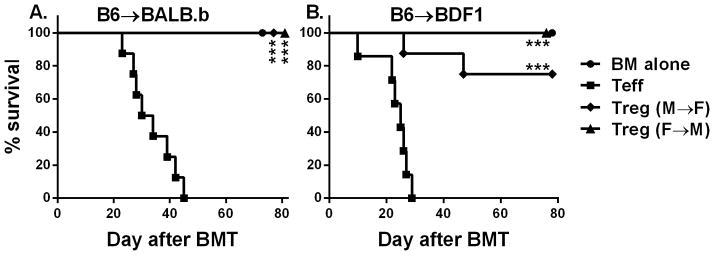
(A) Male → female or female → male BM chimeras were generated using BALB.b mice as described in “Material and Method”. These BM chimeras were lethally irradiated again and divided into 2 cohorts, each of which were transferred with TCD-BM alone or plus 25 x 106/mouse total splenocytes from normal B6 donors. HY-specific iTregs were also included at 4 x 106/mouse into donor graft at the day of BMT for some recipients. Recipient survival is shown, and the data represent 5–8 mice in each group. (B) Male → female or female → male BM chimeras were generated using BDF1 mice as described in “Material and Method”. These BM chimeras were lethally irradiated again and divided into 2 cohorts, each of which were transferred with TCD-BM alone or plus 4 x 106/mouse CD25-depleted total T cells from normal B6 donors. HY-specific iTregs were also included at 2 x 106/mouse into donor graft at the day of BMT for some recipients. Recipient survival is shown, and the data represent 7–8 mice in each group. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001
HY-specific iTregs essentially preserve the GVL effect
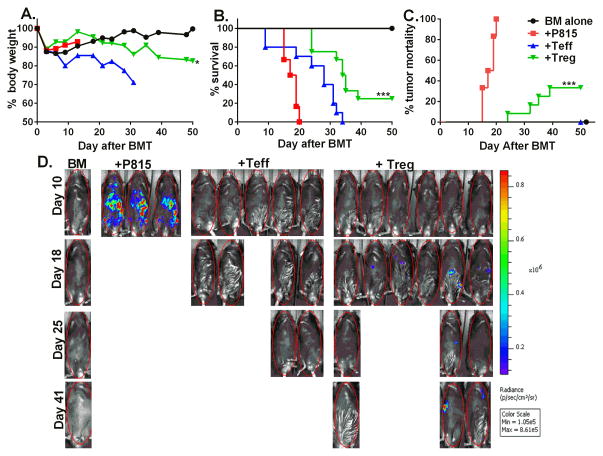
To determine the effect of HY- specific iTregs on the GVL activity, we utilized the clinically relevant B6→BDF1 (haplo-mismatched) BMT model with the injection of p815-luc+ mastocytoma cell line. One day after lethal irradiation, we injected TCD-BM from B6 donors and HY-specific iTregs into male recipients, three days later we then injected B6 Teff cells and p815-luc+ cells. We observed mice receiving BM + p815 alone all succumbed to tumor mortality within 20 days post BMT, as seen by high BLI signal with little weight loss, however mice received an addition of Teff cells died from GVHD indicated by decreased weight loss with little to no BLI signal (Fig. 8). The addition of HY-specific iTregs significantly increased survival (p<0.001) and significantly delayed tumor mortality (p<0.001) as seen by maintained body weight and low BLI signal (Fig. 8).
Fig 8. HY-Specific iTregs spare the GVL effect.
B6D2F1 male recipient mice were lethally irradiated and injected with B6 BM with or without HY-specific iTregs, three days later CD25 depleted Teffs plus 5000 p815-luc mastocytoma cells were injected. Mice were monitored for body weight loss (A), survival (B), and tumor morality (C) using the IVIS 200 imager throughout the course of study. The data depicted in A and B is pooled from 2 replicate experiments, but the imaging shown (D) is from one of these 2 replicate experiments. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001
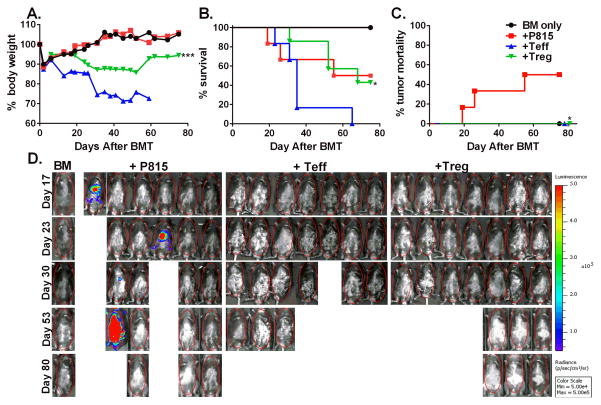
In order to better mimic clinical circumstance where patients have already established tumor, we generated a pre-established tumor model by injecting p815-luc+ cells to the recipients 3 days prior to irradiation and 7 days prior to Teff infusion. One day after irradiation, male recipients were transplanted with BM plus HY-specific iTregs and three days later Teffs were infused. As shown in figure 9, 50% of the recipients of BM plus p815 tumor died within 50 days of BMT without body weight loss and strong BLI signal indicating tumor relapse (Fig. 9, A–C). The recipients of BM plus Teffs also died within 62 days with body weight loss and no detectable BLI signal, indicating GVHD mortality. HY- specific iTreg infusion significantly attenuated GVHD (p<0.05), reflected by higher percentage of survival and no tumor relapse reflected by no BLI signal (Fig. 9A–C, p < 0.05). Taken together, these data indicate the HY- specific iTregs largely preserved the GVL activity mediated by Teffs.
Fig 9. HY-specific iTregs largely preserve GVL effect in pre-established tumor model.
Male BDF1 mice injected with 5000 P815-luc cells three days prior to irradiation. Tumor bearing mice were lethally irradiated and transplanted with 5 x 106 BM and HY-specific iTregs 2 x 106/mouse from B6 donors. Three days later, the recipient mice received 4 x 106 CD25-depleted total T cells. Recipient body weight (A) and survival (B) are shown. Data are from one experiment of two replicates with similar but not the same settings. Mice were imaged once weekly and then once every two-three weeks to monitor tumor growth (C and D) using an IVIS 200 imager. Asterisk indicates statistical significance: *p<0.05, **p<0.01, ***p<0.001
Discussion
Aiming to increase the potency and selectivity of Treg therapy, by using TGFβ-induced Ag-specific iTregs, our previous studies have demonstrated that Ag-specific iTregs, once activated in the recipient, are significantly more effective than expanded polyclonal nTregs in the prevention of GVHD (4, 5). The current study substantially extended our previous work by generating and testing iTregs specific for naturally processed alloantigens. Given the knowledge that female to male transplants occur frequently in the clinic and these patients are at a greater risk of developing GVHD due to miHAg mismatched antigens, like HY, we strove to provide clinical relevance by generating HY-specific iTregs. We found that monoclonal iTregs specific to HY miHAgs were highly effective in preventing GVHD in activation-dependent manner. Furthermore, we observed that HY-specific iTregs largely preserve the GVL effect (Fig. 8 and 9). Given p815 used in our study is a mastocytoma cell line originally derived from male DBA2 mice (24), therefore is susceptible to antigen-specific T cell- rather than NK cell- mediated killing. Our results indicate that miHAg-specific iTregs still permit the GVL activity against the tumor that expresses such a miHAg. This observation is important and clinically relevant, because many miHAgs, such as HY, are ubiquitously expressed.
Unlike freshly isolated nTregs, iTregs are generated from naïve CD4 T cells and thus the number of iTregs is essentially unlimited (Fig. 1). In current nTreg cell expansion protocols for clinical application, long culture period and multiple rounds of expansion are required to reach an optimal number of cells (25, 26) still with potential loss of Foxp3 expression (27). Given iTregs rapid expansion potential (28), this will decrease culture times and in turn resolve the fear of Foxp3 loss in vitro, however there is still concern regarding iTregs stability in vivo. Given our results showing iTregs remain highly stable even under extreme inflammatory conditions (Fig.6), this current work gives strong rationale to move iTreg therapy into the clinic. A potential concern was raised by some studies showing that in vitro generated iTregs were less suppressive than nTregs (29, 30) and failed to prevent GVHD (31, 32). On the other hand, there is also substantial evidence in the literature supporting that iTregs were as or more effective than nTregs in suppressing immune responses in vivo (1, 16, 33–39). Consistent with our previous studies using OVA-specific iTregs4,5, the current work demonstrated that HY-specific iTregs were highly effective in preventing GVHD in clinically relevant murine models of allogeneic BMT in an Ag-dependent manner (Fig. 2 and 3).
The stability and efficacy of iTregs still appears to be controversial with regards to controlling GVHD. However, our results are also supported by the reports from Steinman’s group (40), who demonstrated that iTregs generated with allogeneic DCs in the presence of TGF-β and RA maintained Foxp3 expression and exhibited higher levels of CNS2 demethylation in the Foxp3 gene, a marker for stability. Stability of iTregs generated by us and others may be partially attributed to the presence of RA, which was shown to promote iTregs through increasing histone methylation and acetylation within the promoter and conserved non-coding DNA sequence elements at the Foxp3 gene (41)(38). More strikingly, we interpret that the efficacy of iTregs in the attenuation of GVHD is directly related to TCR-driven activation and expansion in vivo (Fig. 4 and 6). The iTregs used in our studies were ~ 100% Ag-specific and able to expand when recognizing cognate Ag, whereas the iTregs used in studies by others were polyclonal and only a small fraction (e.g. < 1%) of them were able to expand when recognizing alloAgs. Our data clearly shows that iTregs not activated by cognate Ag were unable to expand and were ineffective in the prevention of GVHD. Along this line, Sela et al showed that DC-induced, alloAg-specific iTregs are capable of preventing GVHD (36). However, the therapeutic efficacy of their iTregs was lower than HY-specific iTregs used in our current study but higher than polyclonal iTregs used in other studies (27–29), indicating that the efficacy directly correlates with the frequency of alloreactive cells among different types of iTregs. Taken together, these data provide direct evidence that TCR-driven activation and expansion of iTregs after infusion is essential for their therapeutic efficacy in the control of GVHD.
Since it is commonly accepted that GVHD development requires that donor T cells recognize alloantigens expressed on epithelial tissues, we hypothesized that Tregs must also recognize antigens expressed on epithelia in order to prevent GVHD. A recent study by Tawara et al. proposed that the hosts APCs are necessary and sufficient for GVHD protection by donor Tregs (42). By creating BM chimeras as recipients in which the alloantigens to be recognized by Tregs are expressed on either hematopoietic cells or parenchymal tissues, we observed that HY-specific iTregs were highly capable in preventing GVHD in either type of chimeric recipients (Fig. 7). These results indicate target antigen expressed on either compartment is sufficient for iTregs to exert their suppression in GVHD.
The current work using TCR transgenic T cells clearly provides the evidence that miHAg-specific iTregs were effective in the prevention of acute GVHD. To translate the finding into clinical application, one could generate Ag-specific human iTregs by transducing TCR-gene into CD4 T cells and then induce them into iTregs in vitro. Alternatively and also more practically, miHAg-reactive iTregs could be generated from polyclonal CD4 T cells. In fact, we were able to generate HY-reactive polyclonal iTregs by 2-rounds of stimulation of CD4 T cells with dendritic cells from normal female B6 mice in the presence of HY-peptide (Fig. S4A). These iTregs enriched for HY-specificity exhibited significantly higher efficiency in suppressing B6 CD4 T cells in response to APCs from BDF1 male mice as compared to polyclonal iTregs generated after anti-CD3 stimulation (Fig. S4B). Furthermore, we recently have shown that human nTregs specific for HY miHAg (43) can be extensively expanded ex vivo, which demonstrates the feasibility to acquire sufficient human HY-specific iTregs for clinic trials. In conclusion, the current pre-clinical study provides strong rationale to apply human miHAg-specific iTregs in the clinic for the prevention of GVHD in patients after allogeneic HCT.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants NIH R01 AI080285, CA118116, CA143812 and CA169116 to X.-Z.Y., and CA132197 and HL114994 to C.A.
We thank Dr. Tomomi Toubai for critical reading this manuscript. We wish to acknowledge the technical assistance provided by Jodi Kroeger and Kate Shapland in the Flow Cytometry Core at Moffitt and by Dr. Adam Soloff in Flow Cytometry Core at MUSC. We also appreciate the technical support provided by the Department Lab Animal Research (DLAR), Pathology and Imaging Core at MUSC.
Abbreviations used in this article
- GVHD
graft-versus-host disease
- nTregs
naturally derived regulatory T cells
- iTregs
inducible T regulatory cells
- GVL
graft-versus-leukemia
- BMT
bone marrow transplantation
- miHAg
minor histocompatibility antigen
- RA
retinoic acid
- Teffs
Effector T cells
Footnotes
Disclosures
The authors have no conflict of interest to disclose.
References
- 1.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 2.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. The Journal of experimental medicine. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Podojil JR, Chang J, Luo X, Miller SD. TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. Journal of immunology. 2010;184:6629–6636. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert MH, Liu Y, Anasetti C, Yu XZ. Antigen-dependent suppression of alloresponses by Foxp3-induced regulatory T cells in transplantation. European journal of immunology. 2005;35:2598–2607. doi: 10.1002/eji.200526077. [DOI] [PubMed] [Google Scholar]
- 5.Semple K, Yu Y, Wang D, Anasetti C, Yu XZ. Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:309–318. doi: 10.1016/j.bbmt.2010.12.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347–352. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 7.Zorn E, Miklos DB, Floyd BH, Mattes-Ritz A, Guo L, Soiffer RJ, Antin JH, Ritz J. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133–1142. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogt MH, van den Muijsenberg JW, Goulmy E, Spierings E, Kluck P, Kester MG, van Soest RA, Drijfhout JW, Willemze R, Falkenburg JH. The DBY gene codes for an HLA-DQ5-restricted human male-specific minor histocompatibility antigen involved in graft-versus-host disease. Blood. 2002;99:3027–3032. doi: 10.1182/blood.v99.8.3027. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Meadows LR, den Haan JM, Sherman NE, Chen Y, Blokland E, Shabanowitz J, Agulnik AI, Hendrickson RC, Bishop CE, et al. Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995;269:1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 10.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, Ho V, Soiffer RJ, Antin JH, Ritz J. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 13.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nature reviews. Immunology. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. Journal of immunology. 2008;181:2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Wang D, Liu C, Kaosaard K, Semple K, Anasetti C, Yu XZ. Prevention of GVHD while sparing GVL by targeting Th1 and Th17 transcription factor T-bet and ROR{gamma}t in mice. Blood. 2011;118:10. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 18.Bettini ML, Pan F, Bettini M, Finkelstein D, Rehg JE, Floess S, Bell BD, Ziegler SF, Huehn J, Pardoll DM, Vignali DA. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity. 2012;36:717–730. doi: 10.1016/j.immuni.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darce J, Rudra D, Li L, Nishio J, Cipolletta D, Rudensky AY, Mathis D, Benoist C. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36:731–741. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 21.Bailey DW, Mobraaten LE. Estimates of the number of loci contributing to the histoincompatibility between C57BL-6 and BALB-c strains of mice. Transplantation. 1969;7:394–400. doi: 10.1097/00007890-196905000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asakura S, Hashimoto D, Takashima S, Sugiyama H, Maeda Y, Akashi K, Tanimoto M, Teshima T. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. The Journal of clinical investigation. 2010;120:2370–2378. doi: 10.1172/JCI39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colmenero P, Liljestrom P, Jondal M. Induction of P815 tumor immunity by recombinant Semliki Forest virus expressing the P1A gene. Gene therapy. 1999;6:1728–1733. doi: 10.1038/sj.gt.3301004. [DOI] [PubMed] [Google Scholar]
- 25.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Science translational medicine. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty R, Mahendravada A, Perna SK, Rooney CM, Heslop HE, Vera JF, Savoldo B, Dotti G. Robust and cost effective expansion of human regulatory T cells highly functional in a xenograft model of graft-versus-host disease. Haematologica. 2013;98:533–537. doi: 10.3324/haematol.2012.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. European journal of immunology. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 28.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS biology. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beres A, Komorowski R, Mihara M, Drobyski WR. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:3969–3983. doi: 10.1158/1078-0432.CCR-10-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Forster R, Prinz I. Alloantigen-specific de novo-induced Foxp3(+) Treg revert in vivo and do not protect from experimental GVHD. European journal of immunology. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 33.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, Steinman RM, Suthanthiran M. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;179:11–1390. [PubMed] [Google Scholar]
- 36.Zang W, Lin M, Kalache S, Zhang N, Kruger B, Waaga-Gasser AM, Grimm M, Hancock W, Heeger P, Schroppel B, Murphy B. Inhibition of the alloimmune response through the generation of regulatory T cells by a MHC class II-derived peptide. J Immunol. 2008;181:7499–7506. doi: 10.4049/jimmunol.181.11.7499. [DOI] [PubMed] [Google Scholar]
- 37.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 38.Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, Golshayan D, Muehlbacher F, Wekerle T. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant. 10:751–762. doi: 10.1111/j.1600-6143.2010.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong N, Lan Q, Chen M, Zheng T, Su W, Wang J, Yang Z, Park R, Dagliyan G, Conti PS, Brand D, Liu Z, Zou H, Stohl W, Zheng SG. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Annals of the rheumatic diseases. 2012;71:1567–1572. doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. The Journal of experimental medicine. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W, Quesniaux V, Ryffel B, Brand D, Li B, Liu Z, Zheng SG. All-trans retinoic acid promotes TGF-beta-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PloS one. 2011;6:e24590. doi: 10.1371/journal.pone.0024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tawara I, Shlomchik WD, Jones A, Zou W, Nieves E, Liu C, Toubai T, Duran-Struuck R, Sun Y, Clouthier SG, Evers R, Lowler KP, Levy RB, Reddy P. A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease. Journal of immunology. 2010;185:3866–3872. doi: 10.4049/jimmunol.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veerapathran A, Pidala J, Beato F, Betts B, Kim J, Turner JG, Hellerstein MK, Yu XZ, Janssen W, Anasetti C. Human regulatory T cells against minor histocompatibility antigens: ex vivo expansion for prevention of graft-versus-host disease. Blood. 2013;122:2251–2261. doi: 10.1182/blood-2013-03-492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.