Abstract
Biomicrofluidics is an emerging field at the cross roads of microfluidics and life sciences which requires intensive research efforts in terms of introducing appropriate designs, production techniques, and analysis. The ultimate goal is to deliver innovative and cost-effective microfluidic devices to biotech, biomedical, and pharmaceutical industries. Therefore, creating an in-depth understanding of the transport phenomena of cells and biomolecules becomes vital and concurrently poses significant challenges. The present article outlines the recent advancements in diffusion phenomena of cells and biomolecules by highlighting transport principles from an engineering perspective, cell responses in microfluidic devices with emphases on diffusion- and flow-based microfluidic gradient platforms, macroscopic and microscopic approaches for investigating the diffusion phenomena of biomolecules, microfluidic platforms for the delivery of these molecules, as well as the state of the art in biological applications of mammalian cell responses and diffusion of biomolecules.
I. INTRODUCTION
Microsystems emerged in the 1980s and microfluidics appeared at the same time1 dealing with the science and engineering of devices, which are able to handle fluid volumes in the nano- and sub-nanoliter ranges, make explicit use of the effects typical for fluids interacting with microstructures and enhance surface-to-volume ratios with respect to macrosystems. Specific issues resulting from the importance of “surface-related” phenomena which govern microfluidic systems are laminar flow, surface tension, fast thermal response times, and electrokinetics. The construction and design of these devices with minimum dimensions typically on the 100-μm scale significantly differs from conventional hardware. Hence, it is not generally possible to scale down macrodevices and expect them to work in microfluidic applications.2 The main advantage of microfluidics is utilizing scaling laws for new effects and better performance,3 which results in entirely new ways of acquiring chemical, biological, and physical information. Consequently, processes are more rapid than macroscopic systems and concurrently require much lower amounts of reagents and solvents.4
Microfluidic devices have found diverse applications in chemical, biochemical conversions, synthesis, particle formation, encapsulation, emulsification, protein crystallization, high-throughput screening, and point-of-care analysis. New application fields are expected to emerge due to the unique flow characteristics, reduced sizes and diffusion paths through microfabrication, enhanced heat and mass transfer rates, reduced reaction volumes resulting in lower amounts of reagents and chemicals, controlled sealed systems avoiding contamination,2 possibility for continuous synthesis and operations under high pressure combining the superior properties of supercritical fluids and microfluidics.
II. PHYSICAL CHARACTERISTICS OF MICROFLUIDIC DEVICES
A. Size
Microfluidic devices incorporate structures having internal diameters in the range of 10–500 μm for the transport of gases or liquids. Microscale duts such as channels and slots, pores, and also larger geometries that cause fluids to flow can be incorporated to these platforms.5 High heat transfer performance can be achieved with heat transfer coefficients up to 25 000 W/(m2.K) due to the small diameters of these structures and high specific surface area in the order of 10 000–50 000 m2/m3.6,7 Moreover, the small size and the high rates intensify the reactions, shorten the residence time, and consequently favor having higher throughputs.8
B. Geometries and material
Microfluidic devices are fabricated from a range of materials including glass, metals, ceramics, elastomers, silicon, quartz, and fluoropolymers applying techniques such as photolithography, laser ablation, micromachining, dry and wet chemical etching, deep reactive ion etching, molding, embossing, casting, and milling. The most commonly used material is glass since it is chemically inert and transparent which allows the visual inspection of the channels.9
In microfluidic culture system, the materials comprising the microfluidic channels and the materials where the cells attach must be non-cytotoxic. Poly-dimethylsiloxane (PDMS) is the most popular material for cell culture studies due to its desirable properties such as being autoclavable, gas permeable, and non-toxic to cells. Thin PDMS membranes having thicknesses around 100 μm can be used as gas exchange surfaces in microfluidic culture systems to support cell culture.10 In addition, it has excellent optical properties, including low autofluorescence and optical transparency for imaging applications. In terms of technical aspects, ease of molding makes it suitable for rapid prototyping of microfluidic designs and it is quite cheap.11
Geometries may range from simple tubular structures, where perhaps two reagents are introduced to form a product to more sophisticated multicomponent circuits based on the objectives of application.5 The flexibility in geometry enables the generation of stable concentration gradients. This is particularly useful for controlling the cell microenvironment in chemotaxis experiments where spatial and temporal concentration gradients can induce cell responses such as migration.11 Simple geometries are preferred when one or two reagents are fed in to form a product. If a number of different functionalities are needed such as mixing of reagents, incubation, reaction and separation, then more complex geometries are required. Furthermore, cell culture chambers are also designed to simulate the mass transfer properties of the in vivo tissue environment.10
C. Numbering-up
The numbering-up approach can be referred to achieve an increase in the throughput of microchips which in most of the cases ensures that the desired properties of a basic unit are kept while the size of the total system is increased. The increase in the number of units leads to a higher flexibility in terms of adoption of the production rate to varying demands. It is the strength of the approach that certain number of units can be switched off while further units can be simply added to the whole platform as well. This flexibility may be supported by a considerably broader range of operating conditions of a microchip compared with a macroscopic system.12
It is crucial to culture cells up to larger cell numbers and tissue sizes for obtaining physiological functions and capacity as a specific tissue. In order to utilize microfluidic devices for such kind of larger scale cultures, a scalable method is required to ensure the comfortable conditions for the cells. Numbering-up approach is the major strategy to scale up the reaction volume in the field of microfluidic devices.13
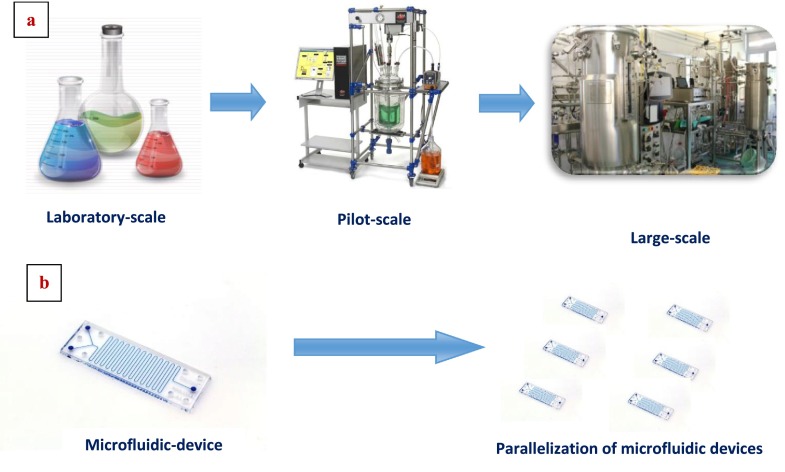
The parallel organization of microchannels is called internal numbering-up which is the most frequent way to enhance the throughput of a device. The parallelization of microdevices is called external numbering-up applied to bypass the flow distribution problems within the equipment. Overall, the numbering-up approach facilitates the scale-up process.14 (Figure 1) One of the biggest advantages of this approach in comparison to scale-up is the uninterrupted continuous processing even in the sense that if one of the units fails, it can easily be replaced without affecting the whole process. In regards to engineering efforts for the development of the setup and testing, much less time will be required compared to the efforts devoted to the development of traditional technologies. As long as the process is deployed to a single chip, the capacity can be increased by combining the same units.15
FIG. 1.
Scale up versus numbering-up approach in microfluidic devices. (a) Scale up concept: complex and expensive. (b) Numbering-up concept: simple and inexpensive.
III. TRANSPORT PRINCIPLES
When using microfluidics for cell culture, keeping shear stresses below a level detrimental for cells is so important. In this regard, it may be necessary to know the shear stress caused by different flow rates.16 The local shear stress in a microfluidic device is a function of the device geometry, flow rate, and fluid properties. Both the maximum shear stress and the shear stress gradient can significantly affect the cell viability. Therefore, the shear field and the geometry of surfaces with which target cells interact must be considered.17
Understanding flow characteristics (laminar or turbulent) and interfacial phenomena at micro scale is of prime importance in order to design and optimize microfluidics devices for bio-based applications. In this section, transport phenomena principles were examined from an engineering perspective.
A. Fluid flow
The flow phenomena can be delineated by dimensionless numbers demonstrating the interplay between inertial and viscous forces. The Reynolds Number (Re) and the Peclet Number (Pe) are the most commonly used dimensionless numbers where the former dictates the flow regime being laminar or turbulent and is defined as the ratio of inertial to viscous force densities which can be determined as
where ρ is the density, is the velocity of the fluid, Dh is the hydraulic diameter of the channel, and μ is the viscosity of the fluid. Mixing in microchannel is hindered due to the low Reynolds number in the microfluidic domain. Mixing by diffusion is often the sole possible mechanism in microchannels in comparison to the macro scale flows where mixing can be traced to the chaotic eddies born out of the turbulence in most of the cases.
The dimensionless number that represents the relative strength of convection over diffusion is called Peclet number (Pe) which is expressed as
where is the width of the microchannel and D is the diffusion co-efficient of the solute particles.18
The transport phenomenon in monophasic fluid flow in microchips is characterized by diffusion. Fick's law of diffusion states that
where n is the particle density or concentration, D is the diffusion coefficient, and is the Laplace operator. The diffusion time t is defined as the time taken by a molecule to travel distance x by diffusive processes
This means that for reactions limited by diffusion, reaction time is proportional to the square of the rate-limiting distance. Therefore, a reaction in a 10 cm diameter flask could take 1 000 000 times less if undertaken in a 100 μm diameter microreactor. Dramatically reduced reaction times have, arguably, been the most potent driving force behind research in microchip technology.5
B. Mixing mechanisms
The mixing process can be based on two principles, namely, diffusion and convection. Diffusion between short distances, establishing high concentration gradients, was initially the most frequently applied principle by simply making the channels smaller. Convection was an alternative considering robustness and costs associated with complex microfabrication of smaller channels. As convection can enlarge mixing interfaces, it is effective for mixing and can be achieved by ultrasound, electrokinetic, and acoustic means apart from micromixers. On the other hand, turbulent mixing can be utilized at high Reynolds numbers as well. However, this may not be practical, as it implies the achievement of unrealistically high flow velocities. Special equipments known to use turbulence rely either on free-guided flows or guide liquid flow through meso-scale channels.12 In addition to these, laminar flow may be exploited under such circumstances that the flow streams moving in parallel may contain reagents that are caused to interact by controlling the flow rate and variations in the microchip geometry. A range of passive and active techniques are reported where complex geometries are introduced within microfluidic manifolds to cause repeated fluid twisting and flattening, acoustic streaming, resonant response, and acoustic cavitation microstreaming. The limitation of the passive techniques such as split and recombine is the requirement that fluids must usually be in a state of flow, whereas active methodologies enable mixing where there is no flow, such as in microwell reactors and under temporary stopped-flow conditions in microchannel reactors.5
C. Surface interactions
Surface effects are of prime importance in microfluidic applications as the surface area to volume ratio is the inverse of the device dimension. A very good example for this is the generation of controlled monodisperse microdroplet between two immiscible fluids. A stream of liquid can easily be confined, controlled or strategically driven by the static or dynamic manipulation of solid-liquid or liquid-liquid surface energies as the effective surface force is significantly pronounced over other competing effects.17 Indeed, there are a number of novel devices designed relying on the dominant consequences imparted by the surface tension force. Moreover, surface energies have been exploited by creating virtual walls as well as pumping mechanisms.18
D. Molecular transport
From a holistic perspective, two major forms of molecular transport exist, one of which is diffusion based and the other, convective transport.
Diffusion is a statistical transport phenomenon which occurs in the presence of a concentration gradient of a solute within a fluid. The statistical movement of a single molecule in a fluid can be described as random. The movement is characterized by Einstein-Smoluchowski relation:
where x is the average distance moved after an elapsed time t between molecule collisions and D is a diffusion constant that is characteristic for the given molecule.19 Diffusion can be described by Fick's second law
where C is the chemical concentration and D is the diffusion coefficient of the molecule of interest. The solution of the concentration field using diffusion equation and the boundary conditions can be obtained using Comsol Multiphysics Analysis software. Diffusion coefficient D can be estimated using Einstein-Stokes equation
where kB is Boltzmann's constant, T—the temperature, —the solvent viscosity, and rH—the hydrodynamic radius of the molecule for diffusion of spherical particles through liquid.20
In the presence of fluid flows, molecular transport process can be described by
where U is the fluid flow velocity, whereas the second term on the right side represents the convective transport.21
IV. CELL RESPONSES IN MICROFLUIDIC DEVICES
A. Mammalian cell culture
Culturing mammalian cells in microfluidic devices is a basic tool for applications such as toxicological studies, drug discovery studies, and cell and tissue engineering efforts. Recent reports have shown that many novel microfluidic systems, including cell culture on a chip, are worthy of scientific and industrial attention.22 As microfluidics allow the delivery of various stimuli with the spatial precision comparable to sub-cellular dimensions, cellular problems can be tackled even at single cell level. The cells can be exposed to the gradient streams of stimuli such as chemo-attractants, hormones, cytokines, and growth factors within microfluidic devices where the responses can be monitored and measured. Macro scale cell culture systems have limitations in terms of accurate quantification of the cell-cell, cell-biomolecule, and cell-extracellular matrix (ECM) interactions which have encouraged the development of microfluidic-based cell culture systems.17 Among the multitude of approaches for manufacturing microfluidic devices, soft lithography of PDMS has become widely applicable for cell culture studies. Micromechanical membrane valves made from PDMS are used to manipulate fluids at the microliter scale which allow precise spatial and temporal control of the flow and delivery of compounds of interest to cells.23 Parallelized fabrication using optical lithography and careful alignment of flow and control layers allow rapid construction of various types of channels, chambers and valves in fully integrated, compact devices (Figure 2).
FIG. 2.

Some examples of commercially available microfluidic cell culture devices fabricated by soft lithography using PDMS: (a) μ-slide chemotaxis for chemotaxis assays in 2D and 3D applications, (b) μ-slide 8 well for cell culture and immunofluorescence staining, (c) μ-slide I for flow applications, and (d) μ-slide III for flow assays and immunofluorescence. Reproduced with permission from Desen Mikrotek. Copyright 2015 Desen Mikrotek.94
Microfluidic technology can be used to supply and transfer media, buffers, and even air while the waste products by cellular activities are drained in a way resembling the human circulatory system. In addition, many studies have focused on analytical microsystems that are integrated into a microfluidic platform that carries out sample mixing, buffer exchange, as well as cell seeding, transferring and separation in a microchannel. Therefore, microfluidic systems can provide an in vivo-like environment for a cell culture as well as a reaction environment for a cell-based assay.22 The advantages of microfluidic cell culture include the ability to more closely mimic a cell's natural microenvironment, for example, by continuous perfusion culture or by creating chemical gradients, and to study low numbers of cells or single cells in high temporal and/or spatial resolution via automation, parallelization, on-chip analysis or direct coupling to downstream analytical chemistry platforms. At the same time, microfluidic cell culture offers reduced consumption of reagents, reduced contamination risk, and efficient high throughput experimentation.24,25
When compared with conventional techniques, microfluidic cell culture (Figure 3) allows controlling fluid flow in the micrometer and nanoliter scale in precisely defined geometries and facilitates simultaneous manipulation and analysis starting from a single cell level, to larger cell populations and up to tissues cultured on fully integrated, automated chips due to the flexibility in the design which allows tailoring the needs of individual cell types and cellular co- cultures implemented on the same chip.26
FIG. 3.
Comparison of cell culture systems from micro to macro scale.
On the contrary of advantages, there are some challenges and limitations in microfluidic cell culture as well. Non-standard culture protocols, novel culture surfaces, complex operational controls, and chip designs are the main challenges to be tackled.24
B. Diffusion-based microfluidic gradient platforms
In a microfluidic device, pure diffusion through the membrane or hydrogel can be utilized for generating diffusion-based gradients. This technique has some merits, such as shear stress is negligible, secreted molecule is maintained, gradients can be generated in a 3-D scaffold, and fluid flows do not come in direct contact with the cells.26 On the other hand, the diffusion-based microfluidic devices are not capable of generating rapid and dynamic gradient profiles.27 From an engineering perspective, it is worth to mention that diffusion-based microfluidic gradient platforms are characterized with Pe < 1.
C. Flow-based microfluidic gradient platforms
Flow-based microfluidic platforms enable the control of concentration gradients by utilizing diffusive mixing of parallel flowing streams of different concentrations to instantly generate a chemical gradient in the direction perpendicular to the flow.28 These devices are consisted of microchannels and cell culture area where two or three solutions are mixed and splitted in a microchannel, resulting in generating stable concentration gradients in perpendicular to the flow direction.27 To enable general use of flow-based gradient generators, the ideal platform should be easy to use and be portable.29,30 This device has been widely used to create molecular gradients in the bulk fluid of the cell chamber or on the surface of a substrate. Establishment of short gradient time can be regarded as a strength, whereas the unwanted fluid flows in the cell chamber cause problems such as the shear stress exerting on the cells which in turn alters cellular behaviors, possible wash out of the signal molecules, and remodeling of the three dimensional matrix.20
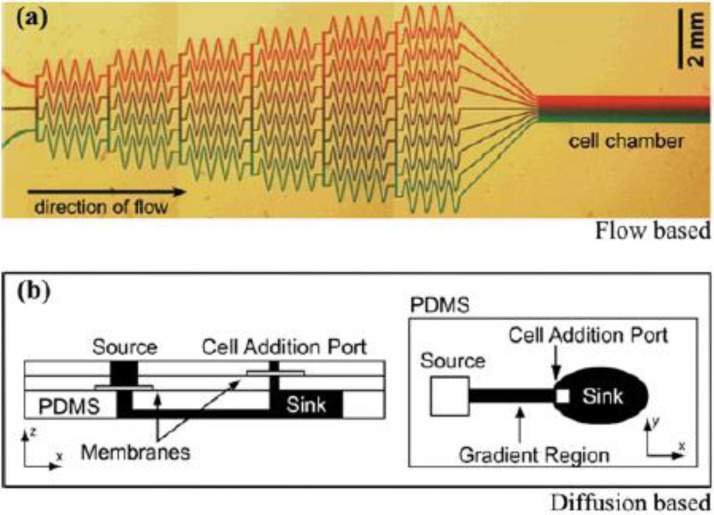
Both the flow-based and diffusion-based microfluidic platforms are used to generate concentration gradients in a tempo-spatial manner and utilized in various biological applications.27 The comparison of a flow based and a diffusion based microfluidic devices are depicted in Figure 4. In the schematic representation for flow-based microfluidic platform, fluid flows with different chemical concentrations are introduced to the inlets of the device. These flows merge and mix through a series of channels and form a gradient when exiting to the cell chamber, where a direct contact of the flow and the cells is maintained (Figure 4(a)). For diffusion based platform, chemicals and buffers are pipetted to the source and sink, or the inlet and outlet of the channel, whereas the cells are added through the cell addition port avoiding direct contact with the flow by introducing a membrane to the microchip. Subsequently, a chemical gradient is generated through diffusion along the center channel (Figure 4(b)).
FIG. 4.
A convective flow based microfluidic device made of PDMS (a), a diffusion based microfluidic device (b). While flow-based microfluidic platforms provide concentration gradient by utilizing diffusive mixing in microchannels, diffusion-based microfluidic platforms provide concentration gradient by embedding chemicals into membrane or hydrogel. Reproduced with permission from B. J. Kim and M. Wu, Ann. Biomed. Eng. 40, 1316–1327 (2012).20 Copyright 2012 Springer.
D. State of the art in biological applications of mammalian cell responses
The concentration gradient of biomolecules such as growth factors, cytokines, chemokines, and hormones results in responses such as directed cell chemotaxis,31 which is a key biological process involved in a wide range of physiological and pathological phenomena. Understanding the molecular mechanisms behind such fundamental process can yield better insight into cancer metastasis, wound healing, and immune responses leading to treatment of the diseases that involve cell migration.32 The Boyden chamber has traditionally been the key instrument for mammalian cell chemotaxis,33 but it has limitations, especially the lack of real-time cell monitoring.27–34 Microfluidic devices such as valved and microarrays, gradient devices have recently emerged as physiologically relevant alternatives35,36 to investigate the phenomena in biological applications. Moreover, microfluidic devices are appropriate platforms for designing 3D in vitro models to study cell-biomolecule interactions. As the cells can be embedded in an extra cellular matrix type of a material, an improved spatial organization can be maintained while providing better functionality than the traditional 3D systems such as transwells for instance.
Considering engineering and design aspects, multiple PDMS layers, embedding a membrane or hydrogel, have been employed in order to form diffusion-based gradient platforms, whereas simple microchannel networks have been used for the flow-based counterparts. Various biological applications investigating cell migration and responses to biomolecule and chemical gradients were summarized in Table I to shed a light on current development of microfluidic-based gradient platforms.
TABLE I.
State of art in microfluidic diffusion-based gradient devices and their biological applications.
| Biological application | Cell type | Stimuli | Microfluidic platform | Reference |
|---|---|---|---|---|
| Cancer chemotaxis | Breast cancer cells (MDA-MB-231) | EGF | Microfluidic cell-motility-based sorting device, compartmented microfluidic device | 37, 39, and 40 |
| Prostate cancer cell line (PC-3) | EGF | PDMS microfluidic device | 41 and 42 | |
| Migration of smooth muscle cells | Primary smooth muscle cells (SMC) | Platelet-derived growth factor (PDGF) | PDMS microfluidic device | 43 |
| Migration of fibroblast cells | Connective tissue-derived fibroblast cells | EGF | PDMS microfluidic device | 44 |
| Stem cell differentiation | Rat adipose-derived stem cells (ASCs) | EGF | “Y” shaped microfluidic gradient device | 46 |
| Neutrophil chemotaxis | HL-60 cells (human promyelocyticleukemia cells) | Chemoattractant f-MLP | Hydrogel based microfluidic device | 45 |
| Dendritic cell chemotaxis | Bone marrow dendritic cells | mCCL21 and mCCL19 chemokines | Agarose-based microfluidic chemotaxis device | 51 |
There is a need to search for alternative chemotherapeutics of biologic origin that could be used in the treatment of various cancer types. Microfluidic devices prove to be perfect platforms to investigate the interactions between cancer cells and various stimuli. A microfluidic chamber was developed to study chemotaxis of MDA-MB-231 metastatic breast cancer cells in epidermal growth factor (EGF) gradients of well-defined profiles.37 In one of our previous studies,38 we focused on the cytotoxic activity of carnosic acid and reported 70% inhibition at 19 μM in MCF-7 cell line, whereas a 2-fold higher inhibition was observed in estrogen independent breast cancer cell, MDA-MB-231. As carnosic acid can be a potential candidate for estrogen independent aggressive cancer cells, it would have been very interesting to investigate the ability of carnosic acid as a chemoattractant. Recently, we initiated some pre-trials in order to observe the interaction between MDA-MB-231 and carnosic acid. In another study, a microfluidic cell-motility-based sorting device was designed where EGF was used to generate a concentration gradient in order to induce the migration of MDA-MB-231 cells and the migratory response of cells were reported to depend not only on the gradient profile but also on EGF concentration as well.39 However, as the concentration of EGF was increased, after a certain value the overall chemotactic response was decreased which was evident in the results of an other study as well.37 The same interaction between EGF and MDA-MB-231 was attempted by another group with a different three compartmented geometry which generated two extreme regions, a steady-state gradient and control for real time monitoring of cellular behavior.40 A PDMS microfluidic device that featured two wells connected by 10 microchannels was designed to quantitatively assess the migration of lung metastasized prostate cancer PC3-ML cells to specific concentrations of epidermal growth factor,41 and similar results were obtained where a concentration of 100 ng/ml enhanced the migration of cells whereas concentrations above this value significantly decreased the migration. All of the developed designs may find useful application in creating a better understanding in regards to cancer cell chemotaxis that require analyzing subpopulations of chemotactically heterogeneous cells.
A novel microfluidic device was fabricated in order to assess chemotaxis and chemokinesis in the same setup, and, as an application, the cellular migration of smooth muscle cells in a platelet-derived growth factor gradient was evaluated.43 Research related to the behavior of cells derived from soft connective tissues has received growing interest in order to enhance the slow rates of repair and regeneration in these tissues. Principally, many tissue engineering studies have focused on the microenvironment experienced by the cells as a strategy for developing more effective soft tissue biomaterials that can be used for surgical dressing and sutures.44 In this regard, microfluidic devices were utilized to measure the migratory responses of ligament-derived fibroblasts to a range of concentration gradients generated by attractants at different concentrations. Such studies suggest that expanded study of the chemotactic abilities of fibroblast cells within low specific gradient fields will provide a distinctive strategy for the development of more effective tissue biomaterials.
Diffusion-based gradient devices were also utilized for stem cell applications as they are one of the key building blocks for tissue regeneration due to their self-renewal ability and multi-potential to differentiate into different specialized cell types45 and hold great promise for treating various degenerative diseases and conditions. Therefore, there is a need to develop effective approaches for stem cells to migrate to the target organs and to isolate effective stem cell populations. In this regard, migration of rat adipose-derived stem cells to a well-defined gradient of EGF was carried out using a Y-shaped microfluidic device for the on-chip selection of cells with higher chemotactic ability.46 The results of this study might be deployed to enhance stem cell homing to target organs and improve the cell therapy. Microfluidic devices can be used to create controllable and steady concentration gradients for quantitative evaluation of chemotaxis on neutrophils as well47 as neutrophils migrate to specific locations to perform immune functions when sensing gradients of chemotactic factors.48 A hydrogel based microfluidic device was designed to assess chemotaxis of human promyelocytic leukemia cells (HL-60) towards chemoattractant formyl-Met-Leu-Phe (f-MLP). Obtained results showed that this device was suitable for both suspended and adherent cell chemotaxis along with providing nutrients and gases required for cell survival via diffusion through the hydrogel from the side channels.49 Dendritic cells (DCs) are considered to be the most potent antigen-presenting cells. DCs are positioned throughout the periphery and migrate to lymphatic vessels and into lymph nodes upon activation, then upregulate the C-C chemokine-receptor CCR7, which allows them to sense and home towards CCR7 ligand-secreting lymphatic vessels and lymph nodes.50 The two known ligands of CCR7, namely, CCL21 and CCL19, are secreted by stromal cells in the lymph node which is important for adaptive immunity. Therefore, it is of great interest to create an understanding on how DCs interpret gradients of these chemokines in a complex 3D microenvironment. In this regard, the agarose-based microfluidic device was developed to assess DC chemotaxis towards stable CCR7 ligand gradients in 3D matrices.51 This study provided concrete basis on CCR7-driven DC chemo invasion along with a quantitative leukocyte chemotaxis.
Flow-based microfluidic gradients are frequently used to investigate responses of cells that are subjected to flow-induced shear stress in their native microenvironments.52 Various biological applications using current development of microfluidic flow-based gradient platforms were reviewed in Table II.
TABLE II.
State of art in microfluidic flow-based gradient devices and their biological applications.
| Biological application | Cell type | Stimuli | Microfluidic platform | Reference |
|---|---|---|---|---|
| Cancer chemotaxis | Prostate cancer cells (PCC) | Chemokine gradient | A PDMS microfluidic device | 54 |
| Breast cancer cells (MDA-MB-231) | EGF | Parallel gradient microfluidic chamber | 55 and 63 | |
| Dendritic cell migration | Murine dendritic cells | Lymphoid chemokine CCL-19 | Agarose-based microfluidic platform | 56 |
| Neutrophil chemotaxis | Peripheral blood neutrophils | N-fMLP | Kit-on-a-lid-assay (KOALA microfluidic devices) | 57 |
| Endothelial cell chemotaxis | Human umbilical vein endothelial cells (HUVEC) | Vascular endothelial growth factor (VEGF) | PDMS microfluidic device | 58 |
| Stem cell differentiation | Human bone marrow derived MSCs | Platelet-derived growth factor BB (PDGF-BB) | Microfluidic gradient device combined with ALZETH osmotic pumps | 62 |
| Migration of fibroblast cells | HFF-1 fibroblasts (SCRC-1041) | EGF | PDMS microfluidic device | 63 |
Cancer metastases are associated with the migration of cancer cells guided by various environmental factors including extracellular matrix, cytokines and growth factors.27,29,53 As the metastasic cells get into the blood stream, they face shear stresses; therefore, it will be much more appropriate to investigate the behavior of these type of cells using flow-based microfluidic devices. In one of the studies, the PDMS microfluidic device was seeded with prostate cancer cells and demonstrated that different chemokine gradient profiles can be obtained by changing the flow rate or the number of inlets or both, using54 parallel gradient microfluidic chambers developed to compare the migration of the human metastatic breast cancer cell line MDA-MB-231 in different gradients of EGF,55 providing a platform to investigate the effects of drug molecules on cell migration and to evaluate their efficiency in regards to metastasis. In another publication, the chemotactic response of murine dendritic cells to a gradient of lymphoid chemokine CCL19 was studied in an agarose-based 3D microfluidic device, in which the flow control unit was separated from the cell-containing matrix by an agarose gel wall.56 Comparisons were made with PDMS stating that microfluidic device fabricated from agarose as a material is quick and easy to assemble and does not require tools such as plasma cleaner.
Kit-on-a-lid-assay (KOALA) for chemotaxis of peripheral blood neutrophils was developed57 to perform neutrophil purification from nanoliter volumes of blood in minutes which generated repeatable chemotactic gradients. This platform permits the users to probe endothelial-leukocyte interactions, thereby giving the possibility to study any disease where neutrophil adhesion and migration are crucial to the pathogenesis of the disease. Another study focused on endothelial cell polarization in a microfluidic device which was capable of producing a stable concentration gradient while minimizing fluid convection inside the cell chamber in order to study chemotaxis of shear-sensitive endothelial mammalian cells towards vascular endothelial growth factor58 which much shed a light on angiogenesis.59–61 Stem cell differentiation was studied in flow-based platforms as well. The microfluidic gradient device combined with two commercially available ALZET® osmotic pumps was utilized to assess the short- and long-term migration of human bone marrow derived mesenchymal stem cells (MSCs) toward a gradient of platelet-derived growth factor BB,62 which stands as a good example for a simple and portable platform. As for fibroblasts, the potential of sigmoidal concentration profiles for controlling the chemotactic activity of human HFF-1 fibroblasts towards EGF in PDMS microfluidic device was investigated.63 A detailed analysis of the chemotactic response of slowly migrating cells was possible by the production of the sigmoidal concentration profiles in microfluidic channels.
V. DIFFUSION OF BIOMOLECULES IN MICROFLUIDIC DEVICES
A. Brownian motion
The movement of the molecules in a gas or liquid is known as Brownian motion. A molecule moves in straight line until it collides with another molecule, and the average linear displacement between two collisions is called the mean free path. In life sciences, we deal with macromolecules and microparticles larger than the fluid molecules. The basic scheme of displacement is the same; the molecules of the carrier liquid collide with the macromolecules to make them perform a random walk. Brownian motion and the random walk theory is the basis of diffusion. If a very small volume is imaged where the diffusing particles are initially confined, these particles will be subjected to a random walk with the time and dispersed in the buffer liquid.
B. Macroscopic and microscopic approaches for diffusion phenomena
There are two main approaches to calculate the diffusion of these macromolecules and microparticles. The first one is the concentration approach based on the continuum hypothesis, and the second one is discrete methods where particles are followed individually.64
1. Macroscopic (concentration) approach
Using Fick's second law and evaluating the mass balance of a substance in an elementary volume of carrier liquid yields the diffusion equation
The term S stands for a source or sink term of concentration. For example, if there is a biochemical reaction in some part of the domain, concentration of substance may locally appear or disappear. In every subdomain where D is constant (does not depend on the spatial coordinates), the equation may be rewritten as
showing that the diffusion equation is of parabolic type with a solution that describes the concentration of a system as a function of time and position. The solution depends on the boundary conditions of the problem as well as on the parameter D. If the concentration c in diffusing particles or molecules is small—which is the most usual case—the diffusion coefficient D does not depend on c, and the equation becomes linear. It is worth to mention that the magnitude of D is 10−9 m2/s for diffusion of the molecules and typically 10−11 m2/s for colloidal substances.
2. Microscopic (discrete) approach
Microscopic approach assumes that in every elementary volume of liquid, there are a number of microparticles sufficiently important to define a concentration. This approach is very convenient because it introduces a partial differential equation that can be solved by ordinary discretization techniques, like the finite element method. Contrary to the preceding continuum approach, this approach is discrete, meaning that the displacement of every particle is calculated. However, very complex geometries of the diffusion domain may not be easily treated with such methods.
The Monte Carlo method can be used to mimic the random walk of biomolecules. Longer linear displacement steps under the condition where the biomolecules remain small compared to the free space defined by the surrounding geometry is possible without changing the randomness as the mean free path is very short. This can be discussed based on two perspectives where 2 and 3D cases are considered.
In the 2D case, a particle moves in a time step from the location (x, y) to the location (, ), where the space increments are defined by the following equations where the validity of the method depends on the quality of randomness of the angle α:
In the 3D case, a particle moves in a time step from the location (x, y, z) to the location (, , ). The random walk algorithm is the following:64
C. Microfluidic platforms for diffusion of biomolecules
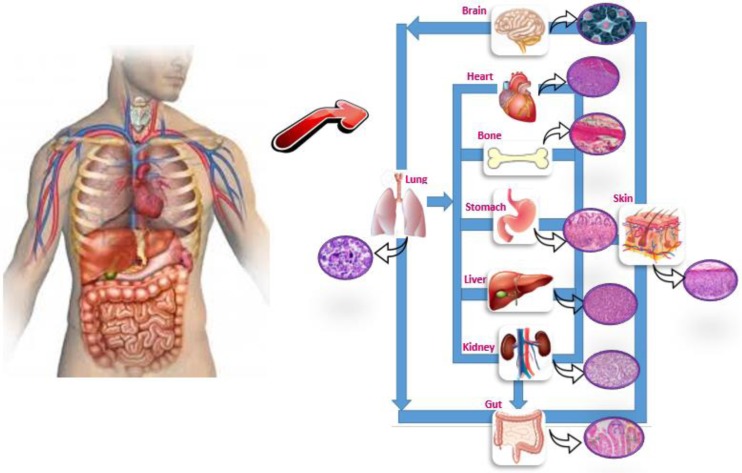
Microfluidic systems allow more accurate modelling of physiological situations for both fundamental research and drug development, and enable systematic high-volume testing for various aspects of drug discovery. Microfluidic systems not only model biological environments but also mimic biological tissues and organs physically as in the case of “organs on a chip” approach which can help to expedite early stages of drug discovery by creating disease models, reduce animal testing studies65,66 and accelerate the clinical translation.67 Just as “Lab on a single device” with the capability of chip, technologies are scaling down entire laboratories performing complex biochemical assays.68 Current research in the field of miniaturization has resulted in novel in vitro models for the capture, analysis, and biomolecule response evaluation of cancer cells which are advantageous in comparison to standard methods as the cell environment can be manipulated and controlled.69 The integration of advanced 3D tissue engineered constitutes with microfluidic channels, termed “multi-organ-chip,” provides a potential new tool for systemic substance testing. These platforms with co-cultures can maintain human originated tissues over long culture periods.70 Many organ-on-a-chip platforms such as cardiac, vascular, brain, liver, lung, kidney, intestine, spleen, gut, muscle, and tumor have been introduced to test free and encapsulated forms of biomolecules as drug carrier systems.71–74 (Figure 5). The objective of microfluidic in vitro 3D cancer models is to generate in vivo-like structures and functions in order to provide a more comprehensive understanding of complex interactions in the tumor microenvironment. These cancer models can be utilized as drug screening platforms to estimate human responses towards cancer drug.75 The continued integration of microfabrication, 3D biology, and microfluidics has led to the development of an “organ-on-a-chip.”76 An organ-on-a-chip is a 3D microfluidic cell culture device linked by a microfluidic circulatory system in a physiologically relevant manner to model a drug absorption, distribution, metabolism, and excretion, and also evaluate drug efficacy and toxicity. As shown in Figure 5, an integrated system of engineered organs representing lung, heart, kidney, liver, gut, and bone can be utilized to investigate the absorption of inhaled aerosol formulations and to measure their toxicity to heart and kidney and metabolism in the liver. Pharmaceutical compounds can also be directly introduced into the gut.77 These systems offer numerous advantages through reducing the number of in vivo experiments along with lowering costs associated with expensive tests and clinical trials. In the long term, reliable living system platforms may be able to characterize drug formulations before proceeding to the in vivo tests, which in turn may enhance the translation of these formulations to the pharmaceutical industry.78
FIG. 5.
Conceptual schematic depiction of an organ-on-a-chip platform which is a 3D microfluidic cell culture device mimicking some of the functions of biological organs and tissues.
D. State of the art in diffusion of biomolecules
The controlled fluidic microenvironments are suitable platforms for studying interesting biological applications allowing real-time screening.79 Detailed analysis of mechanisms in regards to biomolecule interactions with biological systems makes it possible to observe the correlations between in vitro and in vivo studies. There is a variety of microfluidic devices having different structures in order to investigate these interactions. One of these devices is pressure-driven perfusion culture micro-chamber designed by80 for parallel drug cytotoxicity assay. The micro-chamber constructed with independent perfusion channels provided a unique hydrodynamic platform for preventing cross contamination of neighboring chambers, permitting the removal of air-bubbles, allowing uniform cell loading and pressure-driven long-term perfusion culture for quantitative cell-based assays. A parallel cytotoxicity assay was successfully carried out to analyze the effects of seven anticancer drugs, namely, carboplatin, cisplatin, cytarabine, doxorubicin hydrochloride, etoposide, paclitaxel, and tamoxifen citrate on HeLa cells (human cervical carcinoma) and reported that the integration of the microfluidic gradient generator with the pressure-driven perfusion culture chip allowed the simultaneous delivery of multiple drug samples which can serve as an appropriate platform for high throughput drug screening. The authors used Chemical Engineering Module of COMSOL Multiphysics software package to build the Navier-Stokes finite element model. Indeed, computational fluid dynamics is a significantly efficient tool to describe microfluidic flow behaviors and transport of biomolecules and nutrients81,82 as the integration of experimental methods and computational simulations generate more cohesive data sets to study diffusion related topics. Especially, with the emergence of non-regular shaped microfluidic devices, incorporation of the initial and boundary conditions to the models becomes difficult, which in turn raises the need for advanced image processing approaches. COMSOL Multiphysics, OpenFOAM, STAR-CD, ANSYS-Fluent, and ANSYS-CFX are some commonly used packages based on both finite volume and finite element methods.83
Another design was a microfluidic device containing three parallel channels separated by narrowly spaced posts84 in order to develop a 3D in vitro tumor model using melanoma cells. Viability, density, and morphology of cells as well as transport properties of sodium fluorescein which is a cell membrane-impermeable molecule, were investigated. Developed tumor model displayed low extracellular space and high resistance to diffusion of small molecules, which might be a promising platform for quantitative assessment of transport and cellular uptake in tumor tissues. Another study dealt with a microfluidic device containing three-dimensional tumor tissue subjected to continuously flowing media to mimic microenvironment gradients surrounding blood vessels in tumors,85 where old spheroids exhibiting long-term viability by packing at 11 to 21 days were inserted. Subsequently, the tissue microenvironment reproducibly rearranged with viable cells next to the channel and apoptotic and necrotic cells deep into the chamber. The transport of doxorubicin was modelled in the microfluidic device with Fickian diffusion through a semi-infinite solid and the diffusion coefficient was quantified using time-lapse fluorescence imaging which was reported as 8.75 × 10−11 m2/s. Obtained results showed that this device was simple to construct, easy to image, and stable for long-term growth with predictable microenvironment gradients, where the diffusion and localization of any cancer therapeutics can be assessed. Yet another study was related to microfluidic array developed for cultivation of long-term multicellular spheroid HT-29 human carcinoma cells and evaluation of anticancer drug activity.86 A cytostatic drug, 5-fluorouracil was introduced into the microsystem at different frequencies and concentrations in order to observe the effects on HT-29 cells. The construction of the device enabled observation of decreasing spheroid diameter as an indicator of cell death, thereby providing a unique opportunity to evaluate the cytotoxicity of repeated drug doses. In terms of cost issues, a quantitative determination of a three-dimensional cellular model response to an applied drug based on the alterations in the tumor spheroid dimensions is very advantageous.
Diffusion studies were carried out by using 3D microfluidic devices in order to evaluate the diffusion of platelet-derived growth factor and transforming growth factor β1 across collagen and fibrin gels.87 Computer simulations based on a reaction-diffusion transport model were generated for a deeper understanding of the fundamental processes. Another study was related to the diffusional transport and precipitation of an analgesic compound, bupivacaine hydrochloride in a biodegradable microfluidic device comprised of a drug reservoir and microchannels.88 Although the residual amount of bupivacaine was not quantitatively determined in the reservoir during diffusion, a qualitative estimation was used to assess the functioning of the device. In another study, calcein AM was used as a model compound having a diffusion coefficient value of 2.6 × 10−10 m2/s to investigate the transport into cells based on hydrodynamic focusing.89 Subsequent to the diffusion of calcein AM, the enzymatic conversion into fluorescent calcein during and after the diffusion was monitored, where a higher flow rate ratio resulted in lower fluorescence intensity implying that fewer molecules were diffused into the cells.
Considering biocatalytic conversions, diffusion of substrates in microfluidic devices are of prime importance as well.90 In one of our on-going projects, hydrolyses of a number of saponin molecules to pharmacologically active aglycone counterparts are carried out in both PDMS and glass-silicon-glass microchips. Biocatalysis of ginseng RB1 was enhanced under continuous flow at 1 μl/min flow rate.91 Apart from increasing the residence times in the microfludic devices, the proximity of the biomolecules to the enzymes are critical raising the necessity of innovative designs which can reduce the substrates' diffusion time to active sites of the enyzmes as in the case of Cu(i) catalyst immobilized chip for the development of biomolecule based imaging agents.92 Packed-bed designs were reported to enhance the reaction performance as the diffusion path is reduced, but on the other hand, large pressure drop problems were experienced.93
VI. CONCLUSIONS
The development of microfluidic devices will continue to evolve for the exploration of cell-cell, cell-biomolecule, and cell-biomolecule-extracellular matrix interactions in order to develop strategies for the diagnosis and treatment of various diseases. At the forefront of this are the requirements for new transparent materials which allow in situ monitoring of diffusional processes along with innovative functionalities and design. The presented know-how can contribute to the early stages of translational medicine where organ-on-a-chip and human-on-a-chip models can be further improved to replace animal testing. Additionally, diffusion studies will be of great value in terms of investigating the response of lab animals to various biomolecules which will enhance our understanding in terms of stimuli trigger and detection mechanisms.
From a holistic perspective, the increasing trend is expected to continue and the presented state-of-the-art will pave the way for deployment of this knowledge to the industry in order to market new products demonstrating economic and societal benefits. But, it should be kept in mind that the escalating health care costs will force the companies to offer inexpensive, reliable, and easy-to-use devices.
ACKNOWLEDGMENTS
The research support by the Scientific and Technical Research Council of Turkey (TUBITAK) 113M050 project is highly appreciated.
References
- 1. Colin S., “ Microfluidics,” British Library Cataloguing-in-Publication Data ( John Wiley & Sons, Inc., 2010). [Google Scholar]
- 2. Ducrée J. and Zengerle R., “ Flow map microfluidics roadmap for the life sciences,” FlowMap consortium & EC ( Books on Demand GmbH, Norderstedt, Germany, 2004). [Google Scholar]
- 3. Nguyen N. T. and Wereley S. T., “ Fundamentals and applications of microfluidics,” British Library Cataloguing in Publication Data ( Artech House, Inc, 2002). [Google Scholar]
- 4. Pihl J., Karlsson M., and Chiu D. T., “ Microfluidic technologies in drug discovery,” Drug Discovery Today 10(20), 1377–1383 (2005). 10.1016/S1359-6446(05)03571-3 [DOI] [PubMed] [Google Scholar]
- 5. Wirth T., Microreactors in Organic Synthesis and Catalysis ( Wiley-VCH Verlag GmbH&Co. KgaA, Weinheim, 2008). [Google Scholar]
- 6. Weibel D. B. and Whitesides G. M., “ Applications of microfluidics in chemical biology,” Curr. Opin. Chem. Biol. 10, 584–591 (2006). 10.1016/j.cbpa.2006.10.016 [DOI] [PubMed] [Google Scholar]
- 7. Minsker L. K. and Renken A., “ Microstructured reactors for catalytic reactions,” Catal. Today 110, 2–14 (2005). 10.1016/j.cattod.2005.09.011 [DOI] [Google Scholar]
- 8. Nguyen N. and Wereley S. T., Fundamentals and Applications of Microfluidics ( Artech House, Norwood, 2006). [Google Scholar]
- 9. McCreedy T., “ Fabrication techniques and materials commonly used for the production of microreactors and micro total analytical systems,” TrAC, Trends Anal. Chem. 19, 396–401 (2000). 10.1016/S0165-9936(99)00176-4 [DOI] [Google Scholar]
- 10. Kim L., Toh Y. C., Voldman J., and Yu H., “ A practical guide to microfluidic perfusion culture of adherent mammalian cells,” Lab Chip 7, 681–694 (2007). 10.1039/b704602b [DOI] [PubMed] [Google Scholar]
- 11. Young E. W. K. and Beebe D. J., “ Fundamentals of microfluidic cell culture in controlled microenvironments,” Chem. Soc. Rev. 39(3), 1036–1048 (2010). 10.1039/b909900j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Némethné-Sóvágó J. and Benke M., “ Microreactors: A new concept for chemical synthesis and technological feasibility (Review),” Mater. Sci. Eng. 39(2), 89–101 (2014). [Google Scholar]
- 13. Andersson H. and v. d. Berg A., Lab-on-Chips for Cellomics, Micro and Nanotechnologies for Life Science ( Springer, The Netherlands, 2004). [Google Scholar]
- 14. Kockmann N., “ Micro process engineering: fundamentals, devices, fabrication and applications,” Advanced Micro & Nanosystems ( WILEY-VCH, Verlag GmbH&Co. KGaA, Weinheim, 2013). [Google Scholar]
- 15. Šalić A., Tušek A., and Zelić B., “ Review: Application of microreactors in medicine and biomedicine,” J. Appl. Biomed. 10, 137–153 (2012). 10.2478/v10136-012-0011-1 [DOI] [Google Scholar]
- 16. Stone S. D. and Hollins B. C., “ Modeling shear stress in microfluidic channels for cellular applications,” 29th Southern Biomedical Engineering Conference, 2013. [Google Scholar]
- 17. Beebe D. J., Mensing G. A., and Walker G. M., “ Physics and applications of microfluidics in biology,” Ann. Rev. Biomed. Eng. 4, 261–286 (2002). 10.1146/annurev.bioeng.4.112601.125916 [DOI] [PubMed] [Google Scholar]
- 18. Das T. and Chakraborty S., “ Biomicrofluidics: Recent trends and future challenges,” Sadhana 34(4), 573–590 (2009). 10.1007/s12046-009-0035-8 [DOI] [Google Scholar]
- 19. Ong S. E., Zhang S., Du H., and Fu Y., “ Fundamental principles and applications of microfluidic systems,” Front. Biosci. 13, 2757–2773 (2008). 10.2741/2883 [DOI] [PubMed] [Google Scholar]
- 20. Kim B. J. and Wu M., “ Microfluidics for mammalian cell chemotaxis,” Ann. Biomed. Eng. 40(6), 1316–1327 (2012). 10.1007/s10439-011-0489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith J. P., Barbati A. C., Santana S. M., Gleghorn J. P., and Kirby B. J., “ Microfluidic transport in microdevices for rare cell capture,” Electrophoresis 33, 3133–3142 (2012). 10.1002/elps.201200263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeon J. H. and Park J. K., “ Microfluidic cell culture systems for cellular analysis,” Biochip J. 1(1), 17–27 (2007). [Google Scholar]
- 23. Mehling M. and Tay S., “ Microfluidic cell culture,” Curr. Opin. Biotechnol. 25, 95–102 (2014). 10.1016/j.copbio.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 24. Halldorsson S., Lucumi E., Gomez-Sjöberg R., and Fleming R. M., “ Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices,” Biosens. Bioelectron. 15(63), 218–231 (2014). 10.1016/j.bios.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 25. Fridley K. M., Kinney M. A., and McDevitt T. C, “ Hydrodynamic modulation of pluripotent stem cells,” Stem Cell Res. Ther. 3(45), 2–9 (2012). 10.1186/scrt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El-Ali J., Sorger P. K., and Jensen K. F., “ Cells on chips,” Nature 442, 403–411 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- 27. Chung B. G. and Choo J., “ Microfluidic gradient platforms for controlling cellular behavior,” Electrophoresis 31, 3014–3027 (2010). 10.1002/elps.201000137 [DOI] [PubMed] [Google Scholar]
- 28. Cimetta E., Cannizzaro C., James R., Biechele T., Moon R. T., Elvassore N., and Vunjak-Novakovic G., “ Microfluidic device generating stable concentration gradients for long term cell culture: Application to Wnt3a regulation of β-catenin signaling,” Lab Chip 10, 3277–3283 (2010). 10.1039/c0lc00033g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keenan T. M. and Folch A., “ Biomolecular gradients in cell culture systems,” Lab Chip 8, 34–57 (2008). 10.1039/B711887B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paguirigan A. L. and Beebe D. J., “ Microfluidics meet cell biology: Bridging the gap by validation and application of microscale techniques for cell biological assays,” Bioessays 30, 811–821 (2008). 10.1002/bies.20804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunze A., Pushkarsky I., Kittur H., and Carlo D. D., “ Research highlights: Measuring and manipulating cell migration,” Lab Chip 14, 4117-4121 (2014). 10.1039/C4LC90091J [DOI] [Google Scholar]
- 32. Kim S., Kimz H. J., and Jeon N. L., “ Biological applications of microfluidic gradient devices,” Integr. Biol. 2, 584–603 (2010). 10.1039/c0ib00055h [DOI] [PubMed] [Google Scholar]
- 33. Toetsch S., Olwell P., Prina-Mello A., and Volkov Y., “ The evolution of chemotaxis assays from static models to physiologically relevant platforms,” Integr. Biol. 1, 170–181 (2009). 10.1039/B814567A [DOI] [PubMed] [Google Scholar]
- 34. Sai J., Walker G., Wikswo J., and Richmond A., “ The IL sequence in the LLKIL motif in CXCR2 Is required for full ligand-induced activation of erk, akt, and chemotaxis in HL60 cells,” J. Biol. Chem. 281(47), 35931–35941 (2006). 10.1074/jbc.M605883200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hulkower K. I. and Herber R. L., “ Review: Cell migration and invasion assays as tools for drug discovery,” Pharmaceutics 3, 107–124 (2011). 10.3390/pharmaceutics3010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nandy B., “ Theoretical studies of the chemotaxis of biological cells,” Ph.D thesis (University of Duisburg-Essen, 2008). [Google Scholar]
- 37. Wang S. J., Saadi W., Lin F., Nguyen C. M. C., and Jeon N. L., “ Differential effects of EGF gradient profiles on MDA-MB-231 breast cancer cell chemotaxis,” Exp. Cell Res. 300, 180–189 (2004). 10.1016/j.yexcr.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 38. Yesil-Celiktas O., Sevimli C., Bedir E., and Vardar-Sukan F., “ Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of human cancer cell lines,” Plant Foods Hum. Nutr. 65, 158–163 (2010). 10.1007/s11130-010-0166-4 [DOI] [PubMed] [Google Scholar]
- 39. Tai W., Yeh C. F., Wu C. C., and Hsu C. H., “ A microfluidic device for sorting cancer cells based on cell motility,” 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, October 2-6, 2011, Seattle, Washington, USA, 2011. [Google Scholar]
- 40. Rhee S. W., “ Compartmented microfluidic device for positioning and chemotactic migration of cells,” BioChip J 5(2), 129–136 (2011). 10.1007/s13206-011-5205-1 [DOI] [Google Scholar]
- 41. Tata U., Rao S. M. N., Sharma A., Pabba K., Pokhrel K., Adhikari B., Lin V. K., and Chiao J-C, “ Study of lung-metastasized prostate cancer cell line chemotaxis to epidermal growth factor with a BIOMEMS device,” Adv. Nat. Sci.: Nanosci. Nanotechnol. 3, 035007 (2012). 10.1088/2043-6262/3/3/035007 [DOI] [Google Scholar]
- 42. Rao S. M. N., Lin V. K., Tata U., Raj G. V., Hsieh J. T., Nguyen K., and Chiao J. C., “ Research paper: Demonstration of cancer cell migration using a novel microfluidic device,” J. Nanotechnol. Eng. Med. 1(2), 021003 (2010). 10.1115/1.4001280 [DOI] [Google Scholar]
- 43. Zhang C., Jang S., Amadi O. C., Shimizu K., Lee R. T., and Mitchell R. N., “ Clinical study: A sensitive chemotaxis assay using a novel microfluidic device, Hindawi publishing corporation,” BioMed Res. Int. 2013, Article ID 373569. 10.1155/2013/373569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kong Q., Majeska R. J., and Vazquez M., “ Migration of connective tissue-derived cells is mediated by ultra-low concentration gradient fields of EGF,” Exp. Cell Res. 317, 1491–1502 (2011). 10.1016/j.yexcr.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chong J. J. H. and Murry C. E., “ Cardiac regeneration using pluripotent stem cells—Progression to large animal models,” Stem Cell Res. 13, 654–665 (2014). 10.1016/j.scr.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Natarajan K., Tian C., Xiang B., Chi C., Deng J., Zhang R., Freed D. H., Arora R. C., Tian G., and Lin F., “ Selection of chemotactic adipose-derived stem cells using a microfluidic gradient generator,” RSC Adv. 5, 6332–6339 (2015). 10.1039/C4RA12863J [DOI] [Google Scholar]
- 47. Lin F., Nguyen C. M., Wang S. J., Saadi W., Gross S. P., and Jeon N. L., “ Neutrophil migration in opposing chemoattractant gradients using microfluidic chemotaxis devices,” Ann. Biomed. Eng. 33(4), 475–482 (2005). 10.1007/s10439-005-2503-6 [DOI] [PubMed] [Google Scholar]
- 48. Servant G., Weiner O. D., Herzmark P., Balla T., Sedat J. W., and Bourne H. R., “ Polarization of chemoattractant receptor signaling during neutrophil chemotaxis,” Science 287(5455), 1037–1040 (2000). 10.1126/science.287.5455.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cheng S. Y., Heilman S., Wasserman M., Archer S., Shulerac M. L., and Wu M., “ A hydrogel-based microfluidic device for the studies of directed cell migration,” Lab Chip 7, 763–769 (2007). 10.1039/b618463d [DOI] [PubMed] [Google Scholar]
- 50. Randolph G. J., Angeli V., and Swartz M. A., “ Dendritic-cell trafficking to lymph nodes through lymphatic vessels,” Nat. Rev. Immunol. 5, 617–628 (2005). 10.1038/nri1670 [DOI] [PubMed] [Google Scholar]
- 51. Haessler U., Pisanoa M., Wub M., and Swartz M. A., “ Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19,” Proc. Natl. Acad. Sci. USA 108(14), 5614–5619 (2011). 10.1073/pnas.1014920108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Toh A. G. G., Wang Z. P., Yang C., and Nguyen N. T., “ Engineering microfluidic concentration gradient generators for biological applications,” Microfluid. Nanofluid. 16, 1–18 (2014). 10.1007/s10404-013-1236-3 [DOI] [Google Scholar]
- 53. Friedl P. and Wolf K., “ Tumour-cell invasion and migration: Diversity and escape mechanisms,” Nat. Rev. Cancer 3, 362–374 (2003). 10.1038/nrc1075 [DOI] [PubMed] [Google Scholar]
- 54. Rao S. M. N., Huggins C., Rahimi M., Nguyen K., and Chiao J. C., “ Chemokine gradient formation in microfluidic devices to investigate prostate cancer cell migration,” Proc. SPIE 7270, 727015 (2008). 10.1117/12.810757 [DOI] [Google Scholar]
- 55. Saadi W., Wang S. J., Lin F., and Jeon N. L., Chemotaxis of Metastatic Breast Cancer Cells in Parallel Gradient Microfluidic Chambers ( NSTI-Nanotech, 2005), Vol. 1. [Google Scholar]
- 56. Haessler U., Kalinin Y., Swartz M. A., and Wu M., “ An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies,” Biomed. Microdev. 11, 827–835 (2009). 10.1007/s10544-009-9299-3 [DOI] [PubMed] [Google Scholar]
- 57. Sackmann E. K., Berthier E., Young E. W. K., Shelef M. A., Wernimont S. A., Huttenlocher A., and Beebe D. J., “ Microfluidic kit-on-a-lid: A versatile platform for neutrophil chemotaxis assays,” Blood 120(14), e45–e53 (2012). 10.1182/blood-2012-03-416453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shamloo A., Ma N., Poo M., Sohn L. L., and Heilshorn S. C., “ Endothelial cell polarization and chemotaxis in a microfluidic device,” Lab Chip 8, 1292–1299 (2008). 10.1039/b719788h [DOI] [PubMed] [Google Scholar]
- 59. Hotchkiss K. A., Ashton A. W., Klein R. S., Lenzi M. L., Zhu G. H., and Schwartz E. L., “ Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration,” Cancer Res. 63, 527–533 (2003). [PubMed] [Google Scholar]
- 60. Hsu S., Thakar R., Liepmann D., and Li S., “ Effects of shear stress on endothelial cell haptotaxis on micropatterned surfaces,” Biochem. Biophys. Res. Commun. 337(11), 401–409 (2005). 10.1016/j.bbrc.2005.08.272 [DOI] [PubMed] [Google Scholar]
- 61. Wang Y., Chang J., Chen K. D., Li S., Li J. Y. S., Wu C., and Chien S., “ Selective adapter recruitment and differential signaling networks by VEGF vs. shear stress,” Proc. Natl. Acad. Sci. USA 104(21), 8875–8879 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu C., Poh Y. K. C., Roes I., O'Cearbhaill1 E. D., Matthiesen M. E., Mu L., Yang S. Y., Miranda-Nieves D., Irimia D., and Karp J. M., “ A portable chemotaxis platform for short and long term analysis,” PLoS One 7(9), e44995 (2012). 10.1371/journal.pone.0044995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Renner A., Jaeger M. S., Lankenau A., and Duschl C., “ Position-dependent chemotactic response of slowly migrating cells in sigmoidal concentration profiles,” Appl. Phys. A 112, 637–645 (2013). 10.1007/s00339-012-7507-0 [DOI] [Google Scholar]
- 64. Berthier J. and Silberzan P., Microfluidics for Biotechnology ( Artech House, Norwood, MA, 2010). [Google Scholar]
- 65. Neužil P., Giselbrecht S., Länge K., Huang T. J., and Manz A., “ Revisiting lab-on-a-chip technology for drug discovery,” Nat. Rev. Drug Discovery 11, 620–632 (2012). 10.1038/nrd3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selimovic S., Dokmeci M. R., and Khademhosseini A., “ Organs-on-a-chip for drug discovery,” Curr. Opin. Pharmacol. 13, 829–833 (2013). 10.1016/j.coph.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 67. Bhise N. S., Ribas J., Manoharan V., Zhang Y. S., Polini A., Massa S., Dokmeci M. R., and Ali K., “ Organ-on-a-chip platforms for studying drug delivery systems,” J. Controlled Release 190, 82–93 (2014). 10.1016/j.jconrel.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Razzackia S. Z., Thwara P. K., Yanga M., Ugazb V. M., and Burns M. A., “ Integrated microsystems for controlled drug delivery,” Adv. Drug Delivery Rev. 56, 185–198 (2004). 10.1016/j.addr.2003.08.012 [DOI] [PubMed] [Google Scholar]
- 69. Håkanson M., Cukierman E., and Charnley M., “ Miniaturized pre-clinical cancer models as research and diagnostic tools,” Adv. Drug Delivery Rev. 69–70, 52–66 (2014). 10.1016/j.addr.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wagner I., Materne E. M., Brincker S., Sussbier U., Fradrich C., Busek M., Sonntag F., Sakharov D. A., Trushkin E. V., Tonevitsky A. G., Lauster R., and Marx U., “ A dynamic multi organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture,” Lab Chip 13, 3538–3547 (2013). 10.1039/c3lc50234a [DOI] [PubMed] [Google Scholar]
- 71. Ghaemmaghami A. M., Hancock M. J., Harrington H., Kaji H., and Khademhosseini A., “ Biomimetic tissues on a chip for drug discovery,” Drug Discovery Today 17(3-4), 173–181 (2012). 10.1016/j.drudis.2011.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Williamson A., Singh S., Fernekorn U., and Schober A., “ The future of the patient-specific body-on-a-chip,” Lab Chip 13, 3471–3480 (2013). 10.1039/c3lc50237f [DOI] [PubMed] [Google Scholar]
- 73. Wikswo J. P., Curtis E. L., Eagleton Z. E., Evans B. C., Kole A., Hofmeisterab L. H., and Matloff W. J., “ Scaling and systems biology for integrating multiple organs-on-a-chip,” Lab Chip 13, 3496–3511 (2013). 10.1039/c3lc50243k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim D., Wu X., Young A. T., and Haynes C. L., “ Microfluidics-based in vivo mimetic systems for the study of cellular biology,” Acc. Chem. Res. 47, 1165−1173 (2014). 10.1021/ar4002608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shuler M. L. and Esch M. B., “ Body-on-a chip: Using microfluidic systems to predict human responses to drugs,” Pure Appl. Chem. 82, 1635–1645 (2010). 10.1351/PAC-CON-09-10-44 [DOI] [Google Scholar]
- 76. Huh D., Torisawa Y.-S., Hamilton G. A., Kim H. J., and Ingber D. E., “ Microengineered physiological biomimicry: Organs-on-chips,” Lab Chip 12, 2156–2164 (2012). 10.1039/c2lc40089h [DOI] [PubMed] [Google Scholar]
- 77. Huh D., Hamilton G. A., and Ingber D. E., “ From 3D cell culture to organs-on-chips,” Trends Cell Biol. 21(12), 745–754 (2011). 10.1016/j.tcb.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sei Y., Justus K., LeDuc P., and Kim Y. T., “ Engineering living systems on chips: From cells to human on chips,” Microfluid. Nanofluid. 16, 907–920 (2014). 10.1007/s10404-014-1341-y [DOI] [Google Scholar]
- 79. Björnmalm M., Yan Y., and Caruso F., “ Engineering and evaluating drug delivery particles in microfluidic devices,” J. Controlled Release 190, 139–149 (2014). 10.1016/j.jconrel.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 80. Sugiura S., Edahiro J., Kikuchi K., Sumaru K., and Kanamori T., “ Pressure-driven perfusion culture microchamber array for a parallel drug cytotoxicity assay,” Biotechnol. Bioeng. 100, 1156–1165 (2008). 10.1002/bit.21836 [DOI] [PubMed] [Google Scholar]
- 81. Shen F., Li X. J., and Li P. C. H., “ Study of flow behaviors on single-cell manipulation and shear stress reduction in microfluidic chips using computational fluid dynamics simulations,” Biomicrofluidics 8, 014109 (2014). 10.1063/1.4866358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huanga M., Fana S., Xing W., and Liu C., “ Microfluidic cell culture system studies and computational fluid dynamics,” Math. Comput. Modell. 52, 2036–2042 (2010). 10.1016/j.mcm.2010.01.024 [DOI] [Google Scholar]
- 83. Prakash S. and Yeom J., “ Nanofluidics and microfluidics systems and applications,” British Library Cataloguing in Publication Data ( Elsevier Inc., 2014). [Google Scholar]
- 84. Elliott N. T. and Yuan F., “ A microfluidic system for investigation of extravascular transport and cellular uptake of drugs in tumors,” Biotechnol. Bioeng. 109(5), 1326–1335 (2012). 10.1002/bit.24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Walsh C. L., Babin B. M., Kasinskas R. W., Foster J. A., McGarry M. J., and Forbes N. S., “ A multipurpose microfluidic device designed to mimic microenvironment gradients and develop targeted cancer therapeutics,” Lab Chip 9, 545–554 (2009). 10.1039/B810571E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ziolkowska K., Stelmachowska A., Kwapiszewski R., Chudy M., Dybko A., and Brzozka Z., “ Long-term three-dimensional cell culture and anticancer drug activity evaluation in a microfluidic chip,” Biosens. Bioelectron. 40, 68–74 (2013). 10.1016/j.bios.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 87. Moreno-Arotzena O., Mendoza G., Cóndor M., Rüberg T., and García-Aznar J. M., “ Inducing chemotactic and haptotatic cues in microfluidic devices for three-dimensional in vitro assays,” Biomicrofluidics 8(6), 064122 (2014). 10.1063/1.4903948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee K. J., Yang S. Y., and Ryu W. H., “ Controlled release of bupivacaine HCl through microchannels of biodegradable drug delivery device,” Biomed. Microdevices 14, 583–593 (2012). 10.1007/s10544-012-9637-8 [DOI] [PubMed] [Google Scholar]
- 89. Wang F., Wang H., Wang J., Wang H. Y., Rummel P. L., Garimella S. V., and Lu C., “ Microfluidic delivery of small molecules into mammalian cells based on hydrodynamic focusing,” Biotechnol. Bioeng. 100(1), 150–158 (2008). 10.1002/bit.21737 [DOI] [PubMed] [Google Scholar]
- 90. Yesil-Celiktas O., “Patenting trends in enzyme related microfluidic applications,” Biochem. Eng. J. 92, 53–62 (2014). 10.1016/j.bej.2014.06.017 [DOI] [Google Scholar]
- 91. Karabulut D., Akay S., Kazan A., Sargin S., Cetin B., and Yesil-Celiktas O., “ A miniaturized device for hydrolysis of ginseng RB1,” Proceedings of 2nd Novel Fluidic Technologies and Applications Workshop, 9-10 April, Izmir, Turkey, 2015 (2015), pp. 34–35. [Google Scholar]
- 92. Li H., Whittenberg J. J., Zhou H., Ranganathan D., Desai A. V., Koziol J., Zeng D., Kenis P. J. A., and Reichert D. E., “ Development of a microfluidic "click chip" incorporating an immobilized Cu(i) catalyst,” RSC Adv. 5(8), 6142–6150 (2015). 10.1039/C4RA15507F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jomeh S. and Hoorfar M., “ Numerical modeling of mass transport in microfluidic biomolecule-capturing devices equipped with reactive surfaces,” Chem. Eng. J. 165(2), 668–677 (2010). 10.1016/j.cej.2010.09.056 [DOI] [Google Scholar]
- 94.See http://www.desenmikrotek.com for commercially available microfluidic cell culture devices; accessed 30 april 2015.