Abstract
Early-life feeding behaviors foretell later dietary habits and health outcomes. Few studies have examined infant dietary patterns and caries occurrence prospectively.
OBJECTIVE
Assess whether patterns in food and drink consumption before age 12 months are associated with caries incidence by preschool age.
METHODS
We collected early-life feeding data within a birth cohort from low-income families in Porto Alegre, Brazil. Three dietary indexes were defined, based on refined sugar content and/or previously reported caries associations: a count of sweet foods or drinks introduced <6-months (e.g., candy, cookies, soft drinks), a count of other, non-sweet items introduced <6-months (e.g., beans, meat), and a count of sweet items consumed at 12 months. Incidence of severe early childhood caries (S-ECC) at age 38 months (N=458) was compared by score tertile on each index, adjusted for family, maternal, and child characteristics using regression modeling.
RESULTS
Introduction to a greater number of presumably cariogenic items in infancy was positively associated with future caries. S-ECC incidence was highest in the uppermost tertile of the “6-month sweet index” (adjusted cumulative incidence ratio, RR, versus lowest tertile: 1.46; 95% CI: 0.97, 2.04) and the uppermost tertile of the “12-month sweet index” (RR: 1.55; 95% CI: 1.17, 2.23). The association was specific for sweet items: caries incidence did not differ by tertile of the “6-month non-sweet index” (RR: 1.00; 95% CI: 0.70, 1.40). Additionally, each one-unit increase on the 6-month and the 12-month sweet indexes, but not the 6-month non-sweet index, was statistically significantly associated with greater S-ECC incidence and associated with more decayed, missing or restored teeth. Results were robust to minor changes in the items constituting each index and persisted if liquid items were excluded.
CONCLUSIONS
Dietary factors observed before age 12-months were associated with S-ECC at preschool age, highlighting a need for timely, multi-level intervention.
Introduction
Feeding behaviors in the first year of life set the stage for dietary habits and preferences later in childhood (1-3), with implications for nutrition-related health. Early exposure to sugar-sweetened water has been shown to predict later preference for sweet tastes (4), and taste preferences for sweet and fat have been associated with overweight status among children (5).
For oral health, overwhelming epidemiologic evidence implicates dietary habits in the development of caries in children, particularly the consumption of sugar-containing snacks and drinks (6). The American Academy of Pediatric Dentistry recommends a first dental examination no later than age 12 months, in part to counsel caregivers regarding dietary habits (7). However, particular feeding practices initiated earlier than 12 months have been linked to caries: of several habits evaluated, adding sweeteners and beginning snacking before 6 months were both associated with future caries in rural Thailand (8).
Challenges inherent in relating individual diet components to disease risk include potentially strong inter-correlation between dietary variables, multiple hypothesis testing, and the inability to characterize the diet generally or to detect small effects of single items, even if cumulative effects may be meaningful (9). Dietary indexes are based on a priori expectations of what behaviors constitute a healthy nutritional pattern (9) and are one approach to diet pattern analysis applicable for caries research. For example, higher scores on the Healthy Eating Index were associated with lower prevalence of severe early childhood caries (S-ECC) among U.S. 2- to 5-year-olds (10). In another cross sectional study, liquid cariogenicity scores were highest among U.S. pediatric dental clinic attendees presenting with S-ECC (11). Currently, there is little prospective information regarding dietary patterns in infancy and caries incidence in childhood.
Here, using dietary and dental data from a birth cohort in southern Brazil (12,13), we aimed to assess whether a priori defined patterns in food and drink exposure before age 12 months predicted caries incidence by preschool age. Specifically, we hypothesized that caries incidence would be higher among children introduced to a greater number of sweet and/or presumably cariogenic items before age 6 months and among children consuming more sweet/cariogenic items at age 12 months. Additionally, we hypothesized that this association would be specific to foods and drinks designated as cariogenic, such that there would be no association between caries incidence and exposure to lower-sugar dietary items.
Methods
Ethics
Ethical review committees at the Federal University of Health Sciences of Porto Alegre (UFCSPA) and the University of California Berkeley granted approval for this study. Mothers provided informed consent on behalf of their children. Children with caries, suspected anemia, underweight, or overweight status were referred to their local health center.
Study Design and Participants
This observational cohort study was performed as a secondary analysis of data collected during a cluster-randomized controlled trial in Porto Alegre, Brazil, a city of 1.4 million inhabitants with a fluoridated public water supply. The original trial enrolled a stratified random sample of 20 municipal health centers in 2008: 9 health centers that were randomized to an intervention of healthcare worker training that promoted providing healthful complementary feeding advice to new mothers, and 11 control health centers that continued usual maternal counseling practices, as detailed elsewhere (12,13). To be eligible, health centers must have recorded at least 100 infant patient visits in 2006 and have not been engaged in staff sharing with other facilities or participating in another dietary program. Thirty-one of the city’s 52 health centers met eligibility criteria, and all 20 that were randomly selected and invited to participate agreed to join the study. Services at municipal health centers are available to all city residents, but primarily low-income individuals utilize care at these facilities.
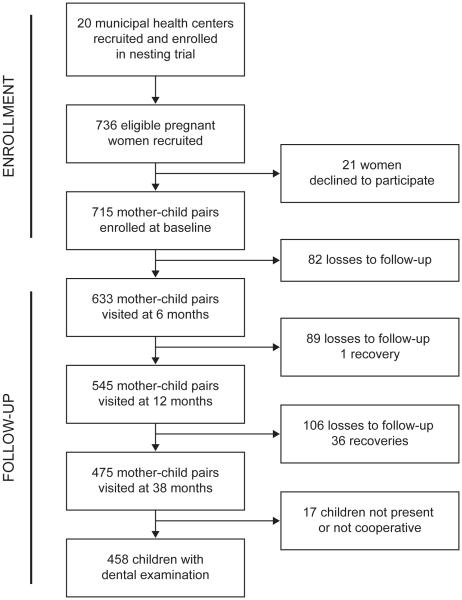
As reported previously, the intervention did not result in a statistically significant reduction in caries incidence (12). Briefly, from April to December 2008, 715 of 736 pregnant women (eligibility: scheduled appointment at participating health center and no history of positive HIV test) were contacted, provided informed consent, and agreed to enroll their children in a birth cohort (Figure 1). Trained, calibrated fieldworkers conducted maternal interviews following structured questionnaires at baseline (in pregnancy) and later assessed child diet, growth, and other health variables at follow-up home-visits corresponding to mean child ages 6 months (range: 5 to 9 months), 12 months (range: 11 to 15 months), and 38 months (range: 31 to 46 months). The analytic sample for this study consisted of the 458 children with dental evaluations at the 38-month visit.
Figure 1. Flow of participants.
Legend: Mother-child pairs recruited from 20 municipal health centers were followed from enrollment (in pregnancy) until child age 38 months.
Food Introduction Measures
We created three indices related to the foods and drinks consumed during the first year of life. At the 6-month assessment, mothers were asked at what age (in months) their child was first introduced to 31 specific items, each later categorized as introduced before 6 months (yes/no). We classified those items into two groups of potential cariogenicity, based on their densities of refined sugar or other simple carbohydrates and/or previously reported associations with caries in the literature. The items categorized as more cariogenic were: added sugar, candy, chips, chocolate, chocolate milk, coffee (sugar added), cookies, fruit-flavored drink, gelatin, honey, ice cream, petit suisse cheese, soft drinks, sweet biscuits, and tea. The 15 items were summed to form an index corresponding to the number sweet foods or drinks introduced to the infant before age 6 months (“6-month sweet index”). The 16 remaining items (beans, cow’s milk, commercial soup, enriched cereal, family food, fried foods, fruit, natural fruit juice, processed meat, red meat, organ meat, salty snacks, savory porridge, simple grain or flour, water, and vegetables) were deemed to contain low sugar and/or have limited cariogenic potential and were combined to form an analogous index (“6-month non-sweet index”).
At the 12-month assessment, mothers were asked specifically about the consumption of high-sugar, low-nutrient density, or high-fat foods in the preceding month (yes/no), of which 17 were summed to form the “12-month sweet index,” based on their presumed cariogenicity: added sugar in a drink, candy, cake, chips, chocolate, chocolate milk, coffee (sugar added), cookies, creamed caramel, fruit-flavored drink, gelatin, honey, ice cream, other confection, petit suisse cheese, soft drinks, and sweet biscuits. Each index demonstrated acceptable internal consistency: 6-month sweet index (Chronbach’s alpha=0.78), 6-month non-sweet index (0.83), and 12-month sweet index (0.69).
Occasionally, classification of dietary items into categories of greater or lower potential cariogenicity relied on incomplete or inconsistent published evidence. For example, coffee and tea were grouped with the cariogenic items, because these drinks are generally sweetened heavily when served to infants (14,15); yet savory porridge, enriched cereals, and simple grains, which are sometimes sweetened, were grouped with less cariogenic items. Natural fruit juice, which contains sugar, was categorized as less cariogenic based on previous literature (16); however, petit suisse cheese and ice cream were grouped with cariogenic items due to sugar added during processing, despite evidence that other dairy products may be caries protective (17). Chips/crisps, although not sweet, were considered potentially cariogenic (18). To assess the robustness of the study results to classification decisions made during index construction, we performed a series of sensitivity analysis in which individual items were individually removed and/or replaced in the 6-month sweet index and 6-month non-sweet index at random with equal probability. Additionally, we repeated the primary analysis with all liquid items excluded.
Dental Caries Measures
At the 38-month visit, in participants' homes, one of two dentist-evaluators, masked to dietary data, assessed caries status following World Health Organization methodology (19), with noncavitated white-spot lesions also recorded. Teeth were brushed without dentifrice, dried with gauze, and then evaluated visually using a lighted intraoral mirror. Interrater unweighted kappa was 0.75; intrarater unweighted kappa was 0.83 for both examiners. Severe early childhood caries (S-ECC) was defined as ≥1 affected maxillary anterior teeth or ≥4 decayed, missing due to caries, or restored tooth surfaces (20). Also calculated was the count of decayed (cavitated), missing due to caries, or restored (filled) primary teeth (dmft).
Analysis
Our primary parameter of interest was the cumulative incidence ratio (relative risk), comparing S-ECC at the 38-month visit according to tertile for each of the three food introduction indexes. Separately for each index, we fitted two different log-linear regression models. The first, the unadjusted model, included only index tertile and allocation status in the nesting trial (i.e., control or intervention) as independent variables. The next model added possible confounders: maternal age (in years), maternal education attainment (≤8 years vs. >8 years), parity (first child vs. other), pre-pregnancy BMI (≤18 vs. >18), smoking status during pregnancy (current vs. former/never), social class (Brazilian Association of Economic Research Institutes classification ≤C vs. >C), child age at dental examination (in months), sex, length-for-age Z-score at 6-months (21), exclusive breastfeeding duration (≥3 months vs. <3 months), and use of a nursing bottle at 6 months (yes/no). To avoid over-adjustment for factors potentially downstream of exposure variables (22), we did not adjust for behaviors recorded after age 12 months, such as oral hygiene practices (daily use of fluoride toothpaste at 38 months, yes/no) and duration of any breastfeeding (<6 months, 6-23 months, ≥24 months). However, as a sensitivity check, we fitted models that did include these variables.
We also fitted separate unadjusted and adjusted models expressing each index as a continuous (count) variable, rather than separated into tertiles. Finally, we fitted analogous negative binomial regression models for the outcome dmft.
The number of participants recruited and retained in the nesting intervention trial determined the sample size available for the present cohort study. With 458 children available, we estimated that this study would have 80% power to detect a 1.6-fold difference in S-ECC incidence (25% vs. 40%) between the lowest and highest tertile for any of the food introduction indexes, assuming 153 participants per tertile (2-tailed alpha=0.05).
Model-based multiple imputation was used to address missing covariate and dietary data (2.7% missing), averaging point estimates over 50 imputations. Nonparametric bootstrap resampling (5000 repetitions) was used to estimate 95% confidence intervals (CI). An association was considered statistically significant (P<0.05) if the 95% CI excluded the parameter value under the null hypothesis. Analyses were completed using software R 3.1.1 (http://r-project.org). Study reporting followed STROBE guidelines (23).
Results
Unmet dental treatment needs in this population were considerable. Approximately one-third of children presented with S-ECC at 38 months (34.3%, 157/458); over 99% of the decayed, missing, filled tooth index was attributable to untreated decay. The study population was predominantly of low socio-economic position. Nearly half the mothers had obtained ≤8 years of formal education (Table 1). The originally recruited and analytic samples were similar in their characteristics (Table 1), with only one statistically significant difference in measured variables: mothers in the analytic sample were older, on average, than mothers lost to follow-up (26.4 vs. 25.2 years at child birth, P=0.02).
Table 1.
Characteristics of the study participants, Porto Alegre, Brazil, 2008-2011
| Initial Cohort (N=715)a | Analytic Sample (N=458)a | |||
|---|---|---|---|---|
| n (%) | mean (SD) | n (%) | mean (SD) | |
| Maternal and Household Variables: | ||||
| Maternal age at birth, years | 26.0 (6.7) | 26.4 (6.7)* | ||
| Maternal education ≤8 years | 340 (47.6) | 214 (46.7) | ||
| Mother has previous children | 397 (55.5) | 259 (56.6) | ||
| Maternal pre-pregnancy BMI ≤ 18 | 106 (15.3) | 73 (16.5) | ||
| Mother is current smoker | 142 (19.9) | 87 (19.0) | ||
| Social classb ≤ C | 569 (79.8) | 358 (78.3) | ||
| Child Variables: | ||||
| Malec | 333 (52.4) | 233 (50.9) | ||
| Length-for-age Z-score at 6-monthsc | −0.1 (1.2) | −0.1 (1.1) | ||
| Use of nursing bottle at 6-monthsc | 375 (61.1) | 263 (59.5) | ||
| Age at dental assessment, years | - | 3.2 (0.2) | ||
| S-ECC | - | 157 (34.3) | ||
| dmft | - | 1.5 (2.8) | ||
Abbreviations: BMI = body mass index; dmft = decayed (cavitated), missing due to caries, filled (restored) primary tooth index; S-ECC = severe early childhood caries; SD = standard deviation
Sample size may be smaller for some variables due to missing data
Brazilian Association of Economic Research Institutes classification system
Data unavailable for 82 children lost to follow-up before the 6-month visit
P < 0.05 for difference between analytic sample and those lost to follow-up, unpaired t-test
Exposure to potentially cariogenic foods and drinks in infancy was widespread: 95% of children had been introduced to at least one item on the 6-month sweet index. Caries at preschool age was associated with exposure to a greater number of presumably cariogenic foods and drinks in the first year of life. Children with S-ECC at 38 months, on average, had been introduced to more sweet foods before age 6 months and had consumed more sweet foods and drinks at 12 months than children without S-ECC (Table 2). In contrast, mean scores on the 6-month non-sweet index were virtually identical between children with and without S-ECC at 38 months (Table 2).
Table 2.
Food and drink index scores by child dental status at 38 months
| Children with S-ECC (N=157) |
Children without S-ECC (N=301) |
Mean differencea (95% CI) |
P-value | |
|---|---|---|---|---|
| 6-month sweet index | 5.61 | 4.85 | 0.77 (0.19, 1.35) | 0.01 |
| 6-month non-sweet index | 7.01 | 6.99 | 0.03 (−0.64, 0.68) | 0.95 |
| 12-month sweet index | 11.16 | 9.99 | 1.17 (0.61, 1.73) | < 0.001 |
Abbreviations: CI = confidence interval; S-ECC = severe early childhood caries
Difference in index values between children with and without S-ECC
The cumulative incidence of S-ECC at 38 months increased with rising score on the 6-month sweet index and the 12-month sweet index. For both the 6-month and 12-month indexes, S-ECC incidence was approximately 1.5-fold greater in the highest tertile of index score compared to the lowest, after adjusting for household socio-demographics and other child variables (Table 3). Similarly, each one-unit increase in score, corresponding to one additional food or drink, was statistically significantly associated with greater S-ECC incidence (Table 3). However, S-ECC incidence did not substantially differ according score on the 6-month non-sweet index, either by tertile or by unit increase in score (Table 3).
Table 3.
Relative incidence of severe early childhood caries and relative number of decayed missing or restored teeth at 38 months by dietary patterns in the first year of life
| Outcome: S-ECC | Outcome: dmft | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Modela | Adjusted Modelb | Unadjusted Modela | Adjusted Modelb | |||||
| RR | 95% CI | RR | 95% CI | Ratioc | 95% CI | Ratioc | 95% CI | |
| 6-month sweet index | ||||||||
| 1st tertile | 1 | reference | 1 | reference | 1 | reference | 1 | reference |
| 2nd tertile | 1.20 | 0.77, 1.61 | 1.18 | 0.71, 1.60 | 1.36 | 0.80, 2.04 | 1.36 | 0.72, 2.12 |
| 3rd tertile | 1.49 | 1.05, 1.99 | 1.46 | 0.97, 2.04 | 1.42 | 0.87, 2.18 | 1.62 | 0.91, 2.70 |
| continuous | 1.05 | 1.01, 1.10 | 1.05 | 1.00, 1.10 | 1.04 | 0.98, 1.10 | 1.07 | 0.99, 1.15 |
| 6-month non-sweet index | ||||||||
| 1st tertile | 1 | reference | 1 | reference | 1 | reference | 1 | reference |
| 2nd tertile | 0.95 | 0.70, 1.27 | 0.94 | 0.69, 1.27 | 0.89 | 0.59, 1.32 | 0.95 | 0.61, 1.47 |
| 3rd tertile | 0.98 | 0.70, 1.35 | 1.00 | 0.70, 1.40 | 0.95 | 0.59, 1.45 | 1.14 | 0.66, 1.97 |
| continuous | 1.00 | 0.96, 1.04 | 1.00 | 0.96, 1.05 | 0.98 | 0.94, 1.04 | 1.01 | 0.96, 1.08 |
| 12-month sweet index | ||||||||
| 1st tertile | 1 | reference | 1 | reference | 1 | reference | 1 | reference |
| 2nd tertile | 1.08 | 0.80, 1.70 | 1.01 | 0.75, 1.60 | 1.13 | 0.76, 1.84 | 1.10 | 0.69, 1.90 |
| 3rd tertile | 1.64 | 1.24, 2.36 | 1.55 | 1.17.2.23 | 1.79 | 1.23, 2.77 | 1.78 | 1.20, 2.90 |
| continuous | 1.09 | 1.05, 1.15 | 1.08 | 1.04, 1.14 | 1.13 | 1.07, 1.21 | 1.14 | 1.08, 1.22 |
Abbreviations: CI = confidence interval; dmft = decayed (cavitated), missing due to caries, restored primary tooth index; RR = cumulative incidence ratio (relative risk); S-ECC = severe early childhood caries
Adjusted for allocation status in nesting trial
Adjusted for allocation status, for maternal age, education, parity, pre-pregnancy body mass index, smoking status, and social class, and for child sex, age at dental assessment, length-forage Z-score (6 months), exclusive breastfeeding duration, and use of nursing bottle (6 months)
Ratio of dmft count compared to reference
Likewise, severity of caries experience at 38 months, measured as the count of decayed, missing or filled teeth, increased with higher scores on both the 6-month and 12-month sweet indexes, albeit not statistically significantly for the 6-month sweet index (Table 3). As with S-ECC incidence, caries severity was not associated with scores on the 6-month non-sweet index (Table 3).
Sensitivity Analyses
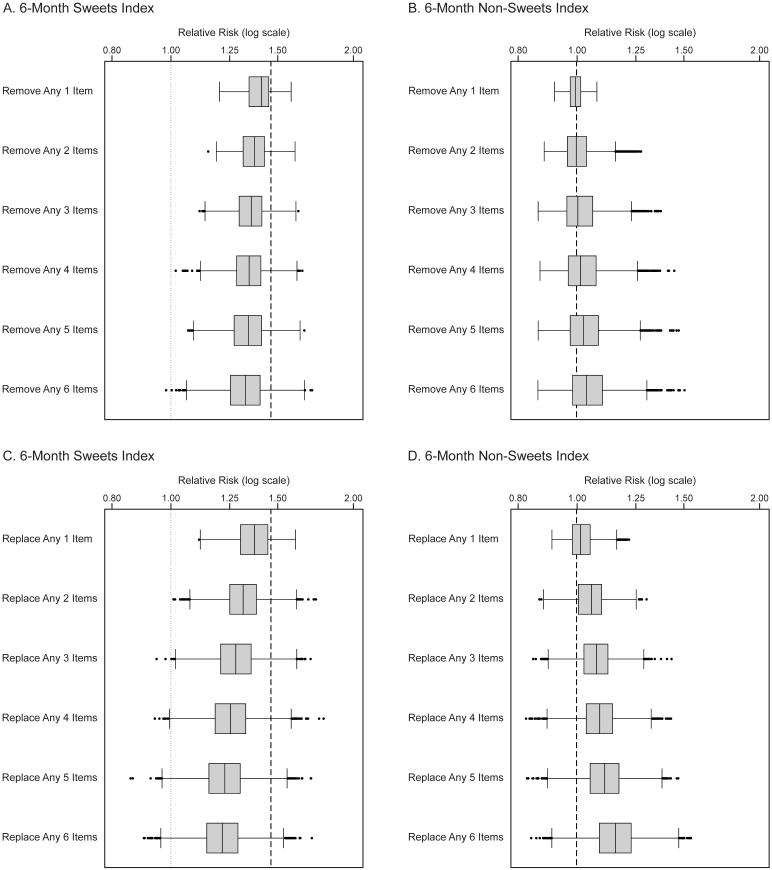
Findings were robust to the removal and/or reclassification of a small number of items between indexes (Figure 2), suggesting that the results reflected overall patterns, rather than strong influence from individual items. The positive association between the 6-month sweet index and S-ECC was somewhat attenuated as items were removed; however, the majority of the subsequent associations remained near magnitude of the association for the originally constructed index (Figure 2A). Similarly, the 6-month non-sweet index remained largely unassociated with S-ECC as items were removed (Figure 2B). Not surprisingly, interchanging items between the 6-month sweet index and the 6-month non-sweet index lessened the magnitude of the caries association for the sweet index (Figure 2C) and induced a weak positive association between caries and the non-sweet index (Figure 2D). Nonetheless, most of the possible trades of single items between the indexes did not greatly alter the direction or magnitude of the associations.
Figure 2. Sensitivity analyses for the 6-month sweet and non-sweet indexes.
Legend: Box plots show variation in point estimates of the adjusted relative risk (log scale) for severe early childhood caries (outcome) comparing the uppermost to lowermost tertile of each index (exposure), under random removal of any set of items from the 6-month sweet index (panel A) and 6-month non-sweet index (panel B), or under random removal from the 6-month sweet index with random replacement from the 6-month non-sweet index (panel C), or under random removal from the 6-month non-sweet index with random replacement from the 6-month sweet index (panel D). Heavy dashed lines show the point estimate for the adjusted relative risk as the indexes were originally constructed: 1.46 for the 6-month sweet index and 1.00 for the 6-month non-sweet index. Boxes reflect the 25th, 50th, and 75th percentile values; whiskers reflect the lower and upper adjacent values.
Results did not meaningfully differ when the indexes were based on solid foods only, excluding all liquid items (Table 4). Without the liquid items, the 6-month sweet index and 12-month sweet index were both positively and statistically significantly associated with S-ECC incidence and positively associated with dmft, but there was no association between the 6-month non-sweet index and caries at age 38 months (Table 4). Finally, adjusting for tooth brushing habits and total breastfeeding duration did not yield substantial changes in results; for example, the adjusted relative risk for S-ECC comparing the third tertile of the 12-month sweet index to the first tertile was 1.56 (95% CI: 1.17, 2.23) with adjustment for these variables and 1.55 (95% CI: 1.17, 2.22) without adjustment (Table 3).
Table 4.
Relative incidence of severe early childhood caries and relative number of decayed missing or restored teeth at 38 months by dietary patterns in the first year of life, all liquid items excluded
| Outcome: S-ECC | Outcome: dmft | |||
|---|---|---|---|---|
| RRa | 95% CI | Ratioa,b | 95% CI | |
| 6-month sweet index | ||||
| 1st tertile | 1 | reference | 1 | reference |
| 2nd tertile | 1.20 | 0.83, 1.63 | 1.27 | 0.80, 1.94 |
| 3rd tertile | 1.63 | 1.11, 2.17 | 1.57 | 0.92, 2.31 |
| continuous | 1.07 | 1.02, 1.13 | 1.06 | 0.98, 1.15 |
| 6-month non-sweet index | ||||
| 1st tertile | 1 | reference | 1 | reference |
| 2nd tertile | 1.03 | 0.75, 1.39 | 1.14 | 0.75, 1.67 |
| 3rd tertile | 0.94 | 0.68, 1.29 | 0.86 | 0.55, 1.30 |
| continuous | 1.00 | 0.96, 1.05 | 0.98 | 0.92, 1.05 |
| 12-month sweet index | ||||
| 1st tertile | 1 | reference | 1 | reference |
| 2nd tertile | 1.17 | 0.80, 1.72 | 1.24 | 0.76, 2.05 |
| 3rd tertile | 1.48 | 1.12, 2.36 | 1.63 | 1.12, 2.67 |
| continuous | 1.11 | 1.05, 1.18 | 1.16 | 1.07, 1.25 |
Abbreviations: CI = confidence interval; dmft = decayed (cavitated), missing due to caries, restored primary tooth index; RR = cumulative incidence ratio (relative risk); S-ECC = severe early childhood caries
Adjusted for allocation status in nesting trial, for maternal age, education, parity, prepregnancy body mass index, smoking status, and social class, and for child sex, age at dental assessment, length-for-age Z-score (6 months), exclusive breastfeeding duration, and use of nursing bottle (6 months).
Ratio of dmft count compared to reference
Discussion
In this low-income Brazilian cohort, dietary patterns in infancy characterized by a greater number of highly sweetened foods and drinks were positively associated with the incidence of dental caries by preschool age. To our knowledge, this is the first prospective study to relate observed infant dietary patterns to caries in early childhood. This finding is consistent with professional guidelines to limit dietary risk factors for early childhood caries from an early age (6), as well as with broader WHO strategy to address dietary intake under a common risk factor approach to chronic disease prevention (24).
Infant-feeding behaviors, including those occurring before or contemporaneously with tooth eruption, could plausibly impact future caries risk by at least two possible pathways. Firstly, early infancy is a critical period in which experiences with various foods and tastes importantly influence food preferences and behaviors later in childhood (3). For example, children regularly fed sweetened water at age 6 months had greater preference for consumption of sweet liquids at age 2 years (4). In a Swedish cohort, habits established at age 1 year, such as intakes of soft drinks and sweet snacks, predicted continuation of those behaviors 1-2 years later (25). Secondly, early dietary patterns may influence bacterial ecology, such as establishment of mutans streptococci, a strong predictor of future caries incidence in young children (26). Total sugar exposure in infancy was positively associated with initial acquisition of Streptococcus mutans in an Australian birth cohort (27), and the adhesion properties of S. mutans may be sensitive to the sucrose concentration of the oral environment (28). Thus, early provision of sweetened foods and drinks may carry significant dental consequences, potentially by setting the foundation for future cariogenic dietary patterns or through shaping bacterial populations in the oral cavity.
One challenge in this analysis was deriving a priori designations regarding whether food items should comprise the “sweet” or “non-sweet” indexes in light of incomplete or potentially conflicting evidence regarding the cariogenicity of certain foods and drinks. In our approach, we constructed defensible categorizations based on outside information and, through sensitivity analyses, demonstrated that re-categorization of a small number of items would be unlikely to strongly affected our findings. Alternatively, had items been selected for the sweet or non-sweet index based on individual associations with caries within this cohort, the external validity of the resulting indexes could have been compromised.
The associations between caries incidence and higher scores on the 6-month and 12-month sweet indexes were not weakened with the exclusion of liquid items, differing from the findings of a recent study, in which S-ECC was associated with liquid cariogenicity scores but not with solid food cariogenicity scores (11). Correlation between the consumption of sweet foods and sweet drinks might have partly accounted for the associations observed in the present study. However, the introduction of more food and drink items, in general, did not drive the caries associations, as suggested by the lack of association with the index of non-sweet items. Importantly, the previously mentioned study (11) measured dietary habits in cross sectional sample of children age 2-6 years, and this difference in the age at which feeding practices were recorded could have contributed to the divergent findings regarding solid items.
This analysis measured the variety and number of sugar-containing items introduced during infancy but did not capture all dietary factors implicated in the caries process, such as feeding frequency. Nonetheless, the sweet food indexes were strongly associated with caries incidence. Similarly constructed variety indexes have been shown to be reasonable indicators of overall diet quality, including nutrient adequacy among young children (29). We speculate that children exposed to a wider variety of cariogenic items will also consume sweet snacks on more daily occasions, but this question requires further study.
Our “investigator-driven” indexes were based on predefined dentally healthy or unhealthy items, unlike “data-driven” approaches that aim to derive dietary patterns a posteriori (30). A combined measure of child sugary snack intake from principal components analysis was associated with caries experience in the United Arab Emirates (31), and patterns of higher soft drink consumption defined through cluster analysis were associated with greater caries increment among low-income children (32). Although the various approaches to pattern analysis address distinct research questions, they often yield similar conclusions, even if the specifics of the analyses vary (30). Our indexes offer the advantages of being straightforward to calculate and easy to translate into clinical messages.
In fact, the uncomplicated dietary measures used in this study augment existing recommendations to introduce complementary foods gradually, after up to 6 months of exclusive breastfeeding, with an emphasis on grains, meats, fruits, and vegetables, while avoiding coffee, soft drinks, candy, and other sweets (33). The graded relationship between caries incidence and the sweet index scores suggests the possibility of some benefit from reducing or delaying exposure to sweet items, even if consumption cannot be eliminated entirely. On the other hand, it has been argued that achieving low caries levels in children and adults might require limiting sugar consumption to well below the proposed WHO target of 10% of total energy intake (34).
Feeding practices are an appealing target for caries prevention, but the results of this study suggest that interventions must address habits that emerge in infancy. The first-year dental visit is an opportunity to provide dietary guidance (7), but significant barriers have been reported in connecting young children with appropriate dental care (35). In Brazil, utilization of dental care for young children is far from universal and marked by socio-economic inequality (36). Actions that can be taken before entry into the traditional dental care delivery system may prove fruitful. Repeated maternal home visits that stressed healthy complementary feeding throughout the first year postpartum reduced S-ECC incidence at age 4 years, even without providing explicit dental health recommendations (37). In Australia, an anticipatory guidance oral health program that enrolled pregnant women also reported S-ECC prevention (38), and in Austria, a program that provided new mothers with dental health counseling was associated with significantly reduced S-ECC incidence at age 5 years (39).
Maternal factors, including educational attainment and the composition of her own diet, strongly predict infant feeding habits (40); yet, efforts to change the knowledge and attitudes of individual mothers might yield greater health improvements if coupled with parallel emphasis on community food environments, such as promoting the availability and affordability of fresh produce (41). Higher purchase costs of nutrient-dense foods have been posed as a barrier to achieving high-quality diets and a contributor to socio-economic inequalities in nutrition-related diseases (42). However, maintaining children caries-free does not necessarily impose greater food expenditures on families (43). On the contrary, presumably cariogenic diets consisting of higher intakes of chocolate, soda, and other sweets were associated with greater child feeding costs among low-income Brazilian households (43).
This study advantageously followed a prospective, community-representative sample and collected caries-related dietary information from an early age. Prospective data collection presumably would be less prone to measurement error than had mothers been asked to recall past infant feeding practices at the time of the dental examination. The related challenges of dental caries and highly sweetened diets are not unique to resource-poor settings, but as a limitation, it is not certain that the results generalize beyond this predominantly low-income cohort in Brazil. Furthermore, losses to follow-up may have influenced results in an unknown direction. Most likely, the impact of such losses was minor, because there was little difference in average characteristics between the initial and analytic samples.
Importantly, this study demonstrated an abundance of sugar-rich foods and drinks in the diet of many infants, with significant consequences for childhood dental health as the number of sugar-rich items increased. The physical and social environments are instrumental in shaping children’s eating patterns (44), and those feeding habits are a possible pathway through which adverse environments become translated into poor health. Evidence that inappropriate feeding patterns emerge as early as the first 6 months of life strongly suggests that effective health-promoting interventions must seek targets earlier in the lifecourse and further upstream.
Acknowledgements
Members of the Nutrition Research Group (NUPEN) at the Federal University of Health Sciences of Porto Alegre assisted in participant recruitment, data collection, and data management. Support was provided from the NIH National Institute for Dental and Craniofacial Research (F30DE022208), the NIH National Center for Advancing Translational Sciences (KL2TR000143), and the Rio Grande do Sul Research Support Foundation (FAPERGS). The information presented is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring organizations.
Footnotes
The authors declare that they have no conflicts of interest related to this research.
References
- 1.Coulthard H, Harris G, Emmett P. Long-term consequences of early fruit and vegetable feeding practices in the United Kingdom. Public Health Nutr. 2010;13:2044–2051. doi: 10.1017/S1368980010000790. [DOI] [PubMed] [Google Scholar]
- 2.Müller LM, de Hoog ML, van Eijsden M, Gemke RJ, Vrijkotte TG. Infant nutrition in relation to eating behaviour and fruit and vegetable intake at age 5 years. Br J Nutr. 2013;109:564–571. doi: 10.1017/S0007114512001237. [DOI] [PubMed] [Google Scholar]
- 3.Ventura AK, Worobey J. Early influences on the development of food preferences. Curr Biol. 2013;23(9):R401–R408. doi: 10.1016/j.cub.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp GK, Moran M. Acceptance of sweet and salty tastes in 2-year-old children. Appetite. 1984;5(4):291–305. doi: 10.1016/s0195-6663(84)80002-1. [DOI] [PubMed] [Google Scholar]
- 5.Lanfer A, Knof K, Barba G, Veidebaum T, Papoutsou S, de Henauw S, et al. Taste preferences in association with dietary habits and weight status in European children: results from the IDEFICS study. Int J Obes (Lond) 2012;36(1):27–34. doi: 10.1038/ijo.2011.164. [DOI] [PubMed] [Google Scholar]
- 6.Tinanoff N, Palmer CA. Dietary determinants of dental caries and dietary recommendations for preschool children. J Public Health Dent. 2000;60:197–206. doi: 10.1111/j.1752-7325.2000.tb03328.x. discussion 207-209. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatric Dentistry Guideline on periodicity of examination, preventive dental services, anticipatory guidance/counseling, and oral treatment for infants, children, and adolescents. Pediatr Dent. 2013;35:E148–E156. [PubMed] [Google Scholar]
- 8.Thitasomakul S, Piwat S, Thearmontree A, Chankanka O, Pithpornchaiyakul W, Madyusoh S. Risks for early childhood caries analyzed by negative binomial models. J Dent Res. 2009;88:137–141. doi: 10.1177/0022034508328629. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nunn ME, Braunstein NS, Krall Kaye EA, Dietrich T, Garcia RI, Henshaw MM. Healthy eating index is a predictor of early childhood caries. J Dent Res. 2009;88:361–366. doi: 10.1177/0022034509334043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans EW, Hayes C, Palmer CA, Bermudez OI, Naumova EN, Cohen SA, et al. Development of a pediatric cariogenicity index. J Public Health Dent. 2013;73:179–186. doi: 10.1111/jphd.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaffee BW, Feldens CA, Vítolo MR. Cluster-randomized trial of infant nutrition training for caries prevention. J Dent Res. 2013;92(7 Suppl):29S–36S. doi: 10.1177/0022034513484331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vítolo MR, Louzada MLdC, Rauber F. Positive impact of child feeding training program for primary care health professionals: a cluster randomized field trial. Rev Bras Epidemiol. doi: 10.1590/1809-4503201400040007. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Engle PL, VasDias T, Howard I, Romero-Abal ME, Quan de Serrano J, Bulux J, et al. Effects of discontinuing coffee intake on iron deficient Guatemalan toddlers' cognitive development and sleep. Early Hum Dev. 1999;53:251–269. doi: 10.1016/s0378-3782(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 15.Stern D, Piernas C, Barquera S, Rivera JA, Popkin BM. Caloric beverages were major sources of energy among children and adults in Mexico, 1999-2012. J Nutr. 2014;144:949–956. doi: 10.3945/jn.114.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall TA, Broffitt B, Eichenberger-Gilmore J, Warren JJ, Cunningham MA, Levy SM. The roles of meal, snack, and daily total food and beverage exposures on caries experience in young children. J Public Health Dent. 2005;65:166–173. doi: 10.1111/j.1752-7325.2005.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Miyake Y, Sasaki S. Intake of dairy products and the prevalence of dental caries in young children. J Dent. 2010;38:579–583. doi: 10.1016/j.jdent.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Johansson I, Holgerson PL, Kressin NR, Nunn ME, Tanner AC. Snacking habits and caries in young children. Caries Res. 2010;44:421–430. doi: 10.1159/000318569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Oral health surveys, basic methods. 4th World Health Organization; Geneva, Switzerland: 1997. [Google Scholar]
- 20.Drury TF, Horowitz AM, Ismail AI, Maertens MP, Rozier RG, Selwitz RH. Diagnosing and reporting early childhood caries for research purposes. A report of a workshop sponsored by the national institute of dental and craniofacial research, the health resources and services administration, and the health care financing administration. J Public Health Dent. 1999;59:192–197. doi: 10.1111/j.1752-7325.1999.tb03268.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO Child Growth Standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 22.Merchant AT, Pitiphat W. Directed acyclic graphs (DAGs): an aid to assess confounding in dental research. Community Dent Oral Epidemiol. 2002;30(6):399–404. doi: 10.1034/j.1600-0528.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . 2008-2013 Action plan for the global strategy for the prevention and control of noncommunicable diseases. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 25.Wendt LK, Hallonsten AL, Koch G, Birkhed D. Analysis of caries-related factors in infants and toddlers living in Sweden. Acta Odontol Scand. 1996;54:131–137. doi: 10.3109/00016359609006019. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara T, Sasada E, Mima N, Ooshima T. Caries prevalence and salivary mutans streptococci in 0-2-year-old children of Japan. Community Dent Oral Epidemiol. 1991;19(3):151–154. doi: 10.1111/j.1600-0528.1991.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 27.Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res. 2003;82:504–508. doi: 10.1177/154405910308200703. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Li W, Lin J, Chen Z, Yu D. Effect of sucrose concentration on sucrose-dependent adhesion and glucosyltransferase expression of S. mutans in children with severe early-childhood caries (S-ECC) Nutrients. 2014;6:3572–3586. doi: 10.3390/nu6093572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatløy A, Torheim LE, Oshaug A. Food variety--a good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur J Clin Nutr. 1998;52:891–898. doi: 10.1038/sj.ejcn.1600662. [DOI] [PubMed] [Google Scholar]
- 30.Reedy J, Wirfalt E, Flood A, Mitrou PN, Krebs-Smith SM, Kipnis V, et al. Comparing 3 dietary pattern methods--cluster analysis, factor analysis, and index analysis--With colorectal cancer risk: The NIH-AARP Diet and Health Study. Am J Epidemiol. 2010;171:479–487. doi: 10.1093/aje/kwp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashim R, Williams SM, Thomson WM. Diet and caries experience among preschool children in Ajman, United Arab Emirates. Eur J Oral Sci. 2009;117:734–740. doi: 10.1111/j.1600-0722.2009.00688.x. [DOI] [PubMed] [Google Scholar]
- 32.Lim S, Sohn W, Burt BA, Sandretto AM, Kolker JL, Marshall TA, et al. Cariogenicity of soft drinks, milk and fruit juice in low-income African-American children: a longitudinal study. J Am Dent Assoc. 2008;139:959–967. doi: 10.14219/jada.archive.2008.0283. [DOI] [PubMed] [Google Scholar]
- 33.Brazilian Ministry of Health and Pan American Health Organization . Guia alimentar para crianças menores de 2 anos [Feeding guide for children less than 2 years of age] Brazilian Ministry of Health; Brasília, Brazil: 2002. [Google Scholar]
- 34.Sheiham A, James WP. A reappraisal of the quantitative relationship between sugar intake and dental caries: the need for new criteria for developing goals for sugar intake. BMC Public Health. 2014;14:863. doi: 10.1186/1471-2458-14-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis CW, Boulter S, Keels MA, Krol DM, Mouradian WE, O'Connor KG, et al. Oral health and pediatricians: results of a national survey. Acad Pediatr. 2009;9:457–461. doi: 10.1016/j.acap.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Ardenghi TM, Vargas-Ferreira F, Piovesan C, Mendes FM. Age of first dental visit and predictors for oral healthcare utilisation in preschool children. Oral Health Prev Dent. 2012;10:17–27. [PubMed] [Google Scholar]
- 37.Feldens CA, Giugliani ER, Duncan BB, Drachler Mde L, Vitolo MR. Long-term effectiveness of a nutritional program in reducing early childhood caries: a randomized trial. Community Dent Oral Epidemiol. 2010;38:324–332. doi: 10.1111/j.1600-0528.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- 38.Plutzer K, Spencer AJ. Efficacy of an oral health promotion intervention in the prevention of early childhood caries. Community Dent Oral Epidemiol. 2008;36:335–346. doi: 10.1111/j.1600-0528.2007.00414.x. [DOI] [PubMed] [Google Scholar]
- 39.Wagner Y, Greiner S, Heinrich-Weltzien R. Evaluation of an oral health promotion program at the time of birth on dental caries in 5-year-old children in Vorarlberg, Austria. Community Dent Oral Epidemiol. 2014;42(2):160–169. doi: 10.1111/cdoe.12072. [DOI] [PubMed] [Google Scholar]
- 40.Robinson S, Marriott L, Poole J, Crozier S, Borland S, Lawrence W, et al. Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. Br J Nutr. 2007;98:1029–1037. doi: 10.1017/S0007114507750936. [DOI] [PubMed] [Google Scholar]
- 41.Capewell S, Graham H. Will cardiovascular disease prevention widen health inequalities? PLoS Med. 2010;7:e1000320. doi: 10.1371/journal.pmed.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monsivais P, Aggarwal A, Drewnowski A. Are socio-economic disparities in diet quality explained by diet cost? J Epidemiol Community Health. 2012;66:530–535. doi: 10.1136/jech.2010.122333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldens CA, Rodrigues PH, Rauber F, Chaffee BW, Vítolo MR. Food expenditures, cariogenic dietary practices and childhood dental caries in southern Brazil. Caries Res. 2013;47:373–381. doi: 10.1159/000348518. [DOI] [PubMed] [Google Scholar]
- 44.Patrick H, Nicklas TA. A review of family and social determinants of children's eating patterns and diet quality. J Am Coll Nutr. 2005;24:83–92. doi: 10.1080/07315724.2005.10719448. [DOI] [PubMed] [Google Scholar]