Abstract
Background
Physical activity is associated with a lower incidence of colorectal cancer (CRC); however, the relationship of physical activity with CRC survival is not yet clear. We evaluated the association between pre-diagnostic physical activity and CRC survival, overall and accounting for tumor-markers associated with CRC survival: BRAF and KRAS mutation status and microsatellite instability (MSI) status.
Methods
Participants were 20–74 year old CRC patients diagnosed between 1998 and 2007 from the population-based Seattle Colon Cancer Family Registry (S-CCFR). Self-reported physical activity in the years preceding CRC diagnosis was summarized as average metabolic equivalent-task hours per week (MET-h/week) (n=1309). Somatic BRAF and KRAS mutations and MSI status were evaluated on a subset of patients (n=1043). Cox regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for overall and disease-specific survival after adjusting for relevant confounders. Stratified analyses were conducted across categories of BRAF, KRAS and MSI, as well as tumor stage and site.
Results
Higher pre-diagnostic recreational physical activity was associated with significantly more favorable overall survival (HR for highest vs. lowest category=0.70, 95% CI 0.52–0.96); associations were similar for CRC-specific survival. Results consistently indicated a favorable association with physical activity across strata defined by tumor characteristics.
Conclusion
Individuals who were physically active prior to CRC diagnosis experienced better survival than those who were inactive or minimally active.
Impact
Our results support existing physical activity recommendations for CRC patients and suggest that the beneficial effect of activity is not specific to a particular molecular phenotype of CRC.
Keywords: physical activity, overall survival, colon-cancer specific survival, BRAF, KRAS, MSI
INTRODUCTION
Epidemiologic studies have established the benefits of physical activity throughout life in lowering the lifetime risk of developing (1–3) or dying (1, 2, 4–6) from several forms of cancer, including colorectal cancer (CRC) (7–9). Several studies have also suggested that overall survival after CRC diagnosis is more favorable in patients with high levels of pre- (10, 11) and post-diagnostic (10–16) physical activity. On the basis of such suggestive findings in CRC survivors, and in survivors of other cancers (17), guidelines for cancer survivors generally recommend 150 minutes of moderate-intensity or 75 minutes of vigorous exercise per week (6, 18).
Similar to general benefits of physical activity with respect to overall survival in CRC patients, there is consistent evidence to support a benefit of post-diagnostic physical activity with respect to CRC-specific survival (10–14). The association between pre-diagnostic physical activity and CRC-specific survival, however, is less conclusive: some studies indicate no association (13, 14) while others report a survival benefit with pre-diagnostic activity (10, 11, 16). Observed inconsistencies in the association between pre-diagnostic physical activity and CRC-specific survival could be partially attributed to differences in study population composition (e.g., age, smoking status) which, in turn, would translate to differences in the distribution of CRC attributes, such as tumor site and tumor-marker status. Thus, to better elucidate the relationship between pre-diagnostic physical activity and survival in CRC patients, with respect to both overall and CRC-specific survival, it is important to consider such potential sources of heterogeneity.
The presence of BRAF (19, 20) or KRAS (21, 22) somatic mutations in CRC has been associated with poorer survival, while the presence of MSI (23–25) has been consistently associated with better survival. Previous studies have also suggested some heterogeneity in the relationship of lifestyle factors with CRC risk and survival according to these tumor-markers (26–31). Thus, consideration of these tumor-markers may provide greater insight into the association between physical activity and CRC survival.
We evaluated the association between pre-diagnostic physical activity and CRC survival with consideration for potential sources of heterogeneity, including BRAF-mutation, KRAS-mutation, and MSI status.
MATERIALS AND METHODS
Study population
The Colon Cancer Family Registry (CCFR) is an international consortium of six study centers (32, 33). The present analysis utilized data on CRC patients in the Seattle CCFR (S-CCFR) site, all of whom were ascertained through the population-based Surveillance, Epidemiology and End Results (SEER) registry serving western Washington State. During the initial recruitment phase (1998–2002), eligible patients included adults aged 20–74 years diagnosed with CRC who resided in the King, Pierce and Snohomish counties at the time of diagnosis. Women aged 50–74 years who were diagnosed with CRC during the same time period and who resided in 10 additional Washington counties were also eligible. In a second recruitment phase (2002–2007), we enrolled individuals diagnosed with CRC between 18–49 years who resided within the 13-county Western Washington SEER area. Only English-speaking participants with publicly available phone numbers were eligible.
In total, 3525 individuals were identified and contacted for recruitment. Of these, 302 (9%) were already deceased, 401 (11%) refused participation, 92 (3%) could not be located, and 24 (1%) only partially completed their interview. Thus, 2706 CRC patients were available for the current analyses. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Exposure and covariate assessment
Patients were enrolled and interviewed an average 8.5 (range 2.3–44.1) months after CRC diagnosis. In a structured telephone interview, they were asked to self-report detailed information on their recreational physical activity during defined age periods prior to diagnosis: ages 20–29, 30–49, and 50+ years. Questions covered different modes of activities (e.g., walking, jogging, running, bicycling, swimming, soccer, tennis, basketball, calisthenics), and the usual duration and frequency of each activity. Evaluation was limited to activities in which the patients were engaged for at least 30 minutes per week, for at least 3 continuous months. The parent questionnaire from which our questionnaire was derived has been previously validated and shown to provide a good measure of the underlying physical activity levels in other studies (34). Standard metabolic equivalent of task (MET) values were assigned to each activity (35, 36) and multiplied by the number of hours per week engaged in that activity to derive MET-hours per week (MET-h/week). Physical activity during the age period of a patient’s CRC diagnosis (i.e., 20–29, 30–49, or 50+ years) was then summarized as average MET-h/week. Henceforth, we refer to these age period-specific physical activity levels as pre-diagnostic physical activity levels. For the present analysis, we categorized this variable as <3.5 (referent group), 3.5-<8.75, 8.75-<17.5, 17.5-<35, ≥35 MET-h/week, which is equivalent to the level of exertion from <1, 1-<2.5, 2.5-<5, 5–10, and >10 hours of brisk walking per week, respectively (35, 36). These cut-points, in addition to being easily interpretable, were based on uniform distribution of participants within each category. We also evaluated the effect of physical activity using a referent cut-point of ≥8.75 MET-h/week reflecting current recommendations of at least 2.5 hours of moderate activity or 75 minutes of vigorous activity per week among cancer survivors (≥8.75 MET-h/week) (6, 18, 37).
Patients also provided information on several other pre-diagnostic exposures, including smoking history (never, former, current), education, height, and weight. Information on tumor site (colon, rectum) was obtained from linkage to SEER. Tumor stage based on the 7th edition of American Joint Committee on Cancer (AJCC) staging system (38) was derived after combining information on TNM stage and SEER summary stage (39) (AJCC Stage 0-IV).
Exclusions and missing data
For the current analyses, we excluded patients missing data on pre-diagnostic physical activity (n=737, 27%). For greater comparability with previous studies that excluded patients with metastatic disease (10, 11, 13), and to account for the possibility of reverse causation such that those with advanced disease may have altered activity patterns prior to diagnosis, we excluded patients with stage IV disease at the time of diagnosis (n=370, 13.9%). Additionally, we excluded individuals with missing data on potential confounders (n=255) and those with somatic mutations in both BRAF and KRAS (n=6) (40). Finally, we excluded individuals in the topmost percentile of physical activity (MET-h/week>140, n=21) to eliminate outliers, leaving a total of n=1309 patients for primary analyses.
Outcomes ascertainment
Vital status and cause of death (classified according to ICD-10 conventions) were determined periodically through linkage to the Puget Sound SEER registry and the National Death Index; most recent linkage to vital status was completed in December 2012 (41). CRC-specific deaths included those with an underlying cause attributed to ICD-10 codes C18.0-C20.9 or C26.0 (41).
Tumor-marker status
Tumor-markers were evaluated from paraffin-embedded formalin-fixed tumor tissue samples. Forward and reverse sequencing was used to identify mutations in the coding sequence of KRAS exon 2 (codons 12/13) in a subset of patients (n=949) (42, 43). We also tested for the c.1799T>A (p.V600E) BRAF-mutation using a fluorescent allele-specific PCR assay (n=949) (44). MSI testing was performed as previously described (n=1040) (24). Most patients (60%) were tested using a 10-marker genetic panel (45, 46); tumors were classified as MSI-H if instability was observed in 30% or more of the markers and as microsatellite stable (MSS) otherwise. Additional patients were tested for MSI using immunohistochemistry (IHC) testing of four markers: MLH1, MSH2, MSH6, and PMS2 (47, 48); those with positive staining for all markers were grouped as MSS, whereas those negative for at least one marker were considered MSI-H. High concordance (97.7%) between IHC and PCR-based MSI methods has previously been demonstrated (49).
Statistical Analyses
We estimated the association between pre-diagnostic physical activity and survival after CRC diagnosis by calculating hazard ratios (HR) and 95% confidence intervals (CIs) using separate Cox models for overall and CRC-specific survival. Days since CRC diagnosis was used as the underlying time metric with left-censoring of patients until study enrollment. Administrative censoring occurred at the last vital status assessment for patients alive until then. For CRC-specific survival, we censored individuals who died of causes other than CRC at the time of death. Confounders were determined a priori by identifying known correlates of both physical activity and survival; associations of these selected confounders with the exposure and outcome were also verified in the analytic dataset. Based on these considerations, we adjusted for age at diagnosis (continuous), sex, BMI (<25, 25–29, 30+), smoking history (never, former, current), education (less than high school, high school, some college, college graduate), and diagnosis year. In separate models, we further adjusted for tumor-marker status and stage. Tests for trend were based on the likelihood-ratio test associated with addition of the categorical physical activity variable in its continuous form (p-trendoverall). We also computed a separate trend test only among those who were physically active (p-trendactive). A two-sided p-value <0.05 was considered statistically significant. The validity of the proportional hazards assumption over time was tested using Schoenfeld’s residuals (50).
In addition to the aforementioned models, we evaluated associations within strata defined by tumor-markers (BRAF and KRAS mutation status, MSI status), and by other tumor (site, stage) and patient characteristics (age, sex). All analyses were performed using STATA, release 13 (StataCorp, College Station, TX).
RESULTS
The distribution of baseline characteristics across categories of pre-diagnostic physical activity levels are presented in Table 1. There were no marked differences across physical activity categories for age at diagnosis, education, stage, tumor site or tumor-marker status. A difference across categories was noted for sex, such that males were more likely to be classified in the highest categories of pre-diagnostic physical activity. BMI appeared modestly elevated for those in the lowest pre-diagnostic physical activity. Patients were followed for a median of 6.1 years (range: 8 days-12.8 years). Of 1309 patients in the primary analytic dataset, 408 (31.2%) died, of whom 229 (56.1%) died from CRC. Compared with patients with complete data, those with missing data on physical activity were older, and were more likely to be female, ever smokers, and of a lower educational attainment (Supplementary table 1).
Table 1.
Baseline characteristics of colorectal cancer patients across categories of prediagnostic physical activity in the Seattle Colon Cancer Family Registry among patients with local and regional stage disease (n = 1309)
| Participant characteristics | Physical activity (MET-hours per week)* | |||||
|---|---|---|---|---|---|---|
| 0-<3.5 (n = 279) |
3.5-<8.75 (n = 253) |
8.75-<17.5 (n = 284) |
17.5-<35 (n = 277) |
≥35 (n = 216) |
Total (n = 1309) |
|
| Age at diagnosis (years), Mean (SD) | 56.9 (12) | 55.8 (12) | 56.2 (12) | 55.2 (12) | 53.8 (13) | 55.7 (12) |
| Males, n(%) | 136 (49) | 121 (48) | 154 (54) | 160 (58) | 139 (64) | 710 (54) |
| BMI (kg/m2), Mean (SD)1 | 28.8 (7) | 27.9 (6) | 27.1 (5) | 27.0 (5) | 27.0 (6) | 27.6 (6) |
| Smoking history2, n(%) | ||||||
| Never | 113 (41) | 130 (51) | 128 (45) | 118 (43) | 77 (36) | 566 (43) |
| Former | 110 (39) | 92 (37) | 112 (39) | 116 (42) | 102 (47) | 532 (41) |
| Current | 56 (20) | 31 (12) | 44 (16) | 43 (15) | 37 (17) | 211 (16) |
| Education level3, n (%) | ||||||
| <High school | 22 (8) | 14 (6) | 6 (2) | 12 (4) | 20 (9) | 74 (6) |
| High school | 79 (28) | 45 (18) | 77 (27) | 52 (19) | 52 (24) | 305 (23) |
| Some college/vocational school | 92 (33) | 98 (39) | 81 (28) | 98 (36) | 73 (34) | 442 (34) |
| College graduate | 86 (31) | 96 (38) | 120 (42) | 115 (41) | 71 (33) | 488 (37) |
| CRC stage at diagnosis (AJCC), n (%) | ||||||
| 0/I | 120 (43) | 116 (46) | 119 (42) | 128 (46) | 93 (43) | 576 (44) |
| II | 49 (18) | 51 (20) | 65 (23) | 51 (19) | 44 (20) | 260 (20) |
| III | 110 (39) | 86 (34) | 100 (35) | 98 (35) | 79 (37) | 473 (36) |
| Tumor site, n (%) | ||||||
| Colon | 187 (68) | 155 (63) | 168 (60) | 177 (64) | 133 (62) | 820 (64) |
| Rectal | 88 (32) | 93 (37) | 111 (40) | 98 (36) | 81 (38) | 471 (36) |
| BRAF & KRAS mutation status4, n (%) | ||||||
| Wildtype | 125 (60) | 114 (62) | 111 (55) | 110 (55) | 95 (60) | 555 (58) |
| BRAF mutated | 24 (12) | 22 (12) | 26 (13) | 24 (12) | 15 (9) | 111 (12) |
| KRAS mutated | 58 (28) | 48 (26) | 65 (32) | 64 (32) | 48 (31) | 283 (30) |
| MSI status5, n(%) | ||||||
| MSS | 199 (88) | 172 (85) | 186 (82) | 180 (84) | 143 (84) | 880 (85) |
| MSI-H | 27 (12) | 31 (15) | 40 (18) | 35 (16) | 27 (16) | 160 (15) |
CRC Colorectal cancer, AJCC American Joint Committee on Cancer
MET hours per week translated into time equivalent spent walking per week: 3.5 = 1 hour; 8.75 = 2.5 hours; 17.5 = 5 hours; 35 = 10 hours; Topmost percentile of physical activity was excluded
BMI assessment was missing in 8 individuals
Smoking history assessed 2 years prior to colorectal cancer diagnosis; smoking data was missing for 3 individuals
Education level was missing for 349 (15.6%) of individuals
858 (38.2%) individuals did not have BRAF-KRAS mutation status evaluated, n = 6 mutated on both BRAF & KRAS dropped
550 (24.4%) individuals did not undergo testing for MSI status
Table 2 presents results for overall and CRC-specific survival according to categories of pre-diagnostic physical activity. Increasing levels of pre-diagnostic physical activity, relative to <3.5 MET-h/week, was associated with a statistically significantly better overall survival in adjusted models [HR(95% CI) for 3.5-<8.75, 8.75-<17.5, 17.5-<35, ≥35 MET-h/week were 0.52 (0.38,0.71), 0.65 (0.49,0.86), 0.63 (0.47, 0.84) and 0.70 (0.52,0.96), respectively]. Adjustment for stage at diagnosis did not appreciably change these results. Results for CRC-specific survival were similar, such that patients with increasing physical activity levels >3.5 MET-h/week had statistically significantly better CRC-specific survival relative to the referent category (HR=0.61 (0.41,0.91) comparing the highest versus lowest activity level). In separate models, we also evaluated the impact of adjusting for BRAF, KRAS, and MSI status among those with available data on these markers. Results were similar to those in primary analyses, indicating statistically significantly better overall and CRC-specific survival in physically active individuals (Supplementary Table 2). When stratified by BRAF mutation status, results did not appreciably differ by MSI status i.e. risk estimates were similar for BRAF-wildtype/MSS vs. BRAF-wildtype/MSI-H and for BRAF-mutated/MSS vs. BRAF-mutated/MSI-H), although numbers were limited (data not shown).
Table 2.
Overall and colon cancer-specific survival by categories of prediagnostic physical activity levels among patients with local or regional disease (n = 1309)
| Physical activitya (MET-hours /week) |
Deaths/CRC | Unadjusted HR (95% CI) |
Adjusted Model 11 HR (95% CI) |
Adjusted Model 22 HR (95% CI) |
|---|---|---|---|---|
| Overall survival | ||||
| <3.5 | 117/279 | REF | REF | REF |
| 3.5-<8.75 | 62/253 | 0.48 (0.35,0.65) | 0.52 (0.38,0.71) | 0.53 (0.39,0.72) |
| 8.75-<17.5 | 86/284 | 0.59 (0.45,0.78) | 0.65 (0.49,0.86) | 0.64 (0.48,0.85) |
| 17.5-<35 | 77/277 | 0.55 (0.41,0.73) | 0.63 (0.47,0.84) | 0.64 (0.47,0.85) |
| ≥35 | 66/216 | 0.62 (0.46,0.84) | 0.70 (0.52,0.96) | 0.70 (0.52,0.96) |
| p-trendoverall3 | 0.004 | 0.04 | 0.04 | |
| p-trendactive3 | 0.22 | 0.15 | 0.13 | |
| CRC-specific survival | ||||
| <3.5 | 69/279 | REF | REF | REF |
| 3.5-<8.75 | 38/253 | 0.52 (0.35,0.77) | 0.56 (0.37,0.83) | 0.58 (0.39,0.86) |
| 8.75-<17.5 | 44/284 | 0.54 (0.37,0.78) | 0.58 (0.39,0.84) | 0.56 (0.38,0.83) |
| 17.5-<35 | 42/277 | 0.54 (0.36,0.79) | 0.58 (0.39,0.86) | 0.60 (0.40,0.88) |
| ≥35 | 36/216 | 0.61 (0.41,0.91) | 0.62 (0.41,0.94) | 0.63 (0.42,0.95) |
| p-trendoverall3 | 0.01 | 0.02 | 0.03 | |
| p-trendactive3 | 0.49 | 0.59 | 0.51 |
CRC Colorectal cancer; HR Hazard ratio; CI Confidence intervals
MET hours per week translated into time equivalent spent walking per week: 3.5 = 1 hour; 8.75 = 2.5 hours; 17.5 = 5 hours; 35 = 10 hours; Topmost percentile of physical activity was excluded
Adjusted for age at CRC diagnosis, sex, body mass index, smoking status, education and diagnosis year
Adjusted for age at CRC diagnosis, sex, body mass index, smoking status, education, diagnosis year and stage at diagnosis
Tests for trend were based on the likelihood-ratio test associated with addition of the categorical physical activity variable in its continuous form (p-trendoverall). We also computed a separate trend test only among those that were physically active (p-trendactive).
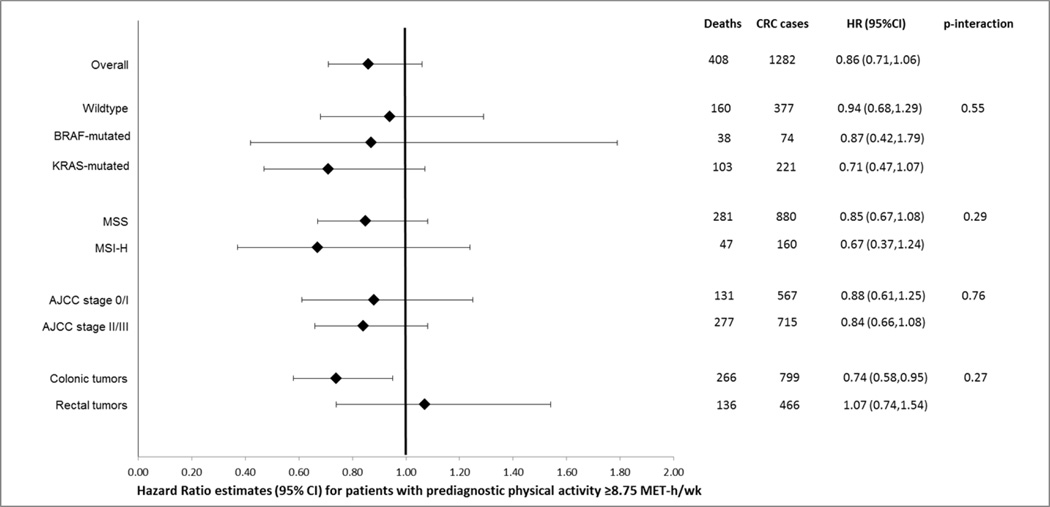
Figure 1 summarizes results for the association between pre-diagnostic physical activity and overall survival according to tumor characteristics (BRAF- and KRAS-mutation status, MSI status, stage, tumor site). In these stratified analyses, those with physical activity ≥8.75 MET-h/week had a better survival, regardless of tumor-marker strata or stage. However, these associations failed to reach statistical significance, likely due to the smaller subgroup sample sizes.. When evaluated by tumor sub-site in the colorectum, physical activity was inversely associated with overall survival among those with colon but not rectal cancers; however, interaction by tumor site was not statistically significant (p-interaction=0.27). There were no meaningful differences observed in overall survival after age or sex stratification (data not shown).
Figure 1.
Overall survival for patients with prediagnostic physical activity at or above recommended levels (≥8.75 MET-h/wk) relative to those with lower activity levels, stratified by tumor characteristics*†
*Risk estimates adjusted for age at diagnosis, sex, body mass index, smoking status, education and diagnosis year.
†Risk estimates based on 1282 CRC patients with local- or regional-stage disease and complete data on confounders.
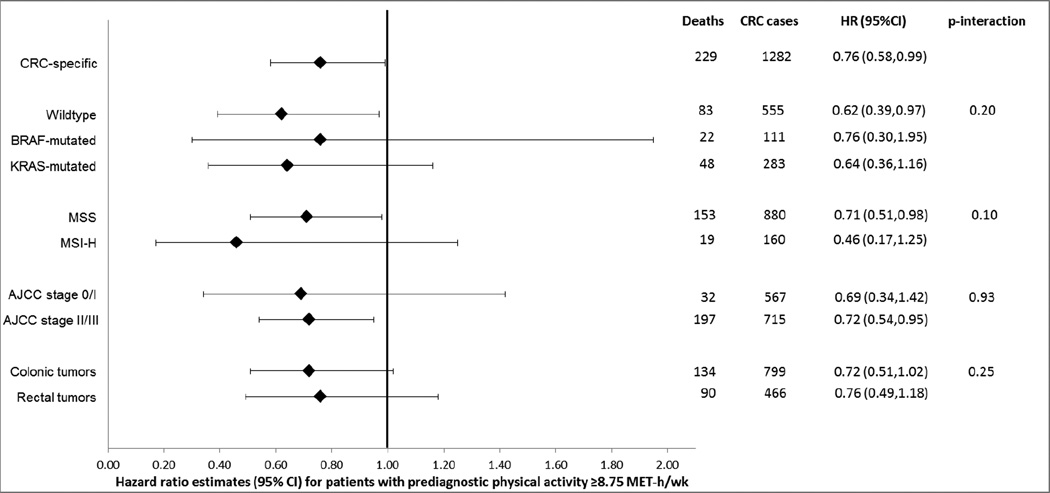
Figure 2 displays the results for stratified analyses in relation to CRC-specific survival. Patients engaged in ≥8.75 MET-h/week of pre-diagnostic physical activity had a better CRC-specific survival, regardless of tumor-marker strata, stage, or tumor site. The inverse association with physical activity was most pronounced among those with MSI-H tumors [HR=0.46 (0.17,1.25)] and weakest among those with BRAF-mutated CRC [HR=0.76 (0.30,1.95)]. Associations did not differ by age or sex for CRC-specific survival (data not shown).
Figure 2.
CRC-specific survival for patients with prediagnostic physical activity at or above recommended levels (≥8.75 MET-h/wk) relative to those with lower activity levels, CRC stratified by tumor characteristics*†
*Risk estimates adjusted for age at diagnosis, sex, body mass index, smoking status, education and diagnosis year.
†Risk estimates based on 1282 CRC patients with local- or regional-stage disease and complete data on confounders.
DISCUSSION
In this cohort of patients with incident CRC, we observed that pre-diagnostic recreational physical activity was associated with favorable overall and CRC-specific survival. This association was unchanged after adjustment for tumor-marker status or stage. Analyses stratified by tumor characteristics consistently indicated more favorable survival in those with physical activity at or above the recommended threshold of ≥8.75 MET-h/week; the inverse association was the strongest among those with MSI-H CRC and weakest among those with BRAF-mutated CRC, although no statistically significant interaction was noted. To the best of our knowledge, this is the first study to explore the associations between physical activity and CRC survival by these tumor-markers.
Five studies, thus far, have assessed the relationship between pre-diagnostic physical activity and overall and CRC-specific survival (10, 11, 13, 14, 16), of which only three reported statistically significant findings (10, 11, 16). A recent meta-analysis summarized the results from these studies and reported a 26% better overall (95% CI, 11–38%) and a 38% better CRC-specific survival (95% CI, 9–38%) for higher versus low physical activity levels (51). In the largest study to-date, persons reporting greater than 8.75 MET-h/week had better overall survival [HR=0.72 (0.58,0.89)]; CRC-specific findings were also suggestive of favorable survival (11). However, none of these studies were able to evaluate differences in associations with physical activity by molecular subtypes of CRC.
CRC is a heterogeneous disease with several distinct molecular subtypes that are suggestive of different pathways of tumorigenesis and progression (52, 53). Thus, consideration of molecular markers that distinguish these pathways, such as BRAF, is important for better understanding the relationship between lifestyle factors and CRC risk and survival. We have previously identified differences in the relationship between smoking and CRC survival according to these markers (28, 29, 31). Additionally, in a previous paper from the S-CCFR, Coghill et al. found that the inverse association between use of non-steroidal anti-inflammatory drugs and survival after CRC diagnosis was restricted to proximal colon cancers, which are more commonly MSI-H and BRAF-mutated than distal CRC (54). Our results instead suggest that the beneficial effect of physical activity is not specific to a particular molecular phenotype of CRC. Although we observe that pre-diagnostic physical activity is broadly beneficial, it may not impact survival in certain case groups, particularly those with rectal cancers. We speculate that the differences in risk factors, natural history, and treatment between colon and rectal cancers, may explain the lack of benefit in overall survival associated with physical activity in rectal cancers in our data (Figure 1). Rectal cancers have been reported to have a greater diagnostic delay and are more likely to be symptomatic at the time of diagnosis (55); both factors may impact a person’s pre-diagnostic physical activity and may explain our observed lack of benefit in rectal cancers.
Several biological mechanisms have been posited for an inverse association between physical activity prior to CRC diagnosis and survival. It is possible that the observed association reflects improved tolerance or decrease in systemic levels of inflammation and/or oxidative stress markers among physically active individuals, which have also been shown to affect CRC risk and survival (17). Physical activity may also increase plasma levels of insulin-like growth factors and C-peptides (17), which have been hypothesized to improve survival among CRC patients (56). Beyond an association with CRC-specific survival, physical activity has been shown to have an impact on risk of death from several other causes, particularly cardiovascular diseases (57, 58). Indeed, in sensitivity analyses we found physical activity to be associated with lower cardiovascular disease mortality in our data. However, as 56% deaths in our study population were CRC-related, we speculate that the overall survival estimates are probably more influenced by beneficial effects of physical activity on reducing CRC deaths than by associations with other causes of death. The breadth of that CRC survival benefit is further supported by the fact that the association we observe is mostly consistent across CRC case groups defined by tumor characteristics. We did not observe any pattern of increasingly favorable survival with increasing levels of physical activity among those who were physically active for either overall or CRC-specific analyses; rather, our results are most consistent with a threshold effect associated with being physically active prior to CRC diagnosis.
There are some limitations to this study. Selection bias in the form of survivor bias is possible if patients who died before they could be enrolled into the study were systematically different from those patients who survived long enough to be interviewed and enrolled into our study. The short lag-time from diagnosis to interview (average 8.5 months; range 2.3–44.1) may limit, but does not preclude, such bias. Additionally, we excluded patients with stage IV disease, as survivor bias is most likely to impact inference within this group. Another important limitation of our study is the high proportion (27%) of missing data on physical activity. Patients with missing physical activity data were slightly different from patients included in the analysis with respect to age, sex, and education level and, thus, may have differed with respect to actual activity levels; such differences may have biased our results. As we were unable to evaluate the differences in survival taking into account the treatment received, we conducted a sensitivity analysis excluding individuals with zero physical activity to eliminate the possibility that such individuals may be different in terms of their care and therefore may have worse prognosis. However, the results did not differ from our reported estimates in Table 2. We also did not have information on disease recurrence, which may also influence survival. As most recurrences occur within 5 years post-diagnosis, we conducted sensitivity analyses restricting follow-up to 5 years after enrollment and the survival estimates were very similar to the ones we reported (data not shown). Lastly, the present analysis was limited to the evaluation of pre-diagnostic physical activity; post-diagnostic activity levels, and changes in physical activity following CRC diagnosis likely also have implications for CRC prognosis and merit further evaluation.
Our study also has several strengths. The population-based design of the S-CCFR cohort, the relatively large sample size, and the long duration of follow-up contribute to the generalizability of our study results. The availability of detailed information on tumor-marker status allowed us to evaluate the role of these molecular variants in the association between physical activity and survival.
In summary, our results, in conjunction with previous studies, suggest that physical activity in the years preceding CRC diagnosis may offer a survival benefit and provide additional support for existing public health recommendations regarding physical activity. Stratified analyses by tumor characteristics revealed a better survival for those with physical activity at or above the previously-recommended threshold irrespective of most measured patient and tumor characteristics. Further studies are needed to better understand the mechanisms through which physical activity may confer its survival benefit, so as to better inform physical activity recommendations for CRC survivors.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grant UM1 CA167551 from the National Cancer Institute and through cooperative agreements with the following CCFR centers: Seattle Colorectal Cancer Family Registry (U01/U24 CA074794) (P.A. Newcomb and J.D. Potter), Australasian Colorectal Cancer Family Registry (U01 CA074778 and U01/U24 CA097735) (D.D. Buchanan, M.A. Jenkins), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01/U24 CA074800) (N.M. Lindor), Ontario Familial Colorectal Cancer Registry (U01/U24 CA074783), University of Hawaii Colorectal Cancer Family Registry (U01/U24 CA074806), USC Consortium Colorectal Cancer Family Registry U01/U24 CA074799). Additionally, National Cancer Institute grants R25CA094880 (S. Hardikar), R03CA165153 (K.W. Makar), K05CA152715 (P. A. Newcomb) and K07CA172298 (A. I. Phipps) have also supported this work. Paraffin-embedded tumor tissue for the Seattle Colorectal Family Registry was provided by The Jeremy Jass Memorial Pathology Bank. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Cerhan JR, Potter JD, Gilmore JM, Janney CA, Kushi LH, Lazovich D, et al. Adherence to the AICR cancer prevention recommendations and subsequent morbidity and mortality in the Iowa Women's Health Study cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13:1114–1120. [PubMed] [Google Scholar]
- 2.McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1089–1097. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- 3.Panel WAE. Food, Nutrition and the Prevention of Cancer: a Global Perspective: American Institute for Cancer Research. 1997 doi: 10.1016/s0899-9007(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 4.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clinic proceedings. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 7.Norat T, Chan DLR, Aune D, Vieira R. The Associations Between Food, Nutrition and Physical Activity and the Risk of Colorectal Cancer. London: World Cancer Research Fund/American Institute for Cancer Research; 2010. [Google Scholar]
- 8.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2005;7:204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014 doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 10.Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer causes & control : CCC. 2012;23:1939–1948. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1410–1420. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 16.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. Journal of the National Cancer Institute. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and science in sports and exercise. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 19.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 21.Phipps AI, Buchanan DD, Makar KW, Win AK, Baron JA, Lindor NM, et al. KRAS-mutation status in relation to colorectal cancer survival: the joint impact of correlated tumour markers. British journal of cancer. 2013;108:1757–1764. doi: 10.1038/bjc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. British journal of cancer. 2001;85:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 24.Phipps AI, Lindor NM, Jenkins MA, Baron JA, Win AK, Gallinger S, et al. Colon and rectal cancer survival by tumor location and microsatellite instability: the Colon Cancer Family Registry. Diseases of the colon and rectum. 2013;56:937–944. doi: 10.1097/DCR.0b013e31828f9a57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. European journal of cancer. 2010;46:2788–2798. doi: 10.1016/j.ejca.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Slattery ML, Anderson K, Curtin K, Ma K, Schaffer D, Edwards S, et al. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutation research. 2001;483:73–81. doi: 10.1016/s0027-5107(01)00228-7. [DOI] [PubMed] [Google Scholar]
- 27.Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. Journal of the National Cancer Institute. 2010;102:1012–1022. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle Colon Cancer Family Registry. Cancer. 2011;117:4948–4957. doi: 10.1002/cncr.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phipps AI, Shi Q, Newcomb PA, Nelson GD, Sargent DJ, Alberts SR, et al. Associations between cigarette smoking status and colon cancer prognosis among participants in North Central Cancer Treatment Group Phase III Trial N0147. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2016–2023. doi: 10.1200/JCO.2012.46.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell PT, Jacobs ET, Ulrich CM, Figueiredo JC, Poynter JN, McLaughlin JR, et al. Case-control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. Journal of the National Cancer Institute. 2010;102:391–400. doi: 10.1093/jnci/djq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Yang SR, Wang PP, Savas S, Wish T, Zhao J, et al. Influence of pre-diagnostic cigarette smoking on colorectal cancer survival: overall and by tumour molecular phenotype. British journal of cancer. 2014;110:1359–1366. doi: 10.1038/bjc.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 33. [cited 2014 July 2];Colon Cancer Family Registry - Data dictionaries. Retrieved from http://coloncfrorg/data-dictionaries Available from:
- 34.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine and science in sports and exercise. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 38.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 39.Young J, Rioffers S, Ries L, Fritz A, Hurlbut A, editors. SEER Summary Staging Manual - 2000: Codes and Coding Instructions, National Cancer Institute, NIH. Pub. No. 01–4969 ed. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 40.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 41.WHO. International Classification of Diseases. 10th ed. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 42.Alsop K, Mead L, Smith LD, Royce SG, Tesoriero AA, Young JP, et al. Low somatic K-ras mutation frequency in colorectal cancer diagnosed under the age of 45 years. European journal of cancer. 2006;42:1357–1361. doi: 10.1016/j.ejca.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Oliner K, Juan T, Suggs S, Wolf M, Sarosi I, Freeman DJ, et al. A comparability study of 5 commercial KRAS tests. Diagnostic pathology. 2010;5:23. doi: 10.1186/1746-1596-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchanan DD, Sweet K, Drini M, Jenkins MA, Win AK, English DR, et al. Risk factors for colorectal cancer in patients with multiple serrated polyps: a cross-sectional case series from genetics clinics. PloS one. 2010;5:e11636. doi: 10.1371/journal.pone.0011636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 46.Newcomb PA, Zheng Y, Chia VM, Morimoto LM, Doria-Rose VP, Templeton A, et al. Estrogen plus progestin use, microsatellite instability, and the risk of colorectal cancer in women. Cancer Res. 2007;67:7534–7539. doi: 10.1158/0008-5472.CAN-06-4275. [DOI] [PubMed] [Google Scholar]
- 47.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 48.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. The Journal of molecular diagnostics : JMD. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cicek MS, Lindor NM, Gallinger S, Bapat B, Hopper JL, Jenkins MA, et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn. 2011;13:271–281. doi: 10.1016/j.jmoldx.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed FE, Vos PW, Holbert D. Modeling survival in colon cancer: a methodological review. Mol Cancer. 2007;6:15. doi: 10.1186/1476-4598-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Des Guetz G, Uzzan B, Bouillet T, Nicolas P, Chouahnia K, Zelek L, et al. Impact of Physical Activity on Cancer-Specific and Overall Survival of Patients with Colorectal Cancer. Gastroenterology research and practice. 2013;2013:340851. doi: 10.1155/2013/340851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jass JR. Molecular heterogeneity of colorectal cancer: Implications for cancer control. Surgical oncology. 2007;16(Suppl 1):S7–s9. doi: 10.1016/j.suronc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 53.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 54.Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60:491–498. doi: 10.1136/gut.2010.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terhaar sive Droste JS, Oort FA, van der Hulst RW, Coupe VM, Craanen ME, Meijer GA, et al. Does delay in diagnosing colorectal cancer in symptomatic patients affect tumor stage and survival? A population-based observational study. BMC cancer. 2010;10:332. doi: 10.1186/1471-2407-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–694. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS medicine. 2012;9:e1001335. doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. American journal of epidemiology. 2010;172:419–429. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.