Summary
Candida albicans and Streptococci of the Mitis group form communities in multiple oral sites, where moisture and nutrient availability can change spatially or temporally. This study evaluated structural and virulence characteristics of Candida-streptococcal biofilms formed on moist or semidry mucosal surfaces, and tested the effects of nutrient availability and hyphal morphotype on dual-species biofilms. Three-dimensional models of the oral mucosa formed by immortalized keratinocytes on a fibroblast-embedded collagenous matrix were used. Infections were carried out using Streptococcus oralis strain 34, in combination with a C. albicans wild-type strain, or pseudohyphal-forming mutant strains. Increased moisture promoted a homogeneous surface biofilm by C. albicans. Dual biofilms had a stratified structure, with streptococci growing in close contact to the mucosa and fungi growing on the bacterial surface. Under semi-dry conditions, Candida formed localized foci of dense growth, which promoted focal growth of streptococci in mixed biofilms. Candida biofilm biovolume was greater under moist conditions, albeit with minimal tissue invasion, compared to semidry conditions. Supplementing the infection medium with nutrients under semidry conditions intensified growth, biofilm biovolume and tissue invasion/damage, without changing biofilm structure. Under these conditions, the pseudohyphal mutants and S. oralis formed defective superficial biofilms, with most bacteria in contact with the epithelial surface, below a pseudohyphal mass, resembling biofilms growing in moist environment. The presence of S. oralis promoted fungal invasion and tissue damage under all conditions. We conclude that moisture, nutrient availability, hyphal morphotype and presence of commensal bacteria influence the architecture and virulence characteristics of mucosal fungal biofilms.
Keywords: Candida, Streptococcus, biofilms, infection models
INTRODUCTION
Candida spp. are opportunistic fungal pathogens that are able to colonize diverse host niches, such as the skin and the urogenital, oral and gastrointestinal tract mucosae of humans (Peleg et al., 2010). At these host sites moisture and nutrient availability can change both spatially and temporally. Candida albicans is the most common oral mucosal fungal pathogen, with infection of otherwise healthy hosts afflicting more frequently denture wearers and the elderly (Lyon et al., 2006).
Reduced salivary flow under denture surfaces and xerostomia, particularly prevalent in the elderly (Johansson et al., 2012), can promote oral fungal infection, especially when combined with poor oral hygiene habits (Lyon et al., 2006) and a high carbohydrate diet (Santana IL et al. 2013). Xerostomia (Lyon et al., 2006), due to radiotherapy at the maxillofacial area, Sjögren's syndrome or the use of xerogenic medications (Guobis Ž et al., 2011) may also predispose the host to this oral infection. However, apart from the reduced amounts of salivary proteins with antimicrobial properties (Meiller et al., 2009), no other mechanisms for the increased virulence of this organism under xerostomic conditions have been proposed.
Under host-permissive conditions (Cassone & Cauda, 2012; Gavaldà et al., 2014; Singh et al., 2014), once C. albicans forms a mucosal biofilm it can invade the superficial strata of the oral mucosa (Williams et al. 2013), disperse to the esophageal mucosa, invade deeper into the submucosa, and potentially disseminate to distant organs haematogenously (Conti et al., 2009). Although much more rare in humans (Lewis et al., 2013), this deep organ dissemination is common in mouse models of oral infection (Conti et al., 2009, Xu et al., 2014b), following the development of severe oropharyngeal and esophageal candidiasis. Germination of fungal cells to form true hyphae is a pre-requisite for epithelial invasion and damage in several experimental systems (Phan et al., 2000; Schlecht et al., 2014; Villar et al., 2004).
More recently, we reported that one of the factors that promote oral mucosal invasion by C. albicans is the presence of commensal streptococci (Diaz et al., 2012, Xu et al., 2014a). Recently, an in vivo study using mice (Xu et al., 2014b) and an in vitro study using oral and esophageal mucosal models (Diaz et al., 2012), both from our group, demonstrated that when C. albicans forms mucosal biofilms with oral streptococci of the mitis group, mixed biofilms acquire a more pathogenic potential, increasing Candida mucosal tissue invasion and amplifying the mucosal inflammatory response. Interestingly, the presence of C. albicans promotes an increase of the streptococcal biofilm biomass, forming communities with direct physical interactions (Diaz et al., 2012). These interactions are likely mediated at least in part by adhesins such as SspA and SspB on streptococci (Peleg et al., 2010; Silverman et al., 2010) and the N-terminal domain of the Als3 protein, an epithelial invasin, in C. albicans (Bamford et al., 2015).
Since interaction of C. albicans with streptococci of the mitis group is an important determinant of fungal pathogenicity, at least in mouse models of mucosal infection by C. albicans,, the aim of this study was to analyze the structural and virulence characteristics of Candida-streptococcal mixed biofilms forming on mucosal surfaces with different degrees of moisture or nutrients to simulate diverse mucosal environments or host colonization sites in vivo. For this we used three-dimensional models of the human oral mucosa formed by immortalized oral keratinocytes on a fibroblast-embedded collagenous matrix to grow mixed biofilms. Infections were carried out using a C. albicans wild-type reference strain, two C. albicans mutants with known hyphal defects (Sellam et al., 2010, Kadosh & Johnson, 2005, Nobile et al., 2012), and Streptococcus oralis 34, a strain with demonstrated pathogenic synergy in a mouse model of oral infection (Xu et al.,2014b). We hypothesized that Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotype.
MATERIALS AND METHODS
Microorganisms used and microbiological media
The microorganisms used in the present study were Candida albicans wild-type reference strain SN425 which forms true hyphae (Noble et al., 2010), an ndt80 homozygous deletion mutant (CJN2412) (Nobile et al., 2012) and its revertant (CJN2328) (Nobile et al., 2012), both derived from this parental wild type strain. A tup1 homozygous deletion mutant (BCa 2–10) (Kadosh & Johnson, 2005) was also used. Strains CJN2412 and BCa 2–10 are defective in true hyphae formation, but are able to form pseudohyphal filaments under most growth conditions (Kadosh & Johnson, 2005; Nobile et al., 2012; Villar et al., 2004), and originated from the same wild type progenitor, strain CAI4. Streptococcus oralis 34 (a kind gift from PE Kolenbrander) was used in these studies since it was shown to form robust mucosal biofilms in the presence of C. albicans (Diaz et al., 2012; Xu et al., 2014b). All C. albicans strains were kept in −80 °C stock cultures, and grown in yeast extract-peptone-dextrose (YPD) agar one week before each experiment. Overnight YPD broth cultures were prepared one day before each experiment, at 70 rpm agitation, aerobically, at room temperature (25°C). The YPD medium consisted of 5 g of yeast extract (Fisher Scientific, Pittsburgh, PA) liter−1, 10 g of peptone (Fisher Scientific) liter−1, and 20 g dextrose (Fisher Scientific) liter−1. Streptococcus oralis was kept in −80 °C stock cultures and reactivated one day before the experiment by overnight growth in brain heart infusion (BHI) medium (Oxoid, Ltd., Cambridge, United Kingdom) under static conditions at 37°C, in a 5% CO2 incubator.
In vitro oral mucosal tissue models
The in vitro oral mucosal models were described in detail previously by Dongari-Bagtzoglou & Kashleva (2006). Briefly, oral mucosa analogues are formed using trans-well inserts which allow limited diffusion of culture media from the bottom of the well. An airlifting growth phase ensures epithelial differentiation and stratification. Analogues consist of human immortalized oral keratinocytes (OKF6/TERT-2) (Wöllert et al., 2012) or SCC15 (ATCC) (Dongari-Bagtzoglou & Kashleva, 2006) seeded (5× 105 cells per well) over a collagen type I matrix, embedded with fibroblasts (3T3 cell line, ATCC). The procedure takes approximately 2 to 3 weeks to complete resulting in a non-keratinizing stratified squamous epithelium. After tissue maturation, culture media with no antibiotics were used for 24h prior to infection with C. albicans and S. oralis.
Inoculation of mucosal tissues with C. albicans and S. oralis
Overnight cultures of C. albicans were washed with PBS and cells were counted on a Neubauer chamber in order to standardize the inoculum. Overnight stationary-phase cultures of S. oralis were inoculated in fresh BHI broth and then allowed to reach the late logarithmic phase for 3–4 h (optical density at 600 nm of 1.0, corresponding to 107 cells/ml). The final inoculum consisted of 106 cells of C. albicans and 107 cells of S. oralis in 20 µL of infection medium (consisting of DMEM, supplemented with L-glutamine, hydrocortisone, ITES, O-phosphorylethanolamine, adenine and triiodothyronine) pipetted in the middle of each well. After a 45 minute incubation at room temperature, infection media were added outside (semidry condition) or inside and outside (moist condition) of the well insert. To rule out the possibility that biofilms growing in a moist environment presented characteristics related to increased nutrient availability, we supplemented the inoculation media with increasingly high concentrations of BHI (0%, 5%, 10%) under semi-dry conditions. Biofilms were then allowed to develop for 16 hours at 37°C, 5% CO2. An uninfected tissue was used as a control to evaluate tissue viability under all conditions.
Determination of biofilm biovolume and structure by confocal laser scanning microscopy
For biofilm biovolume and structure analysis, tissue samples were fixed in 4% paraformaldehyde for 2 hours. Subsequently C. albicans was stained using fluorescein isothiocyanate (FITC)-labeled anti-Candida polyclonal antibody (Meridian Life Science, Saco, ME). For biofilms containing S. oralis, this step was followed by FISH with the Streptococcus-specific oligonucleotide probe STR405 (Thurnheer et al., 2001), labeled with Alexa 546, as previously described (Dongari-Bagtzoglou et al., 2009). Biofilms were visualized using a Zeiss LSM 510 confocal scanning laser microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) with an argon laser (488- and 543-nm), using air Plan-Apochromat ×20/0.8 objective. Stacks of z-plane images from at least nine different fields of view per sample were acquired and then reconstructed into 3-D images using the IMARIS software (Bitplane, Inc., Saint Paul, MN). Isosurface reconstructions using the surpass mode were used to calculate the biovolume (in µm3/microscopic field) of each microorganism.
Determination of mucosal invasion
Determination of tissue invasion was done in samples fixed in 4% paraformaldehyde for 2 hours, followed by a series of ethanol and xylene dehydrations before paraffin embedding. Hematoxylin and eosin stained sections were used to observe tissue architecture, and biofilm distribution and invasion through the tissue layers. To detect invasion of each organism sections were also stained by immunofluorescence for C. albicans and FISH for S. oralis as described above, and counter-stained with nucleic acid stain Hoechst 33258 (Invitrogen, Carlsbad, CA) to visualize the epithelial layers (Diaz et al., 2012). Images were obtained using a Zeiss Axio Imager M1 microscope and an EC Plan-Neofluar ×20 NA 0.5 air objective and further analyzed by the AxioVision LE64 program.
Quantification of tissue damage by lactase dehydrogenase assay
Lactate dehydrogenase (LDH) release into the basal culture media was monitored as an indicator of tissue/cell damage; media from uninfected tissues served as negative controls. The CytoTox-ONE Homogeneous Membrane Integrity Assay kit (Promega, Madison, WI, USA) was used to assay LDH activity using an Opsys MR™ Microplate Reader (Dynex Technologies Inc., Chantilly, VA, USA) and the Revelation QuickLink software (Thermo Labsystems, Chantilly, VA) according to the manufacturer's protocol. The LDH data were expressed as optical density units.
Statistical analysis
Statistical analyses were performed using SigmaPlot 12 software (SigmaPlot v. 12.3, Systat Software Inc., San Jose, USA), using a 5% significance threshold. Data were evaluated by analysis of variance (ANOVA) and when statistical significances were found, all pairwise multiple comparisons were performed with a Bonferroni t-test.
RESULTS
C. albicans-streptococcal biofilm architecture, submucosal invasion and mucosal cell damage are determined by the amount of moisture on the mucosal surface
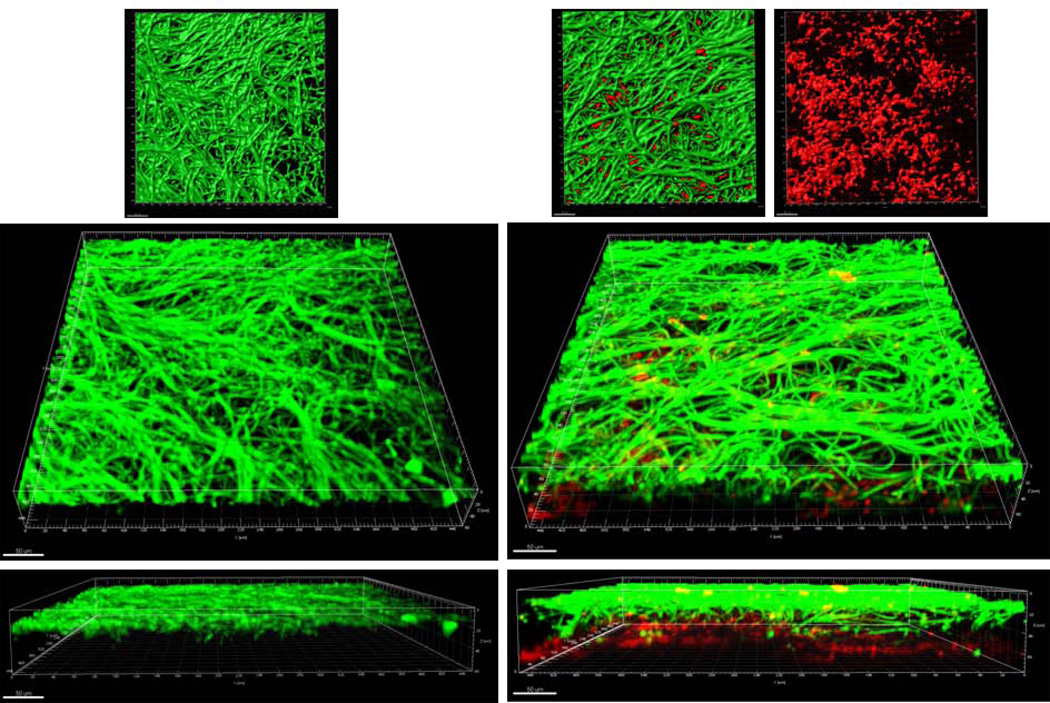
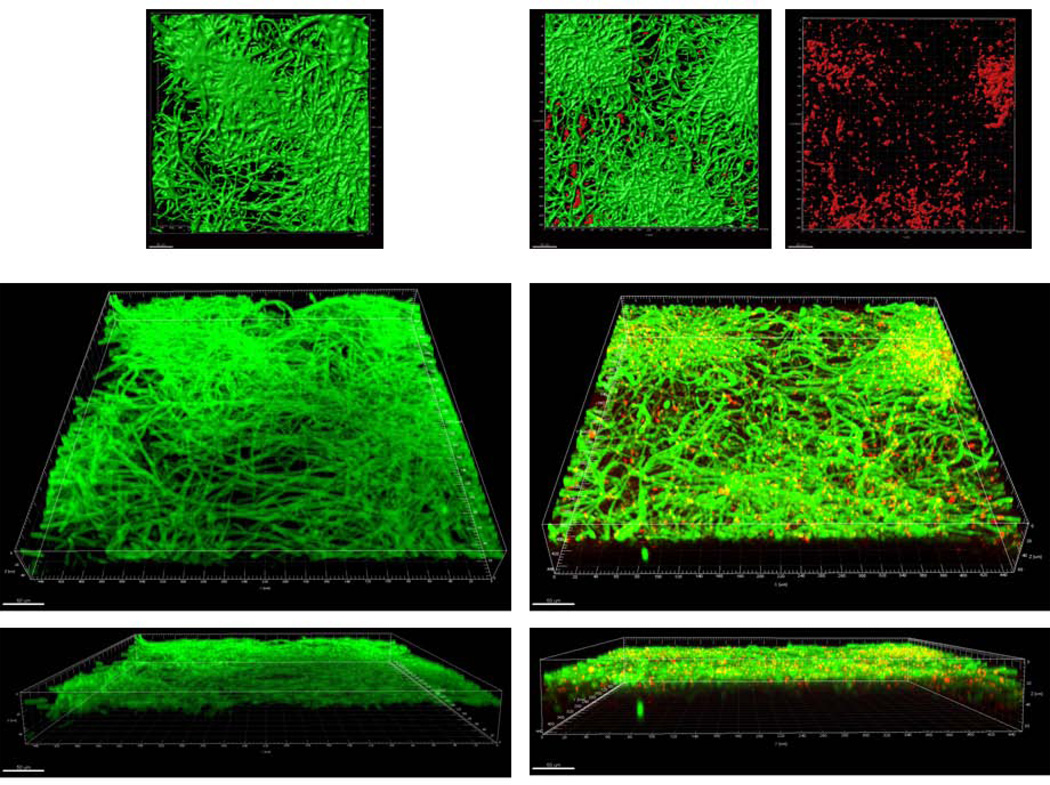
First we compared the structural characteristics of mucosal biofilms formed on wet (media-submerged) versus semidry mucosal surfaces (media limited to inoculum). When grown under moist conditions, the wild-type C. albicans strain extended long intertwined hyphae, to form homogeneous surface biofilm mats in both single and mixed biofilms (Fig. 1A). In mixed biofilms under these conditions, S. oralis also formed a relatively homogeneous biofilm mat which was located under the Candida biofilm in contact with the mucosa, resulting in two distinct strata (fungal biofilm apically, and bacterial biofilm basally). In contrast, when grown under semi-dry conditions, wild-type C. albicans formed localized foci of dense growth from which hyphae extended radially to intertwine with hyphae from adjacent foci (Fig. 1B). In mixed biofilms under semidry conditions, S. oralis grew in close physical contact with the fungal hyphae within the foci, as shown by the yellow co-localization pattern in the 3 dimensional reconstruction of the mucosal biofilm (Fig. 1B). Thus the degree of moisture strongly influenced the structure of the mixed biofilm. In the absence of C. albicans, S. oralis did not form a robust mucosal biofilm under the conditions tested (data not shown), consistent with previous findings (Diaz et al., 2012).
Figure 1.
A: Sixteen-hour mucosal biofilms of C. albicans monospecies (left panel) or C. albicans-streptococci mixed-species (right panel). Biofilms were grown on the surface of three-dimensional models of oral mucosa under wet (media-submerged) conditions. X-Y isosurfaces (top panel) and 3-D reconstructions (bottom panels) of representative confocal laser scanning microscopy images are shown. C. albicans (green) was visualized after staining with a FITC-conjugated anti-Candida antibody. S. oralis (red) was visualized after fluorescence in situ hybridization (FISH) with a Streptococcus-specific probe conjugated to Alexa 546. Scale bar = 50 µm.
B: Sixteen-hour mucosal biofilms of C. albicans monospecies (left panel) or C. albicans-streptococci mixed-species (right panel). Biofilms were grown on the surface of three-dimensional models of oral mucosa under semidry (media limited to inoculum) conditions. X-Y isosurfaces (top panel) and 3-D reconstructions (bottom panels) of representative confocal laser scanning microscopy images are shown. C. albicans (green) was visualized after staining with a FITC-conjugated anti-Candida antibody. S. oralis (red) was visualized after fluorescence in situ hybridization (FISH) with a Streptococcus-specific probe conjugated to Alexa 546. Scale bar = 50 µm.
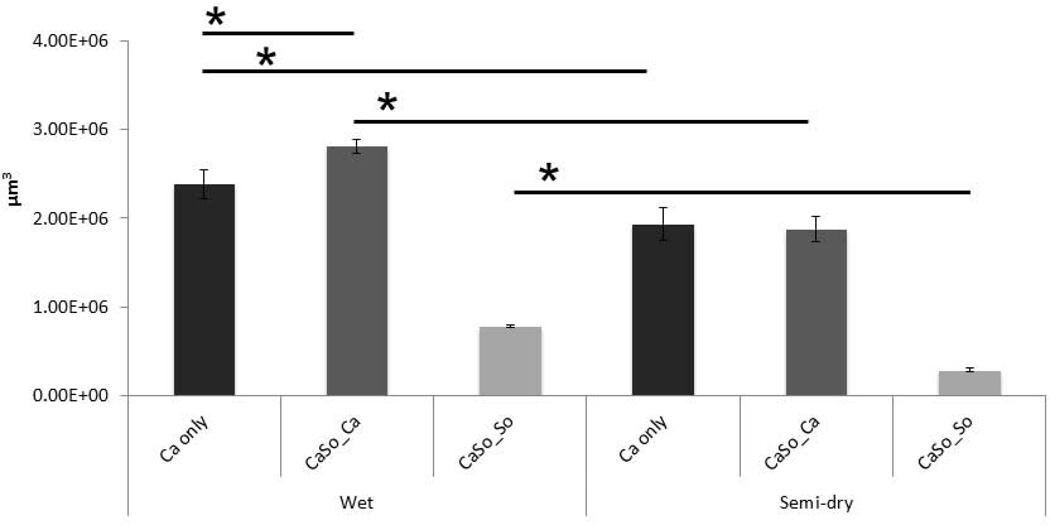
When biofilm biovolumes were compared between moist and semidry conditions, there was a significant increase in the biovolume of wild-type C. albicans in both single and dual biofilms in moist versus semidry biofilms (p≤0.001). There was also a small but statistically significant increase in wild-type C. albicans biovolume in dual- compared to single-species C. albicans biofilms under wet (p≤0.001, Fig. 2), but not under semidry conditions. Finally, wet conditions favored growth of S. oralis in dual-species biofilms (p≤0.001) but did not increase single-species biofilm biovolume (data not shown).
Figure 2.
Biovolumes of sixteen-hour single C. albicans (Ca) or mixed C. albicans-S. oralis (CaSo) mucosal biofilms. Average biovolumes were calculated (in µm3) for each species in eight different confocal laser scanning microscopy images using image stacks from two independent experiments. Average biovolumes of C. albicans in single (Ca only) or mixed (CaSo_Ca) biofilms and S. oralis (CaSo_So) in mixed biofilms, under wet (media-submerged) or semidry (media limited to inoculum) conditions. *P = <0.001 when monospecies biovolumes were compared to mixed-species biovolumes or between wet and semi-dry conditions, using the Bonferroni t-test. The error bars indicate one standard deviation of the mean of eight different images from two independent experiments.
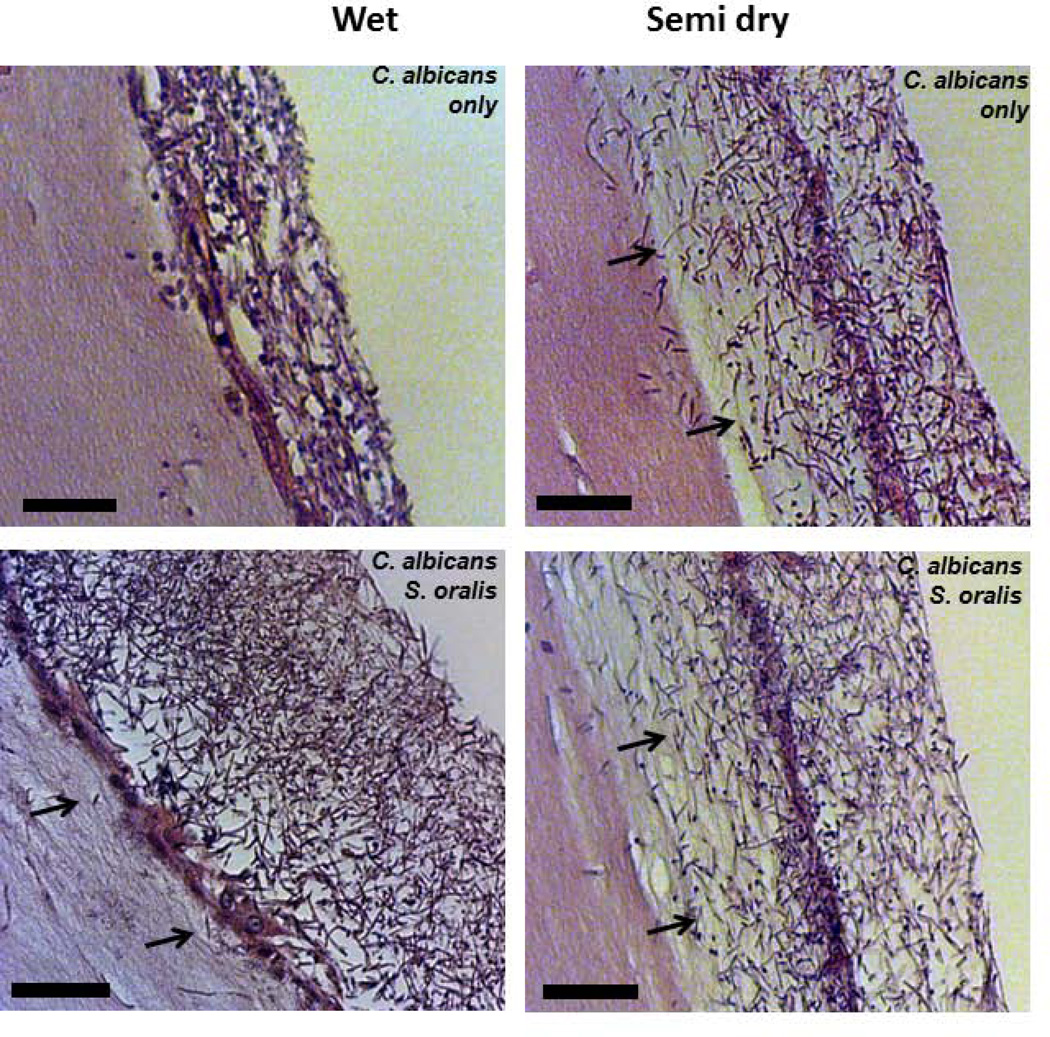
Interestingly, although the wild-type Candida biofilm biovolume was significantly greater under moist conditions, fungal growth in these biofilms was mostly superficial and was associated with minimal tissue invasion compared to semidry conditions where the epithelial barrier was completely breached (Fig. 3). Under wet conditions, S. oralis, which formed a biofilm adjacent to the mucosa layer (Fig. 1A), promoted invasion of wild-type C. albicans past the epithelial barrier (Fig. 3) and increased tissue damage (p≤0.005, Fig. 4). These results are in agreement with our previous findings in a saliva-supplemented mucosal flow cell system (Diaz et al., 2012). Under semidry conditions both single- and dual-species biofilms traversed the entire thickness of the stratified epithelium and entered the submucosal compartment (Fig. 3). Consistent with these findings, tissue damage was highest in dual-species biofilms under semidry conditions (Fig. 4). These findings are also summarized in Table 1.
Figure 3.
H&E-stained tissue sections of 16 hour C. albicans and C. albicans-streptococci mixed-species mucosal biofilms under wet (media-submerged) or semidry (media limited to inoculum) conditions. Arrows indicate invasion of C. albicans through the epithelial barrier formed by OKF6 cells. Scale bar = 50 µm.
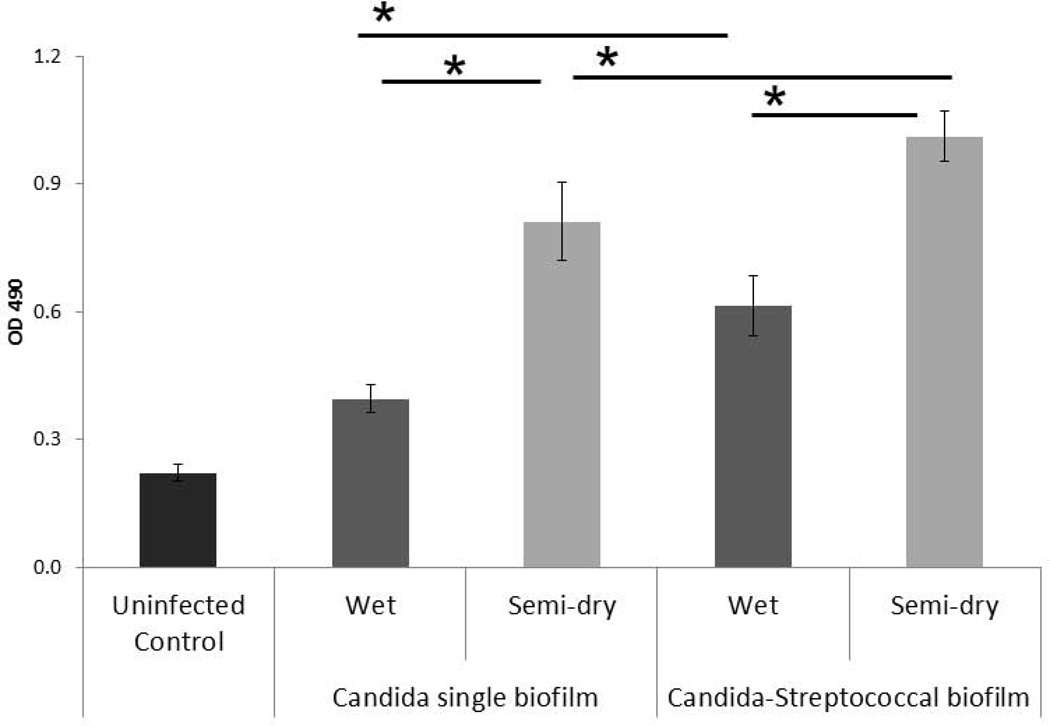
Figure 4.
Lactate dehydrogenase (LDH) released by mucosal cells. Results represent the average OD490 of subnatant samples from triplicate wells in two independent experiments. *P< 0.05 for a comparison with C. albicans and C. albicans-streptococci mixed-species mucosal biofilms under wet (media-submerged) and semidry (media limited to inoculum) conditions, using the Bonferroni t-test. The error bars indicate one standard deviation of the mean of three different wells from two independent experiments.
Table 1.
| Candida biofilm growth conditions |
Biofilm architecture | Dual biofilm biovolume |
Tissue invasion and damage |
|---|---|---|---|
| Wet | Long intertwined hyphae forming a homogeneous surface biofilm | + + + | + |
| Wet, plus S. oralis | S. oralis formed a biofilm mat adjacent to the mucosa under C. albicans | + + + + | ++ |
| Semi-dry | Localized foci of dense hyphal growth | + + + | + + + |
| Semi-dry, plus S. oralis | S. oralis in close physical proximity with hyphae within foci | + + + | + + + + |
| Semi-dry, plus S. oralis, 5% BHI | S. oralis in close physical proximity with hyphae within foci | + + + + | + + + + + |
| Semi-dry, plus S. oralis, 10% BHI | S. oralis in close physical proximity with hyphae within foci | + + + + + | + + + + + + |
Nutrient availability increases mucosal biofilm growth and tissue destruction but does not affect architecture of C. albicans-streptococcal biofilms
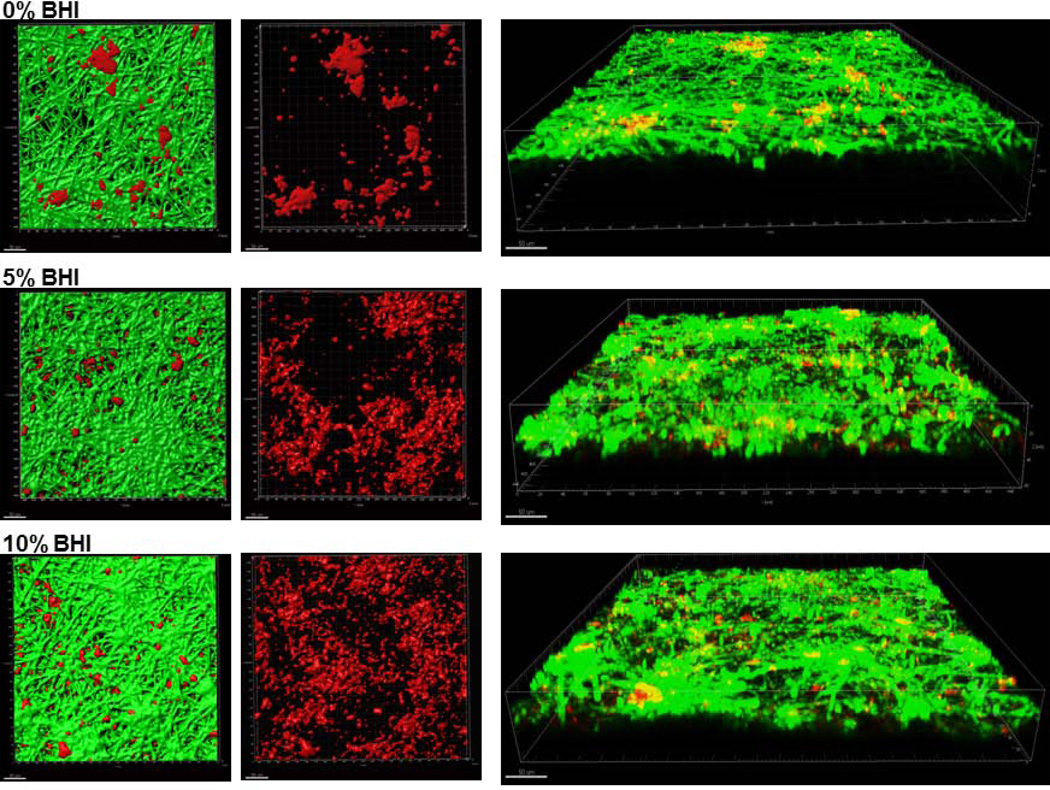
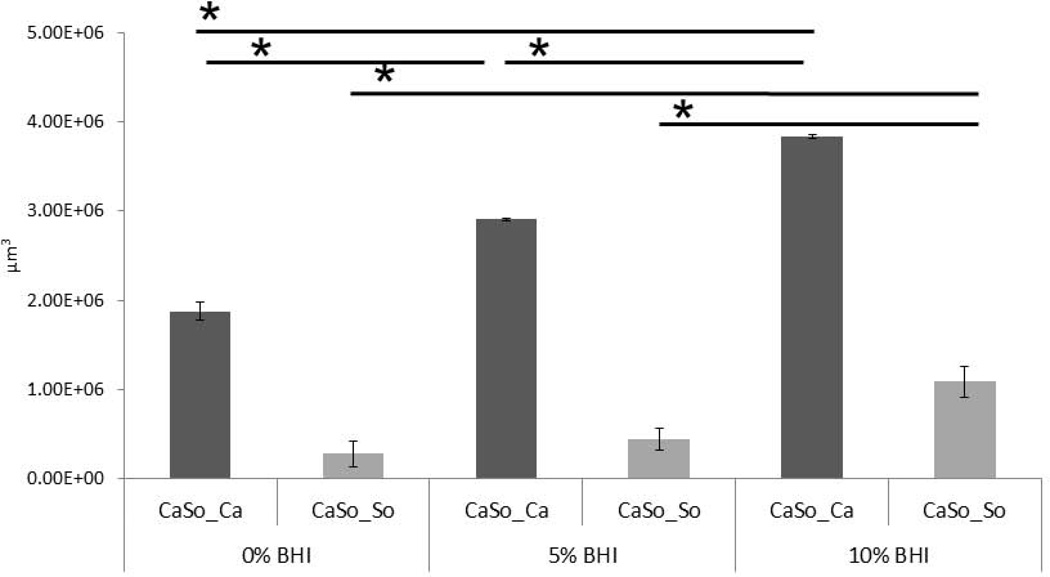
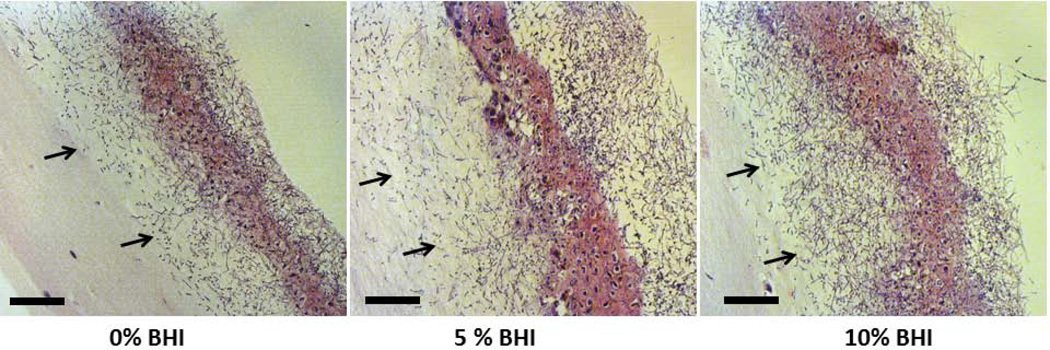
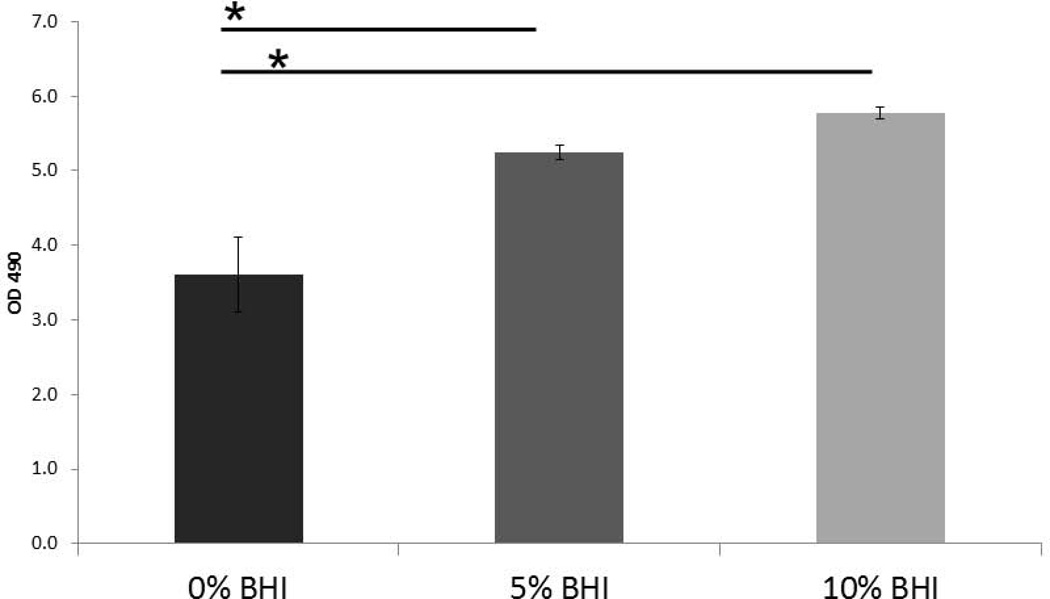
To rule out the possibility that the “wet” biofilm stratified, superficial growth phenotype was attributed to increased nutrient availability we supplemented the microorganism inoculation media with increasingly high concentrations of BHI under semi-dry conditions. We hypothesized that if the “wet” growth phenotype was due to increased nutrient availability in the media, by supplementing the inoculation media with increasing amount of nutrients under semidry conditions, we could promote a more homogeneous superficial growth as opposed to focal, invasive growth. However, supplementing the infection medium with increasing amounts of BHI under semidry conditions did not change the focal biofilm architecture but rather intensified the growth of the foci, which coalesced to form a denser biofilm mat over the entire mucosal surface at the maximum concentration of BHI tested (10%) (Fig. 5A). Increasing the BHI concentration under semidry conditions, led to a dose-dependent increase in wild-type C. albicans biovolumes in mixed Candida-streptococcal biofilms (p≤0.001). The biovolume of S. oralis was also significantly increased in mixed biofilms grown with 10% BHI, compared to 0 and 5% (Fig. 5B). Fungal invasion past the epithelial barrier and tissue damage were also greatest at the highest concentration of nutrients, consistent with the increased biofilm growth and tissue damage (Fig. 6A and 6B). From these experiments we also concluded that semidry conditions using inoculation media supplemented with 10% BHI were optimal for pathogenic synergy and thus these conditions were used for all subsequent experiments.
Figure 5.
A: Sixteen-hour C. albicans-streptococci mixed-species biofilms. Biofilms were grown on the surface of oral mucosa analogues under semidry (media limited to inoculum) conditions and microbial inoculation media were supplemented with 0%, 5% or 10% brain heart infusion broth (BHI). X-Y isosurfaces (left two panels) and 3-D reconstructions (right panel) of representative confocal laser scanning microscopy images of biofilms. C. albicans (green) was visualized after staining with a FITC-conjugated anti-Candida antibody. S. oralis (red) was visualized after fluorescence in situ hybridization (FISH) with a Streptococcus-specific probe conjugated to Alexa 546. Center panel images display the red channel only, showing S. oralis distribution in mixed-species biofilms. Scale bar = 50 µm.
B: Average biovolumes (in µm3) for each species in 16h C. albicans. S. oralis (CaSo) mixed-species biofilms grown under the conditions shown in figure 5A. Biovolumes were measured in eight different CLSM image stacks from two independent experiments. Bars represent average biovolumes of C. albicans (CaSo_Ca) or S. oralis (CaSo_So) when grown together. *P = <0.001 when biovolumes were compared between different BHI concentrations, using the Bonferroni t-test. The error bars indicate one standard deviation of the mean of eight different images from two independent experiments.
Figure 6.
A: H&E-stained tissue sections of 16 h C. albicans-streptococci mixed-species mucosal biofilms. Biofilms were grown on the surface of oral mucosa analogues under semidry (media limited to inoculum) conditions and microbial inoculation media were supplemented with 0%, 5% or 10% BHI. Arrows showing submucosal invasion through the multilayer epithelial barrier formed by SCC15 cells. Scale bar = 50 µm.
B: LDH released by oral mucosa analogues with 16 h C. albicans-streptococci mixed-species mucosal biofilms. Biofilms were grown on the surface of oral mucosa analogues under semidry (media limited to inoculum) conditions and microbial inoculation media were supplemented with 0%, 5% or 10% BHI. Results represent the average of OD490 values in triplicate wells from two independent experiments. *P< 0.05 for a comparison with 0% BHI, using the Bonferroni t-test. The error bars indicate one standard deviation of the mean of three different wells from two independent experiments.
Biofilm architecture and virulence characteristics of Candida-streptococcal biofilms are affected by the hyphal morphotype
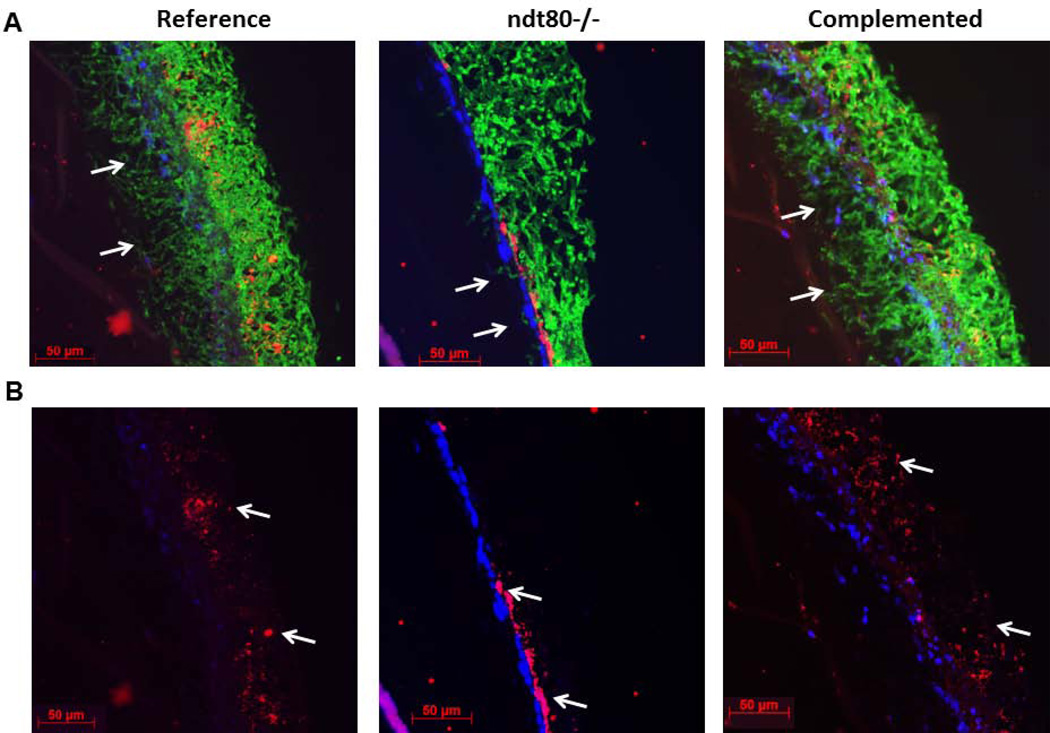
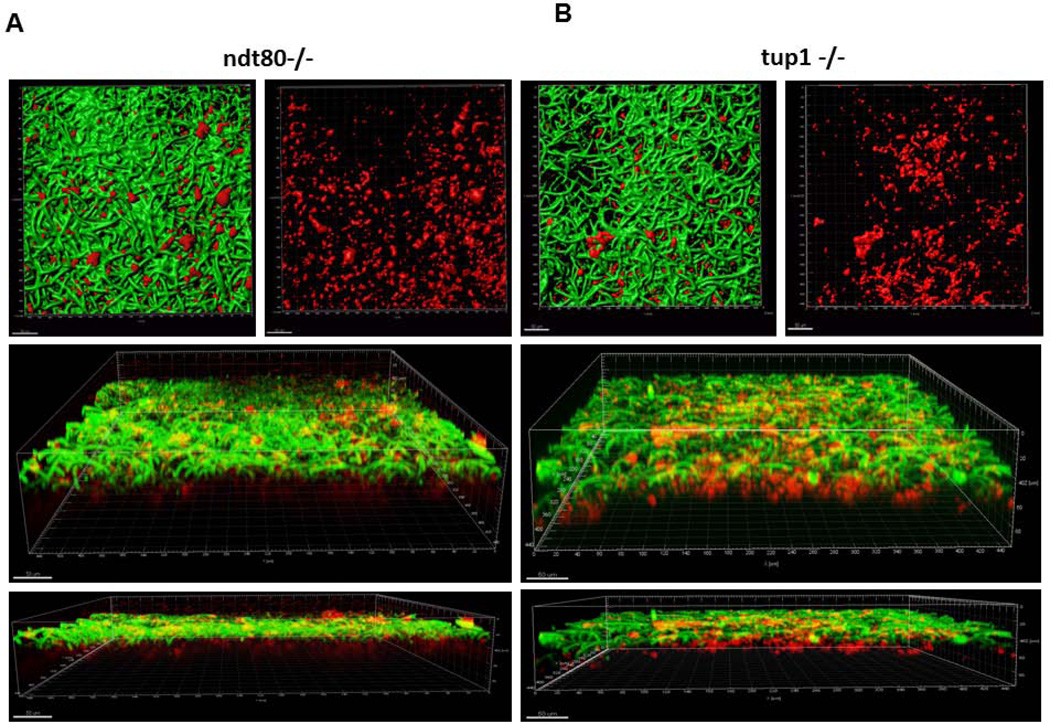
A C. albicans homozygous ndt80 deletion mutant forms defective abiotic surface biofilms composed of long pseudohyphae under several environmental conditions (Nobile et al., 2012). Given the strong co-aggregation interactions between true hyphae and oral streptococci of the Mitis group (Bamford et al., 2015; Silverman et al., 2010; Xu et al., 2014b), we asked whether the biofilm architecture of this strain with S. oralis may be affected by its pseudohyphal morphology. In mixed biofilms with the reference and ndt80-complemented strains, S. oralis grew in close contact with Candida hyphae and was interspersed throughout the thickness of the biofilm (Fig. 7A and 7B). In contrast, when growing with the ndt80 pseudohyphal mutant, S. oralis mostly grew in close contact with the mucosal surface (Fig. 7A and 7B). Three dimensional reconstructions of dual biofilms further showed that the pseudohyphae spread on the surface of the bacterial layer, forming a biofilm with a mixed, partially stratified architecture since some bacteria also co-aggregated with pseudohyphae (please note yellow co-localization, Fig. 8A). To rule out the possibility that the mixed biofilm phenotype of the ndt80 deletion strain was gene-specific we tested the phenotype of another C. albicans transcription factor mutant, tup1, which also forms pseudohyphae under most environmental conditions (Kadosh et al., 2005; Villar et al., 2004). As shown in fig. 8B, similar to the ndt80 mutant, this pseudohyphal strain exhibited a partially stratified biofilm architecture with some bacteria interspersed throughout the pseudohyphal mass but most growing in contact with the epithelial surface.
Figure 7.
C. albicans-streptococci mixed-species mucosal biofilms grown under semidry (media limited to inoculum) conditions. C. albicans reference strain, ndt80 homozygous deletion mutant (ndt80−/−) and its complemented strain were grown in the presence of S. oralis 34 for 16h. Representative tissue sections are shown where C. albicans (green) was visualized after staining with a FITC-conjugated anti-Candida antibody and S. oralis (red) was visualized after fluorescence in situ hybridization (FISH) with a Streptococcus-specific probe conjugated to Alexa 546; Mucosal cell nuclei were counterstained with the nucleic acid stain Hoechst 33258 (blue). A: Overlay of three-color images, showing invasion of each Candida strain into the submucosal compartment (white arrows). B: Red and blue channel overlay, showing S. oralis and epithelial nuclei, respectivelly, highlighting the distinct S. oralis biofilm architecture in mutant vs reference and complemented strain biofilms.
Figure 8.
C. albicans-streptococci mixed-species mucosal biofilms grown under semidry (media limited to inoculum) conditions. C. albicans pseudohyphal strains, ndt80 homozygous deletion mutant (ndt80−/−) and tup1 homozygous deletion mutant (tup1−/−) were grown in the presence of S. oralis 34 for 16h. Representative x-y isosurfaces (top panel) and 3-D reconstructions (bottom panel) of representative CLSM images. C. albicans (green) was visualized after staining with a FITC-conjugated anti-Candida antibody. S. oralis (red) was visualized after fluorescence in situ hybridization (FISH) with a Streptococcus-specific probe conjugated to Alexa 546. Note the partially stratified structure of biofilms with bacteria co-aggregating on the surface of the oral mucosa as well co-localizing with pseudofilaments (yellow). Scale bar = 50 µm.
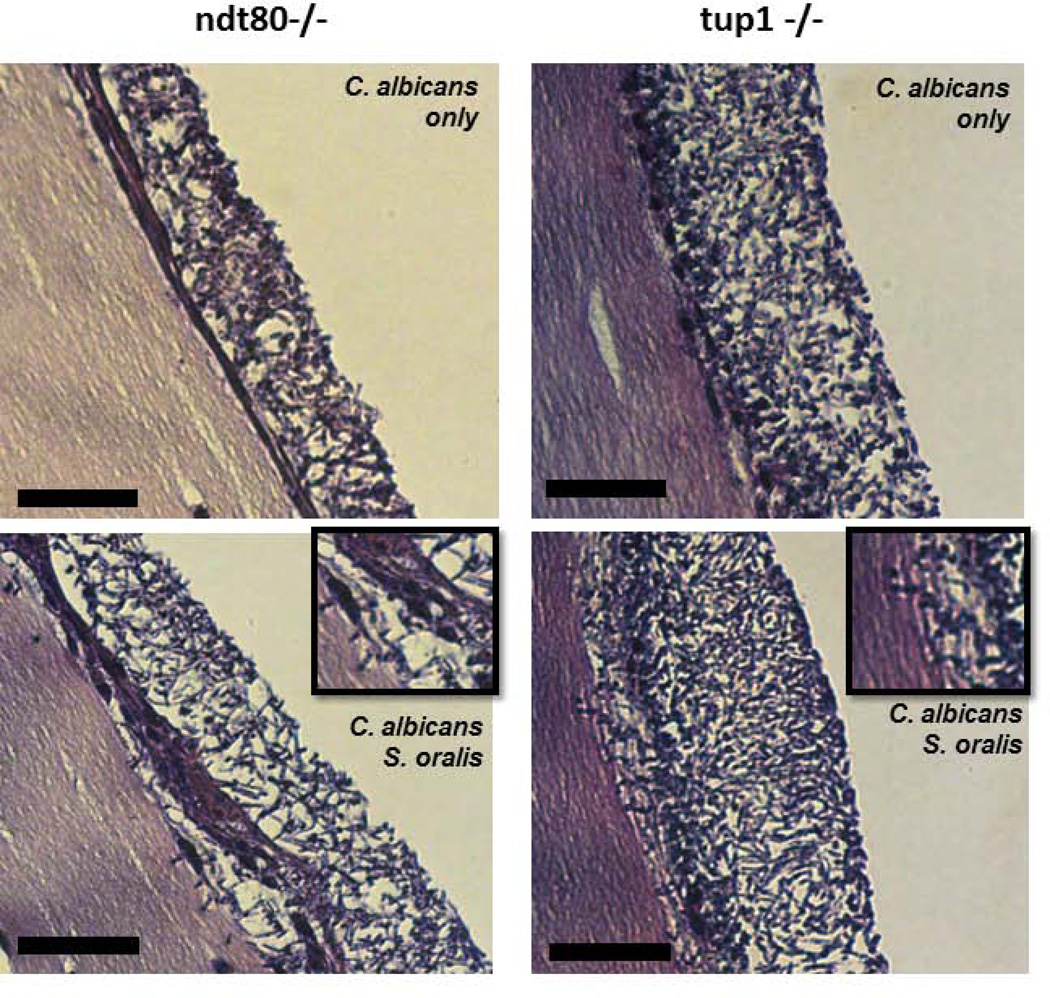
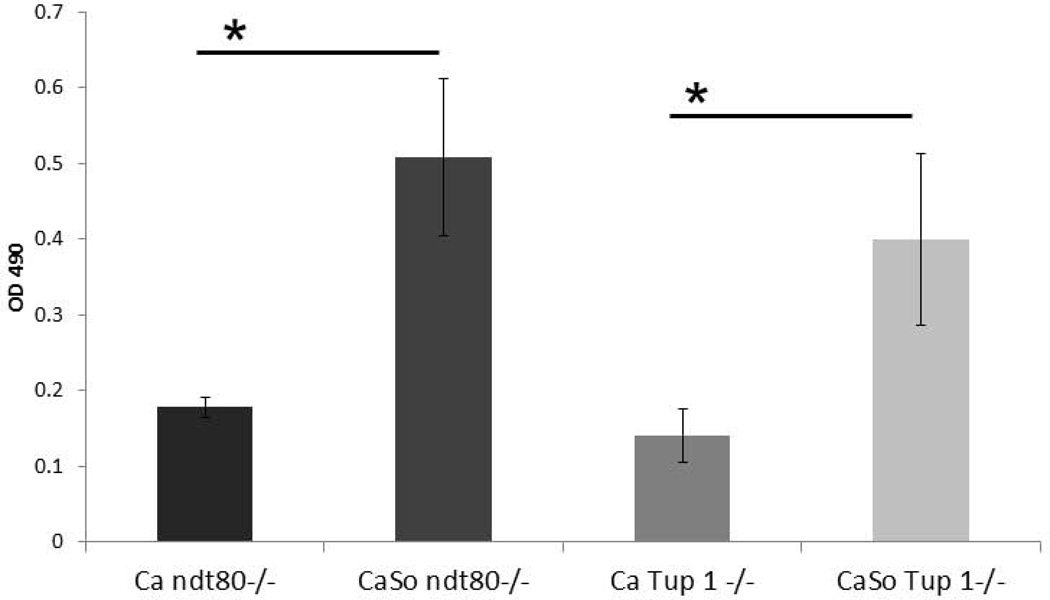
We have previously shown that pseudohyphal organisms are deficient in invading oral epithelium and triggering cell damage (Villar et al., 2004), we thus wondered whether the presence of streptococci could alter this phenotype. As shown in Fig. 9A, in the absence of S. oralis neither pseudohyphal mutant was able to cross the mucosal barrier. However, S. oralis triggered a significant crossing-over of pseudohyphae into the submucosal compartment. This was consistent with the increased tissue damage, as assessed by measuring LDH release, in dual- versus single-species pseudohyphal biofilms (Fig. 9B).
Figure 9.
A: H&E-stained tissue sections of biofilms shown in figure 8. Magnifying squares show invasion of C. albicans pseudohyphae through the epithelial barrier for both mutant strains. Scale bar = 50 µm.
B: LDH released by mucosal analogues inoculated under semidry conditions with pseudohyphal mutants in the presence or absence of S. oralis 34 for 16h. Bars represent the average of the OD490 values in triplicate wells from two independent experiments. *P< 0.05 for a comparison of C. albicans monospecies and C. albicans-streptococci mixed-species mucosal biofilms, using the Bonferroni t-test. The error bars indicate one standard deviation of the mean of three different wells from two independent experiments.
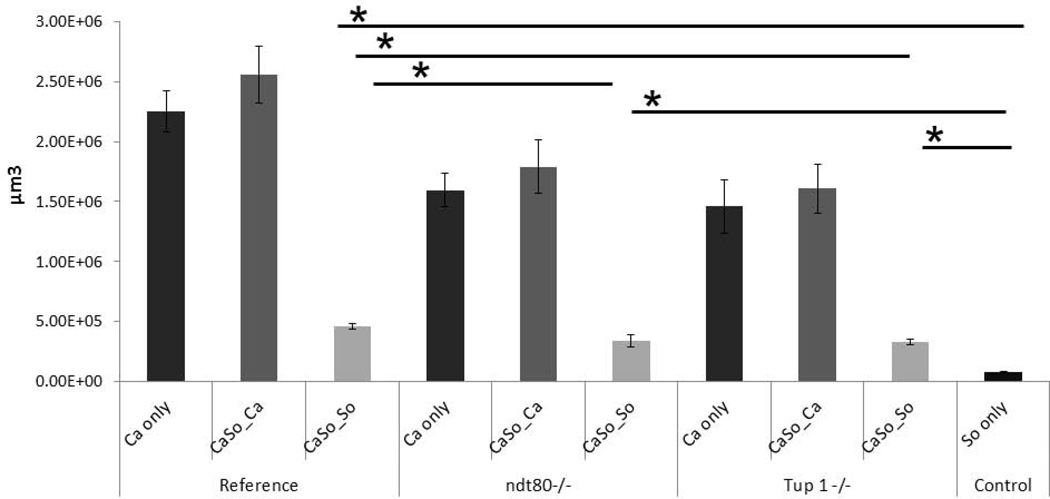
As expected, biofilm biovolume estimates showed that pseudohyphal mutants formed deficient single- and dual-species biofilms compared to the reference strain (Fig. 10). Both pseudohyphal strains were able to promote S. oralis biofilm growth, compared to S. oralis alone, albeit at lower levels compared to the reference strain (p ≤0.001). However there were no significant differences in the biovolumes of C. albicans in a comparison between dual- and single-species biofilms in both pseudohyphal strains. This suggests that pseudohyphal invasion, which was noted only in the presence of S. oralis, was a consequence of the streptococcal presence and not due to increased fungal growth in dual-species biofilms. These findings are also summarized in Table 2.
Figure 10.
Average biovolumes (in µm3) for each species in 16h C. albicans-S. oralis (CaSo) mixed-species biofilms grown under the conditions shown in figures 8–9. Biovolumes were measured in eight different CLSM image stacks from two independent experiments. Bars represent average biovolumes of C. albicans (Ca only) single biofilms, S. oralis (So only) single biofilms and C. albicans (CaSo_Ca) or S. oralis (CaSo_So) when grown together. *P = <0.001 when biovolumes were compared between different strains using the Bonferroni t-test. The error bars indicate one standard deviation of the mean of eight different images from two independent experiments.
Table 2.
| Strain/Hyphal morphotype |
Dual biofilm architecture | Dual biofilm biovolume |
Tissue invasion and damage |
|---|---|---|---|
| Wild type/hyphae | S. oralis in close proximity to hyphae, interspersed throughout the thickness of the biofilm | + + + + + | + + + + + + |
| ndt80 +/+/hyphae | S. oralis in close proximity to hyphae. Interspersed throughout the thickness of the biofilm | + + + + + | + + + + + + |
| ndt80−/−/pseudohyphae | Most S. oralis cells localized adjacent to the mucosal surface, some also co-aggregated with pseudohyphae | + + | C. albicans only + C. albicans+S. oralis + + + |
| tup1−/−/pseudohyphae | Most S. oralis cells localized adjacent to the mucosal surface, some also co-aggregated with pseudohyphae | + + | C. albicans only + C. albicans+S. oralis + + + |
DISCUSSION
In recent years C. albicans-bacterial cross-kingdom interactions have received increased attention (Shirtliff et al., 2009; Morales & Hogan, 2010). Synergistic interactions leading to increased virulence are complex and may be affected by specific environmental host conditions such as mucosal or salivary secretions (Kavanaugh et al., 2014, Edgerton & Koshlukova, 2000). For example, in addition to providing moisture, saliva contains proteins with antifungal properties, such as histatins, that form part of the innate immune system (reviewed by Edgerton & Koshlukova, 2000). In this work we showed that reduced moisture on oral mucosal surfaces promotes fungal invasion and tissue damage, independently of host-derived antimicrobial proteins or of the amount of nutrients present. Importantly, we showed that streptococcal growth promotes C. albicans mucosal invasion and damage both under moist and semi-dry conditions. Surprisingly, we also found that in the presence of streptococci, fungal invasion and tissue damage can bypass the requirement for true hyphal transformation, although the use of a yeast-locked mutant would be required to completely address this question. A certain degree of epithelial invasion by C. albicans has been suggested to be compatible with stable colonization and commensalism, however, significant invasion that leads to tissue damage is the hallmark of the transition of this fungus to pathogenicity (Gow & Hube, 2012). The fact that oral streptococci can trigger further fungal invasion and tissue damage under different experimental conditions is an indication that commensal bacteria may play an important role in the transition of C. albicans from commensalism to pathogenicity.
Presence of sufficient moisture provides adequate growth conditions for C. albicans on human skin without the need of exogenously added nutrients (Leyden, 1984). This also held true in our experimental mucosal system where increased moisture on the mucosal surface promoted robust fungal growth in both single- and dual-species biofilms, in the absence of added microbial nutrients. However, this is the first report to demonstrate that lack of adequate moisture on a host surface can alter biofilm architecture and hyphal orientation, leading to increased tissue invasion. Hyphae respond to polarized environmental signals by altering their axes of growth, thus it is possible that under semi-dry conditions increased moisture within and below the tissue analogue provided a strong environmental cue for vertical hyphal orientation and tissue penetration. In support of our findings, mutants in orientation response genes sustain their hyphal phenotype but have attenuated tissue invasion and epithelial damage properties (Brand et al., 2008). Since physical contact with semisolid substrates triggers invasion of hyphae into these substrates (Brown et al., 1999), it is also reasonable to suggest that reduced hyphal-tissue physical contact in moisture-covered surfaces attenuated tissue penetration. These results provide a novel mechanistic explanation of the increased susceptibility to oral candidiasis in xerostomic patients (Lyon et al., 2006, Torres et al., 2007).
In mixed biofilms the presence of S. oralis promoted C. albicans invasion and tissue damage under both moist and semi-dry conditions, consistent with our prior findings in mucosal biofilms forming under salivary flow (Diaz et al., 2012). One way whereby commensal bacteria can directly increase fungal pathogenicity is by upregulating expression of hyphae-associated fungal genes that have a demonstrated role in mucosal adhesion, invasion and damage. Such candidate genes include the epithelial adhesins hwp1 and als3 as well as secreted aspartyl proteases involved in degradation of epithelial tight junction proteins (Staab et al, 1999, Villar et al, 2007, Cavalcanti et al., 2015).
Pseudohyphal mutants, such as the ndt80 and tup1 transcription factor homozygous deletion mutants, are deficient in the expression of many epithelial adhesins and invasins. This is because transcriptional regulators that control hyphal morphogenesis are also master regulators of most hyphae-associated virulence genes (Sellam et al., 2010, Kadosh & Johnson, 2005). Thus it was not surprising that the ndt80 and tup1 homozygous deletion mutants were deficient in mucosal invasion and damage. However, to our surprise, these pseudohyphal strains acquired a small but significant invasive and tissue damage phenotype in the presence of S. oralis. Since considerable functional redundancy exists among transcriptional regulators in Candida, it is reasonable to suggest that S. oralis may upregulate another transcriptional regulator in the ndt80 or tup1 deletion backgrounds affecting virulence gene expression. A reasonable candidate transcriptional regulator, is Rim101, as we and others have shown that C. albicans utilizes the Rim101 transcription pathway to invade the oral and corneal mucosa (Villar et al., 2005, Villar et al., 2007, Yuan et al., 2010).
Pseudo-hyphal mutants also displayed a different biofilm architecture with S. oralis compared to the reference or complemented strains. Interestingly, when co-cultured with pseudohyphal mutants, S. oralis grew mostly in contact with the mucosal surface, and fewer bacteria co-aggregated with pseudohyphal filaments. This might be due to the fact that these mutants are deficient in activating hypha specific genes encoding for cell wall proteins involved in the recognition of and coaggregation with Streptococcus spp., such as the Als3 or Hwp1 proteins (Conti et al., 2009, Mao et al., 2008, Sellam et al., 2010). More specifically, it has been shown that the ndt80 mutant, is unable to activate both Hwp1 and Als3 genes (Sellam et al., 2010), whereas the tup1 mutant has a two- to three-fold reduction of Hwp1, and almost no Als3 transcribed (Mao et al., 2008). The partially stratified biofilm structure may be explained either by the weak expression of these genes on the surface of the pseudofilaments or by the weak upregulation of an alternative functionally redundant transcriptional regulator triggered by S. oralis in these mutants.
The significant increase of S. oralis biovolume when co-cultured with C. albicans may also suggest a more direct effect of these bacteria on mucosal cells, which could facilitate tissue penetration by hyphae or pseudohyphae. In a mouse S. oralis-C. albicans co-infection model, we have previously shown that Toll-like receptor 2 expression (TLR2) is significantly increased in the oral mucosa and that S. oralis can signal via this receptor to activate a neutrophilic inflammatory response (Xu et al., 2014b). Signaling via TLR2 may also trigger activation of mucosal cell calpains, Ca2+-dependent cysteine proteases which can cleave the epithelial junctional proteins E-cadherin and occludin (Chun & Prince, 2009), thus facilitating filament penetration between cells.
Taken together our data show that moisture, nutrient availability, hyphal morphotype and presence of commensal streptococci strongly affect the architecture and virulence characteristics of mucosal fungal biofilms. Future studies are needed to fully elucidate the specific mechanisms that mediate the changes in the fungal biofilm phenotype triggered by oral streptococci of the Mitis group, which were observed under all environmental conditions tested.
ACKNOWLEDGEMENTS
This work was supported by the NIH/NIDCR grant R01 DE013986, the Brazilian Program Science without Borders # 8810-13-3 and the Brazilian Agency FAPESP # 2012/21011-8 (fellowship) and # 2013/00274-3 (grant), and the NIH NIAID grant R00 AI100896.
REFERENCES
- Bamford CV, Nobbs AH, Barbour ME, Lamont RJ, Jenkinson HF. Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii. Microbiology. 2015;161:18–29. doi: 10.1099/mic.0.083378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Vacharaksa A, Bendel C, Norton J, Haynes P, Henry-Stanley M, Wells C, Ross K, Gow N, Gale CA. An Internal Polarity Landmark Is Important for Externally Induced Hyphal Behaviors in Candida albicans. Eukaryot Cell. 2008;7:712–720. doi: 10.1128/EC.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DH, Jr., Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 1999;34:651–662. doi: 10.1046/j.1365-2958.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- Cassone A, Cauda R. Candida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS. 2012;26:1457–1472. doi: 10.1097/QAD.0b013e3283536ba8. [DOI] [PubMed] [Google Scholar]
- Cavalcanti YW, Morse DJ, da Silva WJ, Del-Bel-Cury AA, Wei X, Wilson M, Milward P, Lewis M, Bradshaw D, Williams DW. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling. 2015;31:27–38. doi: 10.1080/08927014.2014.996143. [DOI] [PubMed] [Google Scholar]
- Chun J, Prince A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe. 2009;5:47–58. doi: 10.1016/j.chom.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, Ikonomou L, Dongari-Bagtzoglou A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80:620–632. doi: 10.1128/IAI.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4:7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Kashleva H. Development of a novel three-dimensional in vitro model of oral Candida infection. Microb Pathog. 2006;40:271–278. doi: 10.1016/j.micpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- Gavaldà J, Meije Y, Fortún J, Roilides E, Saliba F, Lortholary O, Muñoz P, Grossi P, Cuenca-Estrella M. Study Group for Infections in Compromised Hosts. Invasive fungal infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20:27–48. doi: 10.1111/1469-0691.12660. [DOI] [PubMed] [Google Scholar]
- Gow NAR, Hube B. Importance of the C. albicans cell wall during commensalism and infection. Current Opin Microbiol. 2012;15:406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Guobis Ž, Kareivienė V, Basevičienė N, Paipalienė P, Niedzelskienė I, Sabalys G, Kubilius R, Gervickas A. Microflora of the oral cavity in patients with xerostomia. Medicina (Kaunas) 2011;47:646–651. [PubMed] [Google Scholar]
- Johansson AK, Johansson A, Unell L, Ekbäck G, Ordell S, Carlsson GE. Self-reported dry mouth in Swedish population samples aged 50, 65 and 75 years. Gerodontology. 2012;29:e107–e115. doi: 10.1111/j.1741-2358.2010.00420.x. [DOI] [PubMed] [Google Scholar]
- Kadosh D, Johnson AD. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol Biol Cell. 2005;16:2903–2912. doi: 10.1091/mbc.E05-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K. Mucins suppress virulence traits of Candida albicans. mBio. 2014;6:e01911–e01914. doi: 10.1128/mBio.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RE, Cahyame-Zuniga L, Leventakos K, Chamilos G, Ben-Ami R, Tamboli P, Tarrand J, Bodey GP, Luna M, Kontoyiannis DP. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses. 2013;56:638–645. doi: 10.1111/myc.12081. [DOI] [PubMed] [Google Scholar]
- Leyden JJ. Corn starch, Candida albicans, and diaper rash. Pediatr Dermatol. 1984;1:322–325. doi: 10.1111/j.1525-1470.1984.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Lyon JP, da Costa SC, Totti VM, Munhoz MF, de Resende MA. Predisposing conditions for Candida spp. carriage in the oral cavity of denture wearers and individuals with natural teeth. Can J Microbiol. 2006;52:462–467. doi: 10.1139/w05-148. [DOI] [PubMed] [Google Scholar]
- Mao X, Li Y, Wang H, Cao F, Chen J. Antagonistic interplay of Swi1 and Tup1 on filamentous growth of Candida albicans. FEMS Microbiol Lett. 2008;285:233–241. doi: 10.1111/j.1574-6968.2008.01236.x. [DOI] [PubMed] [Google Scholar]
- Meiller TF, Hube B, Schild L, Shirtliff ME, Scheper MA, Winkler R, Ton A, Jabra-Rizk MA. A novel immune evasion strategy of candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One. 2009;4:e5039. doi: 10.1371/journal.pone.0005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DK, Hogan DA. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010;6:e1000886. doi: 10.1371/journal.ppat.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol. 2010;8:340–349. doi: 10.1038/nrmicro2313. [DOI] [PubMed] [Google Scholar]
- Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun. 2000;68:3485–3490. doi: 10.1128/iai.68.6.3485-3490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana IL, Gonçalves LM, de Vasconcellos AA, da Silva WJ, Cury JA, Del Bel Cury AA. Dietary carbohydrates modulate Candida albicans biofilm development on the denture surface. PLoS One. 2013;8:e64645. doi: 10.1371/journal.pone.0064645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht LM, Peters BM, Krom BP, Freiberg JA, Hänsch GM, Filler SG, Jabra-Rizk MA, Shirtliff ME. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology. 2014;161:168–181. doi: 10.1099/mic.0.083485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A, Askew C, Epp E, Tebbji F, Mullick A, Whiteway M, Nantel A. Role of transcription factor CaNdt80p in cell separation, hyphal growth, and virulence in Candida albicans. Eukaryot Cell. 2010;9:634–644. doi: 10.1128/EC.00325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299:1–8. doi: 10.1111/j.1574-6968.2009.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun. 2010;78:4644–4652. doi: 10.1128/IAI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Verma R, Murari A, Agrawal A. Oral candidiasis: An overview. J Oral Maxillofac Pathol. 2014;18:S81–S85. doi: 10.4103/0973-029X.141325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- Thurnheer T, Gmür R, Giertsen E, Guggenheim B. Automated fluorescent in situ hybridization for the specific detection and quantification of oral streptococci in dental plaque. J Microbiol Methods. 2001;44:39–47. doi: 10.1016/s0167-7012(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Torres SR, Peixoto CB, Caldas DM, Akiti T, Barreiros MG, de Uzeda M, Nucci M. A prospective randomized trial to reduce oral Candida spp. colonization in patients with hyposalivation. Braz Oral Res. 2007;21:182–187. doi: 10.1590/s1806-83242007000200015. [DOI] [PubMed] [Google Scholar]
- Villar CC, Kashleva H, Dongari-Bagtzoglou A. Role of Candida albicans polymorphism in interactions with oral epithelial cells. Oral Microbiol Immunol. 2004;19:262–269. doi: 10.1111/j.1399-302X.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Villar CC, Kashleva H, Mitchell AP, Dongari-Bagtzoglou A. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect Immun. 2005;73:4588–4595. doi: 10.1128/IAI.73.8.4588-4595.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007;75:2126–2135. doi: 10.1128/IAI.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Jordan RP, Wei XQ, Alves CT, Wise MP, Wilson MJ, Lewis MA. Interactions of Candida albicans with host epithelial surfaces. J Oral Microbiol. 2013;5:22434. doi: 10.3402/jom.v5i0.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöllert T, Rollenhagen C, Langford GM, Sundstrom P. Human oral keratinocytes: a model system to analyze host-pathogen interactions. Methods Mol Biol. 2012;845:289–302. doi: 10.1007/978-1-61779-539-8_19. [DOI] [PubMed] [Google Scholar]
- Xu H, Jenkinson HF, Dongari-Bagtzoglou A. Innocent until proven guilty: mechanisms and roles of Streptococcus-Candida interactions in oral health and disease. Mol Oral Microbiol. 2014a;29:99–116. doi: 10.1111/omi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz PI, Dongari-Bagtzoglou A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014b;16:214–231. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Mitchell BM, Hua X, Davis DA, Wilhelmus KR. The RIM101 signal transduction pathway regulates Candida albicans virulence during experimental keratomycosis. Invest Ophthalmol Vis Sci. 2010;51:4668–4676. doi: 10.1167/iovs.09-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]