Abstract
Objectives
This study was initiated to elucidate the extent of dietary exposure of children in Korea to two pollutant metals of cadmium (Cd) and lead (Pb). Possible urban–rural difference was also examined.
Methods
Food duplicate and morning spot urine samples were collected from 108 children in 4 kindergartens (KG) (1 KG in Seoul and 3 KGs in Jeju Island), as reported in a previous publication. The samples were analyzed for Cd and Pb by ICP-MS.
Results
Cd and Pb in food duplicate and urine samples were distributed approximately log-normally. Geometric means for Cd and Pb in food duplicate samples were 12.4 and 5.8 μg/day, or 0.58 and 0.27 μg/kg body weight/day, respectively, and the values for Cd and Pb in urine (as observed, i.e., with no correction for urine density) were 0.91 and 1.64 μg/L, respectively. 2.41 and 0.30 μg/day of Cd and Pb (accounting for 19.5 and 5.1 %) came from boiled rice, the staple food. The levels of Cd and Pb burden among the children in the present survey were essentially the same with the levels reported for children in Pusan. The reasons for difference in the rank in Cd-D and Cd-U among the 4 KGs need further study.
Conclusions
The observed levels of Cd and Pb exposure were more or less similar to what were reported for children in Pusan. No apparent urban–rural difference could be detected.
Keywords: Cadmium, Children, Daily foods, Korea, Lead
Introduction
Cadmium (Cd) and lead (Pb) are among the most often studied environmental pollutant metals [1–5]. After a long-term exposure to Cd via food (including water), Cd may adversely affect renal tubules and then calcium metabolism in bones, typically in aged subjects [6]. In contrast, elevated Pb levels in blood may induce retarded development of the central nervous system function in the early stage of life [6, 7]. It is known that the major source of Cd for general population is foods, whereas that for Pb is environmental air in addition to foods due to use of organic lead as an anti-knocking agent in automobile fuels [8, 9]. It would be worthy to note that Cd levels in polished rice, the staple food for general populations in many areas in East and South-East Asia, was the second [10] or sixth highest [11] in Korea among the areas surveyed. Despite the knowledge of ubiquitous presence of these metals, reports on human epidemiology regarding dietary intake of Cd and Pb via foods are still scarce in Korea [12–16]. In fact, only one report is available for intake by children [17].
It is the purpose of this communication to report on dietary intake of children in kindergarten (to be abbreviated as KG) and to alleviate the information shortage. It should be added that the surveys were conducted in Seoul as a representative metropolis, and in Jeju Island as a typical rural area.
Materials and methods
Survey sites and survey subjects
The details of study locations and study populations were described in Part 1 of this series of articles [18]. In short, 108 boys and girls participated in this study. They attended 4 kindergartens (KG) in Korea, i.e., 33 children KG 1 in Seoul, 37 children in KG 2 in Jeju city, 18 children in KG 3 in a village in Jeju Island and 20 children in KG 4 in another village in the island. Their mothers provided informed consent and food duplicate samples (including any drinks) the children took in the past 24 h [19, 20]. Morning spot urine samples were also collected from the children. The study protocol was approved by the Institutional Review Board of Dankook University (DKU2015-03-005), Yongin-si, Gyeonggi-do 448-701, Korea.
Instrumental analysis
The food duplicate, urine and a small portion (ca. 5 g) of boiled rice samples were wet-ashed by heating in the presence of mineral acids as previously described in Part 2 of this series of articles [21]. The wet-ashed liquid samples were subjected to ICP-MS (inductively coupled plasma-mass spectrometry) analyses for Cd and Pb determination. The samples from KG 1 in Seoul were analyzed in an analytical laboratory in Shimadzu Techno-research, Inc., Kyoto, Japan, whereas the samples from KGs 2, 3 and 4 in Jeju Island were analyzed in Kyoto Women’s University [22]. The performances of both laboratories were excellent; the quality of the analysis in the former laboratory was approved by International Organization for Standardization (ISO) 17025, and the performance in the latter laboratory was detailed in Shimbo et al. [22]. Thus, the analysis results from the two laboratories were considered to be compatible to each other. The quantification limits [23] in the former and the latter laboratories were 0.1 and 0.04 ng/g for Cd, and the limits were 1.0 and 0.8 ng/g for Pb, respectively.
Statistical analysis
Both Cd and Pb concentrations in the food duplicate, boiled rice and urine samples were distributed log-normally, and, therefore, geometric means (GMs) and geometric standard deviations (GSDs) were taken as parameters to represent distributions. One-way analysis of variance followed by post hoc test (Scheffe) (taking KGs as independent variables and one of the values for Cd and Pb as dependent variable) was employed to detect possible inter-KG difference in Cd and Pb levels after logarithmic conversion of the values. Correlation analyses were also employed. As body size of the children varied among the KGs (for details, see [18]), analysis was made as measured (i.e., in μg/day) and also as the rate per kg body weight of each child (i.e., in μg/day/kg body weight/day). Correlation coefficients were also calculated. A probability of 5 % (i.e., p < 0.05) was taken as the point of judgment for significant difference.
Results
Daily dietary intake of Cd and Pb
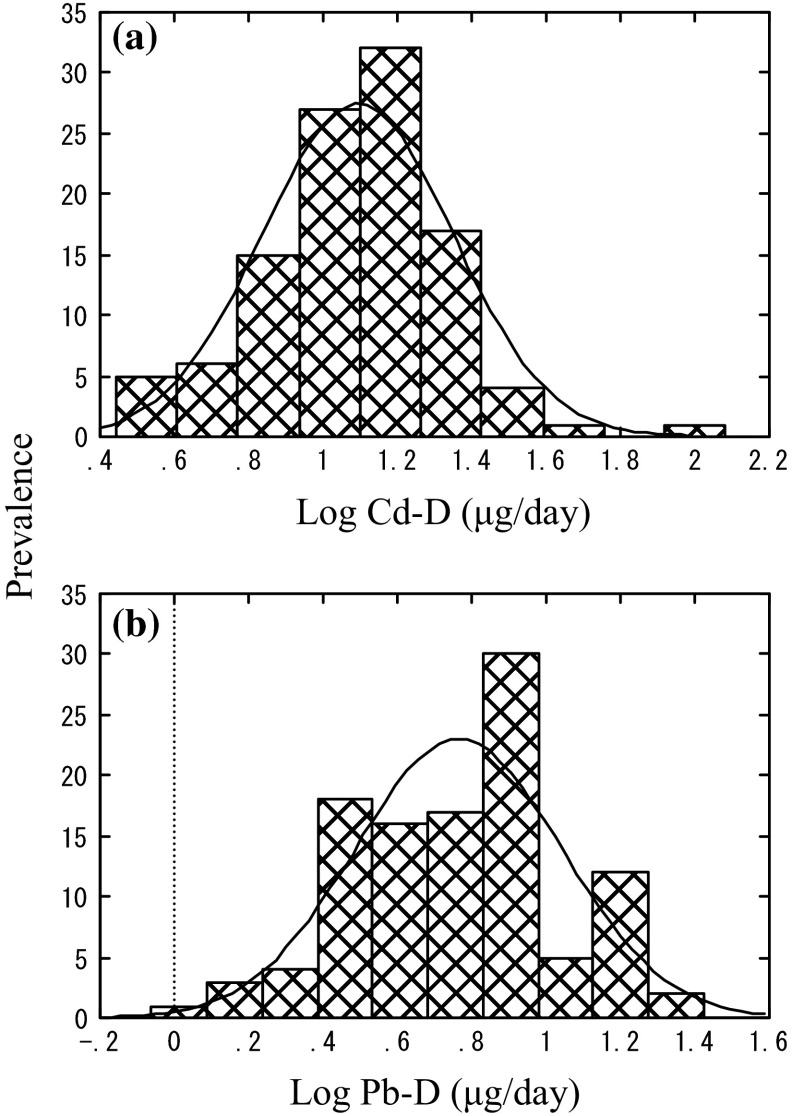
The results of the measurements for dietary Cd and Pb intake (Cd-D and Pb-D) are summarized in Table 1 in terms of the 4 KGs. The results of statistical analysis are also presented to detect possible differences (see Figs. 1 and 2 for distribution of log Cd-D and log Pb-D, respectively). Because body size of children varied among the KGs (as stated above), both daily intake (in μg/day) and the rate of intake/body weight/day were presented. GM for Cd in a total 108 cases was 12.39 μg/day for Cd and 5.82 μg/day for Pb. After adjustment for bodyweight, the values were 0.58 μg/kg/day for Cd and 0.27 μg/kg/day for Pb.
Table 1.
Dietary intake of cadmium and lead by by kindergarten (KG)
| Kindergarten (KG) | No. | Daily intakea (μg/day) | Daily intake by body weighta (μg/kg/day) | Intake from boiled rica (μg/day) | Contribution of boiled rice (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cadmium | Lead | Cadmium | Lead | Cadmium | Lead | Cadmium | Lead | ||
| Total | 108 | 12.39 (1.81) | 5.82 (1.90 ) | 0.58 (1.74) | 0.27 (2.00) | 2.41 (1.55) | 0.30 (4.33) | 19.5 | 5.1 |
| A. KG 1 | 33 | 7.64 (1.60) | 6.88 (1.92) | 0.40 (1.62) | 0.36 (1.98) | 2.67 (1.45) | 0.11 (6.05) | 35.0 | 1.6 |
| B. KG 2 | 37 | 20.46 (1.51) | 4.75 (2.06) | 0.89 (1.56) | 0.21 (2.10) | 2.50 (1.56) | 0.42 (3.21) | 12.2 | 8.9 |
| C. KG 3 | 18 | 11.52 (1.58) | 5.83 (1.54) | 0.52 (1.53) | 0.26 (1.65) | 2.16 (1.45) | 0.40 (2.27) | 18.7 | 6.8 |
| D. KG 4 | 20 | 11.60 (1.56) | 6.40 (1.73) | 0.54 (1.53) | 0.30 (1.69) | 2.11 (1.74) | 0.62 (2.46) | 18.2 | 9.7 |
| Inter-KG comparisonb | B > C ≒ D > A | A ≒ B ≒ C ≒ D | B > A ≒ C ≒ D | A > B ≒ C ≒ D | A ≒ B ≒ C ≒ D | B ≒ C ≒ D > A | |||
aGM (GSD) values are shown
b P > Q shows P was significantly (p < 0.05) larger than Q, whereas P ≒ Q shows that the difference was insignificant (p > 0.05)
Fig. 1.
Distribution of Cd-D and Pb-D. a Cd in food duplicate samples (Cd-D), b Pb in food duplicate samples (Pb-D). The curve in the figure shows a normal distribution
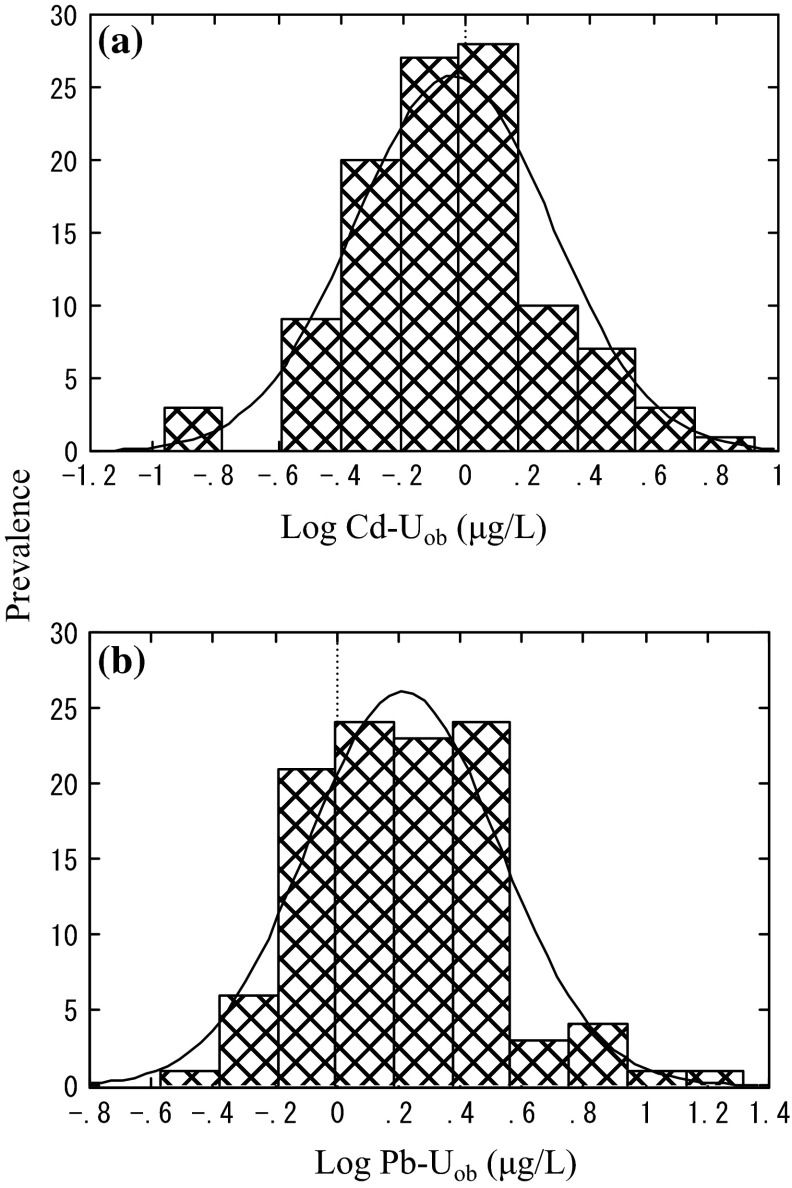
Fig. 2.
Distribution of Cd-Uob and Pb-Uob. a Cd in urine (as observed, i.e., with no correction for urine density) (Cd-Uob), b Pb in urine (as observed, i.e., with no corection for urine density) (Pb-Uob). The curve in the figure shows a normal distribution
Inter-KG comparison revealed that Cd intake was the lowest in KG 1 irrespective of on the daily basis or on the kg/day basis, whereas no inter-KG difference was observed in Pb intake. When Cd and Pb were calculated for the intake via boiled rice, the staple food item, the intakes via boiled rice were 2.41 and 0.30 μg/day for Cd and Pb, respectively. Comparison of the values for boiled rice over total foods revealed that the contributions of boiled rice for Cd and Pb accounted for 19.5 and 5.1 %, respectively. The rate for Cd from rice was highest in KG 1 and lowest KG 2. The reverse was the case for Pb; it was the lowest in KG 1 and the highest in KG 2. Nevertheless, the contribution was much lower for Pb than for Cd.
Concentration of Cd and Pb in urine
Similar analyses were carried out for Cd and Pb levels in urine samples (Table 2). The Cd and Pb levels for grand total were 0.91 μg/L for Cd and 1.64 μg/L for Pb as observed values (i.e., the values without correction for urine density). Inter-KG comparison disclosed that the Cd levels were higher in KG 1 than in any other three KGs. The observation was reproducible irrespective of correction for urine density. It should be noted that the rank for Cd in urine was just the reverse of the rank for dietary Cd intake. In cases of Pb in urine, the rank order was various depending on the correction for urine density. Such may be in agreement with the observation in dietary Pb intake in the sense that there was no clear difference in the rank order.
Table 2.
Cd and Pb levels in urine by kindergarten (KG)
| No. | Creatinine (g/L)1 | Sp.Gr.a,b | Cadmium in urine | Lead in urine | |||||
|---|---|---|---|---|---|---|---|---|---|
| OB (μg/L)c | CR (μg/g)c | SG (μg/L)c | OB (μg/L)c | CR (μg/g)c | SG (μg/L)c | ||||
| Total | 108 | 0.78 ± 0.33 | 21.1 ± 7.2 | 0.91 (2.07) | 1.30 (1.84) | 0.74 (1.85) | 1.64 (2.05) | 2.33 (1.94) | 1.33 (1.90) |
| A. KG 1 | 33 | 0.76 ± 0.35 | 20.1 ± 7.1 | 1.74 (1.96) | 2.54 (1.68) | 1.47 (1.74) | 1.13 (1.73) | 1.66 (1.65) | 0.96 (1.59) |
| B. KG 2 | 37 | 0.73 ± 0.29 | 20.9 ± 7.4 | 0.66 (1.73) | 0.99 (1.38) | 0.54 (1.36) | 1.97 (1.98) | 2.95 (2.08) | 1.62 (1.92) |
| C. KG 3 | 18 | 0.88 ± 0.32 | 22.0 ± 6.4 | 0.83 (1.65) | 1.01 (1.49) | 0.63 (1.42) | 2.11 (1.81) | 2.55 (1.58) | 1.60 (1.72) |
| D. KG 4 | 20 | 0.81 ± 0.37 | 22.4 ± 8.0 | 0.63 (1.77) | 0.88 (1.48) | 0.48 (1.43) | 1.72 (2.50) | 2.41 (2.04) | 1.33 (2.16) |
| Comparisond | A ≒ B ≒ C ≒ D | A ≒ B ≒ C ≒ D | A > B ≒ C ≒ D | A > B ≒ C ≒ D | A > B ≒ C ≒ D | A ≒ B ≒ C ≒ D | B ≒ C ≒ D > A | A ≒ B ≒ C ≒ D | |
aAM ± ASD
bSp.Gr. = (Specific gravity − 1.000) × 1000
cAs observed (OB), as corrected for creatinine concentration (CR), or after correction for a urine specific gravity of 1.016 (SG). GM (GSD) values are shown
dNotes are as under Table 1
Possible correlation between dietary intake and concentration in urine
Correlation analyses were conducted on an individual basis (i.e., on 108 cases) taking dietary intake as an independent variable and urinary concentration as a dependent variable (Table 3). The correlation coefficients were negative (i.e., <0) in cases of Cd and mostly insignificant in cases of Pb. Thus, the observation did not support the expectation that urinary Cd and Pb would correlate with dietary intakes.
Table 3.
Absence of positive correlation of dietary intake of cadmium and lead with urinary concentration
| Dietary intake | Urinary concentration | Corr. Coeff. | p value |
|---|---|---|---|
| Cadmium | |||
| Per day | As observed | −0.489 | <0.01 |
| CR-corrected | −0.430 | <0.01 | |
| Sp.Gr.-corrected | −0.384 | <0.01 | |
| Per body weight per day | As observed | −0.414 | <0.01 |
| CR-corrected | −0.339 | <0.01 | |
| Sp.Gr.-corrected | −0.335 | <0.05 | |
| Lead | |||
| Per day | As observed | −0.122 | ns |
| CR-corrected | 0.141 | ns | |
| Sp.Gr.-corrected | −0.191 | ns | |
| Per body weight per day | As observed | −0.102 | ns |
| CR-corrected | 0.247 | <0.05 | |
| Sp.Gr.-corrected | −0.184 | ns | |
CR corrected corrected for creatinine concentration, Sp.Gr.-corrected corrected for urine specific gravity (1.016)
Food groups as influential sources of dietary Cd and Pb intake
As to be discussed later, potatoes [15, 16], meats, fish (including shellfish) and seaweeds [17] in addition to cereals [8, 9] were suspected as potential sources of Cd and Pb. Possible association of these food groups with Cd and Pb intake was examined by simple regression analysis (Table 4). The results gave significant correlation with Cd intake for cereals and fish, but not for potatoes, meats and seaweeds. No significant correlation was detected for any food groups as influential Pb source.
Table 4.
Relation of food groups with Cd and Pb in daily foods
| Food group | Food intake (g/day)a | Corr. with Cd-Dc | Corr. with Pb-Dc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Name | AM | ASD | Min | Max | MED | r d | p | r d | p |
| 1 | Cereals | 332.8 | 87.6 | 161.9 | 647.7 | 326.9 | 0.25 | <0.01 | 0.13 | ns |
| 2 | Potatoes | 22.0 | 32.4 | 0 | 210.3 | 12.9 | −0.09 | nse | 0.09 | ns |
| 9 | Meats | 42.1 | 38.9 | 0 | 192.6 | 31.6 | −0.04 | ns | 0.13 | ns |
| 11 | Fishb | 32.5 | 25.8 | 0 | 141.0 | 26.9 | 0.35 | <0.01 | 0.09 | ns |
| 12 | Seaweeds | 6.7 | 13.5 | 0 | 80.8 | 2.0 | 0.08 | ns | 0.16 | ns |
a n = 108
bFishes and shellfishes
cCorrelation coefficient between Cd-D (or Pb-D) and the amount consumed of the food group
dCorrelation coefficient
e ns non-significant (p ≧ 0.05)
Discussion
The present study on 108 children in 4 KGs in urban and rural areas made it clear that the overall dietary Cd and Pb intake was 12.39 and 5.82 μg/day, respectively, or 0.58 and 0.27 μg/kg/day after correction for body weight (Table 1). About 20 and 5 % of Cd and Pb, respectively, came from boiled rice, the staple food. It was previously observed that foods for children in KG 4 were found to be specific in the sense that the foods there were richer in meat and poultry than foods in other KGs [18]. However, heavy metal contents in meat and poulry (other than organ meats) are known to be lower than the levels in cereals such as rice, the staple food [1], and the amount (i.e., the content multiplied by intake volume) may not affect daily intake levels substantially as observed in Table 1. The levels in urine (with no correction for urine density) were 0.91 μg/L for Cd and 1.64 μg/L for Pb (Table 2).
There was significant and close correlation between Cd-D and Cd-U, whereas the correlation between Pb-D and Pb-U was essentially insignificant (Table 3). Cd when taken into body will accumulate in renal tubules (in addition to liver), and accumulated Cd and Cd-U are in equilibrium [24]. Pb when absorbed will be bound to erythrocytes in peripheral blood and accumulate in bone tissue [24]. Thus, the close correlation of Cd-U with Cd-D, and poor correlation in case of Pb-D and Pb-U appear to be as expected.
The observation on inter-KG difference that the rank for Cd in dietary intake did not agree with that for Cd in urinary levels was puzzling. No clear explanation for the discrepancy is currently available. From toxicological viewpoint, however, the information from urinary levels should be evaluated with care because Cd-U is an indicator of a long-term exposure to Cd [8]. Nevertheless, Cd in urine also shows significant physiological variation in the levels [25]. In fact, Moon et al. [13] found that both Cd-U and Cd-B (Cd in blood) did not correlate with Cd-D. The additional observation that Pb-U correlated only weakly with Pb-B [13] suggests that Pb-U is a poor indictor of Pb burden, which is in agreement with previous observation [26]. Thus, both Cd-U and Cd-D may need equal attention.
The point of interest is the comparison of the present findings with levels reported in previous publications. In 2000 in Busan (the second largest city in Republic of Korea), GM Cd-D was 11.2 μg/day (or 0.46 μg/kg/day) for 7.5-year-old children and 16.7 μg/day (or 0.30 μg/kg/day) for their mothers [16]. In a survey conducted in 1994 [12], Cd-D intake by adult women in Busan was 21.2 μg/day, and Pb-D intake was 20.5 μg/day as GM. When the average body weight of 56.1 kg for mothers in Busan study [17] was applied, Cd-D and Pb-D were 0.28 and 0.37 μg/kg/day. The present observation for children, 0.58 μg Cd and 0.27 μg Pb/kg/day (Table 1), may be at a similar level with what were reported in these publications [12, 17].
The present survey disclosed that about 20 and 5 % of daily Cd and Pb intake, respectively, came from boiled rice (Table 1). The major source for dietary Cd intake has been discussed by various authors. In a 1994 survey, Moon et al. [12] found that 23 % of Cd and 12 % of Pb in food were attributable to rice. Similar conclusions that cereals and potatoes (in addition to fish and shellfish) are the leading sources of heavy metals in Korean foods were reached in separate studies [15, 16]. Kim et al. [27] in addition pointed out that Cd and Pb contents were high in seaweeds. Unfortunately, no data were presented on daily consumption of seaweeds which assumedly may not be high enough to influence daily intake of Cd and Pb.
As for Cd-U, Lee et al. [28] observed in a nation-wide survey in Korea conducted in 2008 that GM Cd-U was 0.62 μg/L for non-smoking adult Koreans. It appears likely that the present observation of 0.91 μg/L (Table 2) was higher than the national average. Cd-U did not differ significantly (p < 0.05) between those who consumed fish in the latest 3 days (0.64 μg/L) and those who did not (0.66 μg/L) [28]. However, no details were given regarding the types of fish if it was finned fish (with low Cd contents) or small squid (with high Cd contents and edible as a whole).
With regard to urban–rural comparison, Cd-D and Pb-D in Seoul, a metropolis, were 14.4 and 16.7 μg/day, whereas the counterpart values for Haman, a rural village, were 23.48 and 21.84 μg/day. Cd-Uob (i.e., cadmium in urine as observed) were 1.33 and 2.89 μg/L in Seoul and Haman, respectively, and Pb-B (i.e., lead in blood) were 49.8 and 34.8 μg/L in the rank [14]. The dietary intake was the exclusive route for Cd intake whereas both respiratory and dietary intakes are important for Pb burden [8, 9]; atmospheric air was more polluted with Pb in Seoul than that in Haman, whereas dietary Cd burden may be higher in Haman than that in Seoul. Mutlu and Lee [29] estimated that the long-term Pb in air in Seoul was 47 ng/m3. No data on Pb in rural air were presented unfortunately, and it was not possible to make urban–rural comparison on Pb in atmospheric air. Based on large-scale pilot study of Korean children (including adolescents) on pollutant burden, Ha et al. [30] reported that the Pb-B levels were significantly (p < 0.01) higher in urban children (12.9 μg/L) than in rural children (11.6 μg/L), whereas there was no difference in Cd in blood (p > 0.1) between children in urban (0.30 μg/L) and rural areas (0.30 μg/L).
There may be three limitations in the present study. GM Cd-D was the lowest in KG 1 among the 4 KGs whereas the reverse was the case for Cd-U. Although such disagreement in the rank order was also observed previously by Moon et al. [12], this point apparently needs further study for elucidation. The other limitation would be that ICP-MS analyses were conducted in two laboratories, i.e., samples from KG 1 in one and samples from KGs 2, 3 and 4 in another. The quality assurance data suggested data compatibility between the two laboratories. Nevertheless, it was apparently desirable that whole analyses were conducted in a single laboratory to avoid any inter-laboratory difference in analysis performance. No effect markers in urine such as β2-microglobulin [1] was studied in the present survey; the absence may also be a limitation. It is, however, quite unlikely that the Cd and Pb exposures at the observed levels of the present study may induce any health effects.
The results of the present study, conducted some 10 years ago on 108 children in 4 kindergartens in urban and rural areas in the Republic of Korea, showed that the dietary intake of Cd and Pb was 12.4 and 5.8 μg/day (0.58 and 0.27 μg/kg body weight/day), respectively. Cd and Pb levels in urine (as observed, non-corrected value) were 0.91 and 1.64 μg/L. These values were generally close to results of studies conducted in later years. No urban–rural differences could be demonstrated clearly.
Acknowledgments
The authors are grateful to Dr. K. Jung (the General Director) and Dr. J.-H. Kim (the Director of the Food and Drug Division) of Seoul Metropolitan Government Research Institute of Public Health and Environment, Seoul, Korea, for their interest and support to this work. Thanks are also due to the participating children and their guardians, and administrators and staff of the kindergartens that participated in the study. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan: Grant-in-Aid for Scientific Research C: 22500755 (Head Investigator; T. Watanabe for fiscal years 2010–2012), and Grant-in-Aid for Scientific Research C; 26350150 (Head Investigator; H. Nakatsuka for fiscal years 2014–2016).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
E.-S. Kim: Deceased.
References
- 1.International Programme on Chemical Safety (IPCS). Environmental health criteria I34. Cadmium. Geneva: World Health Organization; 1992.
- 2.International Programme on Chemical Safety (IPCS). Environmental health criteria 135. Cadmium—environmental aspects. Geneva: World Health Organization; 1992.
- 3.International Programme on Cheniical Safety (IPCS). Environmental health criteria 3. Lead. Geneva: World Health Organization; 1977.
- 4.International Programme on Chemical Safety (IPCS). Environmental health criteria 85. Lead—environmental aspects. Geneva: World Health Organization; 1989.
- 5.International Programme on Chemical Safety (IPCS). Environmental health criteria 165. Inorganic lead. Geneva: World Health Organization; 1995.
- 6.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analysis of perinatal and postnatal lead exposure and early cognitive development. New Engl J Med. 1987;316:1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 7.Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, Edwards S, Skarupa A, Lisowska-Miszczk I. Very low prenatal exposure to lead and mental development of children in infancy and early childhood. Neuro-epidemiology. 2009;32:270–278. doi: 10.1159/000203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda M, Zhang ZW, Shimbo S, Watanabe T, Nakatsuka H, Moon CS, et al. Urban population exposure to lead and cadmium in east and south-east Asia. Sci Total Environ. 2000;249:373–384. doi: 10.1016/S0048-9697(99)00527-6. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda M, Zhang ZW, Shimbo S, Watanabe T, Nakatsuka H, Moon CS, et al. Exposure of women in general populations to lead via food and air in east and southeast Asia. Am J Ind Med. 2000;38:271–280. doi: 10.1002/1097-0274(200009)38:3<271::AID-AJIM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, Nakatsuka H, Ikeda M. Cadmium and lead contents in rice available in various areas of Asia. Sci Total Environ. 1989;80:175–184. doi: 10.1016/0048-9697(89)90073-9. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Shimbo S, Moon CS, Zhang ZW, Ikeda M. Cadmium contents in rice samples from various areas in the world. Sci Total Environ. 1996;184:191–196. doi: 10.1016/0048-9697(96)05100-5. [DOI] [PubMed] [Google Scholar]
- 12.Moon C-S, Zhang Z-W, Shimbo S, Watanabe T, Moon D-H, Lee C-U, et al. Dietary intake of cadmium and lead among the general population in Korea. Environ Res. 1995;71:46–54. doi: 10.1006/enrs.1995.1066. [DOI] [PubMed] [Google Scholar]
- 13.Moon CS, Zhang ZW, Shimbo S, Watanabe T, Moon DH, Lee CU, et al. Evaluation of urinary cadmium and lead as markers of background exposure of middle-aged women in Korea. Int Arch Occup Environ Health. 1998;71:251–256. doi: 10.1007/s004200050277. [DOI] [PubMed] [Google Scholar]
- 14.Moon CS, Zhang ZW, Shimbo S, Watanabe T, Lee CU, Lee BK, et al. Evaluation of urinary cadmium and lead as markers of background exposure of middle-aged women in Korea: dietary intake as an influential factor. Toxicol Lett. 1999;108:173–178. doi: 10.1016/S0378-4274(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 15.Moon CS, Lee CK, Lee JT, Kim JM, Ikeda M. Time trends in dietary cadmium intake of Korean women. Toxicol Res. 2012;1:145–150. doi: 10.1039/c2tx00002d. [DOI] [Google Scholar]
- 16.Moon CS, Lee CK, Hong YS, Ikeda M. Higher cadmium and lead burden in coastal areas than in inland areas in Korea areas cadmium and lead in blood and urine of middle-aged women in Korea. Asian-Pacific J Clin Nutr. 2014;23:219–224. doi: 10.6133/apjcn.2014.23.2.10. [DOI] [PubMed] [Google Scholar]
- 17.Moon CS, Paik JM, Choi CS, Kim DH, Ikeda M. Lead and cadmium levels in daily foods, blood and urine in children and their mothers in Korea. Int Arch Occup Environ Health. 2003;76:282–288. doi: 10.1007/s00420-002-0415-4. [DOI] [PubMed] [Google Scholar]
- 18.Nakatsuka H, Ko Y, Yang HR, Monn CS, Watanabe T, Kin ES, et al. Food intake survey of kindergarten children in Korea: part 1 food, energy, and nutrient intake. Environ Health Prev Med (in press). [DOI] [PMC free article] [PubMed]
- 19.Acheson KJ, Campbell IT, Edholm OG, Miller DS, Stock MJ. The measurement of food and energy intake in man—an evaluation of some techniques. Am J Clin Nutr. 1980;33:1147–1154. doi: 10.1093/ajcn/33.5.1147. [DOI] [PubMed] [Google Scholar]
- 20.Nakatsuka H, Shimbo S, Watanabe T, Yaginuma-Sakurai K. IkedaM. Applicability of food composition tables as a tool to estimate mineral intake of preschool children in Japan: A validation study. J Trace Elem Med Biol. 2013;27:339–345. doi: 10.1016/j.jtemb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Yang HR, Ko Y, Watanabe T, Nakatsuka H, Moon CS, Kim ES, et al. Food intake survey of kindergarten children in Korea: part 2 increased dietary intake of tin possibly associated with canned foods. Environ Health Prev Med (in press). [DOI] [PMC free article] [PubMed]
- 22.Shimbo S, Zhang Z-W, Watanabe T, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K, et al. Cadmium and lead contents in rice and other cereal products in Japan in 1998–2000. Sci Total Environ. 2001;281:165–175. doi: 10.1016/S0048-9697(01)00844-0. [DOI] [PubMed] [Google Scholar]
- 23.Japan Society for Analytical Chemistry (ed.). Analysis and Reliability of the Analysis Results. Tokyo (Japan): Maruzen; 1998. p. 52 (in Japanese).
- 24.Lauwerys RR, Hoet P. Industrial chemical exposure (3rd ed.) Chap. 2 cadmium, and inorganic lead. Boca Raton et al.: Lewis Publishers; 2001. pp. 54, 104.
- 25.Yamagami T, Suna T, Fukui Y, OhashiF Takada S, Sakurai H, et al. Biological variations in α1-microglobulin, β2-microglobulin and N-acetyl-β-D-glucosaminidase in adult women in a non-polluted area. Int Arch Occup Environ Health. 2008;81:263–271. doi: 10.1007/s00420-007-0206-z. [DOI] [PubMed] [Google Scholar]
- 26.Higashikawa K, Zhang ZW, Shimbo S, Moon CS, Watanabe T, Nakatsuka H, et al. Correlation between concentration in urine and in blood of cadmium and lead among women in Asia. Sci Total Environ. 2000;246:97–107. doi: 10.1016/S0048-9697(99)00415-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Lee JY, Seo JE, Jeong JY, Jung KK. Lead, cadmium and mercury levels in the 2010 Korean diet. Food Add Contamin. 2012;5:260–264. doi: 10.1080/19393210.2012.703699. [DOI] [PubMed] [Google Scholar]
- 28.Lee JW, Lee CK, Moon CS, Choi IJ, Lee KJ, Yi S-M, et al. Korea national survey for environmental pollutants in the human body 2008: heavy metals in the blood or urine of Korean population. Int J Hyg Environ Health. 2012;215:449–457. doi: 10.1016/j.ijheh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Mutlu A, Lee B-K. Air-borne lead levels in the Korea peninsula; characterization of temporal and spatial patterns and cancer risk analysis. Environ Sci Pollut Res. 2012;19:2125–2137. doi: 10.1007/s11356-011-0712-0. [DOI] [PubMed] [Google Scholar]
- 30.Ha M, Kwon HJ, Leem JH, Kim HC, Lee KJ, Park I, et al. Korean Environmental Health Survey in children and adolescents (KorHS-C): Survey design and pilot study results on selected exposure biomarkers. Int J Hyg Environ Health. 2014;217:260–270. doi: 10.1016/j.ijheh.2013.06.001. [DOI] [PubMed] [Google Scholar]