Abstract
Aim
Establish new biocontrol practices with low persistence in the environment against dermatophyte causing mycosis.
Methods
Antimycotic activity of twenty-six plant-derived commercial essential oils (EOs) was evaluated against four dermatophyte keratinophilic fungi (Microsporum canis, Epidermophyton floccosum, Trichophyton rubrum and Trichophyton mentagrophytes). Commercial EOs which showed the strongest mycelial growth inhibitions were selected and re-extracted in vitro from fresh plant samples. Minimal inhibition concentration (MIC) and antifungal index (AI) of pure and combined extracted oils and were evaluated. All samples were collected and examined during the year of 2014.
Results
The results revealed that commercial EOs of Prunus armeniaca, Prunus dulcis var. amara, Olea europaea and Mentha piperita were the most potent antidermatophyte. The mixture of the extracted four oils was the strongest fungicides followed by the alternative two-oil combined extractions then pure extracted oils. MIC was at 50, 25 and 12.5 μg/disc for pure oils, two-oil combinations and four-oil mixture, respectively. Achieved values of AI were found variable.
Conclusion
Using of natural products like plant-derived EOs instead of chemotherapy on pathogens can be regarded as an environmental safety mode of diseases control.
Keywords: Essential oils, Dermatophyte fungi, Antifungal efficacy, Antifungal index, Combined oils, Prunus armeniaca, Prunus dulcis var. amara, Olea europaea, Mentha piperita
Introduction
Millions of people throughout the world are affected by superficial fungal infections, which are the most common skin diseases. These infections, which occur in both healthy and immune-compromised persons, are caused mainly by dermatophytes. The dermatophytes, Trichophyton spp. and Microsporum canis are commonly involved in such infections [1, 2]. They cause common infections in humans which are difficult to control effectively, and the pharmaceutical arsenal currently available against them is rather limited. Hence, plant products that inhibit their growth without harming the host represent potential therapeutic agent [3]. Increasing social and health implications caused by dermatophytes means there is a constant striving to develop safe and new natural antifungal agents to cure human fungal disorders caused by dermatophytes [1].
Plant-derived essential oils (EOs) and extracts are considered as non-phytotoxic compounds and have antimicrobials and antidermatophytic properties [1, 4]. It is known that most of their properties are due to the EOs they contain as products of their secondary metabolism. Therefore, they can be used as a natural therapy to inhibit fungal pathogens causing superficial infections [1, 5]. However, there is only limited information in the literature on the antifungal activity of EOs toward human fungal pathogens.
The objective of this work was to evaluate the antifungal efficacy of 26 plant-derived EOs against four widely spread pathogenic keratinophilic fungal strains that cause superficial skin infections in humans trying to find more safely hygienic natural plant products.
Materials and methods
Study design
Twenty-six commercial EOs of different plant origins were screened for their antifungal efficacy against four filamentous fungal strains of hygienic significance. The most potent toxic commercial EOs were selected and re-extracted in vitro. Minimal inhibitory concentration (MIC) and antifungal index (AI) of plant extracted pure oils, their mixture and their interchangeably two-oil combinations were determined.
Essential oil and plant material
Fresh mint leaves, Mentha piperita, ripe olive drupes, Olea europaea, bitter almond seeds, Prunus dulcis var. amara and apricot seeds, Prunus armeniaca, were collected from local markets in Giza (Egypt) and Alkharj (Saudi Arabia) cities. They were collected during April, May and June, 2014 and subjected to extraction process as soon as collected. At the same period time, twenty-six commercial plant-derived EOs mentioned in Table 1 were purchased from 2 brands “Al-Ahlam for Seeds Oil” (Production Jeddah, Saudi Arabia) and “Al Captain Company” (Cairo, Egypt). 4 months later, all EOs and extracted oils were then bio-assayed.
Table 1.
Mycelial growth density of four dermatophyte references formed on applying 26 plant-derived commercial essential oils at different oil concentrations (μl/ml)
| Essential oils | Microorganisms | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fungal growth density at different oil concentration (μl/ml) | |||||||||||||||||
| Microsporum canis | Epidermophyton floccosum | Trichophyton rubrum | Trichophyton mentagrophytes | ||||||||||||||
| 0.5 | 1.0 | 2.0 | 4.0 | 0.5 | 1.0 | 2.0 | 4.0 | 0.5 | 1.0 | 2.0 | 4.0 | 0.5 | 1.0 | 2.0 | 4.0 | ||
| 1 | Thyme vulgaris | ++++ | ++ | ++ | + | ++++ | ++ | ++ | ++ | ++++ | ++ | ++ | + | ++++ | ++ | ++ | + |
| 2 | Nigella sativa | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++++ | ++ |
| 3 | Prunus armeniaca | ++ | − | − | − | ++ | + | − | − | ++ | + | − | − | ++ | + | − | − |
| 4 | Prunus dulcis var. amara | ++ | − | − | − | ++ | + | − | − | ++ | + | − | − | ++ | + | − | − |
| 5 | Curcume longa | ++++ | ++++ | ++ | ++ | ++++ | ++ | ++ | + | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ | ++ |
| 6 | Olea europaea | ++ | + | − | − | ++ | + | − | − | ++ | + | − | − | ++ | + | − | − |
| 7 | Allium sativum | ++++ | ++++ | ++ | + | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | + |
| 8 | Syzgium aromaticum | ++++ | ++++ | ++ | + | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 9 | Zingiber officinale | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 10 | Cinnamon vulgare | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 11 | Aloe vera barbadensis | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 12 | Mentha piperita | + | − | − | − | + | − | − | − | + | + | − | − | + | + | − | − |
| 13 | Ocimum basilicum | ++++ | ++++ | ++ | + | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | + |
| 14 | Sinapis alba | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 15 | Amygdalus communis | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 16 | Cuminum cyminum | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++++ | ++ |
| 17 | Pimpinella anisum | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++++ | ++ |
| 18 | Elettaria cardamomum | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++++ | ++ |
| 19 | Rosmarinus officinalis | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 20 | Origanum vulgare | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | + | ++++ | ++++ | ++ | ++ |
| 21 | Petroselinum crispum | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ |
| 22 | Crocus sativus | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | + | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | + |
| 23 | Citrus sinensis | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | ++++ | ++++ | ++ | + | ++++ | ++++ | ++ | + |
| 24 | Armoracia rusticane | ++++ | ++ | ++ | + | ++++ | ++ | ++ | + | ++++ | ++++ | ++ | + | ++++ | ++ | + | + |
| 25 | Coriandrum sativum | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 26 | Trigonella foenum | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
++++, good growth; ++, moderate growth; +, weak growth; −, completely inhibited
Oils extraction
The mint leaves, M. piperita, were air dried and then ground to semi-powdered state. The external hard covers of bitter almond, P. dulcis var. amara, and apricot seeds, P. armeniaca, were eliminated. The unshelled seeds were air dried for 2 weeks and then pulverized using laboratory miller into powder form. Fleshy parts of olive drupes, O. europaea, were separated, cut into small parts and minced into a paste. Mint semi-powder (100 g), bitter almond and apricot powders (500 g of each) and olive paste (200 g) were subjected to hydro-distillation for 2 h in case of mint and 3 h for other samples using a Clevenger-type apparatus. Extracted oils were dried over anhydrous Na2SO4, sterilized and preserved in a sealed vial at 4 °C prior to further studies [6].
Fungal strain references
Four pure cultures of local pathogenic dermatophyte isolates were kindly provided by Laboratory of Microbiology, King Khalid Hospital at Riyadh, Kingdom of Saudi Arabia; Microsporum canis, Epidermophyton floccosum, Trichophyton rubrum and Trichophyton mentagrophytes. These clinical keratinophilic references were isolated from patients diagnosed as having various infections. All strains were maintained on Czapek’s dox agar (diffco) at 4 °C as stock cultures.
Antifungal toxicity screening assay
Antifungal toxicity screening of 26 EOs was assayed using method described by Adam et al. [7] with slight modification. Each of the 26 commercial EOs was diluted in 95 % ethanol. It was then mixed and homogenized with Czapek’s dox agar growth medium with a pH of 5.5 to achieve final concentrations of 4, 2, 1, 0.5, 0.25 and 0.125 μl/ml of medium. Twofold serial agar dilution method was applied according to Pandey et al. [4]. Triplicates of petri dishes of 9.0 cm diameter were then poured with aliquots of 25 ml sterilized media containing tested EO at a specific concentration. After solidification of agar, central well of 1.0 cm diameter was aseptically punctured in each Petri dish. Wells were then refilled by Czapek’s dox agar discs of same diameter taken from a culture of 7-day-old reference fungal strains. Negative and positive controls were prepared to control the sensitivity of the tested fungus. In negative control, Czapek’s dox agar was seeded with equivalent amounts of 95 % oil free ethanol. While, in positive control, tested oils was replaced with equivalent amounts of the standard antifungal, ketoconazole (Sigma). All plates were incubated at 28 °C for ≈7–10 days. Antimycotic potential of EO was evaluated by visual monitoring of reference mycelial growth density on agar plate and scored as specified by Abd El Salam and Ibrahim [8]. At the end of incubation period, samples were compared with positive and negative controls. Data were collected and marked down.
Determination of MIC
MIC was surveyed by disc diffusion technique which based on agar diffusion method according to Bansod and Rai [9]. It was determined for extracted mint, olive, bitter almond and apricot pure oils, their interchangeably two-oil combinations (Fig. 1) and a mixture of the four oils. Extracted M. piperita, P. dulcis var. amara, P. armeniaca and O. europaea oil stock solutions were prepared as described by Abd El Salam and Ibrahim [8], 1.0 g extracted oil was dissolved in 10 ml 5 % dichloromethane and was then sterilized by filtration. For two-oil and four-oil mixtures, sterile filter paper discs (Whatman No.1 of 5 mm diameter) were loaded with equal ratios of each of oils in mixture to get final concentrations 100, 50, 25 and 12.5 μg. Also pure oils were loaded separately at the same concentrations. Soaked discs were then aseptically applied to the surface of Czapek’s dox agar plate seeded with equal proportions of 7-day-old target dermatophyte inocula (adjusted at 104 CFU per ml). For each oil concentration, triplicates of petri dishes were prepared. Also, equivalent amounts of ketoconazole were applied in positive controls. All Petri dishes were incubated at 28 °C for 7 days.
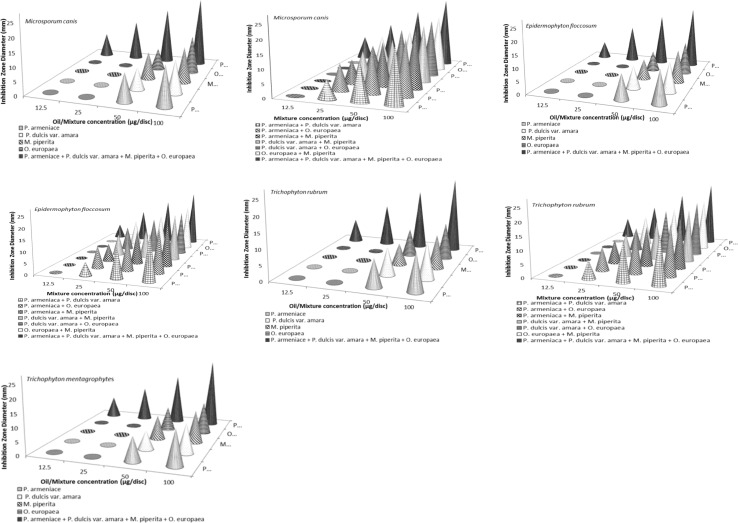
Fig. 1.
Four dermatophyte growth inhibition zones (mm), including disc diameter, formed on applying extracted oils: pure, interchangeably two-oil mixtures and four-oil mixture at different concentrations (µg/disc)
Diameters of dermatophyte mycelial inhibition zones formed surrounding discs (included disc diameter) were measured in mm using a vernier caliper. Diameters mean values were calculated and figured. The MICs were determined as the lowest concentration of oil showing a zone of inhibition. MIC was expressed in μg/disc.
Antifungal index evaluation
For this assay, the method followed by Cheng et al. [10] was employed. Briefly, different concentrations of extracted EOs and their mixtures, 400, 200, 100 and 50 µg/ml, were prepared in triplicates of 9 cm Petri dishes using sterile Czapek’s dox agar. After transferring fungal mycelia, the testing dishes were incubated at 28 °C. When the mycelium of fungus reached the edges of negative control dish (without adding oil or mixed oils) for ≈10–14 days, the antifungal indices were calculated.
where Da is the diameter of growth zone in the experimental dish (cm); Db, the diameter of growth zone in the control dish (cm). AI means and standard deviations were also calculated and tabulated.
Results
Antifungal toxicity screening assay
Variable antimycotic oil effectiveness was registered on screening antifungal toxicity of 26 commercial EOs against keratinophilic M. canis, E. floccosum, T. rubrum and T. mentagrophytes (Table 1). It was found that 24 oils were bioactive, while two oils were inactive.
All screened 26 commercial EOs marked down antifungal inactivity at oil concentrations 0.125 and 0.25 μl/ml (not mentioned in the Table 1). This was detected by visual comparisons of treated reference strains growth; M. canis, E. floccosum, T. rubrum and T. mentagrophytes with their controls. Among the 22 bioactive EOs, four EOs registered the most fungicide toxicity. They caused a complete mycelial growth inhibition of the four references at concentrations of 2.0 and 4.0 μl/ml. These oils were P. armeniace, P. dulcis var. amara, O. europaea and M. piperita EOs (Table 1). On the other hand, all tested concentrations (4, 2, 1, 0.5, 0.25 and 0.125 μl/ml) of Coriandrum sativum and Trigonella foenum EOs allowed normal mycelial growth of four dermatophytes. This reflected the total inactivity of these two oils. Table 1 also demonstrated that 20 commercial EOs showed variable mycelial growth densities of M. canis, E. floccosum, T. rubrum and T. mentagrophytes at concentrations 4, 2, 1, 0.5, 0.25 and 0.125 μl/ml. Maximum antifungal toxicities of the 22 bioactive EOs were recorded at oil concentrations 2.0 μl/ml and/or 4.0 μl/ml.
Both oil concentrations, 2.0 and 4.0 μl/ml, of P. armeniace, P. dulcis var. amara, O. europaea and M. piperita EOs showed the most antidermatophyte efficacy. The two concentrations recorded complete mycelial growth inhibition of M. canis, E. floccosum, T. rubrum and T. mentagrophytes.
Determination of MIC and antifungal index survey
Established MIC values of four-oil mixture, interchangeably two-oil combined mixtures and pure oils are demonstrated in Fig. 1. It was determined by the agar dilution method. Deduced MIC values of all pure extracted M. piperita, P. dulcis var. amara, P. armeniaca and O. europaea EOs were registered at 50 μg/disc. But in case of the two-oil alternative combinations, they were at 25 μg/disc. While, MIC of the four-oil mixture was at concentration of 12.5 μg/disc. Recorded diameters of dermatophyte growth inhibition zones indicated that the fungicidal efficacy of two-oil mixtures against M. canis, E. floccosum, T. rubrum and T. mentagrophytes was higher than that of pure oil extracts. Nevertheless, four-oil mixture registered the highest ever toxicity against four pathogenic references as shown in Fig. 1. Widest fungal inhibition zone diameters (25–26 mm) expressing the most antidermatophyte efficacy were detected on applying four-oil mixture at 100 µg/disc concentration. This was followed by two-oil alternatives inhibition zone diameters (18–21 mm) which were recorded at 100 µg/disc. Pure oils inhibition zones of (11–14 mm) were marked down at 100 µg/disc.
Table 2 demonstrated that among tested pure and mixed oils, four-oil mixture registered the highest, AI values at concentration 50 mg/ml (65.28–69.56 %), while it was the lowest in case of pure oils (26.96–33.46 %). AI of extracted P. armeniaca pure oil recorded the highest values at oil concentration 50 % (30.73–33.46 %) followed by P. dulcis var. amara (28.61–30.73 %), M. piperita (28.88–30.00 %) and then O. europaea (26.96–30.12 %) extracted oils. Antifungal indices of two-oil mixtures were 60.28 and 54.13 %; a percentage of 60.28 % for P. armeniaca and P. dulcis var. amara with M. canis, while it was 54.13 % for a mixture of P. armeniaca and O. europaea with T. mentagrophytes. Moreover, at concentration 100 mg/ml, four-oil mixture (P. armeniaca, P. dulcis var. amara, P. armeniaca and M. piperita) marked down 100 % AI with four studied dermatophytes. All pure extracted oils recorded high antifungal indices (98.00–82.99 %) against all the investigated fungal strains except P. armeniaca oil whose AI value was 100 % against M. canis only. Nevertheless, antifungal indices were equaled to 100 % at concentrations of 200 and 400 mg/ml of all pure oils and oil mixtures (not mentioned in Table 2) with all pathogenic references.
Table 2.
Antifungal index (%) of pure and combined extracted oils at different concentrations (µg/ml)
| Oil extract and oil mixtures concentration (µg/ml) | Dermatophyte fungal strains | ||||
|---|---|---|---|---|---|
| Microsporum canis | Epidermophyton floccosum | Trichophyton rubrum | Trichophyton mentagrophytes | ||
| of antifungal index (%) ± SD | |||||
| P. armeniaca | 50 | 30.11 ± 0.3 | 33.46 ± 0.2 | 30.73 ± 0.29 | 31.91 ± 0.29 |
| 100 | 100 ± 0 | 98.00 ± 0.29 | 97.00 ± 0.5 | 96.01 ± 0.25 | |
| P. dulcis var. amara | 50 | 29.60 ± 0.58 | 30.63 ± 1 | 30.73 ± 0.58 | 28.61 ± 0.25 |
| 100 | 89.34 ± 0.02 | 88.56 ± 0.05 | 90.49 ± 0.29 | 88.00 ± 0.76 | |
| M. piperita | 50 | 30.00 ± 1 | 28.88 ± 1.2 | 29.11 ± 1 | 29.35 ± 0.9 |
| 100 | 88.39 ± 0.23 | 86.99 ± 0.25 | 88.79 ± 0.48 | 87.19 ± 0.74 | |
| O. europaea | 50 | 26.96 ± 1 | 27.98 ± 0.76 | 28.00 ± 0.5 | 30.12 ± 0.9 |
| 100 | 82.99 ± 0.35 | 85.00 ± 0.4 | 84.89 ± 0.45 | 83.45 ± 0.26 | |
| P. armeniaca + P. dulcis var. amara | 50 | 60.28 ± 0.17 | 60.10 ± 0.32 | 58.94 ± 0.47 | 58.11 ± 0.11 |
| 100 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| P. armeniaca + O. europaea | 50 | 55.09 ± 0.15 | 56.66 ± 0.26 | 55.95 ± 0.58 | 54.13 ± 0.36 |
| 100 | 100 ± 0 | 99.54 ± 0.29 | 100 ± 0 | 99.00 ± 0.2 | |
| P. armeniaca + M. piperita | 50 | 56.96 ± 0.29 | 57.98 ± 0.01 | 58.00 ± 0.29 | 60.12 ± 0.48 |
| 100 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| P. dulcis var. amara + M. piperita | 50 | 58.91 ± 0.06 | 60.03 ± 0.76 | 57.88 ± 0.29 | 58.77 ± 1.53 |
| 100 | 100 ± 0 | 100 ± 0 | 99.54 ± 0.33 | 98.98 ± 0.5 | |
| P. dulcis var. amara + O. europaea | 50 | 50.48 ± 0.76 | 50.90 ± 0.29 | 48.64 ± 0.58 | 48.13 ± 0.5 |
| 100 | 100 ± 0 | 100 ± 0 | 99.22 ± 0.11 | 98.99 ± 0.005 | |
| O. europaea + M. piperita | 50 | 40.28 ± 0.58 | 40.10 ± 1.29 | 38.94 ± 0.82 | 38.11 ± 0.39 |
| 100 | 95.23 ± 0.29 | 94.56 ± 1.15 | 95.55 ± 0.58 | 94.00 ± 0.41 | |
| P. armeniaca + P. dulcis var. amara + M. piperita + O. europaea | 50 | 68.56 ± 0.06 | 67.34 ± 0.06 | 69.56 ± 0.06 | 65.28 ± 0.06 |
| 100 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| Z-test | 11.868 | 12.051 | 15.808 | 16.142 | |
| p value | 0.000a | 0.005* | 0.007* | 0.003* | |
* Significant results
Four-oil mixture recorded the lowest MIC and highest AI values against all four dermatophyte references as illustrated in Fig. 1 and Table 2. This was followed by two-combined oil mixtures and then pure oils. The highest MIC and lowest AI values were obtained in case of pure oils.
Discussion
Antifungal toxicity screening assay
A great diversity of plant EOs documented their microbial effectiveness in many investigations. Some were carried out to discover plant products inhibiting pathogenic fungi without environmental health damage. Prakash et al. [11] recommended the use of Ocimum gratissimum EO in spices as a nontoxic antimicrobial and anti-aflatoxigenic agent.
Same tested 26 EOs, except P. dulcis var. amara as investigated by Abd El Salam and Ibrahim [8] whose results were consistent with ours. They screened antimicrobial properties of 39 diversified EOs against Aspergillus niger, Aspergillus flavus, Fusarium lycopersici, and Alternaria solani. On comparing data, relatively same results of antifungal efficacies were noticed; Bansod and Rai established different antimycotic activities on screening EOs extracted from fifteen variable medicinal plants against A. fumigatus and A. niger; Chuang et al. proved that Moringa oleifera Lam crude extracts and EO has antifungal effect on T. rubrum, T. mentagrophytes, E. floccosum, and M. canis; Shin and Lim detected a growth inhibition of six Trichophyton spp by some herbal EOs; Brunia et al. recorded antifungal activity of Ocotea quixos, Lauraceae EOs against dermatophyte T. mentagrophytes; Adam et al. found that EOs of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa exhibited antifungal activities against Malassezia furfur, T. rubrum, and T. beigelii; Kishore et al. screened fungitoxicity of sixteen EOs against T. rubrum and M. gypseum [1, 2, 7, 9, 12, 13]. They documented that Artemisia nelagirica, Caesulia axillaris, Chenopodium ambrosioides, Cymbopogon citratus and Mentha arvensis showed strong activity.
Screening results of 26 EOs in present study were largely supported according to many published findings. Prasad et al. [5] observed variable antidermatophytic activities by Psoralea corylifolia seeds extract against T. rubrum, T. mentagrophytes, E. floccosum and M. gypseum. Bansod and Rai [9] documented variable EOs antimycotic activity against A. niger and A. fumigatus. They found that EOs of Cymbopogon martini, Eucalyptus globulus and Cinnamomum zylenicum were of maximum activity followed by Cymbopogon citratus. While, oils of Mentha spicata, Azadirachta indica, Eugenia caryophyllata, Withania somnifera and Zingiber officinale exhibited moderate activity; and oils of Cuminum cyminum, Allium sativum, Ocimum sanctum, Trachyspermum copticum, Foeniculum vulgare and Elettaria cardamomum showed comparatively low activity. Our study results were also enhanced by Abd El Salam and Ibrahim [8] findings; they deduced that commercial P. armeniaca EO was the most antifungal effective oil among screened 39 EOs at 2000 μg/ml. Zohri et al. [14] studied the inhibitory effect of onion oil against nine different species of dermatophytic fungi. They found that onion oil (200 ppm) completely inhibited the growth of M. canis, M. gypseum and T. simii. While, the growth of both Chrysosporium queenslandicum and T. mentagrophytes was completely inhibited by 500 ppm of onion oil, and the growth of other tested species of dermatophytic fungi was gradually reduced by increasing the concentrations of onion oil.
Determination of MIC and antifungal index survey
Many published studies investigated the effect of different concentrations, determined the MIC and screened AI values of various antimycotic active EOs. Cimanga et al. [15] studied the antifungal activity of EOs from 15 aromatic medicinal plant species. They indicated that all oils from fresh plant materials exhibited an antifungal activity at different levels against Candida albicans, Candida tropicalis, A. niger, T. mentagrophytes and M. canis. They recorded a high antifungal activity of inhibition zone diameter (15–22 mm) followed by (14–17 mm) then (11–17 mm) and (10–12 mm). This finding is consistent with the present study results. Abd El Salam and Ibrahim [8] found that MIC of P. armeniaca extracted oil against F. lycopersici and A. solani was obtained at 500 μg/ml, while MIC was documented with A. niger and A. flavus at 1000 μg/ml. Also, Cheng et al. [10] studied the antifungal activity and evaluated AI of eugenol and cinnamaldehyde against white-rot fungi, Trametesversicolor. At concentration 100 µg/ml, they deduced antifungal indices of 74.5 and 100 %, respectively. Both the materials totally inhibited the growth of Laetiporus sulphureus (brown-rot fungus) and Lenzites betulina at concentration 100 µg/ml. While at the level of 200 µg/ml, AI of both constituents was 100 % against all tested fungal strains.
Many EOs are only fungistatic and high concentrations are needed for fungicidal activity [16]. To enhance the efficacy of EOs, the combined use of different oils has been evaluated recently for potential synergistic effects. The combination of EOs with synthetic antifungals will probably result in a more effective therapy [17–20]. Shin and Lim [2] concluded that the antifungal effects of ketoconazole against Trichophytonspp. were enhanced significantly by administering it in combination with the EO fraction of P. graveolens or its main components. However; no published literatures studied the use of combined EOs with each other as antimicrobial agents. Thus, we decided to investigate this idea in our study by comparing between MIC and AI values of pure and combined extracted oils. We assayed these two parameters to be used as indicators for the fungicidal potential of tested oils. As we studied all achieved AI with MIC data, we deduced that four-oil mixture was the most potent effective fungicide followed by two-combined oil mixtures and then pure oils. This proved the enhancing synergistic effect of the combined use of EOs.
When surveying data obtained in different studies, most publications provide explanations about the active antimicrobial compounds of EOs. They are generally terpenes, which are phenolic in nature and attack the pathogens through cell wall and cell membrane. Thus, active phenolic compounds might have several invasive targets which could lead to the inhibition of human infectious fungal pathogens [21]. The antifungal activity can be also attributed to the presence of some components such as carvacrol, α-terpinly acetate, cymene, thymol, pinene, linalool which are already known to exhibit antimicrobial activity [6, 22–24].
In this study, medical safety point of applying extracted four EOs under investigation on human normal skin was not tested. At the same time, no publications were found examined or even discussed EOs hazards on public health. Accordingly, there was not any evidences showed that these EOs used in this study are not harmful for human normal skin. So, we recommend testing medical safety of these four EOs before applying them in curing of dermal diseases.
Conflict of interest
No conflict of interest declared.
Contributor Information
Sahar Yassin Ibrahim, Phone: 00966 546161900, Email: soo.moussa@yahoo.com.
Magda Magdy Abd El-Salam, Phone: +2- 012- 03615727, Phone: 00966 599869717, Email: mmagdy_hiph@yahoo.com.
References
- 1.Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Antifungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol. 2007;98:232–236. doi: 10.1016/j.biortech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Shin S, Lim S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J Appl Microbiol. 2004;97:1289–1296. doi: 10.1111/j.1365-2672.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 3.Bokhari FM. Antifungal activity of some medicinal plants used in Jeddah, Saudi Arabia. Mycopath. 2009;7(1):51–57. [Google Scholar]
- 4.Pandey DK, Tripathi NN, Tripathi RD, Dixit SN. Fungitoxic and phytotoxic properties of the essential oil Caesulia axillaris Roxb. Angew Bot. 1982;56:259–267. [Google Scholar]
- 5.Prasad NR, Anandi C, Balasubramanian S, Pugalendi KV. Antidermatophytic activity of extracts from Psoralea corylifolia (Fabaceae) correlates with the presence of a flavonoid compound. J Ethnopharmacol. 2004;91:21–24. doi: 10.1016/j.jep.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Knobloch L, Weigand H, Weis N, Schwarn HM, Vigenschow H. Action of terpenoids on energy metabolism. In: Brunke E-J, editor. Progress in Essential Oil Research. Walter de Gruyter, USA; 1985. pp. 429–448.
- 7.Adam K, Sivropoulou A, Kokkini S, Lanaras T, Arsenakis M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739–1745. doi: 10.1021/jf9708296. [DOI] [Google Scholar]
- 8.Abd El-Salam MM, Ibrahim SY. Antimicrobial properties of 39 essential oils against thirteen food-borne microorganisms; efficacy and environmental hygiene of Prunus armeniaca in raw food preservation under cold storage. J Environ Occup Sci. 2014;3:162–169. [Google Scholar]
- 9.Bansod S, Rai M. Antifungal activity of essential oils from indian medicinal plants against human pathogenic Aspergillus fumigatus and A. niger. World J Med Sci. 2008;3:81–88. [Google Scholar]
- 10.Cheng S, Liu J, Hsui Y, Chang S. Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum) Bioresour Technol. 2006;97:306–312. doi: 10.1016/j.biortech.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Prakash B, Shukla R, Singh P, Mishra PK, Dubey NK, Kharwar RN. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res Int. 2011;44:385–390. doi: 10.1016/j.foodres.2010.10.002. [DOI] [Google Scholar]
- 12.Brunia R, Medicib A, Andreottib E, Fantinc C, Muzzolib M, Dehesad M, et al. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem. 2004;85:415–421. doi: 10.1016/j.foodchem.2003.07.019. [DOI] [Google Scholar]
- 13.Kishore N, Mishra AK, Chansouria JPN. Fungitoxicity of essential oils against dermatophytes. Mycoses. 1993;36:211–215. doi: 10.1111/j.1439-0507.1993.tb00753.x. [DOI] [PubMed] [Google Scholar]
- 14.Zohri AN, Abdel-Gawadl K, Saber S. Antibacterial, antidermatophytic and antitoxigenic activities of onion (Allium cepa L.) oil. Microbiol Res. 1995;150:167–172. doi: 10.1016/S0944-5013(11)80052-2. [DOI] [PubMed] [Google Scholar]
- 15.Cimanga K, Apers S, De Bruyne T, Miert SV, Hermans N, Totté J, et al. Chemical composition and antifungal activity of essential oils of some aromatic medicinal plants growing in the democratic Republic of Congo. J Essent Oil Res. 2002;14:382–387. doi: 10.1080/10412905.2002.9699894. [DOI] [PubMed] [Google Scholar]
- 16.Cassella S, Cassella J, Smith I. Synergistic antifungal activity of tea tree (Melaleuca alternifolia) and lavender (Lavandula angustifolia) essential oil against dermatophyte infection. Int J Aromather. 2002;12:2–15. doi: 10.1054/ijar.2001.0127. [DOI] [Google Scholar]
- 17.Yoon SY, Eo SK, Lee DK, Han SS. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch Pharmacal Res. 1994;6:438–442. doi: 10.1007/BF02979122. [DOI] [PubMed] [Google Scholar]
- 18.Suresh B, Sriram S, Dhanaraj SA, Elango K, Chinnaswamy K. Anticandidal activity of Santolina chamaecyparissus volatile oil. J Ethnopharmacol. 1997;55:151–159. doi: 10.1016/S0378-8741(96)01490-0. [DOI] [PubMed] [Google Scholar]
- 19.Giordani R, Trebaux J, Masi M, Regli P. Enhanced antifungal activity of ketoconazole by Euphorbia characias latex against Candida albicans. J Ethnopharmacol. 2001;78:1–5. doi: 10.1016/S0378-8741(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 20.Shin S, Kang CA. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Lett Appl Microbiol. 2003;36:111–115. doi: 10.1046/j.1472-765X.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 21.Tabassum N, Vidyasagar GM. Antifungal investigations on plant essential oils. A review. Int J Pharm Pharm Sci. 2013;5(2):19–28. [Google Scholar]
- 22.Juven BJ, Kanner J, Schved F, Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol. 1994;76:626–631. doi: 10.1111/j.1365-2672.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 23.Harborne JB, Williams CA. Anthocyanins and other flavonoids. Nat Prod Res. 1995;7:639–657. doi: 10.1039/b006257j. [DOI] [PubMed] [Google Scholar]
- 24.Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N, et al. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J Ethnopharmacol. 2002;79:213–220. doi: 10.1016/S0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]