Abstract
The overuse and abuse of antibiotics have contributed to the global epidemic of antibiotic resistance. Current evidence suggests that widespread dependency on antibiotics and complex interactions between human health, animal husbandry and veterinary medicine, have contributed to the propagation and spread of resistant organisms. The lack of information on pathogens of major public health importance, limited surveillance, and paucity of standards for a harmonised and coordinated approach, further complicates the issue. Despite the widespread nature of antimicrobial resistance, limited focus has been placed on the role of environmental factors in propagating resistance. There are limited studies that examine the role of the environment, specifically water, sanitation and hygiene factors that contribute to the development of resistant pathogens. Understanding these elements is necessary to identify any modifiable interactions to reduce or interrupt the spread of resistance from the environment into clinical settings. This paper discusses some environmental issues that contribute to antimicrobial resistance, including soil related factors, animal husbandry and waste management, potable and wastewater, and food safety, with examples drawn mainly from the Asian region. The discussion concludes that some of the common issues are often overlooked and whilst there are numerous opportunities for environmental factors to contribute to the growing burden of antimicrobial resistance, a renewed focus on innovative and traditional environmental approaches is needed to tackle the problem.
Keywords: Antimicrobial, Antibiotic resistance, Environmental health, Water, Sanitation, Hygiene
Introduction
The common bacteria responsible for causing some infectious diseases have steadily developed resistance to antibiotics, resulting in antimicrobial resistance (AMR) escalating into a global health crisis [1]. The greater burden of infectious disease has created a significant need for antimicrobial therapy and has also contributed to the growing burden of resistance [2, 3]. In addition to the paucity of hard evidence for more accurate quantification of the social and economic burdens associated with AMR regionally and globally, the WHO reports that major gaps continue to exist in understanding the brevity of the problem [4]. Although the majority of deaths related to antibiotic resistance occur in hospitals and nursing home settings, antibiotic-resistant infections can happen anywhere, and are most common in the general community [5].
The development of antibiotic stewardship programmes has progressed in industrialised countries such as the UK, Canada and Australia. However, developing countries bear a greater burden from AMR due to the paucity of information on resistance trends, determinants of antibiotic use, limited surveillance and diagnostic capacity [4]. The WHO’s recently published report Antimicrobial resistance: global report on surveillance 2014, focused on surveillance for AMR patterns at country level and in various world regions. The report points to the lack of consensus on methodology, and the gaps in surveillance and data sharing about antibiotic resistance in food-producing animal and the food chain, amongst other issues. It further discusses the clinical outcomes and economic impact of several common infections but laments the lack of data to determine the wider societal impact and economic implications. However, the WHO report did not focus on the underlying risk or contributing factors for AMR [4]. Current evidence suggests that widespread dependency on antibiotics and complex interactions between human health, animal husbandry and veterinary medicine, have contributed to the propagation and spread of resistant organisms [4, 6–8]. The paper is aimed at environmental and preventive health professionals and policy makers and others concerned with the prevention and control of AMR from the environment. It focuses on some environmental risks or contributing factors for AMR; including soil-related factors, animal husbandry and waste management, potable and wastewater, and food safety.
The aim of this paper is to fill the gap in existing literature by shifting the focus from the clinical impacts, such as those highlighted in the WHO report (2014), to some important environmental risk factors that contribute to AMR. The paper does not seek to provide an in-depth scientific analysis but instead, presents a general discussion of some salient environmental surveillance and management issues, with some broad recommendations. Examples from the wider Asia-Pacific region amongst others have been cited to provide some context.
Antimicrobial resistance and the water, sanitation and hygiene problem
This section provides an overview of AMR, an introduction to the issue as a global public health problem and summarises how various environmental issues have contributed to the development of the AMR crisis. It briefly discusses the limited focus placed on the role of environmental factors in propagating resistance, and proposes that various environmental determinants could be playing an important role in the development and spread of AMR.
The development of AMR occurs when a drug is no longer able to inhibit or control the action of a microorganism (bacteria, fungus, virus or parasite) that was previously sensitive to it. As such, the usual treatment regime is no longer effective, resulting in difficulty to control infections, increased risk of spread of infection to others, and in some cases, increased risk of death in some patients when compared with the risk in those infected by non-resistant pathogens [4, 5]. Antibiotics are antimicrobials that are used to treat bacterial infections [5]. AMR develops as part of a natural evolutionary process for microorganisms including bacteria, parasites and viruses [9].
The development of resistance to antibiotics can occur naturally (intrinsic) based on a spontaneous gene mutation in the absence of selective pressure from antibiotics [10]. Bacteria develop resistance to antibiotic (acquired) when at least one bacterium within a heterogeneous colony of bacteria carries the genetic determinant capable of expressing resistance to the antibiotic. The genetic determinants classify the type and intensity of resistance that is eventually expressed by the bacterial cell. Regardless of the way a gene is transferred to a bacterium, resistance develops as a result of the effective expression of the gene, which can be spread and propagated to other bacteria, enabling them to produce a tangible biological effect which inhibits the activity of the antibiotic [9, 10].
It is believed that the origins of antibiotic resistance genes lies with environmental bacteria that produce and release antibacterial to influence microbial populations with which they compete for nutrients [11]. This is in keeping with the discovery of a wide spectrum of antibiotic resistance genes (for e.g. vancomycin resistance element VanA) in 30,000-year-old Beringian permafrost sediments, providing conclusive evidence of the naturally occurring phenomenon that predates the modern selective pressure of clinical antibiotic use [12]. However, it is believed that this naturally occurring resistance can also play a role in the development of acquired resistance; and that both types of resistance can be transmitted horizontally or vertically [10, 12]. It is now relatively well understood at the molecular level, that the same gene can be identified in very unrelated pathogens in humans, clinical settings, as well as among bacteria which grow in agricultural context, meat animals and companion animals; suggesting that ABR grows and is context independent [11]. Resistance however can be accelerated by the selective pressure due to frequent and widespread antibiotic usage [4]. Intrinsic resistance is believed to be relatively less common than the resistance acquired under pressure [10]. Some resistance mechanisms carried by bacteria can be transferred to humans [4]. This results initially in a silent carrier state that may later give rise to seemingly unrelated infections and increased severity of disease, resulting in poorer outcomes for patients [4, 5, 13].
Poor hygiene and lack of compliance with infection prevention and control measures have contributed to the propagation and spread of resistant bacteria strains [4, 6]. Antibiotic resistance such as sulfonamide-resistant Streptococcus pyogenes in the 1930s [14] and penicillin-resistant Staphylococcus aureus in the 1940s [15] were first observed in hospitals where most drugs were being prescribed. Mycobacterium tuberculosis developed resistance to streptomycin soon after the discovery of this antibiotic [16]. Multiple drug resistance emerged a few years later in the 1950s to early 1960s among several gastrointestinal/foodborne pathogens including Escherichia coli, Shigella and Salmonella (FBI) [17]. Multiple drugs resistance has continued to spread among common pathogens, with Haemophilus influenzae developing resistance to ampicillin [18], chloramphenicol and tetracycline [19, 20] and an increased resistance to ampicillin observed, with ampicillin-resistant Neisseria gonorrhoea (STI) emerging mainly in developed countries in the 1970s [9, 21]. Currently, all WHO regions report very high rates of resistance amongst common bacteria (for e.g. E. coli, Klebsiella pneumoniae and S. aureus) that are responsible for common infections like urinary tract infections, wound infections, bloodstream infections and pneumonia, which can be acquired in both health-care and community settings [4].
Antimicrobial resistance has escalated into a major public health issue, with some pathogens being resistant to multiple antibiotics [5]. The WHO surveillance report highlighted the lack of information on pathogens of major public health importance, limited surveillance, and paucity of standards for a harmonised and coordinated approach to address the issue [4]. Some of the greatest burden of antimicrobial resistant pathogens has been reported in the Asian region [4, 9]. This includes widespread distribution of resistant strains of E. coli and vancomycin resistant S. aureus and multi-drug resistant strains of tuberculosis and typhoid fever [4, 9, 22, 23]. Some of the countries with the highest estimated cases of multi-drug resistant tuberculosis (MDR-TB) are located in Asia, including more than half of the nine million people who developed TB in 2013. While the Southeast Asia region appears to be on track to achieve the millennium development goal targets for reductions in TB disease burden (incidence, prevalence and mortality), recent estimates suggest a significant proportion of new cases and previously treated cases are infected with MDR-TB [24, 25]. Extra-drug resistant TB or XDR-TB (i.e. MDR-TB plus resistance to at least one fluoroquinolone and second line injectable), has been reported in at least 100 countries worldwide since 2013 [24]. Antibiotic resistance is not restricted to bacteria only. There is increasing evidence of the development of resistance among enteric protozoa from clinical samples in Australia [26–28].
Despite the widespread and growing evolution of AMR, limited focus has been placed on the role of environmental factors in propagating resistance. However, there are biologically plausible indications that contaminated raw water, wastewater and other environmental determinants may be playing an important role in the development and spread of AMR. It is unclear, the extent to which countries are conducting risk assessments to identify risks and management goals, which can guide the development of environmental management options, with clear indicators of effectiveness [29]. The assessment of the environmental risks is however not straight forward, as naturally occurring resistance genes can be present in the environment [12, 29]. There is a need to understand the environmental factors that contribute to the development of resistant pathogens to identify any modifiable interactions to reduce or prevent further spread of resistance throughout the environment. The WHO global report (2014) suggested that greater emphasis should be placed on public health interventions, such as strengthening hygiene and infection prevention and control measures, improving sanitation and access to clean water [4].
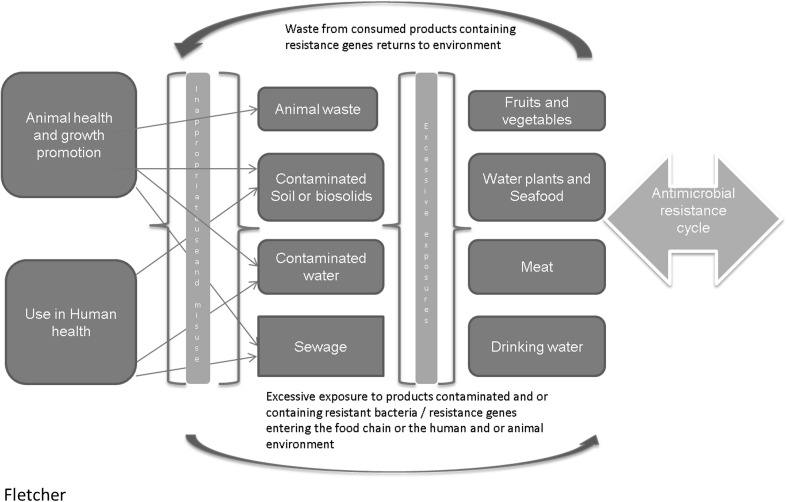
While this paper does not attempt to assess all environmental risks, the following sections will discuss some specific environmental issues that contribute to AMR, including an introduction to horizontal gene transfer, soil-borne resistance, animal husbandry and waste management, drinking water and wastewater, and food safety (Fig. 1). This list is by no means exhaustive, but we hope this paper can contribute to the wider discussion around the common issues that are often overlooked but can be managed without sophisticated interventions. The paper ends by looking at some ways that we can tackle the gaps discussed and proposes some recommendations that may be useful to consider when tackling the AMR issues, especially in resource limited settings.
Fig. 1.
Complex interactions amongst environmental- and health-related factors that contribute to the spread of antimicrobial resistance. The figure summarises how various often interlinked factors contribute to contamination of the environment; with the transfer of antibiotic resistance genes and antibiotic-resistant bacteria going in both directions, perpetuating the cycle of antimicrobial resistance
Horizontal gene transfer
Horizontal gene transfer (HGT) is one of the key factors associated with environmental propagation of AMR. There are numerous studies and reports that describe the characterisation and quantification aspects of HGT in bacterial evolution [30–33]; hence these aspects will not be covered here. However, we briefly introduce the concept of HGT to contextualise the remainder of this discussion.
The acquisition of foreign genes or mobile genetic elements by organisms or between species, is referred to as horizontal gene transfer (HGT) [32]. Horizontal gene transfer depends critically upon internal and environmental factors and is therefore considered to be a non-random genome innovation process [33]. The HGT processes involve several successive steps including the uptake of foreign DNA, its genetic recombination and regulatory integration, and establishment in the host population or the exchange community [33, 34]. The exchange community is defined as ‘a collection of organisms that can share genes by HGT, but does not need be in physical proximity’ [33].
The importance of HGT was initially recognised when bacteria were found to have acquired ‘infectious heredity’ of multiple antibiotic resistances [17, 35]. Many studies have described antibiotic resistance genes as part of self-transferable plasmids or of transposable elements, with evidence of genetic transfer occurring between both related and unrelated bacteria [32, 36]. It has been postulated that some organisms produce and release antibiotics into their environment to influence and control competing bacteria [11]. This occurs when some of the naturally occurring antibiotic resistance genes becomes captured by a mobile genetic element like an integron, transposon, phage, plasmid, or chromosomal island, which then plays a key role in the introduction of natural resistance genes into clinically relevant bacterial species from environmental sources (for e.g. water, sewage, air) [11, 33]. It has been suggested that approximately 20 % of a typical bacterial genome can be acquired from other species [31].
While there have been changing views about the importance of HGT over time, recent advances in whole genome sequencing of bacteria has furthered our understanding that HGT is an important factor in bacterial evolution [30–32, 35]. Several factors including genome size, genome composition, oxygen tolerance, carbon utilization and modularity of genetic units are common features of bacterial exchange communities, where genes are readily exchanged between species [33, 35]. However, there is adequate evidence to suggest that genes that encode resistance have been identified in many different contexts; even in untouched ecological environments and in a single host [11]. The modularity of genetic units supports their spread by HGT [35]. Jain et al. [33] suggest that HGT significantly accelerates the pace of genome innovation, which provides functional modules instead of creating new genes by mutations. There is growing evidence to suggest that HGT is an on-going process that plays a significant role in the real-time ecological adaptation of prokaryotes, through the shaping of bacterial genomes, promoting intra-species variability and distributing functional genetic modules within exchange communities [33, 35]. The combination of microbial characteristics, such as the selective pressure of antimicrobial use, and social and technical changes that enhance the transmission of resistant organisms contributes to the increased transfer of antibiotic resistance genes from their natural environment into pristine and clinical settings [37]. As outlined earlier, ARB can grow in the environment [10, 11] and HGT can occur in the environment independently of each other [10, 12].
Acquisition of resistance genes by bacterial communities may or may not occur in response to selection pressure. The presence of antibiotics in the environment exerts pressure which induces stress response from the microbial community promoting HGT, which induces the sharing of resistance genes as a bacterial adaptation response [10, 11]. For example, the hospital environment provides ample opportunities for the intermingling of bacteria of diverse genera and the mobile genetic elements, providing the opportunity for gene exchange events that initiate the formation of complex multi-drug resistance loci [11]. There is evidence that the genetic determinants which specify resistance to different antimicrobial drugs spread in strongly selective environments. Selective pressure imposed by antibiotics is notably evidenced among pathogenic bacteria in clinical settings, the community and throughout the commercial food chain [34]. Interestingly, while selective pressure occurs from environmentally persistent antibiotics, [38, 39] the presence of intrinsic resistance genes in the environment (for e.g. genes present in manure; sewage; hospital waste) significantly contributes to environmental pollution with resistance genes [40]. A study of watersheds in Colorado identified that resistance levels were determined by the level of resistance genes in manure released from farms rather than the development of resistance due to selective pressure from antibiotics [41].
Soil-borne resistance
Soil serves as the primary nutrient base and habitat for plants and organisms, and plays a vital role as a giant bioreactor for degrading pollutants and facilitating nutrient transformation. This section briefly discusses soil as a potential hot spot for the development and transfer of microbial resistance.
There is insufficient evidence of human exposure to soil-borne resistance; however, this could be severely underestimated. Reports have shown that antibiotic resistance genes and the same genetic platforms (plasmids, integrons) which are currently present in human pathogens, have been detected in pristine environments and in humans and animals populations that have never been in contact with antibiotics. This suggests that antibiotic resistance genes which have integrated in successful gene-transmission elements could persist and spread in the environment even in the absence of antibiotics [7, 42, 43]. Soil receives a large portion of excreted antibiotics through application of manure and sewage sludge as fertilizers [44]. Soil inevitably becomes a hot spot for antibiotics to affect indigenous microbes. Additionally, genetic exchange is encouraged by the higher density of microbes in the soil environment, contributing to the development of microbial resistance in the presence of antibiotics [45]. Bacterial resistant genes adapted to soil or other habitats can be transferred horizontally, resulting in independent transmission further into the environment [46]. For example, soil containing resistance genes and genetic platforms or resistant bacteria can be introduced into clinical environments, and even after disinfection, pathogenic organisms resident in organic soil and residues in drains may rebound if the primary sources of colonisation are not persistently removed [11].
The application of animal waste (biosolids) to agricultural soils (as soil conditioner) is commonly practised in the United States, Europe and Australia [47], and this acts as an entry point of both antibiotics and genetic resistance determinants into the environment [7, 29, 48]. Enteric bacteria can remain persistent in the environment for weeks to months, depending on species and temperature. However, regardless of cell viability some genetic elements can persist in the environment [42, 43]. Studies have demonstrated the absorption of antibiotic residues in food crops grown in manure-applied soil, with the increasing detection of antibiotic residues at increased concentrations of antibiotics in the manure-applied soil [47]. Clear standards and application procedures for the growth of food crops in manure-applied soil should be provided to guide this practise.
Animal husbandry and waste management
The WHO report does not discuss the role of animal husbandry and animal waste management in the development of AMR. The foregoing section however points to the concerns about transmission of AMR from soil and manure to food and clinical environments. This section looks briefly at some high risk animal husbandry practises that contribute to the development of antibiotic resistance genes, which can be transferred from farming environments to other environments and impact human health.
Due to the poor adsorption of antibiotics in the gut of the animals, significant amount of antibiotic residue is excreted in faeces and urine [49]. Since animal waste does not usually undergo the level of secondary treatment that human waste undergoes, higher concentration of antibiotics from animal waste enters the environment [46]. One example is the integrated agriculture–aquaculture farming system known as vegetation, aquaculture, and cage (VAC) which is widely used throughout Asia. ‘The VAC system is a recycling farm, typically consisting of a vegetable field, an aquaculture pond, and caged animals, where livestock manure (usually from pigs, chickens, and ducks) is directly transported to fish ponds and to vegetable and rice fields. This untreated sewage and wastewater from the livestock operations is used for fish culture and for fertilization of the vegetable fields. The animal manure contributes to the eutrophication of pond water, which enhances phytoplankton growth. The VAC system is considered a very economical method of recycling farming [50].
Unfortunately, due to the high rate of antibiotic use on these types of farms, the concentration of antibiotic residues in farm waste can lead to an increase in the number of antibiotic-resistant bacteria in the environment. This results in further selection and transfer of antibiotic resistance genes within the microbial community, within in the surrounding environment [38, 51]. A study of pigs in VAC systems, revealed high levels of resistance to tetracycline, nalidixic acid and enrofloxacin, among enteric bacteria [52]. This suggests that as a result of exposure to high concentrations of various antibiotics, the risk of colonisation with antibiotic-resistant bacteria and genes increases in the intestinal tracts of livestock. The antibiotic resistance genes can then be transferred to other environments and humans through water and the food supply [39]. Antibiotic resistance selection occurs among gastrointestinal bacteria, which are also excreted in manure and stored in waste holding systems. Manure then acts as a reservoir for resistant bacteria and antibiotic compounds [39, 47].
It is therefore necessary to prevent or limit the transfer of antibiotic resistance genes from farming environments to humans and other environments, through water and the food supply. Based on the foregoing risk to the environment and humans, environmental monitoring and enforcement of public health regulations for waste and manure handling and disposal should be reviewed and enforced where applicable by veterinary and public health officials; where mixed farming and application of animal manure to food crops are practised or considered, clear standards and guidelines based on scientific evidence should be developed and monitored. Collaborative efforts amongst farming societies and between governments can provide opportunities for sharing of best practise experiences and lessons learned. Countries could take advantage of existing facilities available through global surveillance efforts. One example of this is the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR) which has been established to support international efforts to minimize the public health impact of AMR associated with the use of antimicrobial agents in all food-producing animals. Collaborative efforts have begun between the WHO-AGISAR and the FAO to implement integrated foodborne pathogen and AMR surveillance in the poultry, beef, pig and aquaculture value chains across Asia and Africa. The aim of these international collaborative efforts is to strengthen national capacities for AMR surveillance and to fill some of the data and information gaps to inform the development of appropriate national policies for several industries including good animal husbandry, health and hygiene [53].
Drinking water and wastewater (sanitation) linkages
Water plays an important role in the transmission of many infectious and non-infectious organisms to humans. This section discusses some of the evidence around the dissemination of resistance elements and antibiotic-resistant organisms through water environments.
Antibiotic-resistant organisms can be disseminated through drinking water produced from surface water sources, which provide the route through which resistance genes are introduced in natural bacterial ecosystems. Non-pathogenic bacteria in this environment can act as a reservoir of resistance genes and platforms [39, 45, 54]. With the progressive accumulation of antimicrobial agents, detergents, disinfectants, and residues from industrial pollution, as heavy metals introduced into the environment, the evolution and spread of such resistant organisms in the water environment is facilitated [55].
Rapid industrial development and economic growth has far outpaced investment in public infrastructure in several developing countries in the Asia-Pacific region. Inadequate infrastructure has resulted in sanitary issues, including contaminants such as antibiotics in wastewater [39]. As previously outlined, antibiotics can easily be released into the environment from numerous source, resulting in the acquisition of resistance genes by bacterial communities in response to selection pressure [11, 33]. One study reported on the presence of quinolones, sulfonamides, and tetracycline in aquatic environments of Indochina and the prevalence of bacteria resistant to them [39].
Antibiotics have been detected in animal wastewater, pond water, sewage sludge, animal effluents, and rivers in China [39, 55, 56]. The most frequently detected antibiotics in China include sulfamethazine (75 %), oxytetracycline (64 %), tetracycline (60 %), sulfadiazine (55 %), and sulfamethoxazole (51 %) [39]. Contamination of the environment with tetracyclines and sulfonamides has been reported in Korea [39]. Several antibiotics, associated with livestock-source contamination, including sulfamethoxazole, sulfapyridine, trimethoprim, erythromycin-H2O, azithromycin, clarithromycin, and roxithromycin were detected at concentrations ranging from 4 to 448 mg/L in the Tamagawa River, Japan [57]. Imipenem resistance has been detected on mobile bathing utensils in Japan, indicating a risk of colonisation with resistant bacteria through this medium [58]. High concentrations (15–328 mg/L) of sulfamethazine, used as a veterinary medicine, have been detected in Vietnamese waters associated with pig and poultry farming [57].
There is evidence that suggests the conventional wastewater treatment process is inadequate in removing resistant bacteria from municipal wastewater. The demonstration of the pandemic and multi-resistant E. coli clone B2-O25b-ST131 being frequently detected in treated wastewater, underscores the risk of transmission of clinically important multi-resistant bacteria strains through wastewater [59]. Another study where survival experiments were conducted found that resistant E. coli strains had a higher survival rate in wastewater treated in lagoons, compared with other E. coli strains which were susceptible to several antibiotics [60].
Various studies have evaluated the effectiveness of wastewater treatment on the removal of antibiotic-resistant bacteria and resistance determinants. However, there is evidence suggests that the efficiency of removal is dependent on antibiotics’ physicochemical properties and the operating conditions of the treatment process [8]. One study has reported no significant reduction in antibiotic resistance genes (ARG) and antibiotic-resistant bacteria (ARB) through chlorination and UV radiation disinfection processes; with no significant difference in ARGs log concentration in pre- and post-disinfected effluent between UV and chlorination disinfection processes [61].
Sewage treated by Membrane Biological Reactor (MBR) facility proved more effective in removing higher concentrations of ARGs and ARB (range of removal 2.57–7.06 logs) compared to conventional treatment plants (range of removal 2.37–4.56 logs) (p < 0.05). Both Chlorination and UV Disinfection processes proved ineffective in significantly reducing ARGs and ARB (p > 0.05) from sewage. Advanced biosolid treatment methods (i.e. anaerobic digestion and lime stabilization) was found to be more effective in reducing the log concentrations of ARGs (except tetW) and ARB when compared with the conventional methods of dewatering and gravity thickening [61].
Another study compared antibiotic resistance of 870 E. coli strains isolated from domestic raw sewage, in the effluent from aerobic lagoons and activated sludge plants and evaluated the efficiency of both treatment systems in removing faecal coliforms (FC). The aerobic lagoon (99.99 % in summer) was found to be more effective than the activated sludge system (91.30 % in summer) to reduce faecal coliforms from domestic raw sewage. However, a significantly higher concentration of antibiotic-resistant E. coli strains (34.66 % were isolated from the effluent of the aerobic lagoon compared with the domestic sewage (23 %) [59].
Based on the foregoing risk to the environment and humans, environmental monitoring and enforcement of public health regulations for waste and manure handling and disposal should be reviewed and enforced where applicable. Clear standards should be developed to define limits for ARG and ARB in raw water and sewage effluents.
Food safety
Numerous studies have been dedicated to the contribution of food safety and animal husbandry to the development of AMR in the past decade. There are studies that cover a range of issues including but not limited to the uncertainties about the use of antibiotics in food animals and the risk posed to human health [62, 63]; the epidemiological and molecular evidence for the spread of resistance from food animals to man [53, 60, 61, 64]; and the benefits and risks of antimicrobial use in food-producing animals [65, 66]. Despite the numerous studies focused on these issues, the WHO surveillance report (2014) indicated that there are major gaps associated with the surveillance and sharing of data around resistance in foodborne bacteria and its relationship with animal and human health [4]. The benefits and risks of the use of antibiotics in the food animals remain a complex issue, and present a major challenge, and appear to be a double-edged sword [66]. This section touches briefly on some of the continued challenges for food safety.
While human antimicrobial use accounts for most of the resistance problem in humans, there is increasing concern that the use of antibiotics in food-producing animals, particularly, the long-term use of antibiotics as prophylaxis or for growth promotion, significantly contributes to the emergence of antibiotic-resistant bacteria in animals [4, 62, 67]. The significant risks to human health arise as resistant bacteria may spread from animals to humans via the food chain [4, 54]. They may also transfer their antibiotic resistance genes into human pathogenic bacteria, leading to failure of antibiotic treatment for some, possibly life-threatening, human conditions. Examples are urinary tract or abdominal infections caused by E. coli that could have been transmitted via the food chain [65]. The use of quinolones and fluoroquinolones in veterinary practise has resulted in a dramatic increase in resistance among Campylobacter strains, resulting from a mutation in the quinolone resistance-determining region of gyrA (Thr-86 to Ile) [62, 68]. Resistance to antimicrobial drugs among foodborne bacteria increases severity of disease and results in poorer outcomes for patients [4]. Some may argue that the danger and risk from the low dosages used for animal growth promotion is relatively small; however, there might be disadvantages to human and to animal health that are yet to be recognised. Bacterial resistance can develop in food animals resulting in the contamination of animal-derived food. The frequency with which resistant bacteria colonize the human gut, and resistance genes are transferred is yet to be determined [54, 62]. While modernized food production has placed emphasis on reducing antibiotic use for agriculture, animal husbandry and fish farming, this is likely to be less controlled in smaller scale traditional production, especially in countries where legislation is lacking and control measures are not enforced [46].
Additionally, there is also growing concern about transmission to human populations of multi-resistant S. aureus (MRSA) related to high-density swine production [4]. Human cross-infection with zoonotic salmonellosis is common; suggesting that resistant bacteria from animals are often passed to humans. People can also be exposed to resistant bacteria through raw or undercooked vegetables contaminated with soil [43]. In Southeast Asia large amounts of antibiotics are used for human medicine, livestock farming, and aquaculture. For example, there have been reports of cases where antibiotics are reportedly added to ice to prevent the decay of fish for sale in markets. People are then exposed to antibiotics through the consumption of the ‘treated’ fish [39]. Whilst adequate cooking of contaminated food can effectively destroy many resistant microorganisms, the antibiotic residue in food cannot be destroyed by cooking [39, 47].
Despite the emerging evidence for the contribution of food animal production and the food chain to the development of AMR, a widespread lack of cooperation and limited implementation of harmonised global standards, has hampered surveillance efforts. Limited access to and data around resistance in foodborne bacteria and its relationship with animal and human health remains a major challenge.
According to the WHO (2014), integrated disease surveillance could be used to further explore the linkages between AMR in food-producing animals and in the food chain [4]. This could include a centralised data repository that allows the collation and sharing of information between sectors. For example, routine AMR monitoring from the veterinary sector could be compared with data on human AMR trends and inform joint interventions. An expert committee could be convened at the national or regional level to discuss the data trends, inform policy development and to make recommendations for the harmonisation of policies across various sectors.
Tackling the problem
The foregoing discussion has highlighted that there are still many gaps in knowledge about the links between AMR and the environment. However, there is sufficient evidence pointing to several environmental risks to human and animal health and the need for coordinated efforts to tackle the problems. The lack of quantitative data has been identified by several sources as a key factor hindering appropriate AMR surveillance efforts. While the assessment of the environmental risks is not straight forward, inter-agency and inter-jurisdictional risk assessments could help to identify risks and management goals with clear indicators of effectiveness, to aid the development of environmental management options [12, 29]. Ashbolt and colleagues (2013) postulated that in addition to traditional risk assessment methods various novel aspects must be considered in the assessment of environmental antibiotic resistance. They suggested that added selective pressure on the environmental resistome that allows for development of antibiotic-resistant bacteria over time, must be considered and accounted for; the extent of horizontal gene transfer (HGT) in the relevant environmental hot spots must be identified and described; and traditional dose–response approaches should be modified to assess the contribution of antibiotic resistance to certain health outcomes and pathways [29]. Risk assessment studies are needed to provide accurate estimates of the level of resistant bacteria in wastewater effluents that would not pose risks for human and environmental health. Wastewater treatment technologies that are capable of producing effluents with an acceptable level of resistant bacteria are also needed [8]. Routine environmental sampling of organic soil and residues in hospital drains can allow for the estimation of the changes in environmental ecosystems in clinical settings, and enhance existing antimicrobial stewardship efforts over time.
Well-resourced laboratories with duly qualified and skilled scientists are needed to evaluate the magnitude of the problem. Inter-country or regional collaboration is encouraged in areas where there is limited technical capacity to detect and manage AMR. This could include sharing of centralised laboratory facilities, research expertise and data. It is also important to build upon existing gains achieved through successfully implemented strategies and measures. To do this, adequate financial and human resources, and infrastructure capabilities are necessary. However, various basic WASH initiatives including water, sanitation and hygiene approaches have proven effective in reducing the burden of infectious diseases. According to the WHO, these initiatives such as hand washing, safe sewage disposal and boiling of drinking water cannot be underestimated and should be revisited and promoted. This includes sharing of best practise approaches and how these can be sustained and built upon, to curtail the emergence and spread of AMR particularly in developing settings [4]. Close partnerships and collaboration should be fostered between policy makers, academia, managers, and interest groups, including collaboration between disciplines within and between sectors. For example, sharing of veterinary ABR data with public health to inform and support shared decision making for food safety.
Conclusion
This discussion has highlighted the fact that there are numerous opportunities for environmental factors to contribute to the growing burden of AMR. The foregoing suggests that the inappropriate use and misuse of antibiotics result in contamination of various aspects of the environment and this can result in the introduction of resistance genes and resistant bacteria into the human food chain and clinical environments. These factors are often interlinked with gene transfer going in both directions, perpetuating the cycle of AMR (Fig. 1). There is however scope for both innovative and traditional environmental public health approaches, including the provision and enforcement of adequate standards and guidelines, basic food safety, secondary treatment of water and wastewater/waste products, basic sanitation and hygiene practises, to eliminate or reduce the problem. Based on the complex interaction between health, veterinary and water and wastewater sectors, careful assessment of the situation is needed at the community and national level to determine the needs of individual countries. The contributory factors are multi-faceted, hence a multi-disciplinary, and intersectoral approach among the various players in the WASH sector is needed.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.World Health Organization . The evolving threat of antimicrobial resistance: options for action. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Sosa AdJ, Byarugaba DK, Amábile-Cuevas CF, Hsueh P-R, Kariuki S, Okeke IN., editors. Antimicrobial resistance in developing countries. New York: Springer; 2010. [Google Scholar]
- 3.Bejarano MT. The global public health threat of ABR: REACT. 2011.
- 4.World Health Organization . Antimicrobial resistance global report on surveillance. Geneva: Switzerland; 2014. [Google Scholar]
- 5.Centers for Disease Control and Prevention US . Antibiotic resistance threats in the United States. Atlanta: US Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 6.Levy SB. The antibiotic paradox: how the misuse of antibiotics destroys their curative powers. 2. Cambridge, MA, USA: Da Capo Press, Perseus Publishing; 2002. [Google Scholar]
- 7.Martinez JL. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut. 2009;157:2893–2902. doi: 10.1016/j.envpol.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy M, et al. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ. 2013;447:345–360. doi: 10.1016/j.scitotenv.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 10.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Djordjevic SP, Stokes HW, Chowdhury PR. Mobile elements, zoonotic pathogens and commensal bacteria: conduits for the delivery of resistance genes into humans, production animals and soil microbiota. Front Microbiol. 2013;4:86. doi: 10.3389/fmicb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Costa VM, King CE, Kalan L, Morar M, Sung WWL, Schwarz C, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 13.Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493. doi: 10.1136/bmj.f1493. [DOI] [PubMed] [Google Scholar]
- 14.Levy S. Microbial resistance to antibiotics: an evolving and persistent problem. Lancet. 1982;320:83–88. doi: 10.1016/S0140-6736(82)91701-9. [DOI] [PubMed] [Google Scholar]
- 15.Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;252:641–644. doi: 10.1016/S0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 16.Crofton J, Mitchison D. Streptomycin resistance in pulmonary tuberculosis. BMJ. 1948;2:1009. doi: 10.1136/bmj.2.4588.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963;27:87. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaves NI, Dimopoulou I, Hayes I, Kerridge S, Falla T, Secka O, et al. Epidemiological studies of large resistance plasmids in Haemophilus. J Antimicrob Chemother. 2000;45:599–604. doi: 10.1093/jac/45.5.599. [DOI] [PubMed] [Google Scholar]
- 19.Levy SB, Buu-Hoi A, Marshall B. Transposon Tn10-like tetracycline resistance determinants in Haemophilus parainfluenzae. J Bacteriol. 1984;160:87–94. doi: 10.1128/jb.160.1.87-94.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elwell LP, Roberts M, Mayer LW, Falkow S. Plasmid-mediated beta-lactamase production in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977;11:528–533. doi: 10.1128/AAC.11.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harish B, Menezes G. Antimicrobial resistance in typhoidal salmonellae. Indian J Med Microbiol. 2011;29:223. doi: 10.4103/0255-0857.83904. [DOI] [PubMed] [Google Scholar]
- 23.WHO-SEARO. Laboratory based surveillance of antimicrobial resistance. Report of regional workshop. Chennai: World Health Organization Regional Office for South-East Asia. 2013. 17–21 June 2013.
- 24.World Health Organization. Drug-resistant tuberculosis surveillance and response: supplement to the global tuberculosis report. France: World Health Organization. 2014.
- 25.World Health Organization . Global tuberculosis report Geneva. Switzerland: World Health Organization; 2014. [Google Scholar]
- 26.Nagata N, Marriott D, Harkness J, Ellis J, Stark D. In vitro susceptibility testing of Dientamoeba fragilis. Antimicrob Agents Chemother. 2012;56:487. doi: 10.1128/AAC.05125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts T, Ellis J, Harkness J, Marriott D, Stark D. Treatment failure in patients with chronic Blastocystis infection. J Med Microbiol. 2014;63:252–257. doi: 10.1099/jmm.0.065508-0. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher S, Caprarelli G, Merif J, Andresen D, Van Hal S, Stark D, et al. Epidemiology and geographical distribution of enteric protozoan infections in Sydney, Australia. J Public Health Res. 2014;3:298. doi: 10.4081/jphr.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashbolt NJ, Amézquita A, Backhaus T, Borriello P, Brandt KK, Collignon P, et al. Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ Health Perspect. 2013;121:993–1001. doi: 10.1289/ehp.1206316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koonin EV, Makarova KS, Aravind L. Horizontal gene transfer in prokaryotes: quantification and classification1. Annu Rev Microbiol. 2001;55:709–742. doi: 10.1146/annurev.micro.55.1.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 32.Kristiansson E, Fick J, Janzon A, Grabic R, Rutgersson C, Weijdegård B, et al. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS One. 2011;6:e17038. doi: 10.1371/journal.pone.0017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain R, Rivera MC, Moore JE, Lake JA. Horizontal gene transfer accelerates genome innovation and evolution. Mol Biol Evol. 2003;20:1598–1602. doi: 10.1093/molbev/msg154. [DOI] [PubMed] [Google Scholar]
- 34.Skippington E, Ragan MA. Lateral genetic transfer and the construction of genetic exchange communities. FEMS Microbiol Rev. 2011;35:707–735. doi: 10.1111/j.1574-6976.2010.00261.x. [DOI] [PubMed] [Google Scholar]
- 35.Heuer H, Smalla K. Horizontal gene transfer between bacteria. Environ Biosaf Res. 2007;6:3–13. doi: 10.1051/ebr:2007034. [DOI] [PubMed] [Google Scholar]
- 36.Courvalin P. Transfer of antibiotic resistance genes between gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 1994;38:1447. doi: 10.1128/AAC.38.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dzidic S, Bedeković V. Horizontal gene transfer-emerging multidrug resistance in hospital bacteria. Acta Pharmacol Sin. 2003;24:519–526. [PubMed] [Google Scholar]
- 38.Heuer H, Smalla K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environl Microbiol. 2007;9:657–666. doi: 10.1111/j.1462-2920.2006.01185.x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki S, Hoa PTP. Distribution of quinolones, sulfonamides, tetracyclines in aquatic environment and antibiotic resistance in Indochina. Front Microbiol. 2012;3:67. doi: 10.3389/fmicb.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heuer H, Schmitt H, Smalla K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol. 2011;14:236–243. doi: 10.1016/j.mib.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Storteboom H, Arabi M, Davis JG, Crimi B, Pruden A. Tracking antibiotic resistance genes in the South Platte River basin using molecular signatures of urban, agricultural, and pristine sources. Environ Sci Technol. 2010;44:7397–7404. doi: 10.1021/es101657s. [DOI] [PubMed] [Google Scholar]
- 42.Chee-Sanford JC, Mackie RI, Koike S, Krapac IG, Lin Y-F, Yannarell AC, et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual. 2009;38:1086–1108. doi: 10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- 43.Marti R, Scott A, Tien Y-C, Murray R, Sabourin L, Zhang Y, et al. Impact of manure fertilization on the abundance of antibiotic-resistant bacteria and frequency of detection of antibiotic resistance genes in soil and on vegetables at harvest. Appl Environ Microbiol. 2013;79:5701–5709. doi: 10.1128/AEM.01682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci. 2003;166:145–167. doi: 10.1002/jpln.200390023. [DOI] [Google Scholar]
- 45.Ding C, He J. Effect of antibiotics in the environment on microbial populations. Appl Microbiol Biotechnol. 2010;87:925–941. doi: 10.1007/s00253-010-2649-5. [DOI] [PubMed] [Google Scholar]
- 46.Sørum H, L’Abée-Lund TM. Antibiotic resistance in food-related bacteria—a result of interfering with the global web of bacterial genetics. Int J Food Microbiol. 2002;78:43–56. doi: 10.1016/S0168-1605(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher SM, Stark D, Harkness J, Ellis J. Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev. 2012;25:420–449. doi: 10.1128/CMR.05038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jindal A, Kocherginskaya S, Mehboob A, Robert M, Mackie RI, Raskin L, et al. Antimicrobial use and resistance in swine waste treatment systems. Appl Environ Microbiol. 2006;72:7813–7820. doi: 10.1128/AEM.01087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarmah AK, Meyer MT, Boxall A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 50.Hop LT. Programs to improve production and consumption of animal source foods and malnutrition in Vietnam. J Nutr. 2003;133:4006S–4009S. doi: 10.1093/jn/133.11.4006S. [DOI] [PubMed] [Google Scholar]
- 51.Petersen A, Dalsgaard A. Species composition and antimicrobial resistance genes of Enterococcus spp., isolated from integrated and traditional fish farms in Thailand. Environ Microbiol. 2003;5:395–402. doi: 10.1046/j.1462-2920.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- 52.Dang STT, Petersen A, Van Truong D, Chu HTT, Dalsgaard A. Impact of medicated feed on the development of antimicrobial resistance in bacteria at integrated pig-fish farms in Vietnam. Appl Environ Microbiol. 2011;77:4494–4498. doi: 10.1128/AEM.02975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manges AR, Smith SP, Lau BJ, Nuval CJ, Eisenberg JN, Dietrich PS, et al. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathog Dis. 2007;4:419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- 54.Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Ind. 2008;8:1–13. doi: 10.1016/j.ecolind.2007.06.002. [DOI] [Google Scholar]
- 55.Baquero F, Martínez J-L, Cantón R. Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol. 2008;19:260–265. doi: 10.1016/j.copbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Wei R, Ge F, Huang S, Chen M, Wang R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere. 2011;82:1408–1414. doi: 10.1016/j.chemosphere.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 57.Managaki S, Murata A, Takada H, Tuyen BC, Chiem NH. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ Sci Technol. 2007;41:8004–8010. doi: 10.1021/es0709021. [DOI] [PubMed] [Google Scholar]
- 58.Sakurai-Komada N, Hirano M, Nagata I, Ejima Y, Nakamura M, Koike K. Risk of transmission of imipenem-resistant Pseudomonas aeruginosa through use of mobile bathing service. Environ Health Prev Med. 2006;11:31–37. doi: 10.1007/BF02898205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mezrioui N, Baleux B. Resistance patterns of E. coli strains isolated from domestic sewage before and after treatment in both aerobic lagoon and activated sludge. Water Res. 1994;28:2399–2406. doi: 10.1016/0043-1354(94)90056-6. [DOI] [Google Scholar]
- 60.Threlfall E, Ward L, Frost J, Willshaw G. Spread of resistance from food animals to man—the UK experience. Acta Vet Scand Suppl. 1999;93:63–68. [PubMed] [Google Scholar]
- 61.Munir M, Wong K, Xagoraraki I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011;45:681–693. doi: 10.1016/j.watres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 62.Phillips I, Casewell M, Cox T, De Groot B, Friis C, Jones R, et al. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53:28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- 63.Teale C. Antimicrobial resistance and the food chain. J Appl Microbiol. 2002;92:85S–89S. doi: 10.1046/j.1365-2672.92.5s1.20.x. [DOI] [PubMed] [Google Scholar]
- 64.Lazarus B, Paterson DL, Mollinger JL, Rogers BA. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis. 2015;60:439–452. doi: 10.1093/cid/ciu785. [DOI] [PubMed] [Google Scholar]
- 65.Salisbury JG, Nicholls TJ, Lammerding AM, Turnidge J, Nunn MJ. A risk analysis framework for the long-term management of antibiotic resistance in food-producing animals. Int J Antimicrob Agents. 2002;20:153–164. doi: 10.1016/S0924-8579(02)00169-3. [DOI] [PubMed] [Google Scholar]
- 66.Hao H, Cheng G, Iqbal Z, Ai X, Hussain HI, Huang L, et al. Benefits and risks of antimicrobial use in food-producing animals. Antimicrob Resist Chemother. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 68.Mukherjee P, Ramamurthy T, Bhattacharya MK, Rajendran K, Mukhopadhyay AK. Campylobacter jejuni in hospitalized patients with diarrhea, Kolkata, India. Emer Infect Dis. 2013;19:1155. doi: 10.3201/eid1907.121278. [DOI] [PMC free article] [PubMed] [Google Scholar]