Abstract
Purpose
Recent studies have explored the relationship between ABO blood type and serum markers of ovarian reserve, specifically follicle-stimulating hormone (FSH) and anti-mullerian hormone (AMH). The primary objective of this study is to investigate whether there is an association between ABO blood type and ovarian stimulation response in patients with serum markers of diminished ovarian reserve (DOR).
Methods
This is a retrospective study of all patients undergoing controlled ovarian stimulation (COS) for in vitro fertilization (IVF) between May 2010 and July 2013. Patients were sub-grouped, a priori, based on serum AMH levels: ≤1 ng/mL, ≤0.5 ng/mL and ≤0.16 ng/mL. Within each sub-group, demographic, baseline IVF characteristics and COS response parameters based on ABO blood types were compared. The number of mature oocytes retrieved was considered the primary outcome. Analysis of variance (ANOVA) and Chi-square tests were used to compare means and percentages between ABO blood types within groups.
Results
Complete data was available for 2575 patients. The mean (± SD) age and BMI of the study cohort was 38.9 (±3.97) years, 23.4 (±5.91) kg/m2, respectively. The distribution of ABO blood types in the cohort was as follows: 36.8 % (A), 6.56 % (AB), 17.3 % (B), and 39.3 % (O). The demographics and baseline IVF characteristics were comparable among patients with blood types A, AB, B, and O within each AMH group. Within each AMH sub-group, no difference was found in the total days of COS, total gonadotropins administered, peak estradiol level, or number of mature oocytes retrieved based on blood type.
Conclusions
Our results suggest no association between ABO blood type and ovarian stimulation response in patients with DOR. The predictive value of ABO blood type in determining ovarian stimulation response in such patients is currently limited.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0485-3) contains supplementary material, which is available to authorized users.
Keywords: ABO, Blood type, Ovarian reserve, Antimüllerian hormone, Ovarian stimulation
Introduction
Ovarian reserve is a commonly used term that reflects the reproductive potential of a woman, primarily based on the number and quality of remaining oocytes [1]. Most often, ultrasonographic measurements of an antral follicle count (AFC) and serum measurements of early follicular levels of follicle-stimulating hormone (FSH), anti-mullerian hormone (AMH) and inhibin-B are used as clinical markers for ovarian reserve [1, 2]. These tests have not only become a part of the routine evaluation of infertility, but have also been used in predicting ovarian stimulation response and in vitro fertilization (IVF) outcomes [2–6]. There has been a growing interest in investigating the relationship between ABO blood types and serum markers of ovarian reserve [7–12]. The results of these studies have been contradictory; while some have suggested that certain blood types may be associated with diminished ovarian reserve [7, 11], others have failed to show such a relationship [8–10, 12]. Despite these associations, there is a dearth of literature regarding ovarian stimulation response and oocyte yield in patients with diminished ovarian reserve (DOR) based on ABO blood type. Because response to ovarian stimulation and oocyte yield is considered the gold standard of a woman’s ovarian reserve [13–15], we set out to investigate whether ABO blood type is associated with ovarian stimulation response in patients with DOR, as indicated by serum AMH levels.
Materials and methods
Inclusion criteria
The Weill Cornell Medical College institutional review board approved our study protocol. We began using the second-generation AMH assay [GenII Beckman ELISA assay (Beckman Coulter, Inc)] as part of the evaluation of infertility in April 2010. Thus, all women initiating fresh IVF cycles at the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine between May 2010 and July 2013 resulting in oocyte retrieval were analyzed for potential inclusion. Women meeting criteria for DOR as demonstrated by serum AMH ≤1 ng/mL were included in the study cohort. Patients with unilateral oophorectomy, known polycystic ovarian syndrome, or adrenal disease were excluded. Cycles cancelled prior to oocyte retrieval, or those utilizing donor oocytes, or with incomplete records were also excluded.
Clinical and laboratory protocols
All serum AMH measurements were performed at our center’s laboratory. Serum samples were assayed using the GenII Beckman ELISA assay (Beckman Coulter, Inc). A single serum AMH level was measured per patient prior to initiating ovarian stimulation. The intra- and interassay variability for AMH were 5.8 and 13.4 %, respectively [6].
Ovarian stimulation was carried out to maximize follicular response while minimizing the risk of ovarian hyperstimulation syndrome (OHSS). Controlled ovarian stimulation (COS), human chorionic gonadotropin (hCG) trigger and oocyte retrieval were performed per standard protocols [6]. Patients undergoing COS were down-regulated in the preceding luteal phase with either oral contraceptive pills (Ortho-Novum, Janssen Pharmaceuticals) or 0.1 mg (Estradiol) E2 patches (Climara, Bayer Healthcare Pharmaceuticals) followed by ovarian stimulation with gonadotropins (Follistim, Merck; Gonal-F, EMD-Serono; and/or Menopur, Ferring). Ovulation was suppressed with a GnRH antagonist (Ganirelix Acetate, 0.25 mg [Organon]; or Cetrotide, 0.25 mg [EMD-Serono]) after ovarian stimulation was initiated. GnRH-agonist based flare protocols were used as clinically appropriate. Initial gonadotropin dosages were based on patient age, weight, antral follicle count, day 3 follicle stimulating hormone (FSH), and previous response to stimulation.
hCG was used as the ovulation trigger. Ovidrel (EMDSerono), Novarel (Ferring Pharmaceuticals) or Pregnyl (Schering-Plough) was administered according to a sliding scale (10,000 IU for E2 < 1500 pg/mL, 5000 IU for E2 1501–2500 pg/mL, 4000 IU for E2 2501–3000 pg/mL, and 3300 IU for E2 > 3001 pg/mL). In general, the hCG trigger was given when the two lead follicles attained a mean diameter ≥17 mm. Oocyte retrieval was performed under conscious sedation using transvaginal ultrasound guidance approximately 35–37 h after hCG administration. After stripping of the cumulus complex, oocyte maturity was determined by the presence of a polar body.
Study variables
Demographic characteristics recorded included age, gravidity, parity, BMI (kg/m2), ABO blood type and Rhesus factor type. Baseline IVF characteristics included FSH (mIU/mL) and E2 (pg/mL) level at cycle start. COS parameters recorded were as follows: protocol type (GnRH antagonist vs. GnRH agonist), total days of ovarian stimulation, total dosage of gonadotropins administered (IU), peak estradiol (E2) level (pg/mL), peak endometrial stripe (mm), total number of oocytes retrieved, and total number of mature oocytes. Patients were sub-grouped into 3 groups based on serum AMH levels: ≤1 ng/mL, ≤0.5 ng/mL and ≤0.16 ng/mL. At our center, serum AMH levels ≤1 ng/mL, ≤0.5 ng/mL, and ≤0.16 ng/mL are considered the cut-offs for diminished, very low, and undetectable ovarian reserve, respectively.
Statistical analysis
Continuous variables were checked for normality and expressed as mean ± standard deviation (SD), while categorical variables were expressed as number of cases (n) and percentage of occurrence (%). Analysis of variance (ANOVA) and chi-square tests were used to compare means and percentages of recorded parameters between ABO blood types within AMH sub-groups. The number of mature oocytes retrieved was considered the primary outcome. Statistical significance was set at P < 0.05. All statistical analyses were performed using STATA version 13 (College Station, TX: StataCorp LP).
Results
Complete data was available for 2575 patients. The overall mean (± SD) age and BMI of the study cohort were 38.9 (±3.97) years and 23.4 (±5.91) kg/m2, respectively. The majority of patients were Caucasian (2239, 87 %). The median [interquartile range (IQR)] AMH level was 0.45 ng/mL (IQR 0.25–0.69). The overall distribution of ABO blood types in the cohort was as follows: 36.8 % (A), 6.56 % (AB), 17.3 % (B), and 39.3 % (O). Of the 2575 patients, 2329 (90.4 %) patients were Rhesus factor positive, while 246 (9.6 %) patients were Rhesus factor negative.
Table 1 compares the demographic and baseline IVF characteristics of patients with AMH ≤1 ng/mL by blood type. There was no statistical difference in the mean age, gravidity, parity, BMI, basal FSH level, basal E2 level, or distribution of Rhesus factor types. Table 2 compares COS response based on blood type in the same group. The overall mean (± SD) number of oocytes retrieved was 6.72 (±3.81), and comparable across all blood types. Furthermore, there was no difference in the total days of COS, total gonadotropins administered, peak E2 level, peak endometrial stripe, or total number of oocytes retrieved.
Table 1.
Baseline characteristics of patients with AMH < 1.0 ng/mL distributed by blood type (n = 2575)
| Parameter | Blood Type A (n = 948) | Blood Type AB (n = 169) | Blood Type B (n = 446) | Blood Type O (n = 1012) | P |
|---|---|---|---|---|---|
| Age (years) | 38.3 (±3.79) | 38.1 (±3.72) | 38.4 (±3.84) | 38.2 (±3.88) | 0.74 |
| Gravidity | 1.27 (±0.21) | 1.29 (±0.28) | 1.26 (±0.31) | 1.27 (±0.26) | 0.64 |
| Parity | 0.63 (±0.38) | 0.65 (±0.41) | 0.62 (±0.45) | 0.64 (±0.39) | 0.77 |
| BMI (kg/m2) | 23.4 (±4.81) | 23.2 (±3.76) | 23.4 (±4.73) | 23.5 (±4.78) | 0.87 |
| Rhesus Factor Positive Negative |
855 (90.2 %) 93 (9.8 %) |
153 (90.5 %) 16 (9.5 %) |
401 (89.9 %) 45 (10.1 %) |
914 (90.3 %) 98 (9.7 %) |
0.98 |
| Basal FSH (mIU/mL) | 5.57 (±3.71) | 5.38 (±3.48) | 5.41 (±3.60) | 5.60 (±3.44) | 0.73 |
| Basal E2 (pg/mL) | 76.1 (±35.6) | 79.5 (±40.4) | 76.4 (±38.7) | 74.3 (±36.4) | 0.31 |
Data are presented as mean ± standard deviation and n (%)
BMI: Body Mass Index
FSH: Follicle Stimulating Hormone
E2: Estradiol
Table 2.
Ovarian stimulation outcomes of patients with AMH < 1.0 ng/mL distributed by blood type (n = 2575)
| Parameter | Blood Type A (n = 948) | Blood Type AB (n = 169) | Blood Type B (n = 446) | Blood Type O (n = 1012) | P |
|---|---|---|---|---|---|
| Protocol GnRH agonist based GnRH antagonist based | 73 (8.11 %) 827 (91.9 %) |
15 (8.93 %) 153 (91.1 %) |
45 (10.3 %) 393 (89.7 %) |
104 (9.73 %) 965 (90.3 %) |
0.96 |
| Total stimulation days | 9.94 (±2.39) | 9.97 (±2.24) | 9.67 (±2.59) | 9.89 (±2.49) | 0.27 |
| Total gonadotropins administered (IU) | 3673.6 (±1078.5) | 3653.1 (±1089.3) | 3637.9 (±1046.9) | 3645.5 (±1066.8) | 0.93 |
| E2 on day of trigger (pg/mL) | 1275.8 (±665.1) | 1287.4 (±747.1) | 1304.2 (±698.6) | 1302.3 (±672.6) | 0.82 |
| Peak endometrial stripe (mm) | 10.5 (±2.57) | 10.6 (±2.32) | 10.2 (±2.79) | 10.3 (±2.68) | 0.11 |
| Number of oocytes retrieved | 7.44 (±4.46) | 7.28 (±4.27) | 7.48 (±4.08) | 7.66 (±4.41) | 0.59 |
| Number of mature oocytes | 6.89 (±3.79) | 6.83 (±3.92) | 6.46 (±3.72) | 6.71 (±3.79) | 0.27 |
Data are presented as mean ± standard deviation and n (%)
GnRH: Gonadotropin releasing hormone
One thousand four hundred thirteen patients with AMH ≤0.5 ng/mL were identified. The distribution of ABO blood types in this group of patients was: 36.3 % (A), 6.16 % (AB), 16.4 % (B), and 41.1 % (O). Supplemental table 1 shows the demographic and baseline IVF characteristics of patients with AMH ≤0.5 ng/mL based on ABO blood type. Overall, no difference in baseline characteristics was found. The overall mean (± SD) number of oocytes retrieved was 4.78 (±2.77). Analysis of supplemental table 2 reveals no difference in the number of mature oocytes retrieved or other COS response parameters in this group based on blood type.
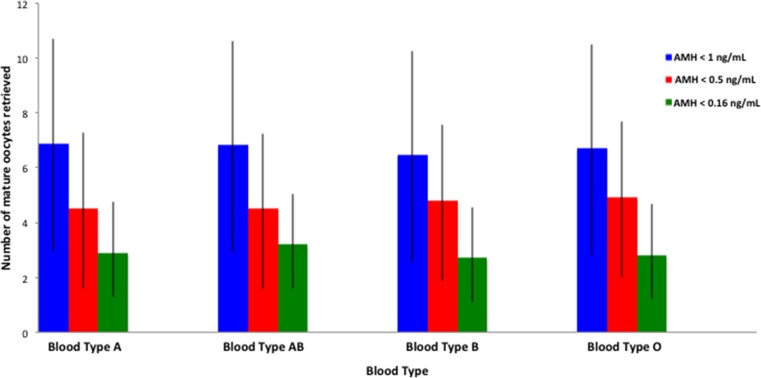
Supplemental table 3 shows the demographic and baseline IVF characteristics of 233 patients with undetectable AMH (≤0.16 ng/mL), stratified by ABO blood type. The distribution of blood types was as follows: 36.1 % (A), 5.58 % (AB), 16.3 % (B), and 42.1 % (O). This was comparable to the overall study cohort and patients with AMH ≤0.5 ng/mL. There was no difference in the demographic or baseline IVF characteristics of patients in this group. The overall mean (± SD) number of oocytes retrieved in this group was 3.01 (±1.79). As evident from supplemental table 4, there was also no difference in the number of mature oocytes retrieved or other COS response parameters. Figure 1 shows no difference in the number of mature oocytes retrieved in each AMH group across all ABO blood types.
Fig. 1.
Comparision of oocyte yield in different AMH sub-groups based on ABO blood type
Discussion
Our findings suggest no association between ABO blood type and ovarian stimulation response in patients with DOR. Our analysis holds true at serum AMH levels ≤1 ng/mL, ≤0.5 ng/mL and ≤0.16 ng/mL, which are considered the cut-offs for diminished, very low, and undetectable ovarian reserve at our center, respectively. Therefore, the predictive value of ABO blood type in determining ovarian stimulation response in patients with DOR is currently limited.
The association of different ABO blood types with gynecologic conditions such as endometriosis [16] and ovarian, endometrial and cervical cancer [17] has been explored in previous studies. Within the realm of assisted reproduction, Binder et al. [18] were the first to report a possible association between blood type A and OHSS. Since then, a few studies exploring the relationship between ABO blood type and serum markers of ovarian reserve have emerged.
Nejat et al. [7] were the first to suggest an association between ABO blood type and DOR. The authors found that patients with blood type O were significantly more likely to exhibit diminished ovarian reserve (defined as FSH >10 mIU/mL) than those with blood types A or AB (OR 2.14, 95 % CI 1.22–3.80). This relationship was stronger when analyzing the subgroup of patients with FSH >12 mIU/mL (OR 2.44, 95 % CI 1.22–5.04). Further, the authors suggested that the A antigen was protective against diminished ovarian reserve by citing a significantly higher representation of blood type A and AB in the group with normal ovarian reserve. The authors theorized that an enzyme associated with the A antigen (A transferase) may play a vital role in oocyte accrual and attrition. While these results generated new interest regarding the relationship of ABO blood type and ovarian reserve, the study had limitations, most notably the variability in the timing of FSH sampling, possibly creating a bias from the falsely increased values for samples collected beyond the early follicular phase of the cycle.
In a retrospective analysis of 35,479 women undergoing IVF and embryo transfer cycles, Lin et al. [11] reached a contradictory conclusion. They showed that their study population had more patients with blood type O with FSH <10 mIU/mL than with FSH >10 mIU/mL (OR 1.41, 95 % CI 1.30–1.54). Additionally, the study found significantly higher percentages of patients with blood type B and type AB with FSH >10 mIU/mL (OR 0.82, 95 % CI 0.76–0.84 for blood type B; OR 0.84, 95 % CI 0.75–0.94 for blood type AB). The authors posited that racial and ethnic differences in blood type distributions might account for the differences in their study’s findings when compared to Nejat et al.
Subsequent investigations have failed to highlight any association between blood type and serum FSH as a marker of ovarian reserve. In their multivariate analysis of 305 patients undergoing IVF, Timberlake et al. [9] found no association between a woman’s blood type and a serum FSH level > 10 mIU/ml after controlling for race, ethnicity, BMI, smoking status, history of endometriosis, or ovarian surgery. Similarly, in another study, Șengül et al. [10] found no statistical difference in the distribution of blood groups in patients with a serum FSH level > 10 mIU/ml compared to patients with a FSH level < 10 mIU/ml.
Although a serum FSH level > 10 mIU/ml is commonly accepted as a cut-off for diminished ovarian reserve [7, 15], cycle-to-cycle variations in FSH levels are frequently encountered in the early follicular phase [1, 2]. Also, AMH levels better correlate with the number of early antral follicles compared to other hormonal markers [19, 20]. Given these advantages of AMH over FSH, subsequent investigations have used AMH as a marker of ovarian reserve. Using AMH as a marker for ovarian reserve, de Mouzon et al. [8] showed no relationship between ABO blood type and ovarian reserve in a cohort of 1020 women. Pereira et al. [12] also showed no significant relationship between ABO blood type and DOR in their retrospective study of 2394 patients.
Despite the aforementioned findings, serum FSH and AMH levels are only indirect biomarkers for ovarian reserve. For women undergoing COS for IVF, quantitative ovarian response to gonadotropins and oocyte yield ultimately reflects ovarian reserve. In a retrospective study of 1889 IVF cycles, Spitzer et al. [21] investigated the association between ABO blood type and ovarian reserve through measurements of cumulus oocyte complexes (COCs) and metaphase II (MII) oocytes collected after ovarian stimulation. The authors found similar number of COCs and MII oocytes across all blood types and ages. Our findings are consistent with the results from Spitzer et al.’s study, showing no relationship between ABO blood type and DOR. Our study may also be an improvement over the former study because it specifically evaluated serum markers of DOR.
Potential strengths of this study are its large sample size and the inclusion of several quantitative parameters to assess response to exogenous gonadotropins and subsequent oocyte yield. In addition, our analyses included response to both GnRH-antagonist and GnRH-agonist based flare protocols. It is also encouraging to note that COS in patients with very low and undetectable ovarian reserve (as indicated by AMH levels) yielded 4.78 (±2.77) and 3.01 (±1.79) oocytes, respectively. We also acknowledge two main limitations. First, our study did not account for confounders such as smoking and ovarian surgery. Previous studies have indicated that these confounders may spuriously lower AMH levels [9]. However, whether smoking or ovarian surgery (specifically cystectomy or endometrioma excision) truly decreases oocyte yield in response to gonadotropins remains contentious. Second, we remain uncertain whether the findings of our retrospective study would hold true in a prospective setting, particularly in a larger cohort of patients with DOR. Though our study cohort, like the Spitzer et al. study, consisted of mostly Caucasian patients, it is also possible that these results may vary in different racial and ethnic groups.
In conclusion, our study adds to the emerging body of literature exploring the association of ABO blood type with serum markers of ovarian reserve and outcomes of ovarian stimulation in IVF cycles. While our current study suggests no association between ABO blood type and oocyte yield in patients with DOR, prospective data may elucidate mechanisms that underlie previously reported associations between blood type and ovarian reserve.
Electronic supplementary material
(DOC 45 kb)
(DOC 46 kb)
(DOC 45 kb)
(DOC 45 kb)
Acknowledgments
Conflict of interest
There is no conflict of interest
Footnotes
Summary capsule
Our findings suggest no association between ABO blood type and ovarian stimulation response in patients with diminished ovarian reserve.
References
- 1.Ovarian reserve testing. Committee Opinion No. 618. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;125:268–73. [DOI] [PubMed]
- 2.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 3.Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651–4. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 4.Toner JP, Philput CB, Jones GS, Muasher SJ. Basal follicle-stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril. 1991;55:784–91. doi: 10.1016/s0015-0282(16)54249-6. [DOI] [PubMed] [Google Scholar]
- 5.Yih MC, Spandorfer SD, Rosenwaks Z. Egg production predicts a doubling of in vitro fertilization pregnancy rates even within defined age and ovarian reserve categories. Fertil Steril. 2005;83(1):24–9. doi: 10.1016/j.fertnstert.2004.05.096. [DOI] [PubMed] [Google Scholar]
- 6.Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012–8.e1. doi: 10.1016/j.fertnstert.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Nejat EJ, Jindal S, Berger D, Buyuk E, Lalioti M, Pal L. Implications of blood type for ovarian reserve. Hum Reprod. 2011;26(9):2513–7. doi: 10.1093/humrep/der199. [DOI] [PubMed] [Google Scholar]
- 8.de Mouzon J, Hazout A, Cohen-Bacrie M, Belloc S, Cohen-Bacrie P. Blood type and ovarian reserve. Hum Reprod. 2012;27(5):1544–5. doi: 10.1093/humrep/des056. [DOI] [PubMed] [Google Scholar]
- 9.Timberlake KS, Foley KL, Hurst BS, Matthews ML, Usadi RS, Marshburn PB. Association of blood type and patient characteristics with ovarian reserve. Fertil Steril. 2013;100(6):1735–9. doi: 10.1016/j.fertnstert.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Sengül O, Dilbaz B, Yerebasmaz N, Dede S, Altınbaş S, Erkaya S. Only female age, and not blood type, is associated with ovarian reserve. Int J Fertil Steril. 2014;8(2):143–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S, Li R, Chi H, Huang S, Zhang H, Zheng X, et al. Effect of ABO blood type on ovarian reserve in Chinese women. Fertil Steril. 2014;102(6):1729–32.e2. doi: 10.1016/j.fertnstert.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Pereira N, Elias RT, Levine BA, Lekovich JP, Rosenwaks Z, Spandorfer SD. Association of ABO blood type with ovarian reserve. J Nat Sci. 2015;1(2) [Google Scholar]
- 13.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855–64. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 15.Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23. doi: 10.1186/1477-7827-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matalliotakis I, Cakmak H, Goumenou A, Sifakis S, Ziogos E, Arici A. ABO and Rh blood groups distribution in patients with endometriosis. Arch Gynecol Obstet. 2009;280:917–9. doi: 10.1007/s00404-009-1031-2. [DOI] [PubMed] [Google Scholar]
- 17.Yuzhalin AE, Kutikhin AG. ABO and Rh blood groups in relation to ovarian, endometrial and cervical cancer risk among the population of South-East Siberia. Asian Pac J Cancer Prev: APJCP. 2012;13:5091–6. doi: 10.7314/APJCP.2012.13.10.5091. [DOI] [PubMed] [Google Scholar]
- 18.Binder H, Flegel WA, Emran J, Muller A, Cupisti S, Beckmann MW, et al. Blood group A: an overseen risk factor for early-onset ovarian hyperstimulation syndrome? Reprod Biomed Online. 2008;17:185–9. doi: 10.1016/S1472-6483(10)60193-9. [DOI] [PubMed] [Google Scholar]
- 19.Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 20.Barad DH, Weghofer A, Gleicher N. Comparing anti-Mullerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91(4 Suppl):1553–1555. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer D, Corn C, Stadler J, Wirleitner B, Schuff M, Vanderzwalmen P, et al. Implications of blood type for ovarian reserve and infertility - impact on oocyte yield in IVF patients. Geburtshilfe Frauenheilkd. 2014;74(10):928–932. doi: 10.1055/s-0034-1383045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 45 kb)
(DOC 46 kb)
(DOC 45 kb)
(DOC 45 kb)