Abstract
Purpose
To evaluate whether it is a feasible option to target the oocyte (with Ca2+-ionophore) in case that sperm motility cannot be restored in Kartagener syndrome.
Methods
A case of a male Kartagener syndrome with exclusively immotile spermatozoa that did not react to the dimethylxanthine theophylline. Thus, half of the associated oocytes were treated for 15 min with the ready-to-use- ionophore CultActive immediately after ICSI whereas the other 50 % were injected with routine ICSI without artificial oocyte activation. Rates of fertilization, blastulation, pregnancy and live birth were evaluated.
Results
Fertilization check revealed that none of the conventionally injected but 4/6 (66.7 %) of the artificially activated oocytes showed two pronuclei. Three embryos were of good and one of fair quality. Corresponding blastocyst formation rate was 3 out of 4 (75 %). A double embryo transfer led to a healthy twin birth in the 34th week of gestation (two boys with a birth weight of 1724 g and 2199 g).
Conclusions
This case indicates that Ca2+-ionophore treatment in cycles from theophylline-resistant Kartagener syndrome patients is a feasible option. The future will show if routine application of A23187 in Kartagener or primary cilia dyskinesis patients will be of benefit.
Keywords: Ca2+-ionophore, Kartagener syndrome, Live birth, Primary cilia dyskinesis, Twin pregnancy
Introduction
Immotile cilia syndrome, also called primary cilia dyskinesia (PCD), is characterized by defects of motile ciliary and flagellar function and the ultrastructure of ciliated cells. It is an autosomal recessive disorder commonly leading to chronic sinusitis, respiratory distress, bronchiectasis, and male infertility. If a situs inversus is present in parallel its combination with PCD is referred to as Kartagener syndrome [1–3].
In healthy males, a 9 + 2 microtubule arrangement, with 9 outer microtubule doublets encompassing a single central pair, is found. Via the outer microtubule doublets dynein arms provide the motor force required for flagellar movement. Nexin links and radial spokes further stabilize the cilia structure. In patients suffering from PCD or Kartagener syndrome, however, male infertility derives from ultrastructural defects of the flagella, e.g., absence of the inner dynein arms. This scenario automatically leads to severe asthenozoospermia [4–6] or much rather to complete absence of motile gametes [7–14].
Patients suffering from Kartagener syndrome or PCD have almost no chance of achieving spontaneous conception because of the above mentioned limitations in sperm motility. Indeed, before the implementation of ICSI in the field of assisted reproductive technologies, said patients were considered to be sterile, but in the era of ICSI fatherhood became a reality. Any decision how to proceed with such patients should be based on genetic counseling and a correct and clear diagnosis of the cause of their infertility [15].
So far, the major problem with the use of immotile sperm for ICSI has been differentiating between live and dead sperm. Numerous approaches have been published suggesting hypoosmotic swell (HOS) test [11, 16], usage of testicular sperm [5, 17, 18] or chemical activation of immotile sperm [12, 14] in order to increase the percentage of viable sperm available for ICSI.
This is the first report of a healthy twin live-birth after usage of ionophore treatment in a patient suffering from Kartagener syndrome who showed failed sperm activation with the dimethylxanthine theophylline.
Case report
Patient history
A 32-year-old nulliparous woman (Body Mass Index 27.0) was referred to the Kinderwunsch Zentrum Linz for ICSI treatment. She had been attempting pregnancy for 2.5 years. Her basal hormonal parameters of AMH, LH, and FSH were 4.05 ng/ml, 2.69 mU/ml, and 5.3 mU/ml, respectively. She regularly ovulated in a 28-day cycle (4 days of bleeding). An ultrasound examination revealed that she had a normal uterine cavity and ovaries as well as patent fallopian tubes. Like her partner she was sero-negative for hepatitis B and C, HIV and syphilis.
Her husband (32 years of age) was aware of his situs inversus; however, it took until the age of 27 when he was diagnosed with Kartagener syndrome because of his chronic sinusitis and the associated respiratory distress. Thereafter, the adipose man (BMI of 31.7) consulted an andrologist where he was confronted with severe oligoasthenoteratozoospermia (2.6 × 106/ml, no motility, no morphological normal sperm) according to the WHO [19]. Hormonal examination further revealed hypotestosteronemia (116 ng/dl) which was treated with a testosterone gel (25 mg, every second day).
Material & method
When the wish for a baby became relevant testosterone treatment was stopped. After a 3 month period (without testosterone supplementation), sperm count and morphology were 20.4 mio/ml and 1 % normality, respectively. Motility, however, was undetectable (asthenoteratozoospermia). Eosin staining indicated that 54 % of the spermatozoa were viable.
This was the time when the couple sought help at the Kinderwunsch Zentrum Linz, Austria.
Because Kartagener syndrome is a genetically inherited disorder, the couple was offered informative genetic counseling before the fertility treatment. Karyotyping revealed a normal male 46, XY chromosomal set. Neither continuous reduction of period of abstinence (from 3 to 1 day) nor application of a ready-to-use theophylline (SpermMobil, Gynemed, Lensahn, Germany) resulted in motile spermatozoa. Consequently, electron microscope analysis of spermatozoa was recommended in order to quantify the percentage of sperms with normal 9 + 2 microtubule structure [17] which the patient refused because the procedure appeared too time-consuming. For the same reasons testicular biopsy was not accepted; much rather, the couple decided to opt for a cycle of controlled ovarian hyperstimulation (COH) with the husband’s immotile sperms from the ejaculate.
For COH an antagonist protocol was chosen using recombinant FSH (Gonal F, Merck Serono, Vienna, Austria) and Cetrotide (Merck Serono). After 11 days of stimulation and consumption of 1450 IU gonadotrophins, 14 follicles developed whereupon ovulation was induced with 10,000 IU of hCG (MSD, Vienna, Austria).
Transvaginal follicular puncture 36 h post ovulation induction led to 12 cumulus-oocyte-complexes. Analysis of ejaculate was as expected (1.8 × 106/ml, 0 % motility, 2 % normal morphology, vitality 32 %). A random sample was mixed with theophylline again without achieving motility. Thus, HOS-selected sperm was used for ICSI. In detail, immediately prior to ICSI, individual morphologically normal spermatozoa were selected and placed in a 150 mOsm HOS solution [20] for selection for viability. Viable spermatozoa immediately reacted to the hypoosmotic solution and showed coiled tails. After identification sperms were washed in order to remove all traces of the HOS-solution whereupon they were used for injection.
After appropriate consultation, the patients provided written consent to have half of their mature oocytes treated with a ready-to-use ionophore solution (A23187; Cult-Active, Gynemed) in order to maximize chances of fertilization. Three hours after oocyte collection a total of 12 metaphase-II eggs were injected with immotile sperms but only 6 of them (randomly splitted under a binocular microscope) were incubated after ICSI for 15 min in Cult-Active before they were washed (3 × 1 min in EmbryoAssist) and transferred in 30 μl droplets of EmbryoAssist medium (Origio, Berlin, Germany) just like their routinely treated sibling oocytes.
Results
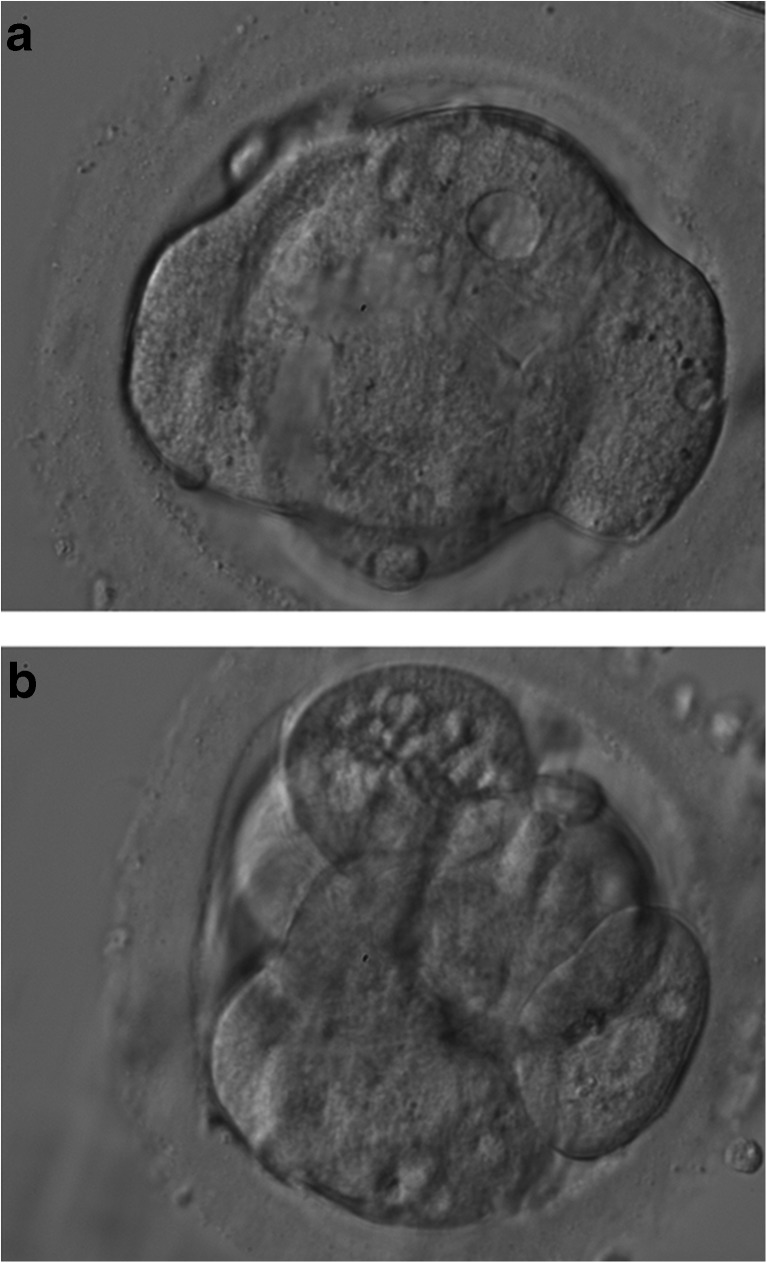
Fertilization check 18 h post ICSI revealed that none of the conventionally but 4/6 (66.7 %) of the artificially activated oocytes showed two pronuclei. Three embryos were of good and one of fair quality according to previously published guidelines [21]. This means that all had stage-appropriate cleavage and timing, no to minimal fragmentation, and no signs of multinucleation. Two embryos (Fig. 1) were transferred on day 3 of preimplantation development using BlastAssist as transfer medium (Origio). The remaining two embryos were further cultured to day 5 when one 511 blastocyst [21] was vitrified which corresponds to a hatching blastocyst showing both an optimal inner cell mass and trophectoderm. A viable intrauterine twin pregnancy (dichorionic-diamniotic) was detected at week 6 of gestation. Consequently, blastocyst formation rate in the present cycle was 75 % (3/4). Two healthy boys (without the presence of a situs inversus) were born in the 34th week of gestation (birth weight of 1724 g and 2199 g). A Cesarean section which was indicated due to premature rupture of the membranes.
Fig. 1.
Day 3 embryos transferred. a. 8-cell embryo with beginning compaction. Vacuoles at 12 and 3 o’clock position should be noted. b. 8-cell embryo with minor compaction and vacuolized cell at the 12 o’clock position
Discussion
Although a few patients with Kartagener syndrome were reported to be fertile [22] the majority of patients is in desperate need of assisted reproductive techniques. Before it was possible to directly inject a single spermatozoon into the ooplasm, subzonal injection (SUZI) of numerous sperms was a successful alternative [23, 24]. However, SUZI required at least minimal sperm motility in order achieve fertilization. ICSI, as the ultimate invasive technique, does not require a motile sperm for subsequent fertilization.
Various sperm motilities have been reported in patients with Kartagener syndrome ranging from 0.3 % [6] to 25 % [4]. Since sperm motility usually reflects sperm viability, patients showing at least some motility will perform better in terms of fertilization. On the other hand, akinetospermia in the presence of normal sperm count and morphology, which is the most likely scenario in Kartagener patients, will bear the risk of selecting non-viable sperms for ICSI.
It has been suggested that the probability of injecting viable (but immotile) spermatozoa was greater when testicular sperms were used [17], while ejaculated sperm, even if tested viable, were less successful [16]. Indeed, several pregnancies were achieved using ICSI with TESE material [5, 17, 18].
However, not every patient wants his testicles to be biopsied, if several methods are available to distinguish between viable and non-viable sperms in the presence of exclusively non-motile ejaculated sperms. With respect to this, HOS test was found to be helpful since several authors reported pregnancies after selection based on osmotic behavior of the sperm membrane which allows estimating sperm viability [9, 11, 16].
In cases in which the native semen already contains spermatozoa showing a HOS-positive phenotype (e.g., curled tail) additional viability assessment methods have to be applied in order to ensure usage of potential viable gametes [13]. Thus, Gerber and co-workers [13] used a 1.48 μm diode laser in order to artificially create a HOS-positive like tail curling as recommended by others [25].
Even using the activation methods discussed so far may not keep embryologists from facing complete fertilization failure [13, 16, 26, 27], fertilization problems [17, 18] or non-appearance of pregnancy [6, 7]. Possible explanations for this unwanted happening are rare but could be related to the quality of the selected spermatozoon. There is indeed evidence that Kartagener patients have sperms showing high level of non-reparable DNA damage [27] and/or aneuploidy, particularly disomies [28]. The latter holds also true for PCD patients with heavily dysmorphic flagella, which would further minimize pregnancy rate in such patients [29, 30].
In PCD (and consequently Kartagener) males with complete absence of sperm motility and low rates of viable spermatozoa it has been suggested to use pentoxifylline in order to restore motility [31], with twitching of the flagellum being the minimal requirement. Hattori et al. [12] were the first to incubate spermatozoa for 10 min in a 0.1 % pentoxifylline solution resulting in “slight sperm movement”. Using these activated sperms for ICSI a fertilization rate of 58 % and a blastocyst formation rate of 57 % was observed. Single blastocyst transfer of a vitrified/warmed blastocyst led to a healthy singleton pregnancy. This approach also worked on vitrified oocytes [14].
Since pentoxifylline has a shorter half-life than other dimethylxanthines (which are inhibitors of phosphodiesterase activity) it was decided to use theophylline in the present case because this agent had turned out to partly restore motility under comparable conditions [32, 33]. Since this approach quite unexpectedly failed, an alternative activation attempt was sought, this time targeting the oocyte. Since ionophore application immediately after ICSI has already proven its potential in severe male factor infertility [34] as well as oocyte-related fertilization problems [35], IRB approval was not required. However, in order to highlight a possible effect of A23187 on fertilization and further development [36] MII-oocytes were split into an ionophore and a non-ionophore half. Although at first glance a benefit of ionophore treatment is probable it has to be stressed that the low number of oocytes did not allow for statistical analysis.
The ionophore A23187 is not only known for its role in sperm acrosome reaction [37] it is also linked to oocyte activation. This holds particularly true if sperm phospholipase C zeta (PLCζ) is aberrantly expressed, localized, and/or destructured [38, 39]. In such cases Ca2+ oscillations, a prerequisite for eventual oocyte activation, cannot be induced in the oocyte naturally but only with the help of an ionophore. To date, it is not clear whether sperms in Kartagener syndrome have a deficiency in PLCζ, the present data, however, support this theory.
To summarize, this a rare report of a twin pregnancy [5, 8, 11, 13] in a Kartagener patient. What makes it unique is that it is the first case showing a certain resistance to conventional sperm selection procedures. Based on our findings it can be said that ionophore treatment in such a given condition could rescue the cycle. The future will show if routine application of A23187 in Kartagener or PCD patients will be of benefit.
Footnotes
Capsule Ca2+-ionophore treatment in cycles from theophylline-resistant Kartagener syndrome patients is a feasible option.
References
- 1.Kartagener M. Zur Pathologie der Bronchiektasien: Bronchiektasien bei situs viscerum inversus. Beitr Klin Tuberk. 1933;83:489–501. doi: 10.1007/BF02141468. [DOI] [Google Scholar]
- 2.Aitken J. Reproductive biology: a clue to Kartagener’s nature. Nature. 1991;353:306. doi: 10.1038/353306a0. [DOI] [PubMed] [Google Scholar]
- 3.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–76. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 4.Kay VJ, Irvine DS. Successful in-vitro fertilization pregnancy with spermatozoa from a patient with Kartagener’s syndrome. Hum Reprod. 2000;15:135–8. doi: 10.1093/humrep/15.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Kaushal M, Baxi A. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener or immotile cilia syndrome. Fertil Steril. 2007;88:497.e9–11. doi: 10.1016/j.fertnstert.2006.11.113. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto Y, Goto S, Hashimoto H, Kokeguchi S, Shiotane M, Okada H. healthy birth after intracytoplasmic sperm injection using ejaculated spermatozoa from a patient with Kartagener’s syndrome. Fertil Steril. 2010;93:2074.e.17–9. doi: 10.1016/j.fertnstert.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Papadimas J, Tarlatzis BC, Bili H, Sotiriadis T, Koliakou K, Bontis J, et al. Therapeutic approach of immotile cilia syndrome by intracytoplasmic sperm injection: a case report. Fertil Steril. 1997;67:562–5. doi: 10.1016/S0015-0282(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 8.von Zumbusch A, Fiedler K, Mayerhofer A, Jessberger B, Ring J, Vogt HJ. Birth of healthy children after intracytoplasmic sperm injection in two couples with male Kartagener’s syndrome. Fertil Steril. 1998;70:643–6. doi: 10.1016/S0015-0282(98)00246-5. [DOI] [PubMed] [Google Scholar]
- 9.Peeraer K, Nijs M, Raick D, Ombelet W. Pregnancy after ICSI with ejaculated immotile spermatozoa from a patient with immotile cilia syndrome: a case report and review of the literature. Reprod Biomed Online. 2004;9:659–63. doi: 10.1016/S1472-6483(10)61777-4. [DOI] [PubMed] [Google Scholar]
- 10.Gerber PA, Kruse R, Hirchenhain J, Krüssel JS, Neumann NJ. Pregnancy after laser-assisted selection of viable spermatozoa before intracytoplasmatic sperm injection in a couple with male primary cilia dyskinesia. Fertil Steril. 2008;89:1826.e9–12. doi: 10.1016/j.fertnstert.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Kordus RJ, Price RL, Davis JM, Whitman-Elia GF. Successful twin birth following blastocyst culture of embryos derived from the immotile ejaculated spermatozoa from a patient with primary ciliary dyskinesia: a case report. J Assist Reprod Genet. 2008;25:437–43. doi: 10.1007/s10815-008-9254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattori H, Nakajo Y, Ito C, Toyama Y, Toshimori K, Kyono K. Birth of a healthy infant after intracytoplasmic sperm injection using pentoxifylline-activated sperm from a patient with Kartagener’s syndrome. Fertil Steril. 2011;95:2431.e9–11. doi: 10.1016/j.fertnstert.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 13.Geber S, Lemgruber M, Taitson PF, Valle M, Sampaio M. Birth of healthy twins after intracytoplasmic sperm injection using ejaculated immotile spermatozoa from a patient with Kartagener’s syndrome. Andrologia. 2012;44(1):842–4. doi: 10.1111/j.1439-0272.2011.01224.x. [DOI] [PubMed] [Google Scholar]
- 14.Montjean D, Courageot J, Altié A, Amar-Hoffet A, Rossin B, Geoffroy-Siraudin C. Normal live birth after vitrified/warmed oocytes intracytoplasmic sperm injection with immotile spermatozoa in a patient with Kartagener’s syndrome. Andrologia. 2014 doi: 10.1111/and.12331. [DOI] [PubMed] [Google Scholar]
- 15.Mendeluk GR, Costa SL, Scigliano S, Menga G, Demiceu S, Palaoro LA. A rare case of respiratory disorders associated with two autosomal recessive diseases and male infertility. Allergy Rhinol (Providence) 2013; 4. e1-5. doi: 10.2500/ar.2013.4.0038. [DOI] [PMC free article] [PubMed]
- 16.Westlander G, Barry M, Petrucco O, Norman R. Different fertilization rates between immotile testicular spermatozoa and immotile ejaculated spermatozoa for ICSI in men with Kartagener’s syndrome: case reports. Hum Reprod. 2003;18:1286–8. doi: 10.1093/humrep/deg240. [DOI] [PubMed] [Google Scholar]
- 17.Cayan S, Conaghan J, Schriock ED, Ryan IP, Black LD, Turek PJ. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener/immotile cilia syndrome. Fertil Steril. 2001;76:612–4. doi: 10.1016/S0015-0282(01)01974-4. [DOI] [PubMed] [Google Scholar]
- 18.Vicdan K, Akarsu C, Vicdan A, Sözen E, Buluç B, Biberoǧlu K, et al. Birth of a healthy boy using fresh testicular sperm in a patient with Klinefelter syndrome combined with Kartagener syndrome. Fertil Steril. 2011;96:577–9. doi: 10.1016/j.fertnstert.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO press; 2010. [Google Scholar]
- 20.Liu J, Tsai YL, Katz, Compton G, Garcia JE, Baramki TA. High fertilization rate obtained after intracytoplasmic sperm injection with 100% nonmotile spermatozoa selected by using a simple modified hypo-osmotic swelling test. Fertil Steril. 1997;68:373–5. doi: 10.1016/S0015-0282(97)81533-6. [DOI] [PubMed] [Google Scholar]
- 21.ALPHA Scientists In Reproductive Medicine; ESHRE Special Interest Group Embryology. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online 2011; 22: 632–46. [DOI] [PubMed]
- 22.Conraads VMA, Galdermans DI, Kockx MM, Jacob WA, van Schaardenburg C, Collen D. Ultrastructurally normal and motile spermatozoa in a fertile man with Kartagener’s syndrome. Chest. 1992;102:1616–8. doi: 10.1378/chest.102.5.1616. [DOI] [PubMed] [Google Scholar]
- 23.Wolf JPH, Feneux D, Escalier D, Rodrigues D, Frydman R, Jouannet P. Pregnancy after subzonal insemination with spermatozoa lacking outer dynein arms. J Reprod Fertil. 1993;97:487–92. doi: 10.1530/jrf.0.0970487. [DOI] [PubMed] [Google Scholar]
- 24.Nijs M, Vanderzwalmen P, Vandamme B, Segal-Bertin G, Lejeune B, Segal L, et al. Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum Reprod. 1996;11:2180–5. doi: 10.1093/oxfordjournals.humrep.a019073. [DOI] [PubMed] [Google Scholar]
- 25.Aktan TM, Montag M, Duman S, Gorkemli H, Rink K, Yurdakul T. Use of a laser to detect viable but immotile spermatozoa. Andrologia. 2004;36:366–9. doi: 10.1111/j.1439-0272.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Musa A, Hannoun A, Khabbaz A, Devroey P. Failure of fertilization after intracytoplasmic sperm injection in a patient with Kartagener’s syndrome and totally immotile spermatozoa: case report. Hum Reprod. 1999;14:2517–8. doi: 10.1093/humrep/14.10.2517. [DOI] [PubMed] [Google Scholar]
- 27.Nuñez R, López-Fernández C, Arroyo F, Caballero P, Gosálvez J. Characterization of sperm DNA damage in Kartagener’s syndrome with recurrent fertilization failure: case revisited. Sex Reprod Healthc. 2010;1:73–5. doi: 10.1016/j.srhc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Rives N, Mousset-Simeon N, Mazurier S, Mace B. Primary flagellar abnormality is associated with an increased rate of spermatozoa aneuploidy. J Androl. 2005;26:61–9. [PubMed] [Google Scholar]
- 29.Stalf T, Sánchez R, Köhn FM, Schalles U, Kleinstein J, Hinz V, et al. Pregnancy and birth after intracytoplasmic sperm injection with spermatozoa from a patient with tail stump syndrome. Hum Reprod. 1995;10:2112–4. doi: 10.1093/oxfordjournals.humrep.a136244. [DOI] [PubMed] [Google Scholar]
- 30.McLachlan RI, Ishikawa T, Osianlis T, Robinson P, Merriner DJ, Healy D, et al. Normal live birth after testicular sperm extraction and intracytoplasmic sperm injection in variant primary ciliary dyskinesia with completely immotile sperm and structurally abnormal sperm tails. Fertil Steril. 2012;97:313–8. doi: 10.1016/j.fertnstert.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Yildirim G, Ficicioglu C, Akcin O, Attar R, Tecellioglu N, Yencilek F. Can pentoxifylline improve the sperm motion and ICSI success in the primary ciliary dyskinesia? Arch Gynecol Obstet. 2009;279:213–15. doi: 10.1007/s00404-008-0671-y. [DOI] [PubMed] [Google Scholar]
- 32.Ebner T, Tews G, Mayer RB, Ziehr S, Arzt W, Costamoling W, et al. Pharmacological stimulation of sperm motility in frozen and thawed testicular sperm using the dimethylxanthine theophylline. Fertil Steril. 2011;96:1331–6. doi: 10.1016/j.fertnstert.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 33.Ebner T, Shebl O, Mayer RB, Moser M, Costamoling W, Oppelt P. Healthy live birth using theophylline in a case of retrograde ejaculation and absolute asthenozoospermia. Fertil Steril. 2014;101:340–3. doi: 10.1016/j.fertnstert.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Ebner T, Köster M, Shebl O, Moser M, van der Ven H, Tews G, et al. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril. 2012;98:1432–7. doi: 10.1016/j.fertnstert.2012.07.1134. [DOI] [PubMed] [Google Scholar]
- 35.Ebner T, Montag M. Oocyte Activation group. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30:359–65. doi: 10.1016/j.rbmo.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Ebner T, Oppelt P, Wöber M, Staples P, Mayer RB, Sonnleitner U, et al. Treatment with Ca2+ ionophore improves embryo development and outcome in cases with previous developmental problems: a prospective multicenter study. Hum Reprod. 2015;30:97–102. doi: 10.1093/humrep/deu285. [DOI] [PubMed] [Google Scholar]
- 37.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20:3459–68. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2þ release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–81. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta in spermatozoa from infertile men. Hum Reprod. 2009;24:2417–28. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]