Abstract
Purpose
To establish a ratio of the P level to the number of follicles (P/F ratio) on the day of human chorionic gonadotropin (hCG) administration and to evaluate whether this ratio is associated with in vitro fertilization (IVF) outcome.
Methods
This study was conducted between January 2012 and June 2013. A total of 337 patients with cleavage-stage day-3 fresh embryo transfer with P levels ≤1.5 ng/mL on the day of hCG administration were included in the study. The main outcome was ongoing pregnancy rate.
Result(s)
The P/F ratio was calculated according to the equation (P[ng/mL]/number of follicles) on the day of final oocyte maturation. Using ROC, we established a cut-off level of 0.075 for the P/F ratio. The area under the curve (AUC) (0.756; 95 % confidence interval [CI]: 0.704–0.807) indicated that it was a good prognostic test. In group 1 (patients under 36 years old), the ongoing pregnancy rates were 57 and 30 % for patients with P/F ratios ≤ .075 and > .075, respectively, (p = 0.003). In group 2 (patients between 36 and 39 years old), the ongoing pregnancy rates were 58 % and 17 % (p = 0.001) for patients with P/F ratios ≤ .075 and > .075, respectively. In group 3 (patients ≥ 40 years old), the ongoing pregnancy rates were 41.7 and 10.9 % (p = 0.001) for patients with P/F ratios ≤ .075 and > .075, respectively.
Conclusions
The P/F ratio is a good prognostic test for predicting IVF outcome that can correlate the P level with ovarian response.
Keywords: Progesterone to number of follicles ratio, Endometrial receptivity, Progesterone elevation
Introduction
Progesterone elevation (PE) has been observed during controlled ovarian stimulation (COS) using gonadotropins and gonadotropin-releasing hormone (GnRH) analogs, occurring mainly at the end of the follicular phase and on the day of human chorionic gonadotropin (hCG) administration. Its frequency varies, but it occurs in up to 35 % of cycles in patients treated with a GnRH agonist and in up to 38 % of cycles in those treated with a GnRH antagonist [1].
A recent meta-analysis has suggested that PE during COS is associated with a decreased probability of pregnancy following fresh embryo transfer (ET), but this elevation is not associated with the outcome of frozen–thawed transfer (FET) [2]. These data suggest that PE may be associated with adverse effects on the endometrium (specifically, advanced endometrial histological maturation and altered gene expression) and that it does not negatively impact embryo quality [3, 4]. Thus, patients with PE during a fresh cycle would benefit from elective FET, for which the entire cohort of embryos is cryopreserved and the embryo transfer is performed later in a natural cycle or in a cycle with hormonal replacement for endometrial priming. [5, 6].
However, deleterious progesterone (P) levels may vary according to ovarian response [7, 8]. Thus, it would be better to define a ratio between P level and ovarian response instead of using a single P level as a prognostic tool. The aim of this study was to determine whether the use of the ratio of P level to the number of follicles (P/F ratio) is optimal compared with an isolated threshold concentration of P on the day of final oocyte maturation for selecting patients to undergo delayed ET rather than fresh ET.
Materials and methods
This study was conducted between January 2012 and June 2013 in a private in vitro fertilization (IVF) unit in Brazil. An institutional review board approved this study, and informed consent was obtained from all patients included in the study.
Patient selection
The following inclusion criteria were established: (1) GnRH antagonist cycles with at least two good-quality embryos (featuring 6–10 cells with no fragmentation and equal blastomere size [grade 1] or up to 20 % fragmentation [grade 2]) for fresh ET; and (2) P < 1.5 ng/mL on the day of trigger. Exclusion criteria included: (1) P ≥ 1.5 ng/mL on the day of trigger; (2) the presence of a uterine pathology (adenomyosis or uterine malformations); (3) recurrent pregnancy loss; (4) previous implantation failure (≥3 previous failed ETs); and (5) severe male factor infertility (oligospermia < 1 million/mL and azoospermia). The patients that fulfilled the inclusion/exclusion criteria (335 patients) were followed for up to 12 weeks of pregnancy when available, via an ultrasound scan in the 7th–8th weekS of pregnancy to confirm clinical pregnancy and contact by telephone in the 12th weeks to confirm ongoing pregnancy.
Study protocol
Patients underwent COS with recombinant follicle-stimulating hormone (FSH) (Gonal-F®; MerckKGaA, Darmstadt, Germany) starting on day 2 or 3 of menses, and levels of up to 450 IU/day were administered using a step-down protocol. The GnRH antagonist cetrorelix (Cetrotide; MerckKGaA) was used for pituitary suppression and was initiated on stimulation day 5 or 6. Final oocyte maturation was induced with 250 mcg of recombinant hCG (Ovitrelle®; MerckKGaA) plus 0.2 mg of triptorelin (Gonapeptyl daily®; Ferring Pharmaceuticals, Saint Prex, Switzerland), referred to as a “dual trigger” [9], when at least two follicles reached a diameter of 18 mm. The patients underwent transvaginal ultrasound-guided oocyte retrieval at 35 h after trigger, followed by intracytoplasmic sperm injection (ICSI). One to four embryos were transferred under ultrasonography guidance on day 3 following oocyte retrieval, and embryo quality was evaluated. Luteal phase support with 90 mg of vaginal progesterone daily (Crinone 8 % vaginal gel; MerckKGaA) was initiated on the day of oocyte retrieval and continued for up to 9 weeks of pregnancy when available.
Hormone analysis and follicle count
Blood samples were collected between 7:00 and 12:00 a.m. on the day of final oocyte maturation, and serum P levels were measured using a chemiluminescent immunoassay for quantitative determination of the hormone (Diagnostics Biochem Canada Inc., Dorchester, ON, Canada) with a sensitivity of 0.1 ng/mL. P concentrations were determined with a coefficient of variation (CV) of <10 %. The P/F ratio was calculated according to the equation (P[ng/mL]/number of follicles) and was defined by the measurements obtained on the day of final oocyte maturation. All ultrasounds were performed at our IVF center, and we included the number of follicles ≥ 14 mm on the day of final oocyte maturation in the ratio calculation.
Outcomes
Pregnancy was defined by hCG titers within 11 days following ET. Clinical pregnancy was defined by the observation of intrauterine embryo heart motion by 7 weeks gestation. Ongoing pregnancy was defined as pregnancy proceeding beyond the 12th weeks of gestation. The implantation rate was regarded as the ratio of the number of observed embryo hearts to the number of transferred embryos.
The main outcome measure was ongoing pregnancy rate, which has been shown to be comparable to live birth as a measure of efficacy [10]. The number of oocytes retrieved, the number of mature oocytes, the fertilization rate, the implantation rate, and the pregnancy rate were the secondary outcome measures.
Statistical analysis
Sample size calculation was performed using pregnancy rate as the base outcome. Using an alpha value of 0.05 and a beta value of 0.2 in a two-sided test, it was determined that 145 exposed subjects and 145 non-exposed subjects were necessary to obtain statistically significant results, with a relative risk of ≥ 0.6.
The data are presented as the mean ± standard deviation or as a percentage. A comparison of the quantitative variables was performed using Student’s t-test for the independent samples. For the comparison of the categorical data, the chi-squared test was performed. Receiver operating characteristic (ROC) analysis was conducted to establish the most efficient cut-off values for the P/F ratio to discriminate between successful and unsuccessful ICSI outcomes of antagonist cycles with day-3 fresh ET. The best cut-off values were determined based on equivalent sensitivity and specificity, and the highest value of the area under curve (AUC) was determined. We also performed the ROC analysis for P levels on the day of oocyte maturation. A p value of <0.05 was considered statistically significant. Statistical analysis was performed with Statistical Package for the Social Sciences software (SPSS version 19.0 for Windows; IBM Corporation, Armonk, NY, USA).
Results
The baseline characteristics of the overall population are presented in Table 1. During the study period, we performed oocyte retrievals on 962 patients. Of them, a total of 335 patients fulfilled the inclusion/exclusion criteria and were included in the study. These patients were followed at least until the 12th weeks of pregnancy or earlier if a negative outcome occurred. The mean age was 36.64 ± 4.58 years, and the mean basal FSH level was 8.26 ± 2.47. The overall implantation and ongoing pregnancy rates were 22.36 and 35 %, respectively.
Table 1.
Baseline characteristics and outcomes of patients undergoing GnRH antagonist cycles and fresh embryo transfer with P < 1.5 ng/mL on day of trigger
| Characteristics | Study group (n = 335) |
|---|---|
| Mean age | 36.64 ± 4.58 |
| Basal FSH (mIU/mL) | 8.26 ± 2.47 |
| AFC | 9.64 ± 4.06 |
| Stimulation days | 10.97 ± 1.17 |
| Total dose of FSH (IU) | 2,405.42 ± 816.75 |
| P on day of trigger (ng/mL) | 0.71 ± 0.28 |
| E2 on day of trigger (pg/mL) | 1610 ± 632.24 |
| No. of follicles (≥14 mm) on day of trigger | 9.06 ± 3.66 |
| No. of oocytes retrieved | 7.63 ± 4.11 |
| No. of MII retrieved | 5.54 ± 3.24 |
| No. of 2PN | 4.3 ± 2.64 |
| No. of transferred embryos | 2.28 ± 0.75 |
| Pregnancy rate (%) | 42 |
| Implantation rate (%) | 22.36 |
| Clinical pregnancy rate (%) | 38 |
| Ongoing pregnancy rate (%) | 35 |
AFC antral follicle count, P progesterone, E 2 estradiol, MII mature eggs
* The results are expressed as the mean ± SD
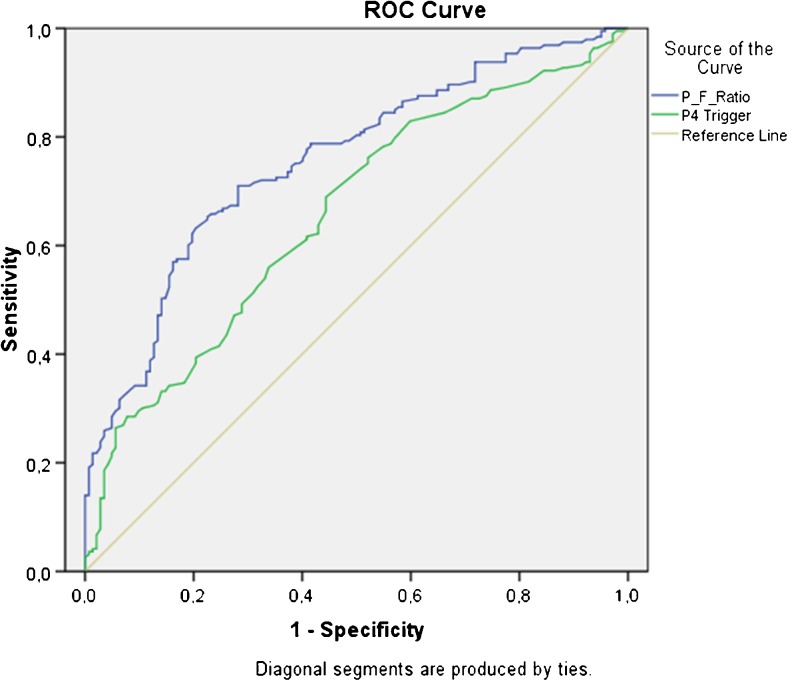
According to the ROC analysis, the P/F ratio was found to be discriminative for achieving versus not achieving pregnancy (p = 0.001; AUC = 0.756; 95 % confidence interval [CI]: 0.704–0.807) with a sensitivity and specificity of 71 % and 71.1 %, respectively (Fig. 1). The optimal cut-off value for the P/F ratio was 0.075. The ROC analysis for P levels on the day of final oocyte maturation for achieving versus not achieving pregnancy showed an AUC = 0.658 (p = 0.001; 95 % CI: 0.599–0.716).
Fig. 1.
ROC curve for P/F ratio (in blue) and for P levels (in green)
To avoid possible bias due the different mean ages between the 2 groups, the patients were stratified by age into three groups. Group 1 included patients under 36 years old; group 2 included those between 36 and 39 years old; and group 3 included those ≥ 40 years old. In all three groups, the IVF outcomes were correlated with the P/F ratio. In group 1 (Table 2), the ongoing pregnancy rates were 57 and 30 % for the patients with P/F ratios of ≤ .075 and > .075, respectively (p = 0.003), and the implantation rates were 35.6 and 19.7 % for the patients with P/F ratios of ≤ .075 and > .075, respectively (p = 0.016). In group 2 (Table 3), the ongoing pregnancy rates were 58 and 17 % (p = 0.001) and the implantation rates were 33.3 and 12.7 % (p = 0.001) for the patients with P/F ratios of ≤ .075 and > .075, respectively. Finally, in group 3 (Table 4), the ongoing pregnancy rates were 41.7 and 10.9 % (p = 0.001) and the implantation rates were 23.3 and 8.9 % (p = 0.001) for the patients with P/F ratios of ≤ .075 and > .075, respectively.
Table 2.
Patient characteristics and IVF outcomes in group 1 (women <36 years old)
| P/F ≤ .075 (n = 88) | P/F > .075 (n = 43) | p value | RR (95 % CI) | |
|---|---|---|---|---|
| Mean age | 31.74 ± 2.71 | 32.4 ± 2.93 | 0.21 | |
| Basal FSH (mIU/mL) | 7.59 ± 1.97 | 8.17 ± 2.60 | 0.16 | |
| AFC | 12.5 ± 4.02 | 9.21 ± 3.57 | 0.001 | |
| Stimulation days | 10.83 ± 0.94 | 10.86 ± 1.37 | 0.89 | |
| Total dose of FSH (IU) | 2037.26 ± 739.89 | 2206.46 ± 756.50 | 0.23 | |
| P on day of trigger (ng/mL) | 0.59 ± 0.23 | 0.98 ± 0.97 | 0.001 | |
| E2 on day of trigger (pg/mL) | 1817.35 ± 610.76 | 1419.42 ± 554.62 | 0.001 | |
| No. of follicles (≥14 mm) on day of trigger | 11.89 ± 3.84 | 7.72 ± 1.98 | 0.001 | |
| No. of oocytes retrieved | 9.75 ± 4.45 | 7.42 ± 3.30 | 0.003 | |
| No. of MII retrieved | 7.09 ± 3.88 | 5.16 ± 2.34 | 0.001 | |
| Fertilized eggs | 5.45 ± 3.29 | 4.09 ± 1.91 | 0.003 | |
| Embryos transferred | 2.06 ± 0.44 | 2.09 ± 0.57 | 0.69 | |
| Implantation rate (%) | 35.6 | 19.7 | 0.016 | |
| Pregnancy rate (%) | 59/88 (67 %) | 18/43 (41.9 %) | 0.006 | 0.624 (0.426–0.914) |
| Clinical pregnancy rate (%) | 57/88 (64.8 %) | 14/43 (32.6 %) | 0.001 | 0.503 (0.318–0.794) |
| Ongoing pregnancy rate (%) | 50/88 (56.8 %) | 13/43 (30.2 %) | 0.003 | 0.532 (0.326–0.868) |
AFC antral follicle count, P progesterone, E 2 estradiol, MII mature eggs
* The results are expressed as the mean ± SD
Table 3.
Patient characteristics and IVF outcomes in group 2 (women between 36 and 39 years old)
| P/F ≤ .075 (n = 45) | P/F > .075 (n = 63) | p value | RR (95 % CI) | |
|---|---|---|---|---|
| Mean age | 37.47 ± 1.06 | 37.78 ± 1.07 | 0.137 | |
| Basal FSH (mIU/mL) | 7.62 ± 1.63 | 8.65 ± 2.61 | 0.014 | |
| AFC | 11.53 ± 3.25 | 8.33 ± 3.57 | 0.001 | |
| Stimulation days | 10.98 ± 0.72 | 11.3 ± 1.17 | 0.08 | |
| Total dose of FSH (IU) | 2287.22 ± 518.59 | 2680.43 ± 634.68 | 0.001 | |
| P on day of trigger (ng/mL) | 0.54 ± 0.19 | 0.87 ± 0.25 | 0.001 | |
| E2 on day of trigger (pg/mL) | 1791.33 ± 528.61 | 1557.63 ± 531.45 | 0.026 | |
| No. of follicles (≥14 mm) on day of trigger | 11.51 ± 3.47 | 7.33 ± 2.19 | 0.001 | |
| No. of oocytes retrieved | 9.98 ± 4.33 | 6.02 ± 3.21 | 0.001 | |
| No. of MII retrieved | 6.89 ± 3.81 | 4.59 ± 2.38 | 0.001 | |
| Fertilized eggs | 5.47 ± 3.01 | 3.38 ± 1.87 | 0.001 | |
| Embryos transferred | 2.69 ± 0.47 | 2.21 ± 0.74 | 0.001 | |
| Implantation rate (%) | 33.33 | 12.7 | 0.001 | |
| Pregnancy rate (%) | 29/45 (64.4 %) | 14/63 (22.2 %) | 0.001 | 0.345 (0.207–0.574) |
| Clinical pregnancy rate (%) | 27/45 (60 %) | 11/63 (17.5 %) | 0.001 | 0.291 (0.168–0.524) |
| Ongoing pregnancy rate (%) | 26/45 (58 %) | 11/63 (17.5 %) | 0.001 | 0.302 (0.167–0.546) |
AFC antral follicle count, P progesterone, E 2 estradiol, MII mature eggs
* The results are expressed as the mean ± SD
Table 4.
Patient characteristics and IVF outcomes in group 3 (women ≥40 years old)
| P/F ≤ .075 (n = 24) | P/F > .075 (n = 73) | p value | RR (95 % CI) | |
|---|---|---|---|---|
| Mean age | 41.46 ± 1.50 | 42.08 ± 1.51 | 0.212 | |
| Basal FSH (mIU/mL) | 7.81 ± 2.24 | 9.30 ± 2.92 | 0.011 | |
| AFC | 8.58 ± 2.75 | 6.82 ± 2.71 | 0.001 | |
| Stimulation days | 11.29 ± 1.60 | 10.78 ± 1.31 | 0.07 | |
| Total dose of FSH (IU) | 2521.87 ± 991.61 | 2759.48 ± 937.7 | 0.06 | |
| P on day of trigger (ng/mL) | 0.50 ± 0.12 | 0.98 ± 1.12 | 0.001 | |
| E2 on day of trigger (pg/mL) | 1744.33 ± 781.59 | 1360.64 ± 671.24 | 0.005 | |
| No. of follicles (≥14 mm) on day of trigger | 8.92 ± 2.34 | 6.41 ± 2.1 | 0.001 | |
| No. of oocytes retrieved | 6.88 ± 2.94 | 5.34 ± 2.90 | 0.01 | |
| No. of MII retrieved | 5.46 ± 2.62 | 3.86 ± 1.95 | 0.001 | |
| Fertilized eggs | 4.58 ± 2.04 | 3.00 ± 1.51 | 0.001 | |
| Embryos transferred | 2.92 ± 0.93 | 2.26 ± 0.97 | 0.001 | |
| Implantation rate (%) | 23.3 | 8.9 | 0.001 | |
| Pregnancy rate (%) | 13/24 (54.2 %) | 9/73 (12.3 %) | 0.001 | 0.228 (0.111–0.465) |
| Clinical pregnancy rate (%) | 11/24 (45.8 %) | 8/73 (10.9 %) | 0.001 | 0.239 (0.109–0.524) |
| Ongoing pregnancy rate (%) | 10/24 (41.7 %) | 8/73 (10.9 %) | 0.001 | 0.263 (0.117–0.589) |
AFC antral follicle count, P progesterone, E 2 estradiol, MII mature eggs
* The results are expressed as the mean ± SD
Discussion
In this study, we observed that the P/F ratio was a better predictor of IVF outcome than the isolated P level during an IVF cycle with fresh ET. There is growing evidence that supra-physiologic hormone levels during COS may jeopardize endometrial receptivity and decrease pregnancy rate during fresh ET. However, embryo quality does not appear to be affected by altered hormone levels [11]. Thus, some patients would benefit from delayed frozen–thawed transfers (FET) [12]. However, it is still a challenge to find a non-invasive test to assess endometrial receptivity that is able to accurately predict IVF outcome, and there is currently no consensus regarding the best way to select those patients who would really benefit from elective FET.
Currently, the best way to select patients for delayed FET is by assessing the P level on the day of final oocyte maturation because PE during the late follicular phase of COS is associated with a decreased probability of pregnancy in patients undergoing fresh ET [2]. In the final follicular phase, the subtle increases in progesterone levels are associated with advancements in the endometrium’s ultrastructural morphology and echogenicity, and these levels seem to have a negative impact on embryo implantation [13, 14]. These alterations may result in embryo–endometrium asynchrony, and they may decrease the implantation rates during ART treatments [6]. The live birth rates of patients undergoing IVF treatments may decrease when there is an increase in P levels on the day of final oocyte maturation [15]. However, we still do not know exactly what the threshold values are when a cycle becomes supra-physiologic [16]. In our study, we correlated the P/F ratio to the IVF outcomes of fresh cleavage-stage ETs in the patients with a P level of <1.5 ng/mL on the day of final oocyte maturation and found that this ratio was a better predictor of pregnancy in all age groups when compared to progesterone alone. The overall IVF outcomes observed in our study are in accordance with previous reports in the literature [17]. However, we found statistically significant differences in outcomes according to ovarian response. When evaluating the ROC analysis in our study, the P/F ratio (which correlates the P level to ovarian response) seems to be a more effective IVF prognostic tool compared with a single P measurement on the day of final oocyte maturation.
It has been suggested that a patient’s hormone profile during COS is related to ovarian response and that the P level increases as the number of follicles and E2 level increase [18]. Some studies have suggested that the progesterone-to-estradiol ratio (P/E2) reflects premature luteinization and that it could be a more accurate predictor of IVF outcome than the isolated P level [19, 20]. However, these studies that have correlated the P/E2 to IVF outcome have reported unsatisfactory values for sensitivity, specificity, and AUC [19, 21, 22]. Conversely, our test showed a moderate level of accuracy with AUC = 0.756, and it exhibited good sensitivity and specificity of 71 % and 71.1 %, respectively. These results for P/F ratio were better than those found for P levels (AUC = 0.658; 95 % CI: 0.599–0.716). Interestingly, we observed that the proportion of patients with a P/F ratio > 0.075 increased with advanced maternal age. A total of 32.8 % of the <36-year-old patients had an elevated P/F ratio in addition to 58.3 % of those that were 36–39 years old, and 75.2 % of those that were >39 years old. We hypothesize that with advanced maternal age, (in addition to worsening oocyte quality) a change in the pattern of ovarian hormonal response could occur. The P/F ratio reflects the average amount of P produced by each follicle ≥ 14 mm on the day of final oocyte maturation. The poorest outcomes observed in patients with higher P/F ratio may be related to a disruption in follicular growth / maturation, and may reflect an increased follicular secretion of P from each mature follicle.[23].
Because this study was not a randomized clinical trial, it may have some weaknesses. There were some differences in the number of retrieved oocytes when comparing the groups with a P/F ratio ≤ .075 with those with a ratio > .075, with a lower number of oocytes noted in the latter group. However, according to the literature [8, 24], both groups of patients have been found to exhibit good prognoses considering the number of retrieved oocytes. When between 6 and 15 oocytes are retrieved, the pregnancy rates for one fresh ET are similar, and when oocyte numbers are either below or above that range, outcomes are compromised [25]. We observed statistically significant differences between the groups with P/F ratios that were under or over the established cut-off, suggesting that these ratios had different consequences on endometrial receptivity according to both ovarian response and P level. The ovarian response, number of retrieved oocytes, number of mature oocytes retrieved, and number of fertilized eggs were significantly lower in the patients with a P/F ratio > .075. Some studies using the P/E2 ratio have found similar results after establishing a cut-off value for P/E2, suggesting that this ratio is associated with lower fertility [19, 26]. Studies attempting to establish cut-off levels or ratios using hormonal measurements may be influenced by the different measurement techniques used or by the lab at which they are measured. These variations may be responsible for the differences between the cutoff levels/ratios reported by the different studies. Thus, based on our findings, we suggest that each IVF center obtain an individual P/F ratio based on their own, location-specific hormonal measurements. There may also be concerns about the transfer in cleavage stage performed in our study, as the practice of blastocyst transfer in increasingly being adopted in many ART clinics over cleavage stage, although there is no clear evidence that this is a safe practice [27]. A recent meta-analysis showed that there is a small advantage in performing blastocyst transfer compared to cleavage stage transfer when considering the live birth rates. However, these results were observed only in good prognosis patients and the cumulative clinical pregnancy rates were higher for cleavage stage transfer when compared to blastocyst transfer [28]. In our IVF center the cleavage stage embryo transfer was a routine procedure in the study period.
To our knowledge, there is only one recent published study to correlate the progesterone levels to ovarian response and IVF outcomes [23], and we still suggest in our study that the P/F ratio is a good prognostic tool for predicting IVF outcome following fresh cleavage-stage ET, even in a select group of patients (P < 1.5 ng/mL on the day of trigger). Further randomized trials are required to evaluate this strategy to validate the results found in this study; however, it seems that the P/F ratio is a better predictor of IVF outcome compared with a single P cut-off level obtained on the day of final oocyte maturation.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest
Footnotes
Capsule The ratio of progesterone-to-number of follicles is a good prognostic test for predicting IVF outcome that can correlate the P level with ovarian response.
References
- 1.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 2.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60000 cycles. Hum Reprod Update. 2013;19:433–57. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 3.Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, Polyzos NP, et al. GnRH-agonist versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod. 2012;6:1822–8. doi: 10.1093/humrep/des066. [DOI] [PubMed] [Google Scholar]
- 4.Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schuit F, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online. 2011;22:263–71. doi: 10.1016/j.rbmo.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–8. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–62. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–7. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Griesinger G, Mannaerts B, Andersen CY, Witjes H, Kolibianakis EM, Gordon K. Progesterone elevation does not compromise pregnancy rates in high responders: a pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil Steril. 2013;100:1622–8. doi: 10.1016/j.fertnstert.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Schachter M, Friedler S, Ron-El R, Zimmerman AL, Strassburger D, Bern O, et al. Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril. 2008;90:1087–93. doi: 10.1016/j.fertnstert.2007.07.1316. [DOI] [PubMed] [Google Scholar]
- 10.Clarke JF, van Rumste MME, Farquhar CM, Johnson NP, Mol BWJ, Herbison P. Measuring outcomes in infertility trials: can we rely on clinical pregnancy rates? Fertil Steril. 2010;94:1647–51. doi: 10.1016/j.fertnstert.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: ready for prime time? Hum Reprod. 2013;28:6–9. doi: 10.1093/humrep/des386. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Kiliçdag EB, Haydardedeoglu B, Cok T, Hacivelioglu SO, Bagis T. Premature progesterone elevation impairs implantation and live birth rates in GnRH-agonist IVF/ICSI cycles. Arch Gynecol Obstet. 2010;281:747–752. doi: 10.1007/s00404-009-1248-0. [DOI] [PubMed] [Google Scholar]
- 14.Huang R, Fang C, Xu S, Yi Y, Liang X. Premature progesterone rise negatively correlated with birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertil Steril. 2012;98:664–670. doi: 10.1016/j.fertnstert.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Venetis CA, Kolibianakis EM, Bosdou JK, Lainas GT, Sfontouris IA, Tarlatzis BC, et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3926 IVF cycles. Hum Reprod. 2015;30:684–91. doi: 10.1093/humrep/deu362. [DOI] [PubMed] [Google Scholar]
- 16.Barnhart KT. Introduction: Are we ready to eliminate the transfer of fresh embryos in in vitro fertilization? Fertil Steril. 2014;102:1–2. doi: 10.1016/j.fertnstert.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, et al. Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod. 2013;28:2318–31. doi: 10.1093/humrep/det278. [DOI] [PubMed] [Google Scholar]
- 18.Kyrou D, Al-Azemi M, Papanikolaou EG, Donoso P, Tziomalos K, Devroey P, et al. The relationship of progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. Eur J Obstet Gynecol Reprod Biol. 2012;162:165–8. doi: 10.1016/j.ejogrb.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Cetinkaya ES, Berker B, Aytac R, Atabekoglu C, Sonmezer M, Ozmen B. The value of the progesterone-to-estradiol ratio on the day of hCG administration in predicting ongoing pregnancy and live birth rates in normoresponders undergoing GnRH antagonist cycles. Eur J Obstet Gynecol Reprod Biol. 2013;170:452–7. doi: 10.1016/j.ejogrb.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Elgindy EA. Progesterone level and progesterone/estradiol ratio on the day of hCG administration: detrimental cutoff levels and new treatment strategy. Fertil Steril. 2011;95:1639–44. doi: 10.1016/j.fertnstert.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 21.Lai TH, Lee FK, Lin TK, Horng SG, Chen SC, Chen YH, et al. An increased serum progesterone-to-estradiol ratio on the day of human chorionic gonadotropin administration does not have a negative impact on clinical pregnancy rate in women with normal ovarian reserve treated with a long gonadotropin releasing hormone agonist protocol. Fertil Steril. 2009;92:508–14. doi: 10.1016/j.fertnstert.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Lee FK, Lai TH, Lin TK, Horng SG, Chen SC. Relationship of progesterone/estradiol ratio on day of hCG administration and pregnancy outcomes in high responders undergoing in vitro fertilization. Fertil Steril. 2009;92:1284–9. doi: 10.1016/j.fertnstert.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Shufaro Y, Sapir O, Oron G, Ben Haroush A, Garor R, Pinkas H, et al. Progesterone-to-follicle index is better correlated with in vitro fertilization cycle outcome than blood progesterone level. Fertil Steril. 2014 doi: 10.1016/j.fertnstert.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 24.van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13:476–80. doi: 10.1016/S1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- 25.Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod. 2013;28:2728–34. doi: 10.1093/humrep/det303. [DOI] [PubMed] [Google Scholar]
- 26.Younis JS, Matilsky M, Radin O, Ben-Ami M. Increased progesterone/estradiol ratio in the late follicular phase could be related to low ovarian reserve in in vitro fertilization-embryo transfer cycles with a long gonadotropin-releasing hormone agonist. Fertil Steril. 2001;76:294–9. doi: 10.1016/S0015-0282(01)01918-5. [DOI] [PubMed] [Google Scholar]
- 27.Dar S, Lazer T, Shah PS, Librach CL. Neonatal outcomes among singleton births after blastocyst versus cleavage stage embryo transfer: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:439–48. doi: 10.1093/humupd/dmu001. [DOI] [PubMed] [Google Scholar]
- 28.Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]