Abstract
Purpose
The presence of Smooth Endoplasmic Reticulum aggregates (SERa) has been reported to be associated with adverse outcomes. An Alpha-ESHRE Consensus was published in 2011, strongly recommending to not inseminating affected oocytes. On the other hand, healthy babies have been born from oocytes presenting this dysmorphism. We surveyed several European IVF centres, to assess their attitudes concerning affected oocytes.
Methods
This survey is based on a computer format and includes questions regarding the fate of affected oocytes.
Results
About 14 % of centres who answered our survey discard SERa+ oocytes. 43 % of centres that do not discard the oocytes, register and follow up neonatal data. About a quarter of centres inform their patients about this dysmorphism. Half of them require an informed consent prior to transferring affected embryos. Twenty-one centres reported having SERa+ births, with one reporting a malformation. 48 % of centres declared having been influenced by the Alpha-ESHRE Consensus, in their management policy of SERa+ oocytes.
Conclusions
Few centres scrupulously respect the recommendations of the Alpha-ESHRE Consensus and discard affected oocytes. Since it is essential to determine if there truly is an impact of this dysmorphism and whether the guidelines are still valid, transfer of affected embryos should only be done when accompanied with data recording and monitoring of all foetal malformations from IVF. Clarifying the situation will allow IVF centres to correctly inform patients about the risk of birth malformations as well as whether a decreased chance of pregnancy exists.
Keywords: Smooth Endoplasmic Reticulum aggregate, Oocyte dysmorphism, Assisted reproduction, Survey
Introduction
The development of ICSI and the possibility of observing the oocyte’s structure prior to fertilization revealed the presence of dysmorphisms in a certain proportion of oocytes [1]. One can distinguish cytoplasmic (granular cytoplasm, vacuoles, Smooth Endoplasmic Reticulum aggregates (SERa)….) or extra-cytoplasmic dysmorphisms (increased peri-vitellin space, fragmented polar body, granular zona pellucida…) [2, 3]. Since several years, it was suggested that oocyte morphology could influence success rates in assisted procreation [1, 4–6]. However, the variety of dysmorphisms and the studied consequences as well as the variety of experimental models and contradictory results did not allow drawing precise conclusions on the global impact of these dysmorphisms on pregnancy outcomes [7]. Moreover, most studies did not focus on the impact of an individual anomaly on IVF outcomes [1, 4–6]. SERa, on the other hand, have been studied independently by several groups and a negative effect of the SERa dysmorphism in terms of fertilization, embryo quality, implantation, pregnancy rates or perinatal complications has indeed been reported [8]. Moreover, neonatal deaths and major foetal malformations have been described, including one case of a Beckwith-Wiedemann syndrome [9]. In view of these results, a European Alpha-ESHRE Consensus was published in 2011 [10], strongly recommending to discard oocytes affected by the SERa dysmorphism. Since then a recent literature review has nevertheless recorded the birth of 22 apparently healthy babies originating specifically from affected oocytes [8]. The data in the literature is contradictory and there is little information on babies born. We therefore decided to conduct a survey amongst European IVF centres in order to investigate their attitude towards this dysmorphism as well as the impact of the Alpha-ESHRE Consensus on current policies.
Material and methods
The survey was based on a computer format. Coordinates of IVF centres in Europe were obtained through national coordinators, whose names and email addresses were provided by ESHRE. The questionnaire was initially submitted via “Monkey Survey”, the 27th of January 2014, to 969 IVF centres from 28 countries. When several email addresses were available for one centre, the survey was sent to the different addresses. Nine reminders were sent between the 3rd of February and the 14th of March 2014. Croatia (13 centres) was added the 10th of February. The survey was terminated the 16th of March 2014. However, Belgian IVF centres were personally contacted (email or phone) in order to obtain a maximum response rate in our country. The last response from Belgium was received on the 9th of April 2014. A letter addressed to participants at the beginning of the survey, informed them, that there are no right or wrong answers, that no judgement would be made on their answers and that the data obtained would be treated as confidential. The survey was divided into two parts. The first part included six questions on demographic data. Information was obtained from each participating centre concerning, the country were the centre is based, the name of the centre (to avoid numerous responses from the same centre), the background of the person answering the questions, his or her experience in the IVF field and the number of cycles annually performed in the centre. A final question, on the activity of the centre allowed us to distinguish between centres that perform embryo culture from those that do not. Indeed, some centres limit their activity to intra-uterine inseminations or oocyte retrievals with transport of the oocytes to another centre. A second part included eight main and six sub questions concerning the fate of SERa+ oocytes, data collection, pregnancy follow up, information communicated to patients and the impact of the Alpha-ESHRE Consensus on IVF policies towards affected oocytes. Participants were also allowed to add any additional comments.
This survey was approved by our local ethical committee.
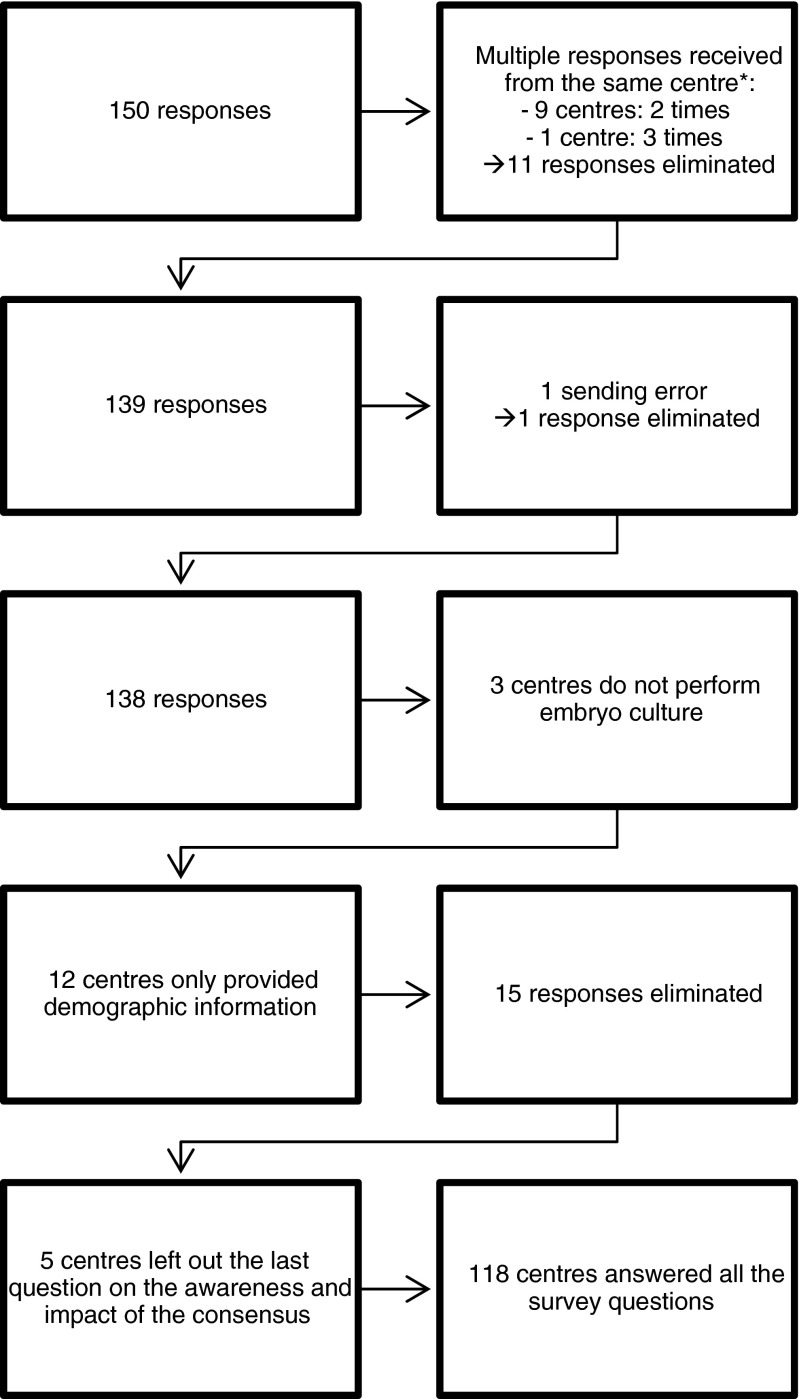
Management of the responses received for the survey is presented in Fig. 1.
Fig. 1.
Flow chart showing how responses to the survey were sorted out.* When multiple answers were obtained from the same centre, the first survey that was fully completed was included
Results
The demographics of participating centres show that the majority (80 %) of them perform less than 1000 cycles a year. Over 86 % of the responses were obtained from embryologists as intended, with an experience in IVF of more than 5 years for 89 % of participants. Amongst the 138 centres who replied to our survey, 135 perform embryo culture. IVF centres from 26 different European countries responded to our survey with a considerable variation of response rates per country. Six countries including; Belgium, France, Spain, the United Kingdom, Germany and Portugal contributed together to 50 % of the total number of answers received. No answer was received for Iceland (one centre), Montenegro (five centres) and Ireland (seven centres). A poor answer rate was obtained from Austria (1/30, 3,3 %), Italy (4/78, 5.1 %) and Hungary (1/17, 5,9 %) but was high in other countries: Slovenia (2/3, 66,7 %), Macedonia (2/3, 66,7 %) and Belgium (18/18, 100 %).
A total of 17 centres out of 123 (13,8 %) discard affected oocytes prior to ICSI or at a later stage of development. Decisions concerning the fate of embryos originating from affected oocytes are presented in Table 1. The majority of SERa+ MII oocytes are included in the ICSI procedure but in more than half of the centres (54 %), transfer of embryos originating from affected oocytes occurs when no other embryo or embryo of sufficient quality is available.
Table 1.
Decisions concerning the fate of affected embryos on the day of embryo transfer
| The day of embryo transfer, an embryo originating from a SERa+ oocyte will be: | n / 123 total answers | % |
|---|---|---|
| Considered for transfer without taking into account the presence of the SERa | 28/123 | 22.8 |
| Only transferred if there are no other embryos or embryos of sufficient quality available for transfer | 66/123 | 53.7 |
| Not transferred in a fresh cycle, but cryopreserved if a good quality embryo is obtained | 4/123 | 3.3 |
| Discarded | 13/123 | 10.6 |
| I don’t know | 4/123 | 3.3 |
| Other (explain) | 8/123 | 6.5 |
Less than half (42.5 %) of the 106 centres who do not eliminate SERa+ oocytes, record and specifically follow up obstetrical and neonatal data. Special pregnancy monitoring is provided in 11 centres (24.4 %). This mainly includes informing the obstetrician as well as more frequent and detailed ultrasounds. Approximately one fourth (30/123) of centres inform patients about SERa, amongst them 53.3 % (16/30) require an informed consent (IC) before transfer of affected embryos. Twenty one centres (17.1 %) reported births from SERa+ embryos, with one declaring a major foetal malformation.
The Alpha-ESHRE Consensus had an impact on management policies of SERa+ oocytes in 56 centres (47.5 %). Forty- eight centres (40.7 %) weren’t influenced, nine (7.6 %) were not aware of the publication, whilst ten either answered “I don’t know” or “other” (4.2 %). The impact of the Alpha-ESHRE Consensus on IVF centres is represented in Table 2. The most important effect of the Alpha-ESHRE Consensus was not to discard affected oocytes (14 centres), but to proceed with ICSI and rather avoid transfer of affected embryos when possible (30 centres).
Table 2.
Impact of the Alpha-ESHRE Consensus on the attitude of IVF centres towards oocytes affected by SERa
| Since the publication of the Alpha-ESHRE Consensus in 2011 concerning embryo assessment (Human Reproduction, Vol 26, No 6, pp. 1270) has your centre’s attitude changed towards the fate of SERa+ oocytes? If yes, in what way? (several answers possible) | 56/118 centres answered yes | |
|---|---|---|
| n / 56 answers | % | |
| We now discard SERa+ oocytes prior to ICSI | 14/56 | 25.0 |
| We now record data and follow up SERa+ oocytes individually | 30/56 | 53.6 |
| We now inform patients about the possible negative outcome of the presence of SERa | 15/56 | 26.8 |
| We now ask patients consent prior to transfer of SERa+ embryos | 6/56 | 10.7 |
| We now record and follow the data of SERa+ oocytes until birth | 9/56 | 16.1 |
| We now transfer SERa+ embryos if there are no other embryos or embryos of sufficient quality for transfer | 30/56 | 53.6 |
| I don’t know | 0/56 | 0.0 |
| Other (explain) | 3/56 | 5.4 |
In Belgium, all centres (18) performing embryo culture answered our survey. Two destroy SERa+ oocytes, seven proceed with embryo transfer without taking into account the presence of the SERa dysmorphism, eight transfer affected embryos only if no other embryo of at least equivalent quality is available and one centre only transfers cryopreserved embryos. Out of the 16 centres that transfer affected embryos, five specifically record obstetrical and neonatal data. No centre informs patients on SERa except one in the case of a transfer cancellation due to no available non affected embryos. Three centres declared births from affected embryos without any reporting malformations. Half the centres were influenced by the Alpha-ESHRE Consensus and the impact was comparable to the rest of Europe.
Discussion
The European survey shows an important heterogeneity in the attitudes of IVF centres towards this dysmorphism and even more in an individual and small country like Belgium. Only a minority of centres systematically destroy affected oocytes as recommended by the Alpha-ESHRE Consensus, whilst 20 % transfer affected embryos without taking into account the presence of this dysmporphism. A variety of less extreme policies are equally observed. For instance, some centres do transfer affected embryos but only when no other good quality non affected embryos are available. This heterogeneity can probably partly be explained by the contradictory data found in the literature. However, some centres were not aware of this dysmorphism, or its impact, whilst others do not record data due to various reasons.
The SER is after mitochondria the most common organelle in the ooplasm. In normal oocytes, two forms are found; vesicular components as well as small aggregates of tubular SER. These aggregates increase during pre-ovulatory maturation and are sensitive to gonadotrophin stimulation [11]. Several studies have observed significantly higher doses of administered gonadotrophins and longer stimulations, when comparing cycles with or without affected oocytes [9, 12, 13]. For patients who display this dysmorphism in a whole cohort of oocytes as well as in subsequent cycles, it is thought that a genetic factor might be involved. Calcium is stored and released by the SER and plays an important role in oocyte maturation, fertilization and embryo development [14, 15]. The presence of the SER dysmorphism has been shown to disturb calcium stores and oscillations [16] which in turn could affect fertilization and embryo development [17]. Generally, SERa are found in metaphase II oocytes and disappear before pronuclear appearance [9]. However, they have also been observed in unfertilized oocytes, aging oocytes and embryos [3, 11, 18]. New evidence demonstrates that the Endoplasmic Reticulum (smooth or rough) plays additional crucial roles in stress responses in the oocyte or embryo [19] as well as in the regulation of the meiotic spindle [20].
Different malformations and pathologies have been described after transfer of an embryo originating from a SERa+ oocyte [21, 22] but equally when a non affected embryo from the same oocyte cohort is transferred [9, 12]. Indeed, some aggregates of smaller size only detectable by electron microscopy could also be pathological [9]. When the Alpha-ESHRE Consensus was published in 2011 [10], it was not clear at that time whether healthy babies had been born from affected oocytes. In 2013, the first births of healthy babies originating from SERa+ oocytes were published by Mateizel and colleagues [23]. A year later, a systematic mini-review of the literature, identified 171 apparently healthy babies from SERa+ cycles with 22 specifically from SERa+ oocytes [8]. Since then a recent publication [13] has added an extra ten healthy births from SERa+ oocytes and our centre 14 (submitted data). The survey shows that one centre out of 21 who transfers SERa+ embryos and follows up the births, reported a malformation.
Interestingly, only half of the centres who responded to our survey were influenced by the Alpha-ESHRE Consensus and amongst those only few scrupulously respect the recommendation to not inseminate SERa+ oocytes. It seems nevertheless, that the general tendency of centres influenced by the publication of the Alpha-ESHRE Consensus is to record data concerning SERa, inform patients and transfer affected embryos only if no other embryo or embryo of equivalent quality is available. Unfortunately, centres that do transfer affected embryos do not always follow up obstetrical and neonatal data. One of the aims of this survey as well as the recent publication of a mini-review [8] is to inform centres on the importance of recording data and publishing the outcomes when affected oocytes are not discarded. Although the birth of healthy babies is encouraging, data is currently lacking to allow an eventual revision of the Alpha-ESHRE Consensus [24]. The systematic recording of such data is the only way to define if there truly is an association between the presence of this dysmorphism and the malformations described by certain studies. Furthermore, in view of the possible implication of epigenetic modifications [9, 16, 25] linked to SERa, follow up of children is mandatory. It would be interesting to follow the fate of all oocytes (with or without dysmorphisms) in order to determine the impact of oocyte morphology on the chances of success in IVF. The collection of obstetrical and neonatal data should be performed in all IVF centres but also at a national and international level. Larger studies would help to clarify the eventual role of SERa in clinical results.
A limited number of centres who transfer affected embryos inform patients about the presence of the dysmorphism and require a specific IC. Centres might consider that a general IC is sufficient since it generally includes the risks associated with the IVF procedure. Certain procedures that are still considered experimental or with an increased risk for patients (artificial oocyte activation, patients with a chronic viral infection, preimplantation genetic diagnosis…) are generally accompanied by a specific IC. In the case of the SER dysmorphism, the fact that the Alpha-ESHRE Consensus advised against using affected oocytes should convince centres who transfer affected embryos to inform patients and obtain a signed consent prior transfer. The current situation concerning SERa is confusing. On the one hand, as discussed above, since the publication of the Alpha-ESHRE Consensus, healthy babies have been born. On the other hand, decreased embryo quality, fertilization and pregnancy rates have been observed in certain studies, but not in others [8]. The initial purpose of an IC, is not to protect doctors from lawsuits but rather to correctly inform patients about the treatment proposed and its associated risks [26]. Informing couples and obtaining their consent does not guarantee though that the patients have understood everything that has been explained to them. In certain cultures, patients trust doctors to do what is best for them. Explaining many details and obtaining consent just before transfer could result in a couple becoming suspicious [26]. Additionally, this could be perceived as very stressful by patients and have a negative impact during their pregnancy. It is important though, that IVF centres willing to obtain patient consent be able to correctly inform couples about the malformation risk associated with this anomaly as well as about the eventuality that pregnancy rates might be reduced when associated with this dysmorphism.
The results of our survey are hampered by several limitations. First of all, the response rate to our survey was very low in some countries and heterogeneously distributed. Nevertheless, we did obtain responses from 26 different European countries and in some countries most IVF centres were covered. Secondly, our study is also limited by the possibility of a misinterpretation of certain questions, of inconsistent answers, of responses distorted by the fear of non-anonymity or by a selection bias. Finally, we assumed that participants are capable of identifying SERa; this however might not be the case for all participants. Despite these limitations, the survey clearly shows that the attitude towards this dysmorphism remains ambiguous and is subject to much debate.
In conclusion, despite a strong recommendation by a group of experts to discard affected oocytes, many IVF centres are transferring affected embryos without adequate data recording and follow up whilst others simply do not take the dysmorphism into account at all. In view of the frequency and recurrence [8] of this anomaly, there is clearly a need to clarify the issue. This would possibly avoid oocyte wastage and more importantly would allow centres to correctly inform couples on their chances of conceiving a healthy child with their own gametes.
Acknowledgments
We thank ESHRE for providing the names and email addresses of national IVF coordinators as well as Chronopoulou Elpiniki for her help in obtaining some IVF centres coordinates.
Conflict of interest
We have nothing to declare.
Footnotes
Capsule IVF centres were surveyed to assess their attitudes towards oocytes affected by the Smooth Endoplasmic Reticulum dysmorphism. Despite a recommendation by the Alpha-ESHRE Consensus to discard these oocytes, many centers are transferring affected embryos without adequate data recording and follow up.
References
- 1.Rienzi L, Ubaldi FM, Iacobelli M, et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90(5):1692–700. doi: 10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12(5):608–15. doi: 10.1016/S1472-6483(10)61187-X. [DOI] [PubMed] [Google Scholar]
- 3.Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990;16(4):324–46. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- 4.Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote. 1995;3(4):283–88. doi: 10.1017/S0967199400002707. [DOI] [PubMed] [Google Scholar]
- 5.Meriano JS, Alexis J, Visram-Zaver S, Cruz M, Casper RF. Tracking of oocyte dysmorphisms for ICSI patients may prove relevant to the outcome in subsequent patient cycles. Hum Reprod. 2001;16(10):2118–123. doi: 10.1093/humrep/16.10.2118. [DOI] [PubMed] [Google Scholar]
- 6.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12(6):1267–70. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 7.Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17(1):34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw-Jackson C, Van Beirs N, Thomas AL, Rozenberg S, Autin C. Can healthy babies originate from oocytes with smooth endoplasmic reticulum aggregates? A systematic mini-review. Hum Reprod. 2014;29:1380–6. doi: 10.1093/humrep/deu101. [DOI] [PubMed] [Google Scholar]
- 9.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19(7):1591–97. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 10.Alpha Scientists in Reproductive Medicine. ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 11.Sathananthan AH. Ultrastructure of the human egg. Hum Cell. 1997;10(1):21–38. [PubMed] [Google Scholar]
- 12.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16(1):113–8. doi: 10.1016/S1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 13.Hattori H, Nakamura Y, Nakajo Y, Araki Y, Kyono K. Deliveries of babies with normal health derived from oocytes with smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2014;31(11):1461–7. doi: 10.1007/s10815-014-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homa ST, Carroll J, Swann K. The role of calcium in mammalian oocyte maturation and egg activation. Hum Reprod. 1993;8(8):1274–81. doi: 10.1093/oxfordjournals.humrep.a138240. [DOI] [PubMed] [Google Scholar]
- 15.Carroll J, Jones KT, Whittingham DG. Ca2+ release and the development of Ca2+ release mechanisms during oocyte maturation: a prelude to fertilization. Rev Reprod. 1996;1(3):137–43. doi: 10.1530/ror.0.0010137. [DOI] [PubMed] [Google Scholar]
- 16.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Tesarik J. Calcium signaling in human preimplantation development: a review. J Assist Reprod Genet. 1999;16(4):216–20. doi: 10.1023/A:1020321024973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makabe S, Naguro T, Nottola SA, Motta PM. Ultrastructural dynamic features of in vitro fertilization in humans. Ital J Anat Embryol. 2001;106(2 Suppl 2):11–20. [PubMed] [Google Scholar]
- 19.Latham KE. Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes. Cell Tissue Res. 2015. doi:10.1007/s00441-015-2124-9. [DOI] [PMC free article] [PubMed]
- 20.De Santis L, Gandolfi F, Pennarossa G, et al. Expression and intracytoplasmic distribution of staufen and calreticulin in maturing human oocytes. J Assist Reprod Genet. 2015. doi:10.1007/s10815-015-0437-y. [DOI] [PMC free article] [PubMed]
- 21.Akarsu C, Cağlar G, Vicdan K, Sözen E, Biberoğlu K. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;92(4):1496.e1–1496.e13. doi: 10.1016/j.fertnstert.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 22.Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96(1):143–9. doi: 10.1016/j.fertnstert.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 23.Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28(8):2111–7. doi: 10.1093/humrep/det241. [DOI] [PubMed] [Google Scholar]
- 24.Ebner T, Shebl O, Oppelt P. Delivery of normal babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28(10):2880. doi: 10.1093/humrep/det318. [DOI] [PubMed] [Google Scholar]
- 25.Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128(6):917–28. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- 26.Macklin R. Informed consent, and assisted reproduction. Ethics, informed consent, and assisted reproduction. J Assist Reprod Genet. 1995;12(8):484–90. [DOI] [PubMed]