Abstract
Purpose
Total body mass impacts reproductive health and infertility which has increased in the United States with rising rates of obesity. Overlapping genetic and environmental factors contribute to obesity and infertility including the androgen receptor (AR), a steroid hormone-activated transcription factor that is key in regulating androgen activity and sensitivity to sex hormones, weight and body composition in both males and females. The AR gene which is X-linked contains a polymorphic CAG trinucleotide repeat which varies in length and inversely correlated with gene expression.
Methods
We examined the AR gene CAG repeat length and measures of weight and body mass index (BMI) in 27 non-syndromic obese and 33 lean controls and for the first time compared with 28 individuals with Prader-Willi syndrome (PWS), a rare obesity-related genetic disorder with natural sex hormone deficits to examine the effects of AR gene CAG repeat length on androgen-mediated response and obesity-related factors relevant to human infertility and reproduction.
Results
Mean CAG repeat length in base pairs (278 ± 7.9) did not significantly differ by subject group (F = 2.6, p = 0.08) but was strongly positively correlated with height standard deviation (SD) among males (r = 0.31, p < 0.05), mainly lean and obese, but not PWS (r = 0.02, p = 0.94). A negative correlation was observed for weight SD among females (r = −0.29, p < 0.04) when grouped together.
Conclusions
The results were consistent with an androgen-mediated effect on height and weight negligible in PWS and supporting the role of sex horomones and AR gene interaction in obesity and infertility, both cardinal features of PWS. CAG repeat length of the AR gene is a marker for increased androgen sensitivity with shorter lengths predicting smaller stature in non-PWS adult males possibly due to accelerating fusion of bone growth plates and reducing the growth phase. Increased androgen effects from shorter CAG repeat lengths in non-PWS females could impact pregnancy-related weight gain and pregnancy outcomes.
Keywords: Androgen receptor (AR) gene, CAG repeat lengths, Obesity, Height, Weight, Body mass index (BMI), Prader-Willi syndrome, Lean, Androgen sensitivity, Testosterone, Fertility
Introduction
About 15 % of all women in the United States are infertile with advanced age playing a role [1, 2]. In addition to age, infertility is related to body mass and weight with over 50 % of adults in westernized societies classified as overweight. Three out of every 10 individuals are obese with a body mass index (BMI) greater than or equal to 30 [1–5] with a life expectancy shortened by 14 years [6]. Obesity not only influences fertility status but also impacts on pregnancy outcomes [7, 8].
Increased weight and obesity status produces physical and biochemical changes including hormone disturbances, preeclampsia, gestational diabetes, fetal growth failure and premature delivery [7, 8]. Obesity and infertility are both influenced by genetic and environmental factors with over 370 obesity genes and 153 infertility and reproductive genes reported [9]. There are over 40 genes including the androgen receptor (AR) gene that plays a role in polycystic ovarian syndrome (PCOS), an endocrine condition that affects 5–10 % of women [9–14]. The androgen receptor is a steroid hormone-activated transcription factor key to regulating androgen activity in both males and females and responsible for secondary sexual characteristics, sex hormone responses and infertility in both sexes. It has been shown to influence body mass and composition [10, 15–19].
The gene coding the androgen receptor is X-linked and localized to the Xq11.2-q12 chromosome region. A polymorphic CAG trinucleotide repeat is located in the AR gene and varies in length from 8 to 35 repeats in normal individuals. An inverse correlation exists between the CAG repeat length and receptor expression impacting on the strength of the androgen actions and sensitivity to sex hormones. Individuals with a lower number of CAG repeats exhibit higher AR gene expression levels and generate more functional AR receptors increasing their sensitivity to testosterone [20–24]. Diminished in vivo androgenicity in males leads to decreased spermatogenesis and smaller seminal vesicles [24–27] thereby impacting fertility and human reproduction [28]. AR gene CAG repeat lengths above 21 correlate with poor pregnancy outcomes in females and recurrent spontaneous abortions [29]. Androgen receptor gene CAG repeat length is also associated with ovarian reserve and may play a role in ovulation but the natural ovarian aging process does not appear to affect ovarian response to gonadotropins [30]. A paucity of studies exists in measuring the effects of the number of CAG repeats in disease (non-oncology) states and impact on body composition but boys with the lower range of AR gene CAG repeat length versus those with intermediate or high repeat lengths had more intra-abdominal fat but not subcutaneous-abdominal fat [20].
Several rare genetic syndromes share obesity and infertility phenotypes with the most classical being Prader-Willi syndrome (PWS) which is recognized as the most common cause of life-threatening obesity in children [31]. PWS is due to loss of paternally expressed genes located in the 15q11–q13 chromosome region, generally from a paternal de novo cytogenetic deletion in about 70 % of cases followed by maternal disomy 15 (both chromosome 15s inherited from the mother) in about 25 % of cases and the remaining individuals with imprinting defects influencing gene expression in the 15q11-q13 region [32–35].
Prader-Willi syndrome is characterized by infantile hypotonia, a poor suck and feeding difficulties, hypogonadism/hypogenitalism and infertility, hormone imbalances including growth hormone deficiency with short stature and small hands/feet, cognitive and behavioral problems and food seeking with hyperphagia in early childhood leading to marked obesity, if not controlled [31–35]. These individuals have low testosterone levels in males and low estrogen levels in females with arrested pubertal development [34]. Natural deficits in sex hormones in both PWS males and females makes them an ideal negative control subject group to examine the effects of AR gene CAG repeat lengths on androgen-mediated responses.
Herein, we examined the AR gene triplet CAG repeat length polymorphism and body composition measures (e.g., height, weight, BMI) in a cohort of non-syndromic obese and healthy lean controls in relationship for the first time in individuals with Prader-Willi syndrome. PWS individuals have obesity and sex hormone disturbances in common. Our goal was to assess the influence of the triplet repeats on obesity-related factors with relevance to human infertility and reproduction.
Materials and method
Subjects
We studied 88 individuals (40 males, 48 females) including 33 with leanness (17 males, 16 females), 27 with obesity (17 males, 10 females), and 28 without growth hormone treatment and genetically confirmed with Prader-Willi syndrome (13 males, 15 females). The age range of participants was 2 to 47 years with an average (±SD) age of 19.0 (±10.2) years (Table 1). Fifteen of the PWS subjects had the 15q11–q13 deletion and 13 had maternal disomy 15/imprinting defects. All participants consented to study investigation approved by the local human subjects institutional review board.
Table 1.
Subject characteristics, growth data and androgen receptor gene CAG repeat length
| Parameter | Lean | Obese | Prader-Willi Syndrome | F test | p-value |
|---|---|---|---|---|---|
| All participants | N = 33 | N = 27 | N = 28 | ||
| Mean age (SD) | 19.6 (9.9) | 18.3 (10.8) | 19.2 (10.2) | 0.12 | 0.89 |
| Mean height (cm) (SD) | 158.6 (21.4) | 152.5 (20.1) | 140.4 (20.8) | 5.9 | 0.004* |
| Mean weight (kg) (SD) | 52.0 (17.0) | 80.5 (40.4) | 61.9 (27.1) | 7.3 | 0.001** |
| Mean BMI (SD) | 19.9 (2.6) | 33.2 (11.2) | 29.3 (8.8) | 21.7 | 0.001*** |
| Mean CAG (SD) | 281 (8.9) | 276 (7.4) | 277 (6.7) | 2.6 | 0.08 |
| Male subjects | N = 17 | N = 10 | N = 13 | ||
| Mean age (SD) | 20.3 (9.0) | 18.3 (15.2) | 20.5 (9.6) | 0.15 | 0.86 |
| Mean height (cm) (SD) | 165.3 (21.1) | 156.5 (22.7) | 143.8 (20.9) | 3.7 | 0.03* |
| Mean weight (kg) (SD) | 58.5 (18.4) | 87.6 (54.3) | 68.8 (29.2) | 2.3 | 0.10 |
| Mean BMI (SD) | 20.6 (2.9) | 34.4 (16.0) | 31.5 (8.7) | 8.2 | 0.001*** |
| Mean CAG (SD) | 280 (9.3) | 275 (10.5) | 276 (8.0) | 1.3 | 0.30 |
| Female subjects | N = 16 | N = 17 | N = 15 | ||
| Mean age (SD) | 18.7 (11.0) | 18.2 (9.6) | 18.1 (11.0) | 0.02 | 0.98 |
| Mean height (cm) (SD) | 151.5 (20.0) | 150.2 (18.7) | 137.5 (21.0) | 2.3 | 0.10 |
| Mean weight (kg) (SD) | 45.1 (12.4) | 76.3 (30.7) | 56.0 (24.7) | 7.2 | 0.002** |
| Mean BMI (SD) | 19.2 (2.0) | 32.5 (7.5) | 27.4 (8.7) | 16.4 | <0.0001*** |
| Mean CAG (SD) | 281 (8.6) | 277 (4.9) | 278 (5.6) | 1.5 | 0.23 |
*Post hoc Bonferroni test indicates a significant difference (p < 0.05) between PWS and lean individuals
**Post hoc Bonferroni test indicates a significant difference (p < 0.05) between obese and lean individuals
***Post hoc Bonferroni test indicates a significant difference (p < 0.05) between both PWS and obese individuals compared to lean
Androgen receptor gene assay
DNA was isolated from peripheral blood as previously described. The number of CAG repeats of the AR gene was determined by using 200 ng of DNA and polymerase chain reaction (PCR) with forward and reverse DNA primers. Each amplified PCR fragment was separated using capillary electrophoresis and an ABI 3100 DNA sequencer (Applied Biosystems, Carlsbad, CA) as previously described [36, 37]. The CAG repeat region is located in the amplified region and length differences reflected CAG repeat sizes. About 90 % of women are known to have an AR gene polymorphism (i.e., have two different AR gene CAG repeat lengths representing each X chromosome per cell) [38].
Statistical analysis
Subject characteristics, growth data and AR gene CAG repeat length are presented as mean ± standard deviation in Table 1. Mean CAG repeat length for females is the mean of the average repeat length from both X chromosomes in females. One-way analysis of variance (ANOVA) with Bonferroni correction was used to test for group differences in age, height, weight and body mass index (BMI). Standard deviational (SD) units were calculated for height and weight to control for variation related to age and normative height and weight data. Findings with p-values of <0.05 were considered significant. Pearson correlation was used to examine the relationship between CAG repeat length and height, weight and BMI for each subject group (lean, obese, PWS) and by gender. Linear regression analysis was used to evaluate the influence of condition and repeat length on height SD, weight SD and BMI, overall and by gender. Statistical analyses including descriptive statistics and Pearson correlations were generated using SAS statistical analysis software version 9.4 (SAS Inc., Cary, NC).
Results
Table 1 shows the subject characteristics for age, height, weight and BMI measures with mean AR CAG repeat lengths in base pair size by condition (lean, obese, and PWS) and gender. PWS subjects were generally shorter and obese individuals were heavier than those with leanness. Post-hoc analysis showed that PWS subjects had significantly lower height SD compared to lean and obese individuals (F = 38.0, df = 2, p < 0.0001) while obese individuals had a significantly greater weight SD than PWS or lean individuals (F = 24.6, df = 2, p < 0.0001). These relationships were independent of the influence of gender. Age was significantly correlated with height, weight and BMI.
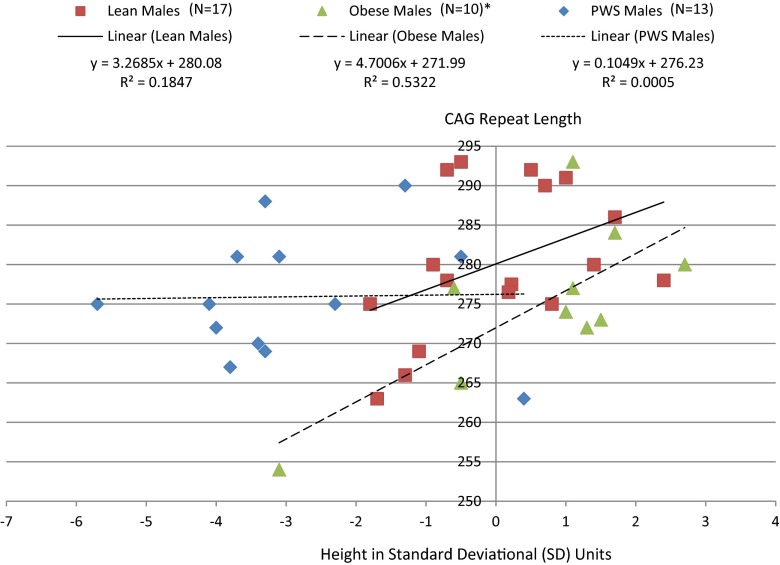
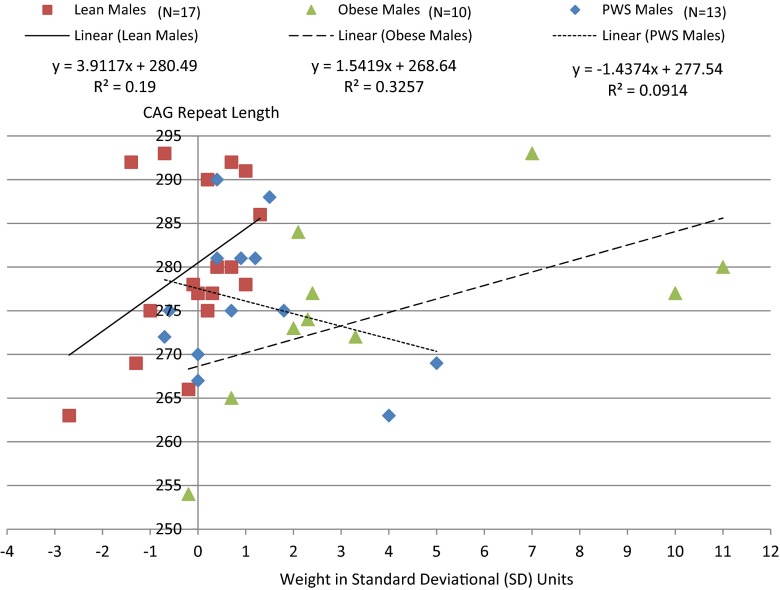
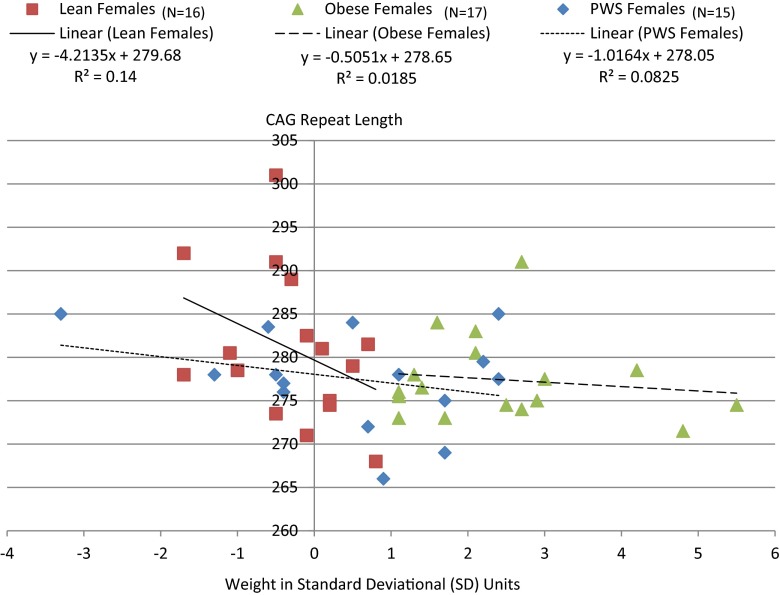
AR gene CAG repeat lengths were significantly positively correlated with height SD (r = 0.31, p < 0.05) among males which appeared to be driven by effects in both lean (r = 0.43, p = 0.08) and obese (r = 0.73, p < 0.02) males but not in PWS (r = 0.02, p = 0.94; Fig. 1). This relationship was supported by linear regression modeling of height SD in males which was significant (F = 18.0, p < 0.0001) and showed a significant association between both subject groups (F = 23.9, p < 0.0001) and CAG repeat length (F = 6.3, p < 0.02) among male subjects. As shown in Fig. 2, similar subgroup effects were noted for weight SD in lean and obese but not in PWS males. A negative correlation was also observed between CAG repeat length and weight SD among females (r = −0.29, p < 0.04) when group together and not observed by group (Fig. 3).
Fig. 1.
Correlation between the AR gene CAG repeat length and height in lean, obese and Prader-Willi syndrome males. *Pearson correlation for obese males alone (r = 0.73, p < 0.02); lean males alone (r = 0.43, p = 0.08); PWS males alone (r = 0.02, p = 0.94). Pearson correlation for all combined males (r = 0.31, p < 0.05)
Fig. 2.
Correlation between the AR gene CAG repeat length and weight in lean, obese and Prader-Willi syndrome males. Pearson correlation for obese males alone (r = 0.57, p = 0.08); lean males alone (r = 0.44, p = 0.08); PWS males alone (r = -0.30, p = 0.32). Pearson correlation for all combined males (r = 0.10, p = 0.55)
Fig. 3.
Correlation between the AR gene CAG repeat length and weight in lean, obese and Prader-Willi syndrome females. Pearson correlation for obese females alone (r = -0.14, p = 0.60); lean females alone (r = -0.37, p = 0.15); PWS females alone (r = -0.29, p = 0.30). Pearson correlation for all combined females (r = -0.29, p = 0.04)
Discussion
The number of trinucleotide CAG repeats of the androgen receptor gene in our study predicted stature among both obese and lean males but not in PWS participants. AR gene CAG repeat lengths among PWS males who are devoid of androgen response showed no relationship to height or weight implicating a primary influence of testosterone as a basis for the difference. Similarly, CAG repeat length in females was inversely related to weight and of interest in the study of human infertility and reproduction impacting maternal pregnancy weight gain and outcomes.
Furthermore, studies in healthy adult males reported by Zitzmann et al. [19] found a positive correlation in the CAG repeat length and body fat content measured by bioimpedance and supported by serum leptin and insulin levels. Pausova et al. [20] also investigated CAG repeat size and found an association with intra-abdominal adiposity, sympathetic modulation of vasomotor tone on heart rate and blood pressure in a cohort of adolescent boys and girls. They reported that intra-abdominal and subcutaneous fat when determined by magnetic resonance imaging (MRI) in males with a lower number of CAG repeat lengths had higher intra-abdominal fat but not subcutaneous fat in those with intermediate or high CAG repeat numbers. Boys with lower CAG repeat numbers had higher blood pressure and higher sympathetic modulation measures of vasomotor tone but no differences were seen among the girls. CAG repeat numbers were also strongly associated with central obesity in older healthy Caucasian male and post-menopausal female adults, particularly in women reported by Gustafson et al. [18] using waist and waist-to-hip measures as study parameters.
Our results paralleled the findings reported by Voorhoeve et al. [17] with an inverse association of CAG repeat length and longitudinal height before and during puberty in young Dutch boys. These studies suggested that longer CAG repeat lengths may decrease testosterone sensitivity and impact levels. This may allow for a longer growth phase and maturation cycle thereby leading to greater height in adulthood which could directly or indirectly impact fertility status. Campbell et al. [39] reported data on androgen receptor gene CAG repeats and body composition measures in Ariaal men from Kenya who were pastoral nomads. They inhabited both upland and lowland regions. They found in this population that CAG repeat length was a significant positive predictor for height, fat free mass, percentage body fat determined by four skinfold measures and waist circumference. The Ariaal men with shorter CAG repeat lengths (<20 CAG repeats) also exhibited significantly greater waist circumferences and waist to hip ratios; fatness patterns more commonly seen in men compared with women. Overall, the Ariaal men sampled were quite lean with an average body fat of 10 %.
In women, polycystic ovary syndrome (PCOS) is a classical obesity-related disorder characterized by menstrual irregularities, obesity, hyperandrogenism and infertility. Obesity is seen in 30–75 % of women with this infertility disorder and over 40 genes have been implicated in the hormonal and metabolic derangements seen in this syndrome [9–14]. Some of these genes are involved in both hormone and related protein production. These include leptin, luteinizing, follicle stimulating and growth hormones, testosterone, estrogen and their related receptors, all playing a role in the development of obesity and infertility in humans. Obesity impacts insulin and insulin-like growth factor levels which can enhance lutenizing hormone production and mediated steroidogenesis in the ovary, thereby increasing production of ovarian androgens [24, 26] leading to hormone imbalances and infertility in women with obesity [40–43]. An increased BMI further reduces the conception rate in females due to higher doses of gonadotrophins required that respond more poorly to ovarian stimulation.
The interpretation of study findings is limited by the small sample size and lack of body composition measurements (e.g., %fat, lean mass and bone density utilizing dual-energy x-ray absorptiometry-DEXA), sex hormone levels and documented infertility status needed to appropriately characterize the relationship between AR gene polymorphisms on BMI, obesity and infertility. Understanding the regulatory role of certain genes and molecular mechanisms contributing to both obesity and infertility in the general population such as the AR gene will increase recognition of at-risk individuals at a young age. This may lead to better management options and eventually prevention strategies for obesity and possibly lessen infertility. Androgen responsivity secondary to AR gene CAG repeat length has a multi-level influence on human reproduction and fertility through effects on male testicular development and spermatogenesis [27, 28] and female pregnancy outcomes [29]. Our study further reports male stature and female weight as androgen responsive modulatory variables with potential influence on fertility. Additional research is needed with larger sample sizes and more measures of body composition (e.g., %fat, lean mass and bone density utilizing dual-energy x-ray absorptiometry-DEXA), sex hormone levels and documented infertility status to characterize the role of AR gene polymorphisms on BMI, obesity and infertility. Our study in the PWS cohort with cardinal features of obesity, sex hormone disturbance and infertility in both PWS males and females but with no difference in CAG repeat lengths further supports the role of sex hormones and the AR gene interaction in relationship with body composition measures and infertility relevant to the general population.
Acknowledgments
We thank Carla Meister and Devin Cox, MS for expert preparation of the manuscript.
Funding
Partial funding support was provided by the Prader-Willi Syndrome Association (USA), the Headley Family Scholarship and the National Institute of Child Health and Human Development (NICHD) grant HD02528.
Conflict of interest
The authors have no conflicts to disclose.
Footnotes
Capsule The number of polymorphic CAG repeats of the androgen receptor (AR) gene impacts androgen activity and sensitivity to sex hormones influencing weight and body composition for both males and females thereby influencing obesity and infertility rates. The number of CAG repeats impacted male stature and female weight in control subjects. However, individuals with Prader-Willi syndrome, a known genetic cause of obesity, growth hormone deficiency and hypogonadism with sex hormone deficits did not show correlations with the CAG repeat size, further supporting AR gene interaction or impact on measures of stature, weight and possibly fertility on the general population.
References
- 1.Marsh CA, Hecker E. Maternal obesity and adverse reproductive outcomes: reducing the risk. Obstet Gynecol Surv. 2014;69:622–8. doi: 10.1097/OGX.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 2.Silber SJ, Barbey N. Scientific molecular basis for treatment of reproductive failure in the human: an insight into the future. Biochim Biophys Acta. 1822;2012:1981–96. doi: 10.1016/j.bbadis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Pandey S, Pandey S, Maheshwari A, Bhattacharya S. The impact of female obesity on the outcome of fertility treatment. J Hum Reprod Sci. 2010;3:62–7. doi: 10.4103/0974-1208.69332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 6.Kitahara CM, Flint AJ, Berrington De Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11:e1001673. doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungheim ES, Travieso JL, Carson KR, Moley KH. Obesity and reproductive function. Obstet Gynecol Clin N Am. 2012;39:479–93. doi: 10.1016/j.ogc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kort JD, Winget C, Kim SH, Lathi RB. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil Steril. 2014;101:1400–3. doi: 10.1016/j.fertnstert.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Butler MG, McGuire A, Manzardo A. Clinically relevant known and candidate genes for obesity and their overlap with human infertility and reproduction. J Assist Reprod Genet. 2015;32:495–508. doi: 10.1007/s10815-014-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–9. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- 11.Barber TM, Franks S. Genetics of polycystic ovary syndrome. Front Horm Res. 2013;40:28–39. doi: 10.1159/000341682. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh T, Suresh PS, Tsutsumi R. New insights into the genetic basis of infertility. Appl Clin Genet. 2014;7:235–43. doi: 10.2147/TACG.S40809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skrgatic L, Baldani DP, Cerne JZ, Ferk P, Gersak K. CAG repeat polymorphism in androgen receptor gene is not directly associated with polycystic ovary syndrome but influences serum testosterone levels. J Sterioid Biochem Mol Biol. 2012;128:107–12. doi: 10.1016/j.jsbmb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Peng CY, Xie HJ, Guo ZF, Nie YL, Chen J, Zhou JM, et al. The association between androgen receptor gene CAG polymorphism and polycystic ovary syndrome: a case–control study and meta-analysis. J Assist Reprod Genet. 2014;31:1211–9. doi: 10.1007/s10815-014-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saute JA, Silva AC, Souza GN, Russo AD, Donis KC, Vedolin L, et al. Body mass index is inversely correlated with the expanded CAG repeat length in SCA3/MJD patients. Cerebellum. 2012;11:771–4. doi: 10.1007/s12311-011-0326-6. [DOI] [PubMed] [Google Scholar]
- 16.Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–53. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 17.Voorhoeve PG, van Mechelen W, Uitterlinden AG, de Waal HA D-v, Lamberts SW. Androgen receptor gene CAG repeat polymorphism in longitudinal height and body composition in children and adolescents. Clin Endocrinol. 2011;74:732–5. doi: 10.1111/j.1365-2265.2011.03986.x. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson DR, Wen MJ, Koppanati BM. Androgen receptor gene repeats and indices of obesity in older adults. Int J Obes Relat Metab Disord. 2003;27:75–81. doi: 10.1038/sj.ijo.0802191. [DOI] [PubMed] [Google Scholar]
- 19.Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46:31–9. doi: 10.1007/s00125-002-0980-9. [DOI] [PubMed] [Google Scholar]
- 20.Pausova Z, Abrahamowicz M, Mahboubi A, Syme C, Leonard GT, Perron M, et al. Functional variation in the androgen-receptor gene is associated with visceral adiposity and blood pressure in male adolescents. Hypertension. 2010;55:706–14. doi: 10.1161/HYPERTENSIONAHA.109.146720. [DOI] [PubMed] [Google Scholar]
- 21.Mohlig M, Arafat AM, Osterhoff MA, Isken F, Weickert MO, Spranger J, et al. Androgen receptor CAG repeat length polymorphism modifies the impact of testosterone on insulin sensitivity in men. Eur J Endocrinol. 2011;164:1013–8. doi: 10.1530/EJE-10-1022. [DOI] [PubMed] [Google Scholar]
- 22.Beilin J, Ball EM, Favaloro JM, Zajac JD. Effect of the androgen receptor CAG repeat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines. J Mol Endocrinol. 2000;25:85–96. doi: 10.1677/jme.0.0250085. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–6. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82:3777–82. doi: 10.1210/jcem.82.11.4385. [DOI] [PubMed] [Google Scholar]
- 25.Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen receptor: possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet. 1995;4:523–7. doi: 10.1093/hmg/4.4.523. [DOI] [PubMed] [Google Scholar]
- 26.Albertelli MA, Scheller A, Brogley M, Robins DM. Replacing the mouse androgen receptor with human alleles demonstrates glutamine tract length-dependent effects on physiology and tumorigenesis in mice. Mol Endocrinol. 2006;20:1248–60. doi: 10.1210/me.2006-0021. [DOI] [PubMed] [Google Scholar]
- 27.Eberhard J, Stahl O, Giwercman Y, Cwikiel M, Cavallin-Stahl E, Lundin KB, et al. Impact of therapy and androgen receptor polymorphism on sperm concentration in men treated for testicular germ cell cancer: a longitudinal study. Hum Reprod. 2004;19:1418–25. doi: 10.1093/humrep/deh231. [DOI] [PubMed] [Google Scholar]
- 28.Ruhayel Y, Lundin K, Giwercman Y, Hallden C, Willen M, Giwercman A. Androgen receptor gene GGN and CAG polymorphisms among severely oligozoospermic and azoospermic Swedish men. Hum Reprod. 2004;19:2076–83. doi: 10.1093/humrep/deh349. [DOI] [PubMed] [Google Scholar]
- 29.Aruna M, Dasgupta S, Sirisha PV, Andal Bhaskar S, Tarakeswari S, Singh L, et al. Role of androgen receptor CAG repeat polymorphism and X-inactivation in the manifestation of recurrent spontaneous abortions in Indian women. PLoS ONE. 2011;6:e17718. doi: 10.1371/journal.pone.0017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lledo B, Llacer J, Turienzo A, Ortiz JA, Guerrero J, Morales R, et al. Androgen receptor CAG repeat length is associated with ovarian reserve but not with ovarian response. Reprod BioMed Online. 2014;29:509–15. doi: 10.1016/j.rbmo.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–32. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler MG, Palmer CG. Parental origin of chromosome 15 deletion in Prader-Willi syndrome. Lancet. 1983;1:1285–6. doi: 10.1016/S0140-6736(83)92745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittel DC, Butler MG. Prader-Willi syndrome: clinical genetics, cytogenetics and molecular biology. Exp Rev Mol Med. 2005;7:1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler MG, Lee PDK, Whitman BY. Management of Prader-Willi Syndrome. 3rd ed. MG Butler, PDK Lee, BY Whitman (eds.) New York: Springer-Verlag Publishers; 2006.
- 35.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 36.Butler MG, Theodoro MF, Bittel DC, Kuipers PJ, Driscoll DJ, Talebizadeh Z. X-chromosome inactivation patterns in females with Prader-Willi syndrome. Am J Med Genet A. 2007;143:469–75. doi: 10.1002/ajmg.a.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bittel DC, Theodoro MF, Kibiryeva N, Fischer W, Talebizadeh Z, Butler MG. Comparison of X chromosome inactivation patterns in multiple tissues from human females. J Med Genet. 2008;45:309–13. doi: 10.1136/jmg.2007.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plenge RM, Stevenson RA, Lubs HA, Schwartz CE, Willard HF. Skewed X-chromosome inactiviation is a common feature of X-linked mental retardation disorders. Am J Hum Genet. 2002;71:168–73. doi: 10.1086/341123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell BC, Gray PB, Eisenberg DT, Ellison P, Sorenson MD. Androgen receptor CAG repeats and body composition among Ariaal men. Int J Androl. 2009;32:140–8. doi: 10.1111/j.1365-2605.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 40.Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev. 2007;8:515–23. doi: 10.1111/j.1467-789X.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 41.Balen AH, Platteau P, Andersen AN, Devroey P, Sorensen P, Helmgaard L, et al. The influence of body weight on response to ovulation induction with gonadotrophins in 335 women with world health organization group II anovulatory infertility. Int J Obstet Gynecol. 2006;113:1195–202. doi: 10.1111/j.1471-0528.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 42.Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306:484–7. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology–a systematic review. Hum Reprod Update. 2007;13:433–44. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]