Abstract
Purpose
We aimed to investigate the effects of FSH for promoting spermatogenesis in mice with low-dose doxorubicin-induced spermatogenesis impairment.
Methods
Eight-wk-old male imprinting control region mice were divided into three groups. Groups D and F received 0.5 mg/kg of doxorubicin twice weekly for 5 weeks. Group C received saline instead of doxorubicin. After inducing spermatogenesis impairment, group D was treated daily with saline for 4 weeks. Group F was given 1 IU of recombinant human FSH daily for 4 weeks. Spermatogenesis recovery was evaluated based on the testis weight, sperm count, histological assessment, and mating. The percentage of sperm with unfragmented deoxyribonucleic acid (DNA) was analyzed by single-cell pulsed-field gel electrophoresis, and the serum FSH levels were measured.
Results
The elevation of serum FSH advanced slowly. The testis weight, sperm count, percentage of seminiferous tubules with spermatogenesis, percentage of sperm with unfragmented DNA and pregnancy rate were significantly increased by the administration of FSH.
Conclusion
Our study findings indicated that the immediate administration of exogenous FSH can promote the recovery from impaired spermatogenesis induced by low-dose doxorubicin before endogenous FSH increases to the maximum level.
Keywords: Chemotherapy, Doxorubicin, FSH, Spermatogenesis impairment
Introduction
The increasing success rate of cancer treatment has improved long-term survival and patients’ quality of life. However, gonadotoxicity is a frequent adverse effect of cancer treatment. The two most common diagnoses in younger age groups are testicular tumor and hematopoietic malignancy. In previous testicular tumor cases, 56 and 20 % of patients did not recover normal sperm count after 1 and 3–5 years, respectively [1]. In lymphoma, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone chemotherapy using cyclophosphamide, doxorubicin, vincristine and prednisone caused azoospermia in almost 100 % of patients, one-third of whom still had the disorder after 5 years [2]. The only method for extracting sperm from azoospermia patients is microdissection testicular sperm extraction. However, in a report by Hsiao et al., the sperm retrieval rate was 37 %, which decreased when the patients received alkylating agents [3]. Henceforth, sperm cryopreservation has been considered the standard pretreatment procedure. However, this option is not available in emergency or prepubertal cases. In fact, 60 % of hematopoietic malignant patients who were referred to our institution underwent remission reduction therapy without a prior cryopreservation procedure (data not published), and most of them had worsening semen analysis results. Moreover, although the exact cause has not been well established, oligozoospermia occurs more frequently in patients with cancer than in healthy men [3, 4]. In our experience, semen cryopreservation is not feasible in patients with poor semen quality even in those who have not yet undergone chemotherapy.
Several trials have reported on the use of hormonal treatment for enhancing the recovery from radiation- or chemical-induced spermatogenesis impairment in animal models. For example, gonadotropin-releasing hormone (GnRH) and exogenous testosterone are commonly used [5–7]; however, these hormones are associated with side effects such as a decreased libido and risk of impaired spermatogenesis caused by negative feedback. In healthy animals, the follicle-stimulating hormone (FSH) and testosterone regulate and play valuable roles in spermatogenesis. In rodents, FSH influences the development of spermatogonia by controlling their survival mechanisms [8]. The activities of FSH and testosterone are important for meiosis progression and spermiation [9]. However, the use of FSH and luteinizing hormone (LH) under a high plasma gonadotropin level is ineffective, because the receptors in the Sertoli and Leydig cells lose their sensitivities [10]. To the best of our knowledge, there are no studies on the effect of FSH therapy on chemically induced spermatogenesis disorders.
Furthermore, in most models, testicular damage is induced by the single administration of a high-dose chemoagent, and the hormones are administered preventively. Doxorubicin (DXR) is an anthracycline antibiotic anticancer agent that is widely used in the treatment of several malignant diseases such as hematopoietic malignancy. The mechanism of action of DXR is the suppression of deoxyribonucleic acid (DNA) synthesis by inhibiting polymerase activity and consequently interfering with cell proliferation. Additionally, other mechanisms such as increasing oxidative stress and inducing cellular apoptosis are known to cause testicular damage [11].
We aimed to investigate whether FSH therapy stimulates the regeneration of spermatogenesis in mice after exposing them to low-dose continuous regimen of DXR in order to induce chronic and reversible spermatogenesis dysfunction. In addition, we analyzed male fertility by assessing sperm quality via DNA fragmentation and mating experiments.
Materials and methods
Reagents
We used DXR (Sandoz Co., Tokyo, Japan), recombinant human FSH (MSD Co., Tokyo, Japan).
The DXR treatment duration and dose were based on a previous report [12], and the findings from our preliminary study showed that DXR causes severe but not irreversible damage to spermatogenesis.
The recombinant human FSH (rhFSH) dose was determined from a previous dose response study in hypogonadal mice [13]. Although their animal model was different from that used in our experiment, they demonstrated that at least 1 IU daily of a FSH injection can stimulate spermatogenesis. Thus, we chose 1 IU daily as our administration dose.
Animals
Eight-wk-old male imprinting control region (ICR) mice weighing 33–35 g were purchased from Sankyo Laboratory (Tokyo, Japan), and they were housed in wire-mesh cages (5 animals per cage) under controlled lighting conditions (12-h light/dark cycle) at a temperature of 20–24 °C. Food and water were provided ad libitum. All the animal housing and surgical procedures were performed in accordance with the guidelines of the institutional animal care and use committee of the Animal Research and Care Committee of the Tokyo Dental College.
Treatment protocol
The animals were divided into three groups (Fig. 1). All the mice, except the controls, were treated intraperitoneally with 0.5 mg/kg of DXR twice weekly (Mondays and Thursdays) for 5 weeks. The control mice received 0.5 mL/kg of saline.
Fig. 1.
Experimental design and group composition. DXR, doxorubicin; F, follicle-stimulating hormone
Group C was the control group with 32 mice that were only treated with saline. Group C was subdivided into four groups (eight mice each). Group C1 received 0.5 mL/kg of saline twice weekly for 5 weeks and was sacrificed to assay hormones. Groups C2, C3, and C4 were treated with saline daily for 4 weeks after the 5 weeks of saline injections. Groups C2 and C3 were sacrificed in the 9th and 13th wk, respectively, to assay hormones. Group C4 was sacrificed in the 21st wk to assess spermatogenesis.
Group D (32 mice) was subdivided into four groups (eight mice each) and received the same regimen of DXR treatment for 5 weeks. Group D1 was sacrificed in the 5th wk to assay hormones. Groups D2 and D3 received saline injections daily for 4 weeks after DXR treatment, and they were sacrificed in the 9th and 13th wk to assay hormones. Group D4 received the same regimen of groups D2 and D3 but was sacrificed in the 21st wk to evaluate spermatogenesis.
Group F (24 mice) was subdivided into three groups (eight mice each) and was treated with the same regimen of DXR for 5 weeks followed by 1 IU of rhFSH daily for 4 weeks. Groups F1 and F2 were sacrificed in the 9th and 13th wk, respectively, to evaluate the hormones, and group F3 was sacrificed in the 21st wk after the mating experiments.
Histological analysis was performed at 21st wk to have enough intervals after DXR treatment (>35 days is the spermatogenesis cycle of mice) [14]. Additionally, we evaluated the recovery of spermatogenesis at this point, according to a previous report that use the same DXR treatment protocol in which the difference in the pregnancy rate was observed [12].
In the mating experiments, each male of groups C4, D4, and F3 was cohabited with two 8-weeks-old ICR virgin female mice for 5 days, starting from 20 week after the initiation of the experiment. The cohabited female mice were sacrificed 2 weeks later and were observed for possible pregnancy, and the implantation rate. The pregnancy rate was calculated as the number of pregnant females per the total number of females in each group (16 females), and the percentage of implantation was calculated as the number of implantations per the number of corpora luteum.
Testis weight and sperm count
The weight of the left testes at 21st wk was measured without peeling the tunica. To determine the sperm counts, sperm samples from the cauda epididymides were collected, as previously reported [15]. Briefly, the epididymides were removed and were placed in 3 mL of normal saline, and then they were minced to allow the sperm to swim for 10 min at 37 °C. The sample were diluted with trypan blue staining solution, and they were transferred into a hemocytometer to determine the cauda sperm count.
Histological evaluation of spermatogenesis
The right testes were fixed in Bouin’s solution and were embedded in paraffin. Each section was stained with hematoxylin and eosin and was examined under a light microscope. To determine the proportion of tubules showing spermatogenesis, we counted the number of seminiferous tubules per section with or without spermatogenesis, as previously reported [16]. Briefly, each tubule was considered positive when at least two layers of germ cells on the whole circumference of the basal membrane were present. This phenomenon represents the regeneration of spermatogenesis, which was promoted by the hormone therapy aimed at repairing the damage resulting from the DXR treatment. For quantitative evaluation, at least three sections with 100 tubules per section were examined in each testis.
Hormone assays
To examine the serum FSH and testosterone levels, 1 mL of blood was obtained from the jugular veins of each mouse before euthanasia. Then the blood sample was centrifuged to isolate the plasma, and it was stored at -80 °C until analysis [17]. The FSH and testosterone levels were measured using the Rodent FSH ELISA TEST Kit (Endocrine Technologies Co., Newark, CA, USA) and Rodent Testosterone ELISA TESE kit (Endocrine Technologies Co.).
Single-cell pulsed-field gel electrophoresis
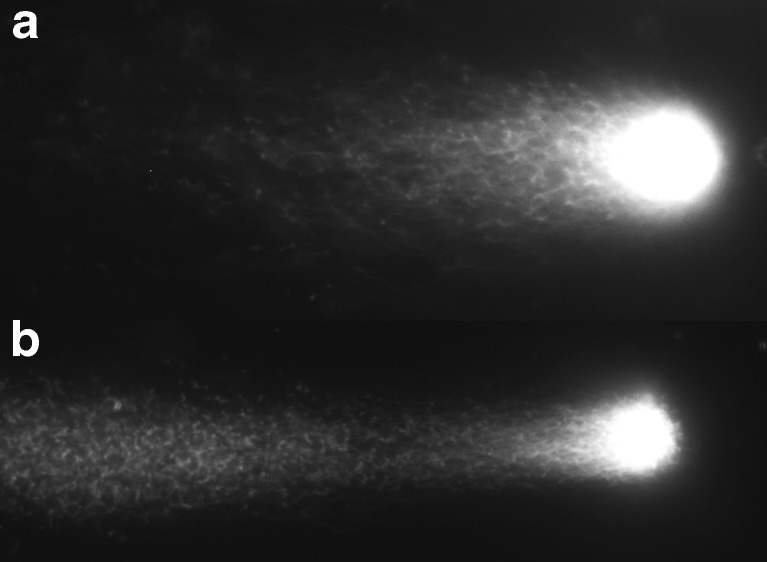
To assess the characterization of sperm quantitatively, single-cell pulsed-field gel electrophoresis (SCPFGE) was performed to observe fragmented DNA derived from single sperm nucleus [18]. Briefly, an aliquot of sperm (2 × 104 cells) was applied to amino propyl-silane-conjugated glass slides, which were embedded in melted 0.56 % agarose mixed with purified trypsin, and they were chilled for 30 min. The gel was incubated in the cell-lysis reagent (30 mM Tris-polyphosphoric acid, 8.2 mM sodium hexa-metaphosphate, 0.05 % Triton X-100, 5.0 mM dithiothreitol, at pH 8.1) at 37 °C for 30 min. SCPFGE was performed at 1.5 V/cm with 3.0 s intervals for 7 min. DNA in the gel was stained with diluted (×104) Cyber-Gold (Molecular Probes, Eugene, OR, USA) and was observed under an epifluorescent microscope. We classified the profiles of DNA according to the sizes of fragmented DNA as long chain fiber elongated from the origin without interruption (Fig. 2a), and the others with granular or fibrous fragments were separated beyond the anterior end of the elongated long chain fiber (Fig. 2b). When DNA fragmentation is advanced, long chain fibers will decrease and shortened, while the granular or fibrous fragments will increase. In each specimen, > 100 sperm were observed and the percentage of sperm that had long chain fiber without containing granular or fibrous fragments was counted.
Fig. 2.
Single-cell pulsed-field gel electrophoresis is performed to observe the fragmented deoxyribonucleic acid (DNA) derived from the single sperm nucleus. (a) The long chain fiber is elongated from the origin without interruption in sperm without impairment of DNA. (b) Granular or fibrous fragments are separated beyond the anterior end of the elongated long chain fiber when the DNA fragmentation is advanced
Statistical analysis
Statistical analyses were performed to analyze differences between the groups. Analysis of variance was used to examine the differences between all the groups, followed by a nonparametric Mann–Whitney U -test to determine the significance of the differences between the pairs of groups. Values were expressed as mean ± standard error of the mean. Results were considered significant at p < 0.05. The analyses were performed using JMP statistical software program, version 5.0 (SAS Institute Inc., Cary, NC, USA).
Results
Body and testis weight and sperm count
All the animals were alive during the DXR treatment and showed increasing body weight. Although body weight decreased in some groups after hormone administration, no statistically significant differences were observed between the groups.
In week 21, the testis weight increased in group F compared to that in group D. In accordance with changes in the testis weight, the epididymis cauda sperm count was statistically increased in group F compared to that in group D (Table 1).
Table 1.
Parameters related to the recovery of spermatogenesis
| Group | C | D | F |
|---|---|---|---|
| Testes weight (mg) | 133 ± 2.73 * | 62 ± 4.98 | 108 ± 6.25 * |
| Sperm concentration (×106/mL) | 6.6 ± 1.6 * | 3.4 ± 0.79 | 6.1 ± 1.7 * |
| % of tubulues with spermatogenesis | 84 ± 5.5 * | 33 ± 6.4 | 56 ± 7.8 * |
| Mating experiment | |||
| % of pregnant female | 93.7 * | 25 | 75 * |
| % of implantations | 82.2 ± 15.8 * | 25.3 ± 38.8 | 48.1 ± 27.9 |
| % of sperm with long chain fragment | 84.1 ± 1.7 * | 29.7 ± 5.2 | 84.0 ± 4.8 * |
Mean ± SE
*P < 0.05 vs group D
Histological analysis of spermatogenesis
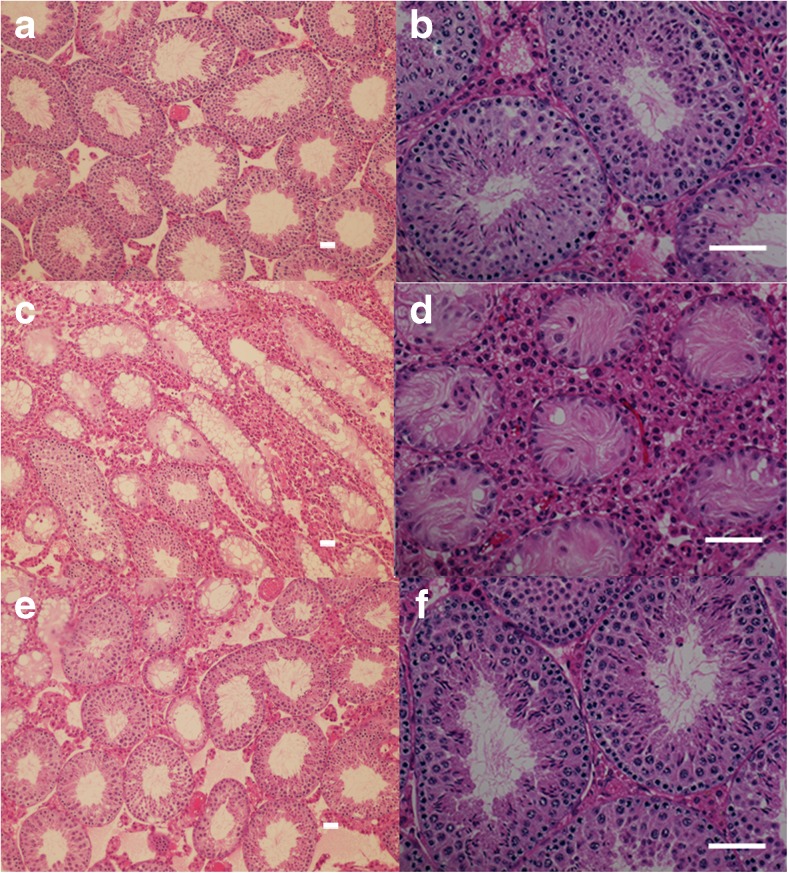
On histological examination, most of the tubules were composed of mature spermatid and sperm cells.in the control group’s testes (group C) (Fig. 3a and b). In the DXR treated testes (group D), the diameters of the testicular tubules were reduced, and the seminiferous tubules showed vacuolization and a reduced germ cell count. As the impairment progressed, the testicular tissues became atrophic and the germ cells in the tubules disappeared, with only Sertoli cells remaining (Fig. 3c and d). The ratio of atrophic tubules was decreased and the number of tubules composed of mature spermatid and sperm cells were increased in the FSH-treated groups’ testes (group F) (Fig. 3e and f).
Fig. 3.
Histopathological findings in the testes that were sacrificed in the 21st week (hematoxylin and eosin staining). (a and b) Most of the tubules are composed of mature spermatid and sperm cells in the control testes shown in (a) under a higher magnification in (b) (paraffin section of group C). (c and d) The doxorubicin-treated testes show shrunken tubules. The thickness of the seminiferous tubules is reduced, and the germ cell layer is decreased. In some seminiferous tubules, vacuolization and a reduced number of germ cells observed. The impairment has progressed, and the testicular tissue has become fibrous and atrophic (paraffin section of group D). (e and f) In the follicle-stimulating hormone-treated testes, the percentage of atrophic tubules is decreased and the number of tubules that compose the of mature spermatids and sperm cells is increased (paraffin section of group F). Scale bar = 100 μm
A quantitative assessment of the recovery from the DXR-induced disorder showed that the proportion was increased in group F compared to that of group D (Table 1).
Sperm quality and fertility
The percentage of sperm that did not containing granular or fibrous fragments was increased in group F (Table 1). Similar to the sperm quality study, the animals in group F showed a significant and drastic recovery in the pregnancy rate (Table 1). Implantation rate decreased significantly in group D compared with control group C. It recovered in group F; however there was no statistical difference between in group D (Table 1).
Hormone assays
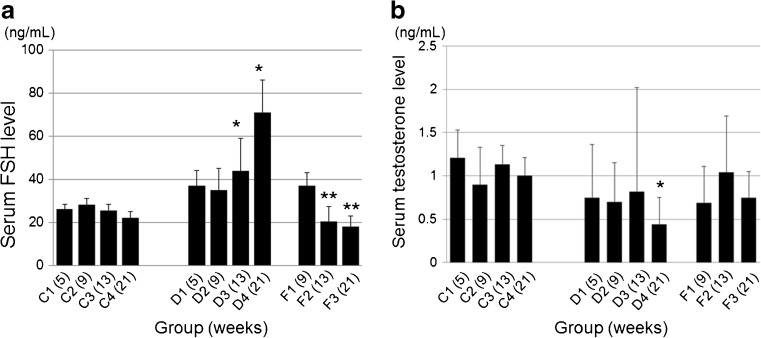
The serum FSH level increased gradually in response to DXR treatment, and the increase became significant at 13 and 21 week compared to the control group (C) (P < 0.05). The serum FSH level increased in response to treatment with rhFSH in week 9, although the difference did not reach statistical significance compared to the control group (C). In week 13 and 21, the FSH level was decreased significantly in group F compared to that in group D (P < 0.05) (Fig. 4a).
Fig. 4.
a Serum follicle-stimulating hormones (FSH) level. In the doxorubicin treated group (D), the FSH levels have slightly increased after DXR treatment and the increase becomes significant in weeks 13 and 21 (*, P < 0.05, vs. in the 13th and 21st weeks of group C). In week 9, there are no differences between the groups. In the group treated with FSH (F), the serum FSH level recovered to the level of the normal controls in weeks 13 and 21. Statistically significant differences are observed between groups F and D (**, P < 0.05 vs. group D). b The serum testosterone level. In the DXR-treated group (D and F), the testosterone levels are slightly decreased compared to the control (C). A significant reduction is observed in week 21 of group D (*; P < 0.05 vs. in the 21st week of group C)
The serum testosterone levels tended to decrease in the DXR-treated groups (D and F) compared to the control (group C); however, the significant difference was only observed at 21 week for group D (Fig. 4b).
Discussion
Treatment methods for hematopoietic malignant disease and testicular cancer have improved in recent years. Sperm cryopreservation provides young patients with the opportunity to pursue marriage and procreation, which are considered important factors that affect quality of life. However, the option to undergo sperm cryopreservation is not available for patients who have been previously treated with chemoagents such as those undergoing remission induction therapy against acute leukemia, and most of these patients have reduced semen parameters. Our present study demonstrated the efficacy of FSH treatment for promoting spermatogenesis after DXR-induced damage in mice. Furthermore, we confirmed sperm quality by using SCPFGE, which has not been performed previously in the same kind of studies.
When spermatogenesis is disturbed by radiation or chemotherapy, endogenous gonadotropins may be produced at high levels. Generally, it is ineffective to administer FSH or LH in the presence of a high plasma gonadotropin level because of the desensitization of the FSH and LH receptors in Sertoli and Leydig cells. Moreover, Meistrich and Shetty described the inhibition of spermatogenesis by testosterone or FSH under pathological conditions [19]. Therefore, the aim of hormone treatment was thought to decrease the FSH level, reset testicular activity and restore the function of the Sertoli and Leydig cells [5, 20]. In the present study, early FSH administration was effective for the recovery of spermatogenesis and fertility. Our findings contradict those of a study that show that the administration of exogenous FSH under conditions of hypergonadotropin did not improve Sertoli cell function [20].
This discrepancy can be attributed to the differences between the methods of testicular impairment used. DXR, an anticancer agent used in several malignant diseases, causes a dose-dependent spermatogenesis disturbance in experimental animals [12, 21–24]. Lu et al. and Meistrich et al. demonstrated that single-dose DXR induced the spermatogenesis disorder [21, 23], whereas at low doses, it caused a mild and chronic spermatogenesis disorder in mouse models [12]. In the present study, the administration of low-dose DXR (0.5 mg/kg twice per week) for a long period (5 weeks) caused a chronic disorder that was different from those previously reported in animal models, in which impairment was induced by a single high-dose injection of chemoagents or radiation. In fact, the serum FSH level did not increase early; it increased gradually in the later weeks of this model (Fig. 4a), which is different from the findings of previous studies in which the FSH was elevated after 6 weeks of procarbazine treatment [25]. Therefore, we assume that if the serum FSH elevated to a peak level at once, the FSH becomes harmful and the Sertoli cells lose their sensitivity. Meanwhile, the FSH sensitization in Sertoli cells would remain before the serum FSH levels increase with the passage of time after DXR treatment, and this may be stimulated by exogenous FSH.
DXR induces testicular impairment through several mechanisms. Reactive oxygen stress causes serious damage and some antioxidant enzymes attenuate the disorder. Xin et al. reported on the increase in the percentage of abnormal spermatozoa in DXR-treated rats [26]. In fact, we showed that DNA fragmentation was severe in the DXR-treated group (D) (Table 1). In group D, the number of pregnant females was low even though the sperm existed. We expected that the reduction in the sperm count and quality may have caused the decrease in pregnant females of the mating study.
DXR may cause the impairment of spermatogonial cells. The spermatogonia are classified as spermatogonial stem cells, proliferative and differentiating spermatogonia. Lu and Meistrich reported that differentiated spermatogonia (A2) were most sensitive; however, stem cells were also killed with doses of DXR [21]. Although the experimental model is different from previous reports, the recovery of spermatogenesis observed in our study suggests that spermatogonial stem cells may remain under the exposure of DXR, and they react to FSH stimulation. However, there were several tubules existing only in the Sertoli cells without germ cells (Fig. 3). Moreover, the sperm count and quality did not increase in group D even though there were enough recovery periods. This suggests that the spermatogonial stem cells were also one of the targets of DXR. In our study, we suppose that DXR widely induced the germ cell disorder among spermatogonial stem cells to differentiated spermatogonia.
Meistrich and Shetty reported that testosterone inhibited spermatogenesis under pathological conditions [19]. However, in past reports on inducing testicular damage by chemoagents or radiation, the level of testosterone did not seem to be directly related to spermatogenesis recovery. The change in testosterone levels after the impairment differed among each report. Meistrich and Kangasniemi reported that the serum testosterone level showed no change by irradiation [5]. Meanwhile, procarbazine reduced the testosterone level after 6 weeks [25]. In fact, in our study, the testosterone level decreased by 21 week after DXR treatment (group D) compared to the control (group C); however there was no significant difference between the FSH-treated group (F) (Fig. 4b).
FSH acts at various stages starting from the regulation of the spermatogonial population through spermiogenesis in rodents [9, 27–30]. In our study, the sperm concentration and percentage of seminiferous tubules with spermatogenesis was elevated in the FSH-treated group (group F). Furthermore, the testis weight, pregnancy rate in the mating study, and recovery of DNA fragmentation of sperm were higher in group F than in the control (group C). Assuming that the testes retained sensitivity to the FSH, efficacy of the FSH in our study suggests that early FSH administration increased spermatogonia and promoted spermatogenesis regeneration. The FSH will not directly act with spermatozoa (i.e., sperm differentiated from the remaining spermatogonia may have had some impairment). However, unimpaired sperm newly differentiated from spermatogonia that were increased by FSH stimulation recovered in their quality.
There are some differences in spermatogenesis between primates and rodents, especially in stem cell populations [31]. Additionally, Lu and Meistrich reported that the effect of DXR on testicular stem cells in humans were less significant than that on mice [21]. Although the sensitivity of chemotherapy is not similar, we hope that our study may become one of the clues for recovering the fertility of young patients who have received chemotherapy.
In conclusion, we showed that early treatment with exogenous FSH before the elevation of endogenous FSH promoted recovery from the damage of spermatogenesis induced by low-dose, long-duration DXR administration in mice.
Acknowledgments
Conflict of interest
The authors declare none.
Footnotes
Capsule The administration of exogenous FSH promotes the recovery from impaired spermatogenesis induced by low-dose doxorubicin.
References
- 1.Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancer. J Clin Oncol. 1997;15:239–45. doi: 10.1200/JCO.1997.15.1.239. [DOI] [PubMed] [Google Scholar]
- 2.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;34:12–7. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao W, Stahl PJ, Osterberg EC, Nejat E, Palermo GD, Rosenwaks Z, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol. 2011;29:1607–11. doi: 10.1200/JCO.2010.33.7808. [DOI] [PubMed] [Google Scholar]
- 4.Chung K, Irani J, Efymow B, Blasco L, Patrizio P. Sperm cryopreservation for male patients with cancer: an epidemiological analysis at the University of Pennsylvania. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S7–11. doi: 10.1016/j.ejogrb.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Meistrich ML, Kangasniemi M. Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J Androl. 1997;18:80–7. [PubMed] [Google Scholar]
- 6.Udagawa K, Ogawa T, Watanabe T, Tamura Y, Kita K, Kubota Y. Testosterone administration promotes regeneration of chemically impaired spermatogenesis in rats. Int J Urol. 2006;13:1103–8. doi: 10.1111/j.1442-2042.2006.01484.x. [DOI] [PubMed] [Google Scholar]
- 7.Aminsharifi A, Shakeri S, Ariafar A, Moeinjahromi B, Kumar PV, Karbalaeedoost S. Preventive role of exogenous testosterone on cisplatin-induced gonadal toxicity: an experimental placebo-controlled prospective trial. Fertil Steril. 2010;93:1388–93. doi: 10.1016/j.fertnstert.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Ruwanpura SM, McLachlan RI, Stanton PG, Meachem SJ. Follicle-stimulating hormone affects spermatogonial survival by regulating the intrinsic apoptotic pathway in adult rats. Biol Reprod. 2008;78:705–13. doi: 10.1095/biolreprod.107.065912. [DOI] [PubMed] [Google Scholar]
- 9.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–31. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 10.Gnanaprakasam MS, Chen CJ, Sutherland JG, Bhalla VK. Receptor depletion and replenishment processes: in vivo regulation of gonadotropin receptors by luteinizing hormone, follicle stimulating hormone and ethanol in rat testis. Biol Reprod. 1979;20:991–1000. doi: 10.1095/biolreprod20.5.991. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi PP, Tripathi DN, Jena GB. Hesperetin protects testicular toxicity of doxorubicin in rat: role of NFkappaB, p38 and caspase-3. Food Chem Toxicol. 2011;49:838–47. doi: 10.1016/j.fct.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Sudo K. An experimental model of Adriamycin-induced spermatogenic disorder in mice (1): histological and functional analysis. J Med Soc Toho. 1991;38:462–75. [Google Scholar]
- 13.Singh J, Handelsman DJ. The effects of recombinant FSH on testosterone-induced spermatogenesis in gonadotrophin-deficient (hpg) mice. J Androl. 1996;17:382–93. [PubMed] [Google Scholar]
- 14.Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–28. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Sueoka K, Tanigaki R, Tajima H, Nakabayashi A, Yoshimura Y, et al. Green tea extracts attenuate doxorubicin-induced spermatogenic disorders in conjunction with higher telomerase activity in mice. J Assist Reprod Genet. 2010;27:501–8. doi: 10.1007/s10815-010-9438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanatsu-Shinohara M, Toyokuni S, Morimoto T, Matsui S, Honjo T, Shinohara T. Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol Reprod. 2003;68:1801–7. doi: 10.1095/biolreprod.102.012575. [DOI] [PubMed] [Google Scholar]
- 17.Zohni K, Zhang X, Tan SL, Chan P, Nagano MC. The efficiency of male fertility restoration is dependent on the recovery kinetics of spermatogonial stem cells after cytotoxic treatment with busulfan in mice. Hum Reprod. 2012;27:44–53. doi: 10.1093/humrep/der357. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko S, Yoshida J, Ishikawa H, Takamatsu K. Single-cell pulsed-field gel electrophoresis to detect the early stage of DNA fragmentation in human sperm nuclei. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meistrich ML, Shetty G. Inhibition of spermatogonial differentiation by testosterone. J Androl. 2003;24:135–48. doi: 10.1002/j.1939-4640.2003.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 20.Foresta C, Bettella A, Spolaore D, Merico M, Rossato M, Ferlin A. Suppression of the high endogenous levels of plasma FSH in infertile men are associated with improved Sertoli cell function as reflected by elevated levels of plasma inhibin B. Hum Reprod. 2004;19:1431–7. doi: 10.1093/humrep/deh255. [DOI] [PubMed] [Google Scholar]
- 21.Lu CC, Meistrich ML. Cytotoxic effects of chemotherapeutic drugs on mouse testis cells. Cancer Res. 1979;39:3575–82. [PubMed] [Google Scholar]
- 22.Imahie H, Adachi T, Nakagawa Y, Nagasaki T, Yamamura T, Hori M. Effects of Adriamycin, an anticancer drug showing testicular toxicity, on fertility in male rats. J Toxicol Sci. 1985;20:183–93. doi: 10.2131/jts.20.183. [DOI] [PubMed] [Google Scholar]
- 23.Meistrich ML, Goldstein LS, Wyrobek AJ. Long-term infertility and dominant lethal mutations in male mice treated with adriamycin. Mutat Res. 1985;152:53–65. doi: 10.1016/0027-5107(85)90046-6. [DOI] [PubMed] [Google Scholar]
- 24.Lui RC, Laregina MC, Herbold DR, Johnson RF. Testicular cytotoxicity of intravenous doxorubicin in rats. J Urol. 1986;136:940–3. doi: 10.1016/s0022-5347(17)45136-6. [DOI] [PubMed] [Google Scholar]
- 25.Meistrich ML, Wilson G, Huhtaniemi I. Hormonal treatment after cytotoxic therapy stimulates recovery of spermatogenesis. Cancer Res. 1999;59:3557–60. [PubMed] [Google Scholar]
- 26.Xin YF, You ZQ, Gao HY, Zhou GL, Chen YX, Yu J, et al. Protective effect of Lycium barbarum polysaccharides against doxorubicin-induced testicular toxicity in rats. Phytother Res. 2012;26:716–21. doi: 10.1002/ptr.3633. [DOI] [PubMed] [Google Scholar]
- 27.Meachem SJ, Wreford NG, Stanton PG, Robertson DM, McLachlan RI. Follicle-stimulating hormone is required for the initial phase of spermatogenic restoration in adult rats following gonadotropin suppression. J Androl. 1988;19:725–35. [PubMed] [Google Scholar]
- 28.Sinha-Hikim AP, Swerdloff RS. Temporal and stage-specific effects of recombinant human follicle-stimulating hormone on the maintenance of spermatogenesis in gonadotrophin-releasing hormone antagonist-treated rat. Endocrinology. 1995;136:253–61. doi: 10.1210/endo.136.1.7828538. [DOI] [PubMed] [Google Scholar]
- 29.McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. The effects of recombinant follicle-stimulating hormone on the restoration of spermatogenesis in the gonadotropin-releasing hormone-immunized adult rat. Endocrinology. 1995;136:4035–43. doi: 10.1210/endo.136.9.7649112. [DOI] [PubMed] [Google Scholar]
- 30.Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology. 2003;144:509–17. doi: 10.1210/en.2002-220710. [DOI] [PubMed] [Google Scholar]
- 31.Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479–93. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]