Abstract
Purpose
To verify if polymorphisms of LH (Trp8Arg/Ile15Thr), LH receptor (insLQ), and FSH receptor (Asn680Ser) are associated with endometriosis and infertility.
Methods
This is a prospective case–control study. Sixty-seven patients with endometriosis and infertility (study group) and 65 healthy fertile patients (control group) were enrolled in the study between July 2010 and July 2013. All patients had their endometriosis diagnosis made or excluded by laparoscopic surgery; study group was submitted to the surgery for infertility investigation and control group for tubal ligation. Day-3 serum hormones were collected from all patients. Analysis of nucleotide mutations for LH polymorphisms (Trp8Arg and Ile15Thr), LHR polymorphism (insLQ), and FSHR polymorphism (Asn680Ser) were performed by PCR.
Results
Day-3 FSH, estradiol and LH serum levels were not different between the groups, while CA-125 was higher in patients with endometriosis and infertility. All polymorphisms studied were in Hardy-Weinberg equilibrium. The prevalence of insLQ was significantly higher in patients with endometriosis and infertility (P = 0.005). Allele occurrence in control group was 0.10 versus 0.25 in infertile endometriosis group (P = 0.001). There was no difference regarding Trp8Arg/Ile15Thr (P > 0.05) and Asn680Ser (P > 0.05) prevalence between groups.
Conclusion
This is the first time that prevalence of insLQ was shown to be higher in patients with endometriosis and infertility than in healthy fertile patients. There was no difference in LH and FSHR polymorphisms’ prevalence between groups.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0477-3) contains supplementary material, which is available to authorized users.
Keywords: Endometriosis, Infertility, Hormones, Polymorphisms
Introduction
Endometriosis is a common disease that affects approximately 10–15 % of premenopausal women in the general population. However, among infertile patients the prevalence can be as higher as 30–60 % [1, 2]. Clinical manifestations of endometriosis usually include dyspareunia, dysmenorrhea, chronic pelvic pain and infertility [3, 4].
The pathogenesis of this disease and its relationship with infertility is not fully understood, but it is already known that it involves hormonal, genetics and immunologic factors [5–10]. Endocrine disorders like altered folliculogenesis, ovulatory disorders and luteal phase abnormalities had also been described. Our research group has demonstrated that this disease is associated with hyperprolactinaemia, diminished ovarian reserve and luteal insufficiency with a subsequent altered pattern of progesterone secretion in the second phase of menstrual cycle [11–13].
It is well known that the women’s fertility is dependent of a normal function of gonadotropins and their receptors. Gonadotropins are glycoprotein hormones, consisting of a common α subunit and a unique β subunit specific for each of the hormones [14, 15]. They act by binding to the extracellular domain of their specific receptors, which belong to the superfamily of G protein-coupled receptors [14, 15]. Furthermore, FSH and LH are responsible for an adequate folliculogenesis, steroidogenesis in the ovary, oocyte maturation, ovulation, and luteinization [16–19]. During luteal phase, LH is responsible for maintaining an adequate progesterone secretion; consequently its adequate function is essential during this period.
Gonadotropins and their receptors’ polymorphisms can affect the hormones’ function [20–22]. For example, the FSH receptor (FSHR) single nucleotide polymorphism (SNP) Asn680Ser affects baseline FSH levels, increases gonadotropin requirements during controlled ovarian hyperstimulation and influences in vitro fertilization outcomes [23–25]. Furthermore, a common genetic variant of LH (V-LH), which is characterized by two amino acid replacements, Trp8Arg and Ile15Thr, in the β subunit gene, gives the hormone an increased in vitro bioactivity and a decreased half-time in vivo [21]. V-LH has also been associated to ovulatory disorders, hyperprolactinemia and luteal insufficiency in infertile patients [26]. Moreover, a polymorphic insertion in exon 1 of LH receptor (LHR) gene (insLQ) increases its activity in vitro, by decreasing the half maximal effective concentration (EC50) and increasing the cell surface expression [22]. This SNP has been associated with early onset and adverse outcome in breast cancer patients [22, 27].
It has been supported by a great number of studies that heritability plays an important role in the development of endometriosis [9, 28–30]. Furthermore, many authors have looked for associations of several polymorphisms and endometriosis [31, 32].
Taking into account that LH, FSH and their receptors contribute profoundly to the regulation of women’s reproductive processes and that endometriosis is commonly associated to infertility and has a hereditary component, the aim of our study is to verify this possible relation between LH, LHR and FSHR polymorphisms, endometriosis and infertility.
Materials and methods
Design
This is a prospective case–control study performed in the Gynecologic Department of Hospital de Clínicas de Porto Alegre. STROBE guideline was used [33].
Subjects
Sixty seven patients with endometriosis and infertility (study group) and 65 healthy fertile patients (control group) were recruited between July 2010 and July 2013 to take part in the study. Diagnosis of infertility was considered when the couple had not conceived after 12 months of contraceptive-free intercourse [34]. We selected all patients who had endometriosis diagnosed during laparoscopic surgery for infertility investigation and matched the including criteria for study group (described below). Endometriosis degree was classified according to revised American Society for Reproductive Medicine [35]. Patients submitted to laparoscopic tubal ligation formed control group. Therefore they had endometriosis diagnosis excluded (by laparoscopy) and they also had previous history of normal fertility.
The inclusion criteria in study and control group were: (i) regular menstrual cycles, (ii) presence of both ovaries, (iii) no endocrine disorder and (iv) no family history of genetic disease. The study group had also a normal sperm analysis. Patients who presented endometrioma or polycystic ovary syndrome diagnosis were excluded from the study.
Hormonal assays
On the early follicular phase (cycle day 3), we collected blood samples to measure serum FSH, LH and CA-125 levels. All samples were centrifuged at 2500 rpm for separation of plasma, which was frozen for later analysis. Hormones were analyzed using chemiluminescence kits (Immulite, Deerfield, USA). The largest inter and intra-kit variation was 6.1 and 6.5 % for FSH; 6.5 and 5.3 % for LH; 2.0 and 1.7 % for CA-125. The detection limit was 1 mIU/mL for FSH and LH, and 1.5 U/ml for CA-125.
DNA extraction
Blood samples were collected in EDTA tubes through venipuncture and DNA was extracted with commercial kit Easy DNA (Invitrogen, Paisley, UK).
Detection of Trp8Arg e Ila15Thr mutations in LH gene
Exon 2 of LHB gene was amplified as described by Elter et al. [36], using primers F1 (5 GAAGCAGTGTCCTTGTCCCA 3) and R1 (5 GAAGAGGAGGCCTGAGAGTT 3). In brief, amplification was performed in a Veriti thermal cycler (Life Technologies) at annealing temperature of 62 °C, using 20 pmol of each primer, 0.5 U Taq DNA Polymerase, 2 mM dNTP and 1.5 mM MgCl2 (all reagents from Invitrogen, Paisley, UK). Amplification was confirmed by the presence of a 662 bp band stained with SYBR® Gold (Invitrogen, Paisley, UK) on electrophoresis in 1.5 % agarose gel.
PCR products were purified with EXO-SAP (GE Healthcare) and submitted to automated sequencing in ABI 3100 (Life Technologies) using BigDye Terminator v.3.0 (Life Technologies, California, USA). For sequencing, 4 μl of purified PCR product, corresponding to 30–50 ng, estimated by visual inspection with Low Mass Ladder (Invitrogen, Paisley, UK) were used with 3.2 pmol of F primer. The results were compared with the reference sequence from GenBank (NCBI) under accession number NG_011464.1. Supplemental Figure 1a shows two sequencing results, one homozygote for LH wild-type and the other heterozygote for V-LH polymorphism.
Detection of insLQ in LHreceptor gene
Exon 1 of LHR gene was amplified with primers GhLHR1 (5 CACTCAGAGGCCGTCCAAG 3) E GhLHR2 (5 GGAGGGAAGGTGGCATAGAG 3) as described by Atger et al. [37]. In brief, amplification was performed in a Veriti thermal cycler (Life Technologies) at annealing temperature of 64 °C, using 20 pmol of each primer, 0.5 U Taq DNA Polymerase, 2 mM dNTP and 1.5 mM MgCl2 (all reagents from Invitrogen, Paisley, UK). Amplification was confirmed by the presence of a 295 bp band stained with SYBR® Gold (Invitrogen, Paisley, UK) on electrophoresis in 1.5 % agarose gel.
PCR products were purified with EXO-SAP (GE Healthcare) and submitted to automated sequencing in ABI 3100 (Life Technologies) using BigDye Terminator v.3.0 (Life Technologies, California, USA). For sequencing, 4 μl of purified PCR product, corresponding to 30–50 ng, estimated by visual inspection with Low Mass Ladder (Invitrogen, Paisley, UK) were used with 3.2 pmol of F primer. The results were compared with the reference sequence from GenBank (NCBI) under accession number NG_008193.1. Supplemental Figure 1b shows two sequencing results, one wild-type sequence (allele N) and the other with insLQ polymorphism (allele V).
Detection of Asn680Ser in FSH receptor gene
Asn680Ser variant, in exon 10 of the FSH receptor gene, was detected by the nested PCR–RFLP (PCR–restriction fragment length polymorphism) method, as described by Sudo et al. [38]. All reagents were purchased from Invitrogen (Invitrogen, Paisley, UK). All the polymerase chain reaction (PCR) reactions were performed in an Eppendorf Personal Cycler Thermocycler (Eppendorf, Hamburg, Germany). Samples were analyzed simultaneously.
For Asn680Ser detection, the FSH receptor gene was amplified by PCR using genomic DNA as the template and a set of primers (5 TTTGTGGTCATCTGTGGCTGC 3 and 5 CAAAGGCAAGGACTGAATTATCATT3), which amplified a DNA fragment of 520 bp at an annealing temperature of 60 °C. Since the A to G transition creates an endonuclease BsrI recognition site, the PCR fragment following BsrI digestion and 2.5 % agarose gel electrophoresis with SYBR® Gold reveals three different patterns. Based on this RFLP analysis, patients were classified into three groups, NN (680Asn/Asn), NS (680Asn/Ser) and SS (680Ser/Ser) (Supplemental Figure 1c).
Sample size calculation
Sample size calculation was performed based on the literature of all the polymorphisms studied. We have calculated 47 women for each group considering the significance level of 5 % and the power calculation for this sample size of 80 %, with an expected difference of at least 10 % between groups [38–41].
Statistical analysis
The statistical analysis was carried out using the SPSS 18.0 software. The measure of central tendency used was the mean and the measure of variability was the standard deviation [42]. Medians and minimum-maximum values were used when normality of data distribution could not be ascertained. Categorical variables in the 2 groups were compared using the 2-sided Pearson Chi2 test. Continuous variables were compared using the t student test. Logistic regression analysis was performed in order to determine the odds ratios and 95 % confidence intervals associated with the endometriosis/infertility, taking the control as the reference group. We considered P < 5 % significant. Genotype distribution was tested for Hardy–Weinberg equilibrium, and the difference in genotype frequencies between the samples was tested using a Chi square-test for independence [43].
Ethical approval
The local ethics committee approved this study and a written informed consent was provided for all subjects prior to the blood sample collection (IRB equivalent).
Results
A total of 67 patients with endometriosis and infertility composed the study group and 65 fertile patients with no endometriosis composed the control group. Demographic characteristics are shown in Table 1. There was statistically significant difference between the study group and the control group regarding color pattern, weight, body mass index (BMI) and pregnancy history. The groups were not different regarding age, height and menstrual characteristics.
Table 1.
Demographic characteristics for women with infertility and endometriosis (study group) and healthy fertile women (control group)
| Control group (n = 65) | Study group (n = 67) | P value | |
|---|---|---|---|
| Age (years) | 33.13 ± 5.15 | 32.0 ± 4.48 | .190a |
| Color | .014b | ||
| White | 48 (73.8 %) | 57 (85 %) | |
| Black | 17 (26.2 %) | 10 (15 %) | |
| Weight (Kg) | 70.11 ± 12.91 | 62.68 ± 11.93 | .011 a |
| Height (meters) | 1.61 ± 0.065 | 1.63 ± 0.054 | .139 a |
| BMI (Kg/m2) | 27.16 ± 4.61 | 23.67 ± 4.27 | .001 a |
| Menarche (years) | 12.19 ± 1.94 | 12.33 ± 1.77 | .790 a |
| Pregnancy | 3.72 ± 2.24 | 0.42 ± 0.78 | .000b |
| Para | 2.66 ± 2.06 | 0.08 ± 0.27 | .000 |
| Abortion | 0.44 ± 0.86 | 0.24 ± 0.55 | .171 |
All values are means ± SD; BMI body mass index, a student t test, b Chi2 test
There was no difference regarding serum LH and FSH levels (data not shown). Nevertheless, the study group’s level of CA-125 was 37.8 ± 36.9 while the control group’s level was 15 ± 9.4, and this difference was statistically significant (P = 0.039).
LH, LHR and FSHR genotype analysis was possible in all patients. Allelic distributions for all polymorphisms studied were in the Hardy-Weinberg Equilibrium [43]. Homozygous and heterozygous carriers for the LH and LHR polymorphisms were combined for statistical reasons, as done in previous papers [26, 39].
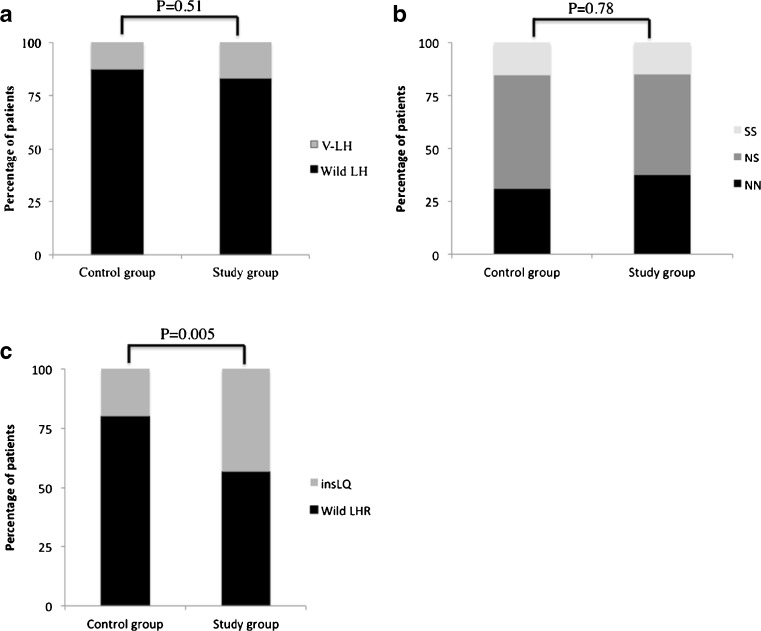
Frequency of V-LH was 16.4 % in the study group and 12.3 % in the control group (Fig. 1a) and this difference was not statistically significant, OR 1.4 (CI 95 % 0.51–3.82). There was also no difference in FSHR polymorphism Asn680Ser prevalence between groups (Fig. 1b). Nevertheless, insLQ’s frequency was 43.3 % in the study group and 20 % in the control group (Fig. 1c) and this difference was statistically significant, OR 3.05 (CI 95 % 1.4–6.64). Moreover, allele occurrence in control group was 0.10 versus 0.25 in infertile endometriosis group (P = 0.001).
Fig. 1.
LH (a), FSH receptor (b) and LH receptor polymorphisms (c) frequencies in women with infertility and endometriosis (study group) and healthy fertile women (control group). V-LH = LH polymorphism Trp8Arg and Ile15Thr; Wild LH = LH with no polymorphism; FSH receptor variants: SS = 680Ser/Ser, NS = 680Asn/Ser and NN = 680Asn/Asn; insLQ = LH receptor with polymorphic insertion in exon 1; Wild LHR = LH receptor with no polymorphic insertion
In the study group, we also divided endometriosis in mild (stages I/II), 65.6 % of patients, and severe (stages III/IV), 34.4 % of patients [35]. There were no statistic differences in LH polymorphisms (Trp8Arg and Ile15Thr), LHR polymorphism (insLQ), and FSHR polymorphisms (Asn680Ser) prevalence between these groups (data not shown). The prevalence of chronic pelvic pain in our study group was 32 %.
In addition, in order to control a possible effect of BMI and skin color as confounding biases, we performed a multivariable analysis (regression) considering insLQ’s as the primary endpoint. This model did not show significance (P > 0.05) for both variables.
Discussion
We have shown for the first time that the prevalence of insLQ SNP is higher in infertile patients with endometriosis than in healthy fertile patients. Nevertheless, we have found no difference in V-LH’s prevalence between these groups. Furthermore, we have also found no difference in FSHR polymorphism Asn680Ser frequency between those groups.
Taking into account that the pathogenesis of infertility in endometriosis patients is related to luteal insufficiency [11] and that insLQ is associated to an altered LHR function [22], our hypothesis that endometriosis and infertility are linked to this SNP was confirmed in this research. Previous studies about insLQ SNP were all related to breast cancer [22, 27]; it was never studied in the reproduction field.
We have speculated that endometriosis/infertility patients may have an impaired LH function, and insLQ increases LHR activity to try a compensatory effect. Moreover, we have already demonstrated that these patients have an abnormal progesterone secretion during luteal phase [11], corroborating with this pathologic LH/LHR function theory.
V-LH gives LH an increased in vitro bioactivity and a decreased half-time in vivo [21], and it was not found to be associated to endometriosis and infertility in our study. Because this SNP is associated to LH dysfunction, many authors have looked for its association with endocrine pathologies. Therefore, V-LH has already been related to infertility [26], premature ovarian failure [44], delayed tempo of pubertal progression in healthy boys [45] and polycystic ovarian syndrome [46, 47]. Furthermore, Alviggi et al. studied 60 IVF cycles retrospectively and found that V-LH was more frequent in women who needed higher doses of FSH [10].
Takahashi et al. studied 97 infertile Japanese females and found V-LH correlated with luteal insufficiency, hyperprolactinaemia and ovulatory disorders [26]. In this study, they found no association with endometriosis, but they had a small number of patients and no fertile controls. In accordance with our results, Gazvani et al. studied 85 patients with endometriosis and found no relationship between V-LH and endometriosis [48].
Regarding FSHR polymorphism, there are several studies claiming that patients homozygous to Asn680Ser polymorphism have more poorly reproductive outcomes. For example, these patients have a longer follicular phase [49], higher day-3 serum FSH level [25], require a higher gonadotrophin dosage to induce ovulation [50] and have fewer mature oocytes in IVF cycle [25]. In men, this polymorphism has been associated to lower testes volume and altered serum reproductive hormone levels [51].
A Chinese study which included 637 women, has shown that women with this polymorphism had a significantly lower risk of endometriosis [52]. However, this study did not specify if those patients were infertile or not. Therefore, this is the first time that this SNP has been studied in infertile/endometriosis patients, and we have found no association between them.
Our study weaknesses include the lack of functional investigation and the heterogeneity of Brazilian population. Nevertheless, endometriosis is also a very heterogeneous disease, which makes it more difficult to find a polymorphism associated to it. Another of our study’s flaw is the fact that we did not have a group of fertile endometriosis patients. On the other hand, it is very difficult to find endometriosis patients with proven fertility after endometriosis diagnosis has been made.
Furthermore, the main focus of our study was endometriosis associated with infertility, not infertility alone. Therefore, it is ethically questionable to submit patients with unexplained infertility to laparoscopy for research purpose. Laparoscopy is mandatory in this case, once we need to exclude endometriosis.
We found differences in weight and BMI between the study group and the control group. Nevertheless, these findings agree with the literature data, once lower BMI is associated with endometriosis [53]. Furthermore, high serum CA-125 levels in endometriotic patients was also an expected result [54].
In conclusion, despite we found no difference in V-LH and FSHR polymorphisms between groups, our study did find an important relation between insLQ and endometriosis/infertility, opening up a new perspective in terms of endometriosis ovulatory-dysfunction. Further studies could investigate insLQ prevalence in other causes of infertility and its influence on treatment’s response.
Electronic supplementary material
(DOC 216 kb)
Acknowledgments
This study was supported by grants from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and from the Fundo de Incentivo a Pesquisa (FIPE) of Hospital de Clínicas de Porto Alegre, RS – Brazil.
Footnotes
Capsule The presence of insLQ polymorphism is higher in patients with endometriosis and infertility than in healthy fertile patients.
References
- 1.Moghissi KS. Medical treatment of endometriosis. Clin Obstet Gynecol. 1999;42(3):620–32. doi: 10.1097/00003081-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561–72. doi: 10.1016/j.fertnstert.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Howard FM. Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol. 2009;16(5):540–50. doi: 10.1016/j.jmig.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 4.ASRM Endometriosis and infertility. Fertil Steril. 2006;86(5 Suppl 1):S156–60. doi: 10.1016/j.fertnstert.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. [PubMed] [Google Scholar]
- 6.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. doi: 10.1016/S0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 7.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90(2):247–57. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 9.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137(3):327–31. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 10.Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. 2009;18(1):9–14. doi: 10.1016/S1472-6483(10)60418-X. [DOI] [PubMed] [Google Scholar]
- 11.Cunha-Filho JS, Gross JL, Bastos de Souza CA, Lemos NA, Giugliani C, Freitas F, et al. Physiopathological aspects of corpus luteum defect in infertile patients with mild/minimal endometriosis. J Assist Reprod Genet. 2003;20(3):117–21. doi: 10.1023/A:1022625106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha-Filho JS, Gross JL, Lemos NA, Brandelli A, Castillos M, Passos EP. Hyperprolactinemia and luteal insufficiency in infertile patients with mild and minimal endometriosis. Horm Metab Res. 2001;33(4):216–20. doi: 10.1055/s-2001-14945. [DOI] [PubMed] [Google Scholar]
- 13.Lemos NA, Arbo E, Scalco R, Weiler E, Rosa V, Cunha-Filho JS. Decreased anti-Mullerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil Steril. 2008;89(5):1064–8. doi: 10.1016/j.fertnstert.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 14.La Marca A, Sighinolfi G, Argento C, Grisendi V, Casarini L, Volpe A, et al. Polymorphisms in gonadotropin and gonadotropin receptor genes as markers of ovarian reserve and response in in vitro fertilization. Fertil Steril. 2013;99(4):970–8.e1. doi: 10.1016/j.fertnstert.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 15.Lamminen T, Huhtaniemi I. A common genetic variant of luteinizing hormone; relation to normal and aberrant pituitary-gonadal function. Eur J Pharmacol. 2001;414(1):1–7. doi: 10.1016/S0014-2999(01)00756-7. [DOI] [PubMed] [Google Scholar]
- 16.Rodini GP, Genro VK, Matte U, Pereira FS, Bilibio JP, Greggianin C, et al. There is no complete linkage between the polymorphisms N680S and T307A of the follicular stimulating hormone receptor gene in fertile women. J Assist Reprod Genet. 2011;28(3):221–4. doi: 10.1007/s10815-010-9503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100(1–2):51–4. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 18.Filicori M. The role of luteinizing hormone in folliculogenesis and ovulation induction. Fertil Steril. 1999;71(3):405–14. doi: 10.1016/S0015-0282(98)00482-8. [DOI] [PubMed] [Google Scholar]
- 19.Filicori M, Cognigni GE, Pocognoli P, Ciampaglia W, Bernardi S. Current concepts and novel applications of LH activity in ovarian stimulation. Trends Endocrinol Metab. 2003;14(6):267–73. doi: 10.1016/S1043-2760(03)00085-7. [DOI] [PubMed] [Google Scholar]
- 20.Gromoll J, Simoni M. Genetic complexity of FSH receptor function. Trends Endocrinol Metab. 2005;16(8):368–73. doi: 10.1016/j.tem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Haavisto AM, Pettersson K, Bergendahl M, Virkamaki A, Huhtaniemi I. Occurrence and biological properties of a common genetic variant of luteinizing hormone. J Clin Endocrinol Metab. 1995;80(4):1257–63. doi: 10.1210/jcem.80.4.7714098. [DOI] [PubMed] [Google Scholar]
- 22.Piersma D, Berns EM, Verhoef-Post M, Uitterlinden AG, Braakman I, Pols HA, et al. A common polymorphism renders the luteinizing hormone receptor protein more active by improving signal peptide function and predicts adverse outcome in breast cancer patients. J Clin Endocrinol Metab. 2006;91(4):1470–6. doi: 10.1210/jc.2005-2156. [DOI] [PubMed] [Google Scholar]
- 23.de Castro F, Ruiz R, Montoro L, Perez-Hernandez D, Sanchez-Casas Padilla E, Real LM, et al. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80(3):571–6. doi: 10.1016/S0015-0282(03)00795-7. [DOI] [PubMed] [Google Scholar]
- 24.Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. 2005;15(7):451–6. doi: 10.1097/01.fpc.0000167330.92786.5e. [DOI] [PubMed] [Google Scholar]
- 25.Boudjenah R, Molina-Gomes D, Torre A, Bergere M, Bailly M, Boitrelle F, et al. Genetic polymorphisms influence the ovarian response to rFSH stimulation in patients undergoing in vitro fertilization programs with ICSI. PLoS One. 2012;7(6):e38700. doi: 10.1371/journal.pone.0038700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Kurioka H, Ozaki T, Kanasaki H, Kohsaka M, Miyazaki K, et al. Increased prevalence of luteinizing hormone beta-subunit variant in Japanese infertility patients. Hum Reprod. 1998;13(12):3338–44. doi: 10.1093/humrep/13.12.3338. [DOI] [PubMed] [Google Scholar]
- 27.Powell BL, Piersma D, Kevenaar ME, van Staveren IL, Themmen AP, Iacopetta BJ, et al. Luteinizing hormone signaling and breast cancer: polymorphisms and age of onset. J Clin Endocrinol Metab. 2003;88(4):1653–7. doi: 10.1210/jc.2002-021585. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff F, Simpson JL. Genetics of endometriosis: heritability and candidate genes. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):219–32. doi: 10.1016/j.bpobgyn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Treloar S, Hadfield R, Montgomery G, Lambert A, Wicks J, Barlow DH, et al. The International Endogene Study: a collection of families for genetic research in endometriosis. Fertil Steril. 2002;78(4):679–85. doi: 10.1016/S0015-0282(02)03341-1. [DOI] [PubMed] [Google Scholar]
- 30.Vigano P, Somigliana E, Vignali M, Busacca M, Blasio AM. Genetics of endometriosis: current status and prospects. Front Biosci. 2007;12:3247–55. doi: 10.2741/2308. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, et al. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14(5):447–57. doi: 10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tempfer CB, Simoni M, Destenaves B, Fauser BC. Functional genetic polymorphisms and female reproductive disorders: part II–endometriosis. Hum Reprod Update. 2009;15(1):97–118. doi: 10.1093/humupd/dmn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ASRM Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60. doi: 10.1016/j.fertnstert.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 35.ASRM Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21. doi: 10.1016/S0015-0282(97)81391-X. [DOI] [PubMed] [Google Scholar]
- 36.Elter K, Erel CT, Cine N, Ozbek U, Hacihanefioglu B, Ertungealp E. Role of the mutations Trp8 = > Arg and Ile15 = > Thr of the human luteinizing hormone beta-subunit in women with polycystic ovary syndrome. Fertil Steril. 1999;71(3):425–30. doi: 10.1016/S0015-0282(98)00491-9. [DOI] [PubMed] [Google Scholar]
- 37.Atger M, Misrahi M, Sar S, Le Flem L, Dessen P, Milgrom E. Structure of the human luteinizing hormone-choriogonadotropin receptor gene: unusual promoter and 5′ non-coding regions. Mol Cell Endocrinol. 1995;111(2):113–23. doi: 10.1016/0303-7207(95)03557-N. [DOI] [PubMed] [Google Scholar]
- 38.Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8(10):893–9. doi: 10.1093/molehr/8.10.893. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson C, Pettersson K, Millar RP, Coerver KA, Matzuk MM, Huhtaniemi IT. Worldwide frequency of a common genetic variant of luteinizing hormone: an international collaborative research. International Collaborative Research Group. Fertil Steril. 1997;67(6):998–1004. doi: 10.1016/S0015-0282(97)81430-6. [DOI] [PubMed] [Google Scholar]
- 40.Rodien P, Cetani F, Costagliola S, Tonacchera M, Duprez L, Minegishi T, et al. Evidences for an allelic variant of the human LC/CG receptor rather than a gene duplication: functional comparison of wild-type and variant receptors. J Clin Endocrinol Metab. 1998;83(12):4431–4. doi: 10.1210/jcem.83.12.5325. [DOI] [PubMed] [Google Scholar]
- 41.Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, et al. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab. 1999;84(2):751–5. doi: 10.1210/jcem.84.2.5500. [DOI] [PubMed] [Google Scholar]
- 42.Lambalk CB, de Koning CH, Flett A, Van Kasteren Y, Gosden R, Homburg R. Assessment of ovarian reserve. Ovarian biopsy is not a valid method for the prediction of ovarian reserve. Hum Reprod. 2004;19(5):1055–9. doi: 10.1093/humrep/deh216. [DOI] [PubMed] [Google Scholar]
- 43.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, et al. Strengthening the reporting of genetic association studies (STREGA): an extension of the strengthening the reporting of observational studies in epidemiology (STROBE) statement. J Clin Epidemiol. 2009;62(6):581–98. doi: 10.1016/j.jclinepi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Ozaki T, Okada M, Kurioka H, Kanasaki H, Miyazaki K. Increased prevalence of luteinizing hormone beta-subunit variant in patients with premature ovarian failure. Fertil Steril. 1999;71(1):96–101. doi: 10.1016/S0015-0282(98)00409-9. [DOI] [PubMed] [Google Scholar]
- 45.Raivio T, Huhtaniemi I, Anttila R, Siimes MA, Hagenas L, Nilsson C, et al. The role of luteinizing hormone-beta gene polymorphism in the onset and progression of puberty in healthy boys. J Clin Endocrinol Metab. 1996;81(9):3278–82. doi: 10.1210/jcem.81.9.8784083. [DOI] [PubMed] [Google Scholar]
- 46.Rajkhowa M, Talbot JA, Jones PW, Pettersson K, Haavisto AM, Huhtaniemi I, et al. Prevalence of an immunological LH beta-subunit variant in a UK population of healthy women and women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1995;43(3):297–303. doi: 10.1111/j.1365-2265.1995.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 47.Tapanainen JS, Koivunen R, Fauser BC, Taylor AE, Clayton RN, Rajkowa M, et al. A new contributing factor to polycystic ovary syndrome: the genetic variant of luteinizing hormone. J Clin Endocrinol Metab. 1999;84(5):1711–5. doi: 10.1210/jcem.84.5.5702. [DOI] [PubMed] [Google Scholar]
- 48.Gazvani R, Pakarinen P, Fowler P, Logan S, Huhtaniemi I. Lack of association of the common immunologically anomalous LH with endometriosis. Hum Reprod. 2002;17(6):1532–4. doi: 10.1093/humrep/17.6.1532. [DOI] [PubMed] [Google Scholar]
- 49.Greb RR, Grieshaber K, Gromoll J, Sonntag B, Nieschlag E, Kiesel L, et al. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamics of the menstrual cycle. J Clin Endocrinol Metab. 2005;90(8):4866–72. doi: 10.1210/jc.2004-2268. [DOI] [PubMed] [Google Scholar]
- 50.Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85(9):3365–9. doi: 10.1210/jcem.85.9.6789. [DOI] [PubMed] [Google Scholar]
- 51.Grigorova M, Punab M, Poolamets O, Sober S, Vihljajev V, Zilaitiene B, et al. Study in 1790 Baltic men: FSHR Asn680Ser polymorphism affects total testes volume. Andrology. 2013;1(2):293–300. doi: 10.1111/j.2047-2927.2012.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang HS, Cheng BH, Wu HM, Yen CF, Liu CT, Chao A. A mutant single nucleotide polymorphism of follicle-stimulating hormone receptor is associated with a lower risk of endometriosis. Fertil Steril. 2011;95(1):455–7. doi: 10.1016/j.fertnstert.2010.07.1092. [DOI] [PubMed] [Google Scholar]
- 53.Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):94–8. doi: 10.1016/j.ejogrb.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Maiorana A, Cicerone C, Niceta M, Alio L. Evaluation of serum CA 125 levels in patients with pelvic pain related to endometriosis. Int J Biol Markers. 2007;22(3):200–2. doi: 10.1177/172460080702200306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 216 kb)