Abstract
Purpose
Researchers have hypothesized that an imbalance of immune cells in the uterine decidua and a dysfunction in cytokines they produce may contribute to recurrent pregnancy loss (RPL). The objective of this study was to determine if IL-22, IL-23 and IL-17 are expressed abnormally in the decidua of patients with RPL compared to those women with a normal pregnancy. We also sought to confirm that uterine natural killer (uNK) cells are lower in the decidua of patients with RPL, as well as identify IL-22 expression by uNK cells.
Methods
After meeting strict inclusion criteria, maternal decidua of nine patients with unexplained RPL and a confirmed euploid fetal loss, and 11 gestational age-matched patients undergoing elective pregnancy termination were included in our analysis. Quantitative real time-polymerase chain reaction (qRT-PCR) was performed to quantify RNA expression, Western blot was performed to quantify protein expression and immunohistochemistry (IHC) was performed to identify IL-22 and uNK cells.
Results
We found that women with unexplained RPL and a euploid fetal loss had significantly less gene and protein expression of IL-22 in the decidua. Additionally, we found that IL-22 is primarily expressed by uNK cells in the decidua.
Conclusions
In conclusion, our results suggest that lower levels of IL-22 in the uterine decidua in patients with unexplained RPL may contribute to a disruption of decidual homeostasis and ultimately lead to early pregnancy loss.
Keywords: Recurrent pregnancy loss, Interleukin-22, Interleukin-17, Interleukin-23, Uterine decidua
Introduction
Spontaneous miscarriage is the most common complication of early pregnancy, affecting 10–25 % of all clinically recognized pregnancies [1, 2]. After one loss, most women will go on to have a normal live birth; however, there is a small subset of women (1–5 %) that will go on to suffer two or more clinical pregnancy losses [3]. After a complete evaluation, including evaluation for fetal aneuploidy, nearly 25 % of all patients with recurrent pregnancy loss (RPL) will remain unexplained [4]. Many hypothesize that specific immune cells, which express various cytokines, at the maternal-fetal interface are critical to early pregnancy survival.
Initial publications reported that patients with RPL had an imbalance of uterine natural killer (uNK) cells, CD4+ T cells and macrophages. Published data on NK cell levels in RPL is contradictory; studies addressing the decidua of pregnancy loss specimens at the time of dilatation and curettage (D&C) have been the most consistent, with the majority of groups reporting lower numbers of uNK cells present in the decidua basalis of RPL specimens compared to normal pregnant controls [5–7]. More recently, the decidua of patients with RPL have also been evaluated for the presence of T helper (Th) 17 cells. Those patients with RPL have been shown to have higher numbers of Th17 cells in the decidua than a fertile control population [8, 9].
Although evidence has validated that patients with RPL have abnormal distributions of immune cells in the decidua, it is also likely that the leukocytes present in this patient population exhibit abnormalities in function [6–9]. This dysfunction may result in abnormal cytokine expression in the decidua of patients with RPL. Although publications on cytokine levels in patients with RPL are abundant, research looking specifically at decidua specimens at the time of a fetal loss (with a confirmed euploid fetus) is limited. Knowing this limitation in the literature, we sought to determine if three cytokines, emerging as important regulators of host defense and excessive inflammation, are altered in patients with unexplained RPL and a euploid loss.
Interleukin-22 has been the focus of emerging research in a variety of systemic inflammatory diseases and it has been identified as a key regulator of host defense acting directly at the mucosal barriers [10, 11]. Little is known about IL-22 and reproductive function. In 2013, Wang et al. reported that fetal trophoblasts express the IL-22 receptor (IL-22R1) and have enhanced proliferation when exposed to IL-22. They also found that in spontaneous abortion specimens there is significantly lower IL-22R1 protein expression compared to normal pregnancies [12]. Although they did show a relationship between IL-22 and IL-22R1 on the trophoblast, their primary objective was to evaluate trophoblast tissues for IL-22R1. To date, there is no published research quantifying gene and protein expression of IL-22 in normal pregnancies or in unexplained RPL specimens in the decidua.
Interleukin-23 and IL-17 are two cytokines that have been shown to interact with IL-22 in various systemic inflammatory conditions. Interleukin-23 is expressed predominantly by activated macrophages and dendritic cells. Upon release from these cells, IL-23 initiates Th17 cell proliferation, as well as stimulates the innate lymphoid cells to proliferate and secrete various cytokines, specifically IL-22 [13]. Once activated, the Th17 cells produce a pro-inflammatory cytokine, IL-17, which acts at the same tissues as IL-22. Previous publications in the reproductive literature have reported higher levels of IL-23 and IL-17 in the decidua of patients with RPL [8, 9]. Given these previous findings, we hypothesized that IL-22 and IL-17 are antagonistic in RPL, where IL-22 is tissue protective and IL-17 is destructive.
In systemic diseases, it appears that IL-22 is primarily produced by T cells, most commonly Th1 and Th17 cells, with less expression from NK cells [10, 14]. Research in reproductive tissues is not as definitive, but a few studies have identified uNK cells that are capable of expressing IL-22 [15]. Although publications have shown a link between uNK cells and IL-22 expression, none have shown that IL-22 is predominantly expressed by uNK cells in the decidua of normal pregnancies, as well as in tissues of patients with unexplained RPL.
The primary objective of this study was to evaluate the gene and protein expression levels of IL-22, IL-23 and IL-17 in the decidua of patients with unexplained RPL and a confirmed euploid fetal loss. Additionally, we sought to confirm that in this well characterized group of unexplained RPL patients, the number of uNK cells is lower in the decidua at the time of fetal loss. We hypothesized that fewer numbers of uNK cells, as well as dysfunctional cytokine production from these leukocytes will result in significantly lower levels of IL-22 in the decidua of patients with truly unexplained RPL compared to those patients with normal pregnancies.
Materials and methods
Participant population and tissue sampling
Patients receiving care at the RPL clinic at Stanford University Fertility and Reproductive Medicine Center and Planned Parenthood were offered enrollment in this study after written informed consent. The protocol was approved by the Institutional Review Board prior to enrollment. Prior to enrollment, RPL patients who presented to the clinic completed an ASRM recommended evaluation for RPL, including a uterine cavity evaluation, anti-phospholipid antibody testing and parental chromosome testing [16]. Those patients with a negative work-up were diagnosed with unexplained RPL and were encouraged to try and conceive spontaneously. The RPL patients were prescribed a daily baby aspirin, which they were instructed to start immediately, and continue throughout the pregnancy. They were also instructed to call the office with a missed menses or a positive home pregnancy test. After pregnancy was confirmed with a serum quantitative human chorionic gonadotropin level, the RPL patients were started on supplemental vaginal progesterone suppositories and were instructed to use it daily, through 12 weeks of gestation.
Twenty-nine established RPL patients with ≥3 clinical miscarriages presented to the RPL clinic for ultrasound guided suction D&C after a diagnosis of spontaneous abortion (Fig. 1). The diagnosis of spontaneous abortion was made when fetal cardiac activity was absent in an appropriately dated pregnancy and it was confirmed at the time of the ultrasound-guided D&C. The D&C was performed within 5 days of the diagnosis of fetal loss. Of the 29 RPL patients that presented, three were excluded from enrollment because they declined to participate or had a positive finding on her ASRM recommended evaluation [16]. After the procedure, chorionic villi were carefully separated from the maternal decidua (described in detail below). The chorionic villi were then sent for cytogenetic analysis. Cytogenetic analysis of the chorionic villi was performed using single nucleotide polymorphism (SNP) array. Of the 26 specimens sent for analysis, nine were confirmed euploid losses, four were 46XY and five were 46XX. The samples that were identified as 46XX were further evaluated for maternal cell contamination using comparative SNP analysis with parental genomic information. After following strict inclusion and exclusion criteria, we had nine samples of unexplained, euploid RPL losses for these experiments.
Fig. 1.
Screening process and exclusion criteria for study participation. Final study population consisted of confirmed unexplained recurrent pregnancy loss (RPL) patients with a euploid fetal loss (4- 46XY and 5- 46XX ruled out for maternal cell contamination with single nucleotide polymorphism microarray analysis) and gestational age-matched controls presenting for elective termination of pregnancy
The control population consisted of patients presenting for elective pregnancy termination at Planned Parenthood. Immediately prior to the procedure, the control patient underwent a transvaginal ultrasound for dating and each patient had a documented fetal heartbeat. Initially, 32 patients were evaluated for inclusion in the study; 15 patients were excluded as they were >10 weeks gestation and six declined to participate or were <18 years of age. A total of 11 gestational age-matched normal pregnancies undergoing an elective termination of pregnancy were included in the control population (Fig. 1).
Tissue processing
At the time of D&C, chorionic villi were carefully dissected from the maternal decidua. The tissue samples were washed repeatedly with sterile normal saline to remove excess blood and mucus. Then, under a dissecting microscope, the maternal decidua was dissected away from the chorionic villi. The chorionic villi from the patients with RPL were sent for cytogenetic analysis; the villi from the patients in the control population were discarded. A portion of the maternal decidua was then snap frozen in liquid nitrogen and stored at −80 °C for mRNA and protein extraction. The remaining tissue was immediately fixed in 4 % paraformaldehyde and embedded in paraffin for section cutting.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was extracted from the samples with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and total RNA concentrations were determined with NanoDrop. About 2 μg of total RNA was used for reverse transcription with Moloney murine leukemia virus reverse transcriptase (Invitrogen) to generate cDNA for quantitative real time-PCR. Water was used as a negative control and the size and sequencing of each PCR product was used for validation of the results.
Quantitative real time-PCR (qRT-PCR) was then completed on all samples with Power SYBR green master mix (ABI biosystems, Foster City, CA) and the primer for IL-22 (forward: 5′-CGACCAGGTTCTCCTTCCCCA, reverse: 5′-CAGATTTCTGCAGGGCGGCCA), IL-17 (forward: 5′-CCGGACTGTGATGGTCAA, reverse: 5′-CTCATTGCGGTGGAGATT), or IL-23 (forward: 5′-CCTTCTCTGCTCCCTGATAGC, reverse: 5′-GACTGAGGCTTGGAATCTGCT). The qRT-PCR samples were run in triplicate using the ABI 7900HT lightcycler (ABI biosystems, Foster City, CA) and GAPDH was used for normalization of the data. Then the IL-17, IL-22 and IL-23 expression was quantified by Comparative CT (∆∆ CT) method.
Protein extraction and western blot
Protein from frozen tissues were extracted with RIPA lysis buffer, protease inhibitor cocktail, and quantified using the Bio-Rad Bradford Assay method. About 20 μg of protein from each sample, loading buffer as a negative control, and a commercial molecular weight marker were resolved on a 12 % SDS-polyacrylamide gel, and then transferred to a nitrocellulose membrane. The membrane was blocked with 5 % non-fat milk in Tris buffer saline (TBS), then cut into two separate membranes at 25kD for probing with IL-22 (ABCAM, rabbit polyclonal, 1:1000 dilution) and beta-actin (Santa Cruz, rabbit polyclonal, 1:400 dilution) overnight at 4 °C, separately. They were then washed with TBST (0.1 % Tween 20 in TBS) and incubated with goat anti-rabbit IgGs conjugated to horseradish peroxidase (Santa Cruz, 1:5000 dilution) for 1 h at room temperature. Then the membranes were visualized with a film after incubation with an ECL solution (Cell Signaling Technology). The IL-22 bands were identified according to its previously reported size in the literature, as well as the antibody sheet provided by ABCAM. The IL-22 expression intensity was semi-quantified by densitometric analysis of bands using ImageJ and normalized to beta-actin in each sample.
Immunohistochemistry
For IHC, 5 μm paraffin sections were deparaffinized and rehydrated and then heated in sodium citrate buffer (pH 6.0) for antigen retrieval. Once cool, the slides were rinsed with 0.1 % Tween 20 phosphate buffer solution (PBS-T) and then incubated mouse anti-CD56 (Zymed, 1:100 dilution) at 4 °C overnight after blocking. Sections incubated without the primary antibody were used as negative controls. Then the sections were visualized by biotin-conjugated horse anti-mouse antibody and Avidin-FITC (VECTOR Lab, Burlingame, CA). For double immunostaining, the sections were first blocked with avidin and biotin blocking reagent, and then incubated with rabbit anti-IL-22 (ABCAM, 1:200 dilution, Cambridge, MA) overnight at 4 °C. The next day, the sections were visualized by biotin-conjugated goat anti-rabbit antibody and Avidin-Texas Red (VECTOR Lab, Burlingame, CA). Then the sections were mounted with mounting medium with DAPI (Vectashield), and observed with a Zeiss Axioskop 2 microscope and images were captured using a Zeiss AxioCam camera.
Statistical analysis
The data were analyzed using Microsoft Office Excel and GraphPad Prism calculator. The mean, standard deviation, median and range were used for calculation of patient characteristics. The median was used when comparing the results from qRT-PCR and western blot in the two groups, as the individual measurements did not follow the normal distribution. The mean values were analyzed using the student t-test and the median values compared with the Mann-Whitney U test. The difference was considered statistically significant when the p-values were <0.05.
Results
Patient populations are similar for both the RPL and control groups
The age, body mass index, gestational age at time of loss and gravidity were not statistically different between the two groups. As expected, the RPL patient population had a significantly greater number of clinical losses. The demographic and patient characteristics are compared in Table 1.
Table 1.
Baseline patient characteristics
| Recurrent pregnancy loss patients (n = 9) | Control patients (n = 11) | p-value | |
|---|---|---|---|
| Age (mean +/− SD) (median years, range) |
37.1 +/− 2.6 39 (33–39) |
33.2 +/− 5.7 33 (22–44) |
NS NS |
| BMI (mean +/− SD) (median, range) |
22.8 +/− 2.3 23 (19–26) |
23 +/− 2.2 23 (20–27) |
NS NS |
| GA at time of loss (mean +/− SD) (median weeks, range) |
7.2 +/− 1.5 7 (6–10) |
7.6 +/− 1.1 8 (6–10) |
NS NS |
| Gravidity (mean +/− SD) (median, range) |
4.3 +/− 1.4 4 (3–7) |
3.4 +/− 2.4 3(1–9) |
NS NS |
| Number of current loss (mean+/− SD) (median, range) |
4.3 +/− 1.4 4 (3–7) |
0.4 +/− 0.7 0 (0–2) |
<0.05 <0.05 |
IL-23 and IL-17 gene expression in the decidua of patients with RPL does not show a statistically significant difference
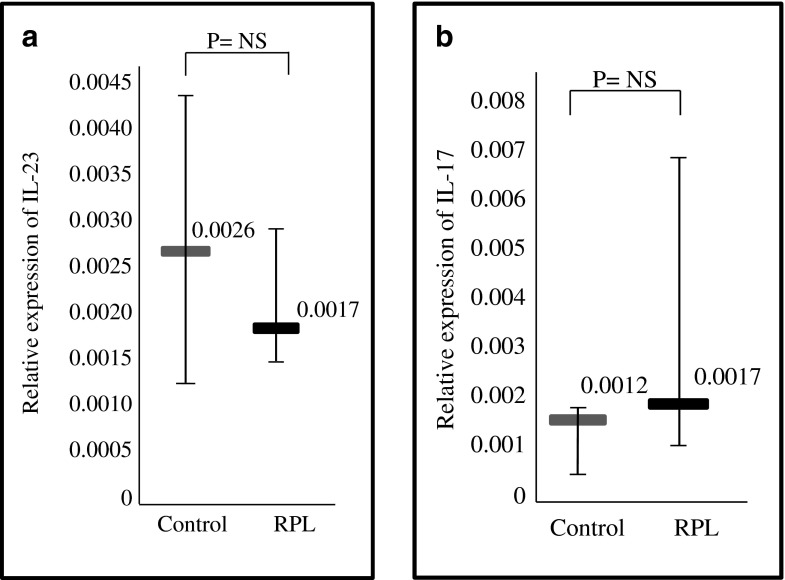
Using qRT-PCR, the gene expression of IL-23 and IL-17 were analyzed in the decidua of patients with RPL and the control group. The gene expression of IL-23 showed a trend towards greater expression in the control group; however, it did not reach statistical significance (Fig. 2a). The gene expression of IL-17 was also similar between the two groups (Fig. 2b).
Fig. 2.
Gene expression of interleukin (IL)-23 and IL-17 in the decidua of normal pregnancies (controls) and patients with recurrent pregnancy loss (RPL). a. Relative gene expression of IL-23, assessed by quantitative real time- polymerase chain reaction (qRT-PCR), in decidua of control patients versus patients with RPL. The relative expression of IL-23 was quantified by Comparative CT (∆∆ CT) method. p = NS b. Relative gene expression of IL-17, assessed by quantitative real time- polymerase chain reaction (qRT-PCR), in decidua of control patients versus patients with RPL. The relative expression of IL-17 was quantified by Comparative CT (∆∆CT) method. p = NS Horizontal lines represent the median, whiskers represent the range
Reduced IL-22 gene and protein expression in the decidua of patients with RPL
Using qRT-PCR, the gene expression of IL-22 was analyzed in the decidua of both groups. The gene expression pattern of IL-22 was significantly reduced in the decidua of patients with RPL (median 2.9 [range 2.0-4.4]), compared to normal controls (median 5.1 [range 3.1–6.8], p < 0.05) (Fig. 3a). Also, the overall expression level of IL-22 was much higher than IL-17 and IL-23 in the decidua from both control and RPL patients.
Fig. 3.
Gene and protein expression of interleukin (IL)-22 in the decidua of normal pregnancies (controls) and patients with recurrent pregnancy loss (RPL). a. Relative gene expression of IL-22, assessed by quantitative real time- polymerase chain reaction (qRT-PCR), in decidua of control patients versus patients with RPL. The relative expression of IL-22 was quantified by Comparative CT (∆∆ CT) method. p < 0.05 b. Representative image of Western blot bands of IL-22 and b-actin to normalize protein levels in decidua of controls and RPL patients. c. The expression of IL-22 was semi-quantified by densitometric analysis of bands using ImageJ and normalized to beta-actin in each sample. p < 0.05 Horizontal lines represent the median, whiskers represent the range
After identifying a down-regulation of gene expression of IL-22 in the decidua of patients with RPL, IL-22 down-regulation was further confirmed at the protein level by western blot. Expression of IL-22 protein in the decidua was detected by western blot for both groups. Beta-actin from the same samples were used to normalize the IL-22 expression. In patients with RPL, the decidua expressed significantly less IL-22 protein (median 0.05 [range 0.005–0.11]) compared to the control population (median 0.19 [range 0.08–0.29], p < 0.05) (Fig. 3b and c). There was a greater than 3-fold reduction in IL-22 protein expression in the RPL decidua.
Uterine natural killer cells express IL-22 in uterine decidua
The uterine decidua of pregnancies, ranging between 6 and 8 weeks gestational age, from both groups were evaluated for CD56 (uNK) and IL-22 using IHC. The slide sections were evaluated at 10×, 20× and 40× magnification. The number of CD56 positive and IL-22 positive cells were counted at 40× magnification on at least three sections from each of the pregnancies. The number of CD56+ cells in the decidua of patients with normal pregnancies (median 50 [range 30–80]) was slightly higher than those women with RPL (median 41 [range 31–58]); however, this finding was not statistically significant (Fig 4a and c). The number of IL-22+ cells in the decidua was statistically higher in the patients with normal pregnancies (median 52 [range 36–65]), compared to those women with RPL (median 36 [range 26–58], p < 0.05) (Fig. 4b and d). When compared to the decidua of patients with RPL, there were consistently more IL-22+ cells present in the decidua of the normal pregnancies.
Fig. 4.
Immunohistochemical analysis of cluster of differentiation (CD)56 (green) and interleukin (IL)-22 (red) in decidua samples from normal pregnancies (controls) (a,b) and patients with recurrent pregnancy loss (RPL) (c,d). Five μm thick paraffin-embedded sections of decidua from controls and patients with RPL were incubated with CD56 and IL-22 antibodies. All images magnified at 20×. a. Control decidua sample, stained for CD56. b. Control decidua sample, stained for IL-22. c. RPL decidua sample, stained for CD56. d. RPL decidua sample, stained for IL-22
Examination of the double-immunostained sections revealed that 90 % (range 78–95 %) of the cells expressing IL-22 in the controls and 93 % (range 89–98 %) in the RPL patients were also immunopositive for CD56, suggesting that uNK cells are the primary source of IL-22 production in the decidua.
Conclusions
The maintenance of early pregnancy involves a unique interaction between the fetal tissue and maternal decidua; one that requires specific leukocyte populations and appropriate cytokine expression. In this study we showed that IL-22 gene and protein expression is reduced in the decidua of women with unexplained RPL and a euploid fetus. In addition, we identified a trend to fewer uNK cells in the decidua of patients with unexplained RPL compared to patients with a normal pregnancy, and also found that IL-22 production appears to be expressed predominantly by uNK cells in the decidua.
IL-22 has emerged as a key regulator of mucosal homeostasis and NK cells are an innate source of this cytokine [17]. Research has shown that IL-22 can help constrain inflammation and protect mucosal sites, and that lower levels of IL-22 are associated with several inflammatory diseases, such as inflammatory bowel disease and acute graft-versus-host disease [18, 19]. Additionally, it has been shown that, patients with worse symptoms and a prolonged disease course have significantly lower levels of IL-22 expression [18, 19]. In this context, IL-22 likely plays a key role in maintaining decidual homeostasis and helps to constrain the inflammation common in early pregnancy. Our finding of lower levels of IL-22 in unexplained RPL may help explain the disruption of homeostasis resulting in recurrent early loss.
In addition, we also sought to confirm findings from earlier research, which indicated that patients with RPL have fewer uNK cells present in the decidua basalis at the time of D&C compared to normal pregnancy controls [5–7]. Although we only saw a trend to fewer uNK cells in the decidua of patients with RPL, we did identify significantly reduced expression of IL-22 in patients with RPL compared to normal pregnancy controls. We also identified that uNK cells appear to be the primary source of IL-22 expression in the uterine decidua. Given the number of uNK cells was not reduced as significantly as the gene and protein expression of IL-22, it is possible that the uNK cells present in the decidua of patients with unexplained RPL are dysfunctional and they do not produce the same level of cytokines as patients with normal pregnancies. Exploring cytokine dysfunction at the level of the immune cells would be an exciting next step in discovering the role of IL-22 in unexplained RPL.
Although these findings are fascinating, we know there are also limitations to this study. The decidua samples from the unexplained RPL population were collected after the diagnosis of fetal loss. It is therefore unfeasible to interpret if our lower levels of IL-22 are a cause of fetal loss or in fact a consequence of fetal death. Additionally, the RPL patients were supplemented with vaginal progesterone suppositories through 12 weeks of gestation. It is highly unlikely that progesterone supplementation would further suppress IL-22 expression below the levels induced by normal, physiologic levels of progesterone during early pregnancy (controls). However, further studies are needed to rule out this possibility. The ideal samples from an unexplained RPL population would be those patients undergoing an elective termination of an early pregnancy; however, volunteers for that study would be extremely challenging to recruit. It would be interesting to investigate the role of IL-22 in early pregnancy in a murine RPL model.
We also evaluated the relationship between IL-23, IL-17 and IL-22 in the decidua of patients with RPL. In past research, both IL-23 and IL-17 were reported to be higher in the decidua of patients with RPL compared to normal pregnancy [8, 9]. Given these past findings, we expected to see an inverse relationship between IL-22 and IL-17, IL-23. Our findings on IL-17 and IL-23 expression were not as robust; we did not find a significant difference in the expression of these two genes in our two patient populations. We did, however see a trend towards a slight increase in IL-17 expression in our patients with RPL. It is possible that with more patient specimens we would have found similar results.
One of the greatest strengths of this study is that we consistently identified lower levels of IL-22 in both gene and protein expression in our unexplained RPL cohort compared to normal pregnancies. These findings suggest that the significantly reduced expression of IL-22 in unexplained RPL patients may play a role in repeat euploid fetal loss or that it may be a marker of immune dysfunction in this cohort.
In conclusion, we are the first group to quantify gene and protein expression of IL-22 in the decidua of normal pregnancies and unexplained, euploid RPL pregnancies. We demonstrated that in patients with unexplained RPL, there is significantly lower IL-22 gene and protein expression in the uterine decidua. We also provide evidence that the IL-22 cytokine is expressed predominantly by the uNK cells of the decidua. It is possible that a slight decrease in uNK cells, in addition to dysfunctional IL-22 expression, contributes to the poor pregnancy outcomes of patients with unexplained RPL.
Acknowledgments
This research was made possible through the support of the Ernest and Amelia Gallo Endowed Postdoctoral fellowship, the Child Health Research Institute, Lucile Packard Foundation for Children’s Health, as well as the Stanford CTSA (grant number UL1 TR000093).
Conflict of interest
There are no known conflicts of interest associated with this publication.
Footnotes
Capsule In patients with unexplained RPL and a euploid fetal loss, lower levels of IL-22 in the decidua may contribute to alterations in decidual homeostasis and play a role in early pregnancy loss.
Contributor Information
Candice O’Hern Perfetto, Phone: 713-790-9900, Email: cperfetto@infertilitytexas.com.
Xiujun Fan, Email: xiujun.fan@gmail.com.
Sabita Dahl, Email: sabitad@stanford.edu.
Sacha Krieg, Email: skrieg@kumc.edu.
Lynn Marie Westphal, Email: lynnw@stanford.edu.
Ruth Bunker Lathi, Email: rlathi@stanford.edu.
Nihar R. Nayak, Email: nayakn@stanford.edu
References
- 1.Jacobs PA, Hassold T. Chromosome abnormalities: origin and etiology in abortions and live births. In: Vogel F, Sperling K, editors. Human genetics. Berlin: Springer; 1987. pp. 233–44. [Google Scholar]
- 2.Warburton D, Fraser FC. Spontaneous abortion risk in man: data from reproductive histories collected in a medical genetics unit. Am J Hum Genet. 1964;116:1–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673–5. doi: 10.1016/0140-6736(90)92159-F. [DOI] [PubMed] [Google Scholar]
- 4.Suguira-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of miscarriage. Hum Reprod. 2012;8:2297–303. doi: 10.1093/humrep/des179. [DOI] [PubMed] [Google Scholar]
- 5.Quack KC, Vassiliadou N, Pudney J, Anderson DJ, Hill JA. Leukocyte activation in the decidua of chromosomally normal and abnormal fetuses from women with recurrent abortion. Hum Reprod. 2001;16:949–55. doi: 10.1093/humrep/16.5.949. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Li YP, Ding B, Zhao YR, Chen ZJ, Xu CY, et al. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J Obstet Gynaecol Res. 2014;40:1288–95. doi: 10.1111/jog.12329. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Takahashi Y, Kase N, Mori H. Decidual natural killer cells in recurrent spontaneous abortion with normal chromosomal content. Am J Reprod Immunol. 1999;41:337–42. doi: 10.1111/j.1600-0897.1999.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, et al. Study on relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–11. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang WJ, Hao CF, Lin Y, Yin GJ, Bao SH, Qiu LH, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84:164–70. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Sabat R, Ouyang W, Wolk K. Therapeutic opportunites of the IL-22-IL-22R1 system. Nat Rev. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–51. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Xu B, Li MQ, Li DJ, Jin LP. IL-22 secreted by decidual stromal cells and NK cells promotes the survival of human trophoblasts. Int J Clin Exp Pathol. 2013;6:1781–90. [PMC free article] [PubMed] [Google Scholar]
- 13.Croxford A, Mair F, Becher B. IL-23: one cytokine in control of autoimmunity. Eur J Immunol. 2012;42:2263–73. doi: 10.1002/eji.201242598. [DOI] [PubMed] [Google Scholar]
- 14.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–9. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 15.Male V, Hughes T, McClory S, Colucci F, Caligiur M, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–8. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Practice Committee of the American Society of Reproductive Medicine Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 17.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339–50. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–57. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]