Abstract
Purpose
Asthenozoospermia is a common cause of human male infertility characterized by reduced sperm motility. The molecular mechanism that impairs sperm motility is not fully understood. This study proposed to identify novel biomarkers by focusing on sperm tail proteomic analysis of asthenozoospermic patients.
Methods
Sperm were isolated from normozoospermic and asthenozoospermic semen samples. Tail fractions were obtained by sonication followed by Percoll gradient. The proteins were extracted by solubilization and subjected to two-dimensional gel electrophoresis (2-DE); then, the spots were analyzed using Image Master 2D Platinum software. The significantly increased/decreased amounts of proteins in the two groups were exploited by matrix-assisted laser desorption-ionization time-of-flight/time-of-flight (MALDI-TOF-TOF) mass spectrometry.
Results
Three hundred ninety protein spots were detected in both groups. Twenty-one protein spots that had significantly altered amounts (p < 0.05) were excised and exploited using MALDI-TOF-TOF mass spectrometry. They led to the identification of the following 14 unique proteins: Tubulin beta 2B; glutathione S-transferase Mu 3; keratin, type II cytoskeletal 1; outer dense fiber protein 2; voltage-dependent anion-selective channel protein 2; A-kinase anchor protein 4; cytochrome c oxidase subunit 6B; sperm protein associated with the nucleus on the X chromosome B; phospholipid hydroperoxide glutathione peroxidase-mitochondrial; isoaspartyl peptidase/L-asparaginase; heat shock-related 70 kDa protein 2; stress-70 protein, mitochondrial; glyceraldehyde-3-phosphate dehydrogenase, testis-specific and clusterin.
Conclusion
Fourteen proteins present in different amounts in asthenozoospermic sperm tail samples were identified, four of which are reported here for the first time. These proteins might be used as markers for the better diagnosis of sperm dysfunctions, targets for male contraceptive development, and to predict embryo quality.
Keywords: Asthenozoospermia, Sperm tail, Two-dimensional gel electrophoresis, Mass spectrometry
Introduction
Approximately 15–20 % of human couples are infertile, and about half of these are cases of male factor infertility [1]. Asthenozoospermia (AS) is a common cause of human male infertility, diagnosed by reduced sperm motility, and has no effective therapeutic treatment [2].
Sperm is a highly polarized cell, and its motility is fully dependent on flagellum. The ability of sperm to move forward is crucial for the successful fertilization of an egg [3]. There is little information about the normal physiology of sperm motility or the proteins responsible for sperm function [4]. In fact, routine semen analysis has its own limitations and does not account for putative sperm dysfunctions. It has become clear that identifying protein markers of sperm motility that could predict fertilizing ability apart from semen analysis criteria is needed [5]. It is possible that individual protein defects in a human might also cause fertilization failure [4].
Several studies have investigated human spermatozoa to understand the role of proteins in sperm function [6–8]. Proteins related to sperm function have been categorized into five groups: 1) sperm movement and structural organization; 2) energy and metabolism; 3) stress response and turn over proteins; 4) signaling and transport proteins; and 5) proteins with antioxidant activity [9]. The altered amounts of these proteins are involved in infertility conditions such as globozoospermia (round-headed sperm) [10], varicocele [11], and oligozoospermia [12].
Proteomics provides new insight into the structural and functional aspects of proteins. This technique could be useful in identifying the proteins responsible for diagnosis of sperm dysfunctions and may assist in predicting further fertilization failure in patients undergoing IVF or ICSI [13]. The strong correlation between progressively motile spermatozoa and IVF success rates is well-proven [13]. A recent study on human sperm proteome demonstrated that some proteins of sperm tail could potentially contribute to embryo quality [14].
The proteomic studies on asthenozoospermic individuals are very limited. To date, 34 proteins responsible for sperm motility have been detected in asthenozoospermic patients, as differentially expressed proteins compared with normozosprmic ones, including Tektin 1, glycerol kinase testis specific, isocitrate dehydrogenase subunit α, heat shock 70 kDa related protein 2, cytochrome c oxidase subunit 6B, prolactin-induced protein, outer dense fiber protein 2, semenogelin, and others [9, 15, 16], and the number of proteins involved in sperm motility identified by proteomic methods is increasing [9, 15].
Sub-cellular fractionation is a good strategy for gleaning more information about sperm motility. It also gives additional knowledge about protein localization and low-abundant proteins compared with whole cell lysates and could provide further information regarding their exact biological functions [17].
The current study aimed to accomplish the proteomic analysis of low motile sperm tails and compare them with normal ones to define the proteins involved in sperm motility and attribute them as probable biomarkers for the better diagnosis of sperm dysfunctions, contraception purposes, and to predict embryo quality.
Materials and methods
Chemicals
Reagents for two-dimensional electrophoresis were purchased from Sigma. IEF strips, Percoll and Coomassie brilliant blue tablets (Phast Gel Blue R-350) were obtained from GE. Silver nitrate, glycerol and glutaraldehide were purchased from Merck (Darmstadt, Germany). Other chemicals like Ham’s F10 (1X, 10X) were purchased from local vendors.
Sample collection and analysis of ejaculates
Semen samples were collected from patients recruited from the Infertility Research and Treatment Center of Khuzestan (ACECR). Included in this study were 35 asthenozoospermic patient sperm samples and 33 sperm samples from normozoospermic individuals. Semen was collected in specific sterile falcon tubes by masturbation after 3–5 days of sexual abstinence and allowed to liquefy for 30–60 min at 37 °C in a CO2 incubator (5 % CO2 in air at 95 % relative humidity).
At least two samples were collected from each patient and control at an interval of 5 days. The normal donors were selected from patients attending the clinic for female factors. After liquefaction of the semen, the sperm parameters (volume, sperm count, percentage of motility, and motion characteristics) were evaluated according to published recommendations of WHO (World Health Organization 2010) guidelines [18] and using a caliber computer-assisted semen analyzer (CASA). Two observers manually checked the sperm parameters. Motility was assigned to the following categories: rapidly progressive (type A), slowly progressive (type B), not progressive (type C), and immotile (type D). Normozoospermic samples (sperm concentration ≥20 × 106/ml, motility grades A and B ≥50 %, normal sperm morphology ≥30 %) and asthenozoospermic semen samples (sperm concentration ≥20 × 106/ml, total of motility grades A and B ≤30 %, normal sperm morphology ≥30 %) were selected for the study. All participants signed consent forms permitting the use of their sperm samples in the study.
Patients with a medical history of depression, diabetes, cancer, hypertension, hyperthyroidism, or sexually transmitted diseases were excluded from sampling as were patients exposed to environmental stress, including radiation or chemicals, smokers, and those with an abnormal body mass index. This study was approved by the Ethics Committee of Ahvaz Jundishapour University of Medical Sciences.
Sample preparation
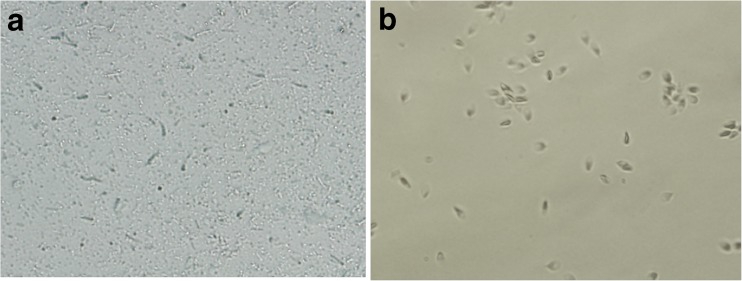
After liquefaction, a single layer of 50 % Percoll (2 ml) was prepared in a Falcon tube as described by Martınez-Heredia et al. [15], overlaid with 2 ml of semen, and centrifuged at 800×g for 20 min at room temperature; the pellet was washed twice with Ham’s F10 medium. After that, 1 mM PMSF was added, and the sample was transferred to a glass tube. Sonication on ice followed with a Dr. HESHELL sonicator (model UP200H, Ultrasonic Corporation, Germany) at 30 % output, three pulses of 15 s each, and at 60-s intervals to prevent excess heating. Then the sample was loaded on 5 ml of 80 % Percoll and centrifuged (650×g, 35 min, 4 °C). The top 1.5 mL was discarded, and the following 1 ml of enriched sperm tail was collected [19] and centrifuged at 16,000×g, for 70 min at 4 °C. It was then washed two times with Tris–HCl 50 mM. The sperm tails were assessed by light microscope and determined to be at least 95 % pure (Fig. 1). They were then stored at -80 C for future use.
Fig. 1.
Image of sperm tail (a) and head (b) after sonication and percoll gradient
Sperm tail preparation for proteomic analysis
Isolated sperm tails were solubilized in lysis buffer(containing 8 M urea, 2 M thiourea, 20 mM DTT, 4 % CHAPS (3-cholamido-propyl)di-methylammonio]-1-propanesulfonate (w/ v), 2 % (v/ v) IPG buffer (PH 3–10), 5 % v/v protease inhibitor cocktail, Tris–HCl 35 mM) by being gently shaken for 1 h at room temperature and centrifuged at 14,000×g for 20 min (to eliminate non solubilized materials).
Protein assay
The total protein concentration of sperm tail was measured by the Bradford method with bovine albumin as the standard.
Each sample was analyzed individually in triplicates [20].
Two-dimensional gel electrophoresis
Sperm tail proteins in quantities of 250 and 1000 μg were assigned to analytical and preparative gels, respectively, for the first dimension electrophoresis. The isoelectric focusing (IEF) was performed in 18 cm IPG strips (pH 3–10, non-linear) using the protean IEF cell (Biorad, Hercules, CA, USA) at 20 °C and a maximum current setting of 50 μA/strip with the following program: 50 V/h with a linear ramp (0–300 V), 50 V/h with a linear ramp (300–600 V), 500 V/h with a linear ramp (600–2000 V), 1000 V/h with a linear ramp (2000–4000 V), 2000 V/h with a rapid ramp (4000–50,000 V) up to 52 kVh. After the IEF, the IPG strips were equilibrated in the equilibration solution (50 mM Tris–HCl (pH 8.8), containing 2 % (w/v) SDS, 1 % (w/v) dithiothreitol (DTT), 6 M urea, and 30 % (w/ v) glycerol for 15 min and then in the same solution containing 5 % iodoacetamide instead of DTT.
The second dimension was carried out in 13.5 % separating gels using the Dodeca Cell System (Bio-Rad Laboratories, Hercules CA, USA) at a constant voltage and 50 mA per gel at 15 °C for 7 h [21].
Silver and coomassie blue staining
The analytical gels were stained with acidic silver nitrate that is very sensitive for protein visualization [22]. Briefly, the gel was fixed in the appropriate first fixation solution (40 % methanol and 7 % acetic acid) and the second fixation solution (5 % methanol and 7 % acetic acid) for 30 min with shaking, respectively. The gel was submerged in 10 % glutaraldehyde for 30 min followed by washes in deionized water. Afterward, it was placed in deionized water containing DTT (5 μg /μL) for 30 min, in 1 % silver nitrate for 30 min with gentle shaking, and then rinsed briefly in deionized water before being placed and developed in developing solution (3 % w/v sodium carbonate and 0.019 % w/v formaldehyde). After the detection of protein spots, the gel was incubated in the stop solution (second fixation solution) for 30 min and finally stored in 7 % acetic acid for future analysis.
The preparative gels were stained by Coomassie blue R-350 (PlusOne Coomassie Tablets, PhastGel Blue, GE Healthcare) for their compatibility with matrix-assisted laser desorption-ionization time-of-flight/time-of-flight (MALDI-TOF-TOF). 200 mL of a 0.1 % staining solution was used. Gels were kept in 7 % acetic acid. Triplicate gels were run for each sample [21].
Imaging and statistical analysis
The gels were scanned with an Image scanner (Amersham Pharmacia, Piscataway, NJ, USA) and saved in TIFF format. Spot detection, quantification, comparisons, and statistical analysis were carried out using ImageMaster 2D Platinum 6.0 software (GE Healthcare). Then images were edited manually. The results were in agreement with the visual inspection. The volume of each spot from three replicate gels was normalized against total spot volume, quantified, and subjected to the Student’s t-test (p < 0.05). Only those spots that were present on all three replicate gels were quantified and enrolled for statistical analysis. The spots with significantly different amounts (p < 0.05) between the two groups were considered to have up- or down-regulation. The ratios of the spot volume values of asthenozoospermic versus normozoospermic were calculated and are listed as the “fold change” column in Table 2. Differences in normalized protein expression ≥1.5-fold were considered as the threshold of expression variation and selected as candidate proteins for MALDI-TOF-TOF analysis.
Table 2.
Proteins identified using MA LDI-TOF-TOF mass spectrometry
| Spot ID a | MASCOT Search Results | Accession Number (SWISS-PROT) | Protein Name | Fold Changef (Asthenospermia/Normozoospermia) | Peptide | ||||
|---|---|---|---|---|---|---|---|---|---|
| Molecular Mass (kDa) | pI b | No. of Matched Peptides | Sequence Coverage c (%) | Score d | |||||
| 24 | 50377 | 6.56 | 3 | 12 | 270 | Q9BVA1 | Tubulin beta-2B chain | −2.3 | R.FPGQLNADLR.K K.GHYTEGAELVDSVLDVVR.K R.SGPFGQIFRPDNFVFGQSGAGNNWAK.G |
| 2 | 32060 | 7.49 | 5 | 28 | 381 | P45880 | Voltage dependent anion selective channel protein 2 | −2.1 | K.WCEYGLTFTEK.WR.NNFAVGYR.T R.TGDFQLHTNVNDGTEFGGSIYQK.V K.VCEDLDTSVNLAWTSGTNCTR.F K.VNNSSLIGVGYTQTLRPGVK.L |
| 88 | 10414 | 6.54 | 4 | 56 | 388 | P14854 | Cytochrome c oxidase subunit | −3.27 | R.FPNQNQTR.N K.GGDISVCEWYQR.V R.NCWQNYLDFHR.C R.VYQSLCPTSWVTDWDEQR.A |
| 29 | 22674 | 8.69 | 6 | 35 | 343 | P36969 | Phospholipid hydroperoxide glutathione peroxidase, mitochondrial | −1.96 | K.TEVNYTQLVDLHAR.Y R.ILAFPCNQFGK.Q K.QEPGSNEEIKEFAAGYNVK.F K.ICVNGDDAHPLWK.W R.YGPMEEPLVIEK.D R.YGPMEEPLVIEK.D |
| 56 | 32376 | 5.84 | 2 | 8 | 172 | Q7L266 | Isoaspartyl peptidase/Lasparaginase | +1.6 | R.LTLFHIEQGK.T K.LHFGIDPDDTTITDLP |
| 224 | 96140 | 7.53 | 6 | 6 | 331 | Q9BVA1 | Outer dense fiber protein 2 | −6.9 | K.NIDLTAIISDLR.S R.KNIDLTAIISDLR.S K.GDLELEIIVLNDR.V K.TRLEADEVAAQLER.C K.LAECQDQLQGYER.K K.EKGDLELEIIVLNDR.V |
| 36 | 95842 | 6.56 | 5 | 7 | 277 | P45880 | A-kinase anchor protein 4 | −6.1 | K.VGDTEGEYHR.A K.VDLYNPEGQQDQDR.K K.VDLYNPEGQQDQDRK.V K.NTNNNQSPSAPPAKPPSTQR.A K.VICFVDVSTLNVEDKDYK.D |
| 113 | 95842 | 6.56 | 4 | 4 | 237 | P14854 | A-kinase anchor protein 4 | −4.6 | K.VDLYNPEGQQDQDR.K K.VDLYNPEGQQDQDRK.V K.NTNNNQSPSAPPAKPPSTQR.A K.VICFVDVSTLNVEDKDYK.D |
| 40 | 44815 | 8.38 | 5 | 23 | 413 | P36969 | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | −3.9 | R.DIVLTNVTVVQLLR.Q K.AEVEPQPQPEPTPVR.E R.NGQLVVDNHEISVYQCK.E R.EEIKPPPPPLPPHPATPPPK.M R.AVGSPYVVESTGVYLSIQAASDHIS AGAQR.V |
| 269 | 53031 | 5.89 | 1 | 3 | 109 | Q7L266 | Clusterin | +3 | K.LFDSDPITVTVPVEVSR.K |
| 123 | 26998 | 5.37 | 1 | 7 | 56 | P21266 | Glutathione S-transferase Mu 3 | −2.3 | K.LTFVDFLTYDILDQNR.I |
| 150 | 70263 | 5.56 | 1 | 2 | 50 | P54652 | Heat shock-related 70 kDa protein 2 | −2.8 | K.GQIQEIVLVGGSTR.I |
| 155 | 73920 | 5.87 | 1 | 2 | 45 | P38646 | Stress-70 protein,mitochondrial | −2.8 | K.LLGQFTLIGIPPAPR.G |
| 188 | 11032 | 5.22 | 1 | 12 | 32 | Q8TAD1 | Sperm protein associated with the nucleus on the X chromosome E | −6.8 | R.TSPEELVNDHAR.E |
| 290 | 66170 | 8.15 | 1 | 4 | 56 | P04264 | Keratin, type II cytoskeletal | +2.85 | R.GGGGGGYGSGGSSYGSGGGSYGSG GGGGGGR.G |
a Spots are numbered according to the 2-DE gel
b Isoelectric point
c Percentage of the protein sequence covered by identified peptides
d The protein score is the sum of all of the ion scores of all of the peptides
e The MASCOT results of MALDI-TOF-TOF
f The fold changes in downregulated and up regulated proteins are shown as negative and positive values respectively
Protein identification by mass spectrometry
Differentially expressed spots were manually excised from preparative Coomassie blue stained gels. Analysis was carried out by the Proteomics Laboratory; University of York, U.K., using MALDI-TOF-TOF mass spectrometry as previously described [21].
Sequence database searching and protein identification
Tandem mass spectral data were submitted to database searching using a locally-running copy of the MASCOT program against the IPI (International Protein Index) human database (Matrix Science Ltd., version 2.1) through the Bruker ProteinScape interface (version 2.1). The following specified parameters were applied for the database search: database (SwissProt), taxonomy (homo sapiens), proteolytic enzyme (trypsin), peptide mass tolerance (±100 ppm), fragment mass tolerance (±0.5 Da), fixed modification (carbamidomethyl (Cys)), variable modification (oxidation (Met)), and max missed cleavage [21].
Statistical analysis
All data were presented as the mean ± standard deviation. The Student’s t-test was used for statistical comparisons. The statistically significant level was p < 0.05.
Results
Semen parameters
The mean rapid progressive motility (%) of normal and asthenozoospermic groups were 27.89 ± 4.45 and 4.82 ± 2.44, respectively, and the mean morphology (%) were 32.6 ± 1.7 and 29.2 ± 2.05 (Table 1).
Table 1.
Basic data of asthenozoospermic and normozoospermic semen samples
| Asthenozoospermia | Normozoospermia | |
|---|---|---|
| Mean age of patients (year) | 33.7 ± 4.69 | 31.05 ± 4.69 |
| Volume(ml) | 3.57 ± 1.25 | 3.73 ± 0.98 |
| Sperm count(×106 sperm/ml) | 53.7 ± 17.1 | 66.9 ± 17.19 |
| Normal morphology (%) | 29.2 ± 2.05 | 32.6 ± 1.7 |
| Motility A (Rapid progressive%) | 4.82 ± 2.44 | 27.89 ± 4.45 |
| Motility B (slow or sluggish %) | 22.52 ± 7.57 | 21.39 ± 4.75 |
| Motility C (non progressive %) | 12.55 ± 5.62 | 2.36 ± 3.13 |
Values are presented as the mean ± SD
Isolation of sperm tail
To obtain a tail fraction from the head segment, sonication followed by Percoll gradient and centrifugation were applied. The tail fraction was obtained from interphase and the heads from pellet. The two collected fractions were enriched in isolated tail pieces and heads, respectively (Fig. 1).
Gel imaging
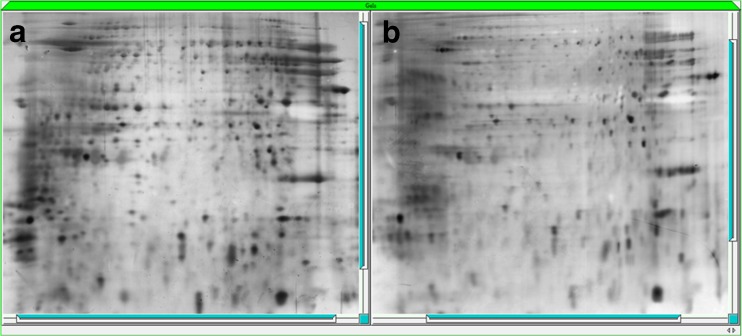
The 2D sperm tail protein patterns were similar between the asthenozoospermia and normal groups (Fig. 2). The mean number of spots detected per gel was 390.
Fig. 2.
Two-dimensional gel electrophoresis of normozoospermic (a) and sthenozoospermic groups (b) (silver staining)
Mass spectrometry assessment and MASCOT report
Twenty-one protein spots (which underwent changes in expression level) were excised from the gels and analyzed by MALDI-TOF-TOF. As a result, 14 unique proteins were identified. MASCOT supporting identification data are listed in Table 2. Sequence similarity is available as an NCBI BLAST search of accession number.
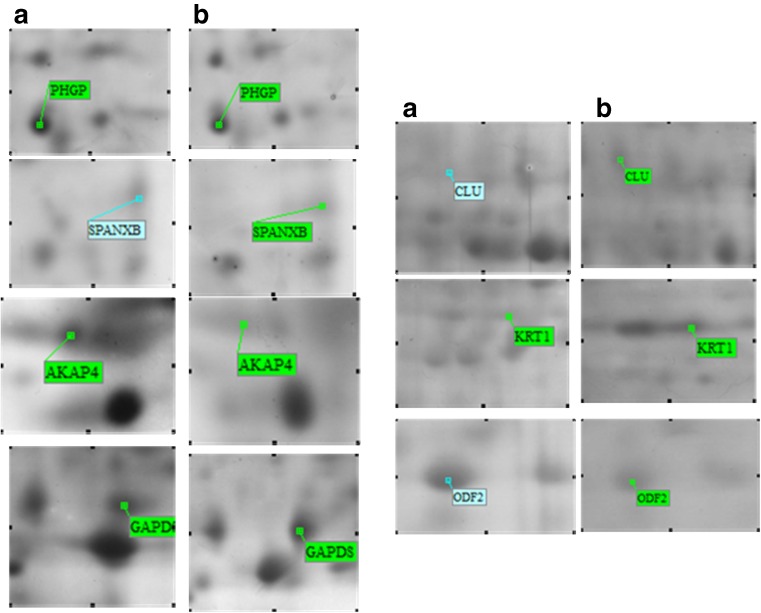
The proteins included: Tubulin beta 2B (spot 24); glutathione S-transferase Mu 3 (GST Mu3) (spot 123); keratin, type II cytoskeletal 1 (KRT1) (spot 226); outer dense fiber protein 2 (ODF2) (spots 216, 217, 227, 290); voltage-dependent anion-selective channel protein 2 (VDAC2) (spot 2); A-kinase anchor protein 4 (AKAP4) (spots 36, 113, 114); cytochrome c oxidase subunit 6B (COX6B) (spot 88); sperm protein associated with the nucleus on the X chromosome B (SPANXB) (spot 188); phospholipid hydroperoxide glutathione peroxidase-mitochondrial (PHGPx) (spot 29); Isoaspartyl peptidase/L-asparaginase (ASRGL1) (spot 56); heat shock-related 70 kDa protein 2 (HSPA2) (spot 150); stress-70 protein, mitochondrial (HSPA9) (spot 177); glyceraldehyde-3-phosphate dehydrogenase, testis-specific (GAPDS) (spots 40, 80, 170), and clusterin (CLU) (spot 269) (Fig. 3).
Fig. 3.
Magnified Two-dimensional gel electrophoresis maps of some representative spots with differential expression between the normozoospermic (a) and asthenozoospermic patients (b)
The majority of these proteins belong to the five following sperm functional groups: proteins related to sperm movement and structural organization: (TUBB2B, ODF2, AKAP4, KRT1, CLU); proteins involved in energy and metabolism: (COX6B, GAPDS, PHGPx); stress response and turn over proteins: (HSPA2, HSPA9); signaling and transport proteins: (VDAC2); and proteins with antioxidant activity: (GST Mu3).
The definite functions of two proteins, namely ASRGL1 and SPANXB, have not yet been clearly identified.
It should be noted that the PI of these proteins ranged between 4.5 and 8.7 with most of them ranging between 4.5 and 6. The molecular weights of the proteins ranged from 10 to 96 KDal, and eight proteins had molecular weights of more than 50 KDa.
Among these, eleven proteins (AKAP4, ODF2, TUBB2B, COX6B, GSTMu3, PHGPx, GAPD-S, VDAC2, HSPA2, HSPA9,and SPANX B) had higher expression levels in normal donors. Results also indicated the increased amounts of three proteins that had higher expression levels in asthenozoospermic patients, namely CLU, KRT1, and ASRGL1. Four spots matched ODF2, three spots corresponded with AKAP4, and three other spots matched with GAPD-S.
Discussion
This study investigated the protein composition of human sperm tail from normozoospermic and asthenozoospermic patients using the MALDI-TOF-TOF technique. The results identified 14 differentially expressed proteins between the two groups.
In their recent proteomic studies of sperm, Baker et al. (2013) and Amaral et al.(2013) identified the proteins belonging to normal sperm head and tail domains [14, 19].
On the basis of the proteome analysis presented in our study, TUBB2B was found to be more abundant in the normal donors than in the asthenozoospermic group. Tubulin is known to play a crucial role in the microtubules of flagellum. Although the organization of microtubules in the tail is well known, the relationship between tubulin changes and pathological conditions of spermatozoa is not fully understood [23]. Significantly lower concentrations of Tubulin beta 2C isomer in asthenozoospermia and globozoospermia patients were shown by Siva et al.(2010) and Liao et al.(2009), respectively [9, 10]. In a previous genetic study on Drosophila, Fackenthal et al.(1993) demonstrated that TUBB2B was expressed in post-mitotic cells in male germ cells and are essential for the formation of meiotic spindles and cytoplasmic microtubules. More particularly, they share in the organization of 9 + 2 of the axonemal microtubule in sperm tail [24]. Chemes et al.(1998) in an ultra structural study on asthenozoospermic patients with dysplasia of fibrous sheat (DFS) showed missing axonemal central pairs and complete distortion of 9 + 2 axonemal structure in many of patients, that not apparent on semen smears [25]. In this regard, reduction of TUBB2B might lead to the formation of defective microtubules of sperm flagellum and, consequently, result in defective sperm motility. Of course, additional studies are needed to establish the actual influence of the TUBB2B protein on sperm motility.
The present study showed four protein spots (spot IDs 216, 217, 227, 290) corresponding to ODF 2. Furthermore, it showed that their expression was significantly decreased in the asthenozoospermic group.
outer dense fibers (ODFs), is an accessory structure that surround the axoneme of the sperm tail and help to preserve the elastic rigidity of sperm flagellum [26]. Numerous polypeptides such as ODF1 and ODF2 constitute the ODF sheath [27]. Petersen et al. (1999) demonstrated that the absence of one or more outer dense fiber proteins could affect sperm motility and cause nonfunctional tails; thus, outer dense fiber proteins may act as markers of male factor infertility [28]. An ultra structural study of asthenozoospermia men with DFS by Chemes et al.(1998) showed the abnormal extension of ODF to the principal piece [25]. The present study demonstrated that ODF2 was less expressed in asthenozoospermic patients that might result in abnormality of outer dense fibers and decreased elasticity of sperm flagellum which could affect sperm motility.
Another finding of this study was the down-regulation of AKAP4 in the asthenozoospermic group. Teddy et al. (2003) found that, in the human fibrous sheath, AKAP3 and AKAP4 are the most abundant structural proteins and anchor cyclic-AMP-(cAMP)-dependent protein kinase A (PKA) to the fibrous sheath that increases the tyrosine phosphorylation of sperm proteins and leads to activation of flagellum [29]. Miki et al. (2004) showed that deleting the AKAP 4 gene in male mice caused incomplete formation of the fibrous sheath, which resulted in loss of motility [30]. Baccetti and co workers (2005) in a study on asthenozoospermia men with DFS showed the badly assembled and thickened of fibrous sheath in TEM, and partial sequence of AKAP4/AKAP3 binding regions by PCR [31]. Taken together, it seems that AKAP4 not only has a pivotal role in normal morphology of sperm, but also could influence the sperm locomotion and might be a biomarker of understanding prefertilization events.
In this study, KRT1 in asthenozoospermic samples was up-regulated. Sperm hyper activation is necessary for zona reaction and regulated by post-translational modifications (PTMs), i.e., thyrosin phosphorylation [13] and KRT1 is a structural protein which requires phosphorylation [32]. Asthenozoospermic samples display lower levels of tyrosine phosphorylation [33]. It seems that the higher expression of KRT1 in asthenozoospermia is a compensatory effect of lower levels of tyrosine phosphorylation. Nevertheless, the local synthesis of KRT1 in sperm tails and its role in regulating sperm function should be well-documented by further studies.
Higher expression of CLU in the asthenozoospermic group was another finding of this research. In bulls, CLU presented on abnormal spermatozoa which correlated negatively with motility and maturity [34]. CLU has been found in seminal fluid as well as on the plasma membrane of sperm heads and tails [15]. Future studies should clarify the relationship between altered amounts of CLU on sperm surfaces and contents of seminal plasma.
The results of the current study also showed that the GAPDS protein (spots 40, 80, and 170) was significantly lower in the asthenozoospermic group. GAPDS is a glycolytic enzyme and its arrangement along the principal piece of sperm tail might be related to ATP production in distal regions of the flagellum [6]. Francavilla et al.(2006) in an Ultrastructural analysis of asthenozoospermic ejaculates reported that constituents of fibrous sheath formed irregular masses around the axoneme and disorganized or missing of mid piece, in cases of DFS [35] Miki et al. (2004) demonstrated that, although no change in mitochondrial oxygen consumption was seen in GAPDS knockout male mice, lower levels of ATP were produced. Also they reported the variation in the spacing between the ribs of the fibrous sheath in the TEM of these GAPDS knockout male mice [6]. In a study by Khan et al. (2009), GAPDS was reported as an immunogenic protein with epididymal origin, and, using an immuno-proteomics approach, this protein could be exploited as a potential target for contraception and biomarkers for infertility [36]. When these results are taken together, it seems that decreased amount of GAPDS, could influence the structure of fibrous sheath and decreased ATP production. In the other hand GAPDS has a crucial role in sperm motility and could be considered as a biomarker of sperm dysfunctions.
The present study found that asthenozoospermic patients had significantly lower concentrations of COX6B in sperm tails, a result similar to that of Martınez-Heredia et al. (2008) [15]. COX6B is the terminal enzyme of the respiratory chain and is necessary for ATP synthesis [15]. Therefore, lower levels of COX6B may be associated with defective sperm motility.
In the current study, PHGPx or GPX4 was present in significantly lower amount in the asthenozoospermic samples than in the normal ones. PHGPx is a major component of capsule, a keratinous structure around the mitochondria of mid piece and play a crucial role in spermatogenesis [37]. Evidence suggests that PHGPx, controls the production of reactive oxygen species (ROS),that produced in small amounts by spermatozoa and maintenance the spermatozoa viability, but at high concentrations, are toxic [38]. Meseguer et al. (2006) demonstrated the poorer embryo development and morphology were related to lower sperm PHGPx expression [39]. From these results, it seems that reduced PHGPx may be correlated with lower production of ATP and production of high levels of ROS which may induce oxidative stress and impair sperm motility. Therefore, proteins responsible for energy production, especially GAPDS and PHGPx, might be considered as core components of diagnostic tests for male factor infertility and IVF outcomes.
Another finding of the current study was the down-regulation of VDAC2 in patients of the asthenozoospermic group. Evidence exists that VDAC family proteins in somatic cells has an ATP binding site which mediates ATP transport through the outer membrane of the mitochondria. VDAC2 probably controls sperm motility by regulating energy metabolism [40]. Kwon et al. (2013) demonstrated that blocking the VDAC2 and VDAC3 gene caused significantly decreased sperm motility, acrosome reaction, capacitation, tyrosine phosphorylation, fertilization, and embryo development, suggesting that VDACs are essential for successful reproduction [41]. It seems that, by regulating energy metabolism, VDAC2 has a pivotal role in male fertility and may be a good candidate for diagnosis of sperm dysfunction, and use as a predictor of embryo quality.
The present study showed lower expression of HspA2 and HSPA9 in the asthenozoospermic patients. HspA2 and HSPA9 are stress response proteins belonging to the heat shock protein family which protect the cell against hyperthermia, oxidative stress, inflammation, and infection [42]. Recent evidence suggests that HSP’s can influence acrosome reaction, penetration of the zona pellucida, and other processes that culminate in fertilization by unknown mechanism [43]. HSPA9 is predominant in the mitochondrial matrix, suggesting the existence of a transport system to transfer HSPA9 from the cytosol into the mitochondria and helps in the refolding of mitochondria proteins [44]. Ergur et al. (2002) demonstrated that HspA2 could be a marker for sperm maturity, and low expression of the HspA2 protein could predict IVF pregnancy failure [5]. Therefore, it seems that decreased HspA2 and HSPA9 could affect the maturity and fertilizing ability of spermatozoa. Additional studies are needed to establish the actual influence of HSPA9 on male fertility and IVF outcome.
In the current study, the GSTMu3 was down-regulated in asthenozoospermia. This enzyme can participate in the detoxification of carcinogens, drugs, toxins, and products of oxidative stress [45]. Rapuling et al. (2010) found that in immature sperm populations, the GSTMu3 was less abundant [13]. It is probable that the lower expression of GSTMu3 led to the accumulation of oxidative stress products and could have influenced sperm motility.
The present study also demonstrated that ASRGL1 was significantly up-regulated in the asthenozoospermic group. ASRGL1 converts the amino acid L-asparagine into L-aspartate and localizes in the mid piece of rat and human sperm tails. It is probably associated with the mitochondria. Bush et al. (2002) found that the antibody against this protein was seen in sera of rat and human post-vasectomized samples [46]. The ASRGL1 epitopes act as autoimmunity antigens and may impair fertility, so it can be a promising candidate for contraceptive purposes, although additional studies are needed to elucidate the exact location and role of ASRGL1 in sperm.
The current study found that expression level of SPANX B was lowered in asthenozoospermic individuals. SPANX B has been detected in both head and tail domains of sperm [19]. The function and exact location of this protein in flagella has not yet been clarified. Shen et al. (2013) showed lower amounts of this protein in idiopathic asthenoospermia [47]. Paasch et al. (2011) demonstrated higher expression levels of SPANX C, a isomer form of protein, in the sperm of diabetic and obese individuals [48]. This point may be clarified further by examining related studies that have shown poor semen quality accompanied by reduced sperm motility in diabetic individuals [49, 50]. SPANX B might be related to energy production in spermatozoa; however further investigation is needed to clarify the exact function of this protein in sperm tail.
Conclusions
The current study evaluated the protein expression of sperm tails in asthenozoospermia patients and identified 14 proteins that had altered amounts. Ten of these proteins have been previously documented in asthenozoospermia patients, such as GAPDS, COX6B, ODF2, AKAP4, PHGPx, and CLU, indicating that these are conserved proteins and probably play a critical role in sperm function. The 4 proteins HSPA9, TUBB2B, SPANX B, and ASRGL1 are reported here for the first time with altered amount in low motility sperm samples.
The approach used in this study was unique as it not only identified the extracted proteins of sperm tail, but also may be useful in finding new therapeutic targets for treating low motility sperm and providing candidates for a sperm contraceptive vaccine and for IVF outcome.
Acknowledgments
We thank all members of the infertility research and treatment center of Khuzestan and in Particular Mrs. Adham and Mrs. Ghalambaz for assistance in semen collection and analysis.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This work is a part of the PhD thesis of Susan Sabbagh and was funded by Grant No. 90, from Cellular and Molecular Research Center (CMRC 90), Ahvaz Jundishapour University of Medical Sciences, Ahvaz, Iran.
Authors’ contributions
Mahmoud Hashemitabar designed the project and revised manuscript.
Susan Sabbagh carried out the experiments, writing of the manuscript.
Mahmoud Orazizadeh participated in its design.
Maryam Bahmanzadeh participated in analysis of data and writing of the manuscript.
Atta Ghadiri helped to draft the manuscript.
All authors read and approved the final manuscript.
Footnotes
Capsule
The molecular mechanism that impairs sperm function is not fully understood. The current study evaluated the protein expression of sperm tails in asthenozoospermia patients and identified 14 proteins that had altered amounts. These are conserved proteins and probably play a critical role in sperm function. These proteins might be used as markers for the better diagnosis of sperm dysfunctions, targets for male contraceptive development, and to predict infertility and embryo quality.
References
- 1.Moore FL, Reijo-Pera RA. Male sperm motility dictated by mother’s mtDNA. Am J Hum Genet. 2000;67(3):543. doi: 10.1086/303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril. 1991;56(2):192–3. [PubMed] [Google Scholar]
- 3.Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev. 2005;18(2):25–38. doi: 10.1071/RD05120. [DOI] [PubMed] [Google Scholar]
- 4.Pixton KL, Deeks ED, Flesch FM, Moseley FLC, Björndahl L, Ashton PR, et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19(6):1438–47. doi: 10.1093/humrep/deh224. [DOI] [PubMed] [Google Scholar]
- 5.Ergur AR, Dokras A, Giraldo JL, Habana A, Kovanci E, Huszar G. Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril. 2002;77(5):910–8. doi: 10.1016/S0015-0282(02)03073-X. [DOI] [PubMed] [Google Scholar]
- 6.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101(47):16501–6. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y-F, He W, Mandal A, Kim Y-H, Digilio L, Klotz K, et al. CABYR binds to AKAP3 and Ropporin in the human sperm fibrous sheath. Asian J Androl. 2011;13(2):266. doi: 10.1038/aja.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, et al. Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol. 2004;24(18):7958–64. doi: 10.1128/MCB.24.18.7958-7964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, et al. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16(7):452–62. doi: 10.1093/molehr/gaq009. [DOI] [PubMed] [Google Scholar]
- 10.Liao T-T, Xiang Z, Zhu W-B, Fan L-Q. Proteome analysis of round-headed and normal spermatozoa by 2-D fluorescence difference gel electrophoresis and mass spectrometry. Asian J Androl. 2009;11(6):683–93. doi: 10.1038/aja.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, Sabbaghian M, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81(2):293–300. doi: 10.1016/j.urology.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Cedenho A, Lima S, Cenedeze M, Spaine D, Ortiz V, Oehninger S. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum Reprod. 2006;21(7):1791–4. doi: 10.1093/humrep/del055. [DOI] [PubMed] [Google Scholar]
- 13.Rapuling L. Proteomic analysis of human sperm proteins in relation to sperm motility, morphology and energy metabolism. Stellenbosch: University of Stellenbosch; 2010. [Google Scholar]
- 14.Amaral A, Castillo J, Estanyol JM, Ballescà JL, Ramalho-Santos J, Oliva R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol Cell Proteomics. 2013;12(2):330–42. doi: 10.1074/mcp.M112.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23(4):783–91. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Huo R, Wang F-Q, Lin M, Zhou Z-M, Sha J-H. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87(2):436–8. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar P, Collier TS, Randall SM, Muddiman DC, Rao BM. The subcellular proteome of undifferentiated human embryonic stem cells. Proteomics. 2012;12(3):421–30. doi: 10.1002/pmic.201100507. [DOI] [PubMed] [Google Scholar]
- 18.Organization WH. WHO laboratory manual for the examination and processing of human semen. 2010. [PubMed]
- 19.Baker MA, Naumovski N, Hetherington L, Weinberg A, Velkov T, Aitken RJ. Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics. 2013;13(1):61–74. doi: 10.1002/pmic.201200350. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Hashemitabar M, Bahmanzadeh M, Mostafaie A, Orazizadeh M, Farimani M, Nikbakht R. A proteomic analysis of human follicular fluid: comparison between younger and older women with normal FSH levels. Int J Mol Sci. 2014;15(10):17518–40. doi: 10.3390/ijms151017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostafaie A, Yari K, Kiani S. A comparative evaluation of rehydration and cuploading sample application for modified twodimensional gel electrophoresis of human serum proteins using immobilized pH gradient. Afr J Biotechnol. 2013;10(55):11711–5. [Google Scholar]
- 23.Peknicova J, Pexidrova M, Kubatova A, Koubek P, Tepla O, Sulimenko T, et al. Expression of beta-tubulin epitope in human sperm with pathological spermiogram. Fertil Steril. 2007;88(4):1120–8. doi: 10.1016/j.fertnstert.2006.12.070. [DOI] [PubMed] [Google Scholar]
- 24.Fackenthal JD, Turner FR, Raff EC. Tissue-specific microtubule functions in drosophila spermatogenesis require the β2-tubulin isotype-specific carboxy terminus. Dev Biol. 1993;158(1):213–27. doi: 10.1006/dbio.1993.1180. [DOI] [PubMed] [Google Scholar]
- 25.Chemes H, Olmedo SB, Carrere C, Oses R, Carizza C, Leisner M, et al. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13(9):2521–6. doi: 10.1093/humrep/13.9.2521. [DOI] [PubMed] [Google Scholar]
- 26.Cao W, Gerton GL, Moss SB. Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics. 2006;5(5):801–10. doi: 10.1074/mcp.M500322-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Brohmann H, Pinnecke S, Hoyer-Fender S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J Biol Chem. 1997;272(15):10327–32. doi: 10.1074/jbc.272.15.10327. [DOI] [PubMed] [Google Scholar]
- 28.Petersen C, Füzesi L, Hoyer-Fender S. Outer dense fibre proteins from human sperm tail: molecular cloning and expression analyses of two cDNA transcripts encoding proteins of ~70 kDa. Mol Hum Reprod. 1999;5(7):627–35. doi: 10.1093/molehr/5.7.627. [DOI] [PubMed] [Google Scholar]
- 29.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61(1):103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 30.Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248(2):331–42. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- 31.Baccetti B, Collodel G, Gambera L, Moretti E, Serafini F, Piomboni P. Fluorescence in situ hybridization and molecular studies in infertile men with dysplasia of the fibrous sheath. Fertil Steril. 2005;84(1):123–9. doi: 10.1016/j.fertnstert.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 32.Chan C-C, Shui H-A, Wu C-H, Wang C-Y, Sun G-H, Chen H-M, et al. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res. 2009;8(11):5382–6. doi: 10.1021/pr9003932. [DOI] [PubMed] [Google Scholar]
- 33.Yunes R, Doncel GF, Acosta AA. Incidence of sperm-tail tyrosine phosphorylation and hyperactivated motility in normozoospermic and asthenozoospermic human sperm samples. Biocell-Mendoza. 2003;27(1):29–36. [PubMed] [Google Scholar]
- 34.Ibrahim NM, Gilbert GR, Loseth KJ, Crabo BG. Correlation between clusterin‐positive spermatozoa determined by flow cytometry in bull semen and fertility. J Androl. 2000;21(6):887–94. [PubMed] [Google Scholar]
- 35.Francavilla S, Pelliccione F, Cordeschi G, Necozione S, Santucci R, Bocchio M, et al. Utrastructural analysis of asthenozoospermic ejaculates in the era of assisted procreation. Fertil Steril. 2006;85(4):940–6. doi: 10.1016/j.fertnstert.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Suryawanshi AR, Khan SA, Gajbhiye RK, Gurav MY, Khole VV. Differential proteomics leads to identification of domain‐specific epididymal sperm proteins. J Androl. 2011;32(3):240–59. doi: 10.2164/jandrol.110.010967. [DOI] [PubMed] [Google Scholar]
- 37.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285(5432):1393–6. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8(5):338–48. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 39.Meseguer M, de los Santos MJ, Simón C, Pellicer A, Remohí J, Garrido N. Effect of sperm glutathione peroxidases 1 and 4 on embryo asymmetry and blastocyst quality in oocyte donation cycles. Fertil Steril. 2006;86(5):1376–85. doi: 10.1016/j.fertnstert.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Wang P, Wang Z, Jia Y, Niu X, Wang W, et al. Analysis and difference of voltage-dependent anion channel mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia. J Assist Reprod Genet. 2010;27(12):719–24. doi: 10.1007/s10815-010-9466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon W-S, Park Y-J, Mohamed E-SA, Pang M-G. Voltage-dependent anion channels are a key factor of male fertility. Fertil Steril. 2013;99(2):354–61. doi: 10.1016/j.fertnstert.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 42.Neuer A, Spandorfer S, Giraldo P, Dieterle S, Rosenwaks Z, Witkin S. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6(2):149–59. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- 43.Dix DJ, Garges JB, Hong RL. Inhibition of hsp70–1 and hsp70–3 expression disrupts preimplantation embryogenesis and heightens embryo sensitivity to arsenic. Mol Reprod Dev. 1998;51(4):373–80. doi: 10.1002/(SICI)1098-2795(199812)51:4<373::AID-MRD3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 44.Schneider H-C, Berthold J, Bauer MF, Dietmeier K, Guiard B, Brunner M et al. Mitochondrial Hsp70/MIM44 complex facilitates protein import. 1994. [DOI] [PubMed]
- 45.Aitken J, Buckingham D, Krausz C. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994;39(3):268–79. doi: 10.1002/mrd.1080390304. [DOI] [PubMed] [Google Scholar]
- 46.Bush LA, Herr JC, Wolkowicz M, Sherman NE, Shore A, Flickinger CJ. A novel asparaginase‐like protein is a sperm autoantigen in rats. Mol Reprod Dev. 2002;62(2):233–47. doi: 10.1002/mrd.10092. [DOI] [PubMed] [Google Scholar]
- 47.Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol. 2013;31(6):1395–401. doi: 10.1007/s00345-013-1023-5. [DOI] [PubMed] [Google Scholar]
- 48.Paasch U, Heidenreich F, Pursche T, Kuhlisch E, Kettner K, Grunewald S, et al. Identification of increased amounts of eppin protein complex components in sperm cells of diabetic and obese individuals by difference gel electrophoresis. Mol Cell Proteomics. 2011;10(8):M110–007187. doi: 10.1074/mcp.M110.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramalho-Santos J, Amaral S, Oliveira PJ. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4(1):46–54. doi: 10.2174/157339908783502398. [DOI] [PubMed] [Google Scholar]
- 50.Mangoli E, Talebi AR, Anvari M, Pourentezari M. Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Iran J Reprod Med. 2013;11(1):53. [PMC free article] [PubMed] [Google Scholar]