Abstract
A cDNA for parafusin, an evolutionarily conserved phosphoglycoprotein involved in exocytosis, has been cloned and sequenced from a unicellular eukaryote, Paramecium tetraurelia. A Paramecium cDNA library was screened with an oligonucleotide probe synthesized to an internal amino acid sequence of isolated parafusin. The insert was 3 kb long with an open reading frame of 1.75 kb. Data base searches of the deduced amino acid sequence showed that Paramecium parafusin had a 50.7% sequence identity to rabbit muscle phosphoglucomutase, although no detectable phosphoglucomutase activity has been detected in isolated parafusin. The deduced parafusin amino acid sequence had four inserts and two deletions, which might confer on the protein specific functions in signal transduction events related to exocytosis. Furthermore, searches for potential phosphorylation sites showed the presence of a protein kinase C site (KDFSFR) specific to parafusin. Southern blot analysis with probes specific for parafusin and phosphoglucomutase suggested that these proteins were products of different genes. We propose that parafusin and phosphoglucomutase are members of a superfamily that conserve homologies important for the tertiary structure of the molecules.
Full text
PDF

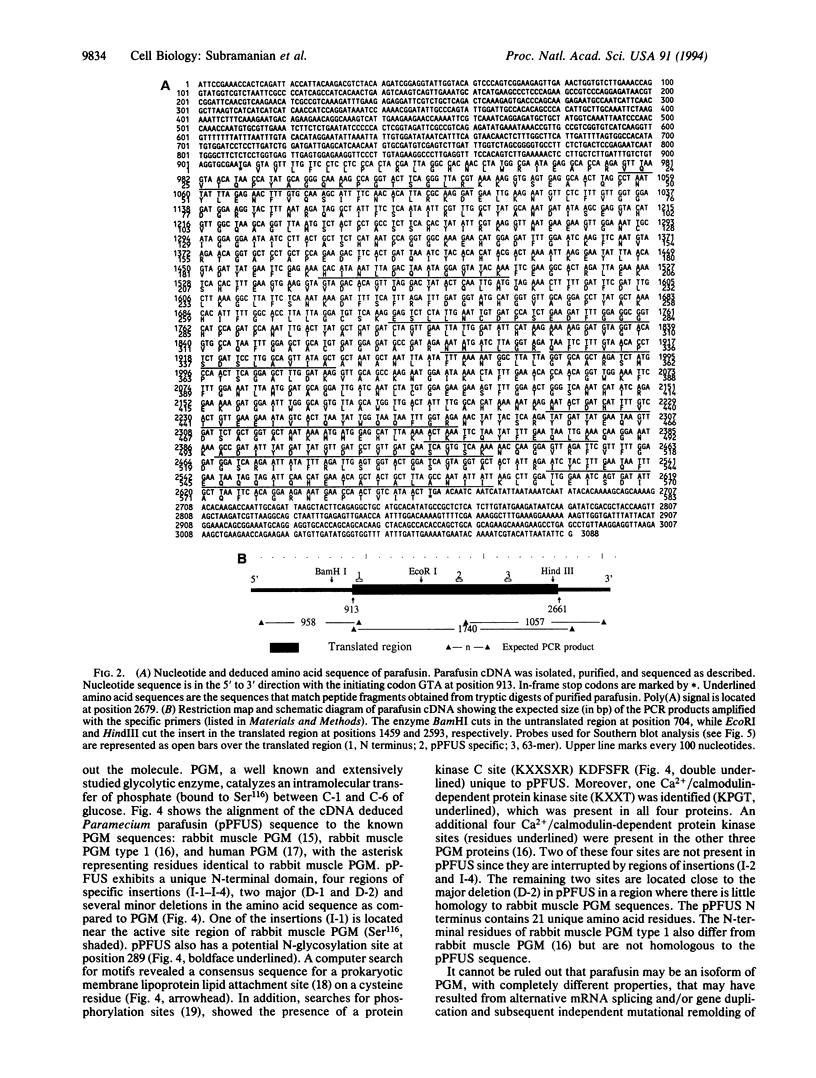
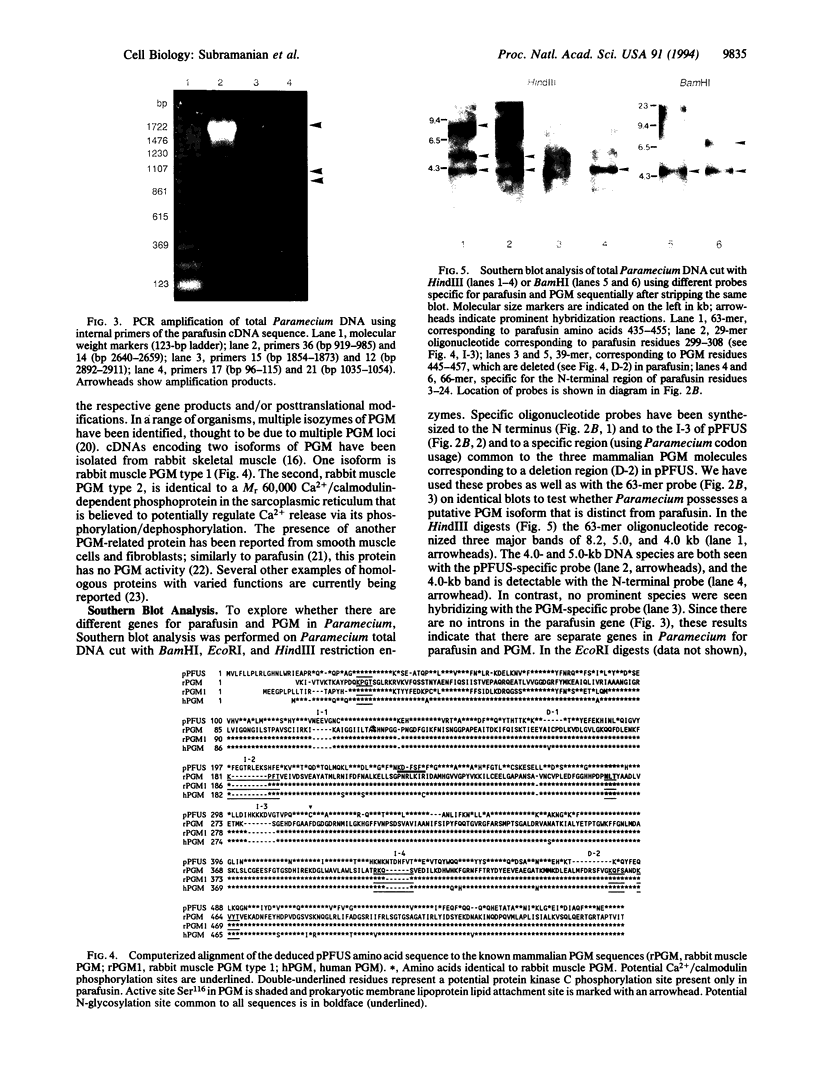

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. P., Wyroba E., Reichman M., Zhao H., Satir B. H. The activity of parafusin is distinct from that of phosphoglucomutase in the unicellular eukaryote Paramecium. Biochem Biophys Res Commun. 1994 May 16;200(3):1353–1358. doi: 10.1006/bbrc.1994.1600. [DOI] [PubMed] [Google Scholar]
- Belkin A. M., Klimanskaya I. V., Lukashev M. E., Lilley K., Critchley D. R., Koteliansky V. E. A novel phosphoglucomutase-related protein is concentrated in adherens junctions of muscle and nonmuscle cells. J Cell Sci. 1994 Jan;107(Pt 1):159–173. doi: 10.1242/jcs.107.1.159. [DOI] [PubMed] [Google Scholar]
- Gilligan D. M., Satir B. H. Protein phosphorylation/dephosphorylation and stimulus-secretion coupling in wild type and mutant Paramecium. J Biol Chem. 1982 Dec 10;257(23):13903–13906. [PubMed] [Google Scholar]
- Godiska R., Aufderheide K. J., Gilley D., Hendrie P., Fitzwater T., Preer L. B., Polisky B., Preer J. R., Jr Transformation of Paramecium by microinjection of a cloned serotype gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7590–7594. doi: 10.1073/pnas.84.21.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Marks A. R., Gureckas N., Lacro R., Nadal-Ginard B., Kim D. H. Purification, characterization, and molecular cloning of a 60-kDa phosphoprotein in rabbit skeletal sarcoplasmic reticulum which is an isoform of phosphoglucomutase. J Biol Chem. 1992 Oct 15;267(29):21080–21088. [PubMed] [Google Scholar]
- Linz J. E., Lira L. M., Sypherd P. S. The primary structure and the functional domains of an elongation factor-1 alpha from Mucor racemosus. J Biol Chem. 1986 Nov 15;261(32):15022–15029. [PubMed] [Google Scholar]
- Mahalingam R., Seilhamer J. J., Pritchard A. E., Cummings D. J. Identification of Paramecium mitochondrial proteins using antibodies raised against fused mitochondrial gene products. Gene. 1986;49(1):129–138. doi: 10.1016/0378-1119(86)90392-6. [DOI] [PubMed] [Google Scholar]
- Martin R., Hoover C., Grimme S., Grogan C., Höltke J., Kessler C. A highly sensitive, nonradioactive DNA labeling and detection system. Biotechniques. 1990 Dec;9(6):762–768. [PubMed] [Google Scholar]
- Martindale D. W. Codon usage in Tetrahymena and other ciliates. J Protozool. 1989 Jan-Feb;36(1):29–34. doi: 10.1111/j.1550-7408.1989.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Murtaugh T. J., Gilligan D. M., Satir B. H. Purification of and production of an antibody against a 63,000 Mr stimulus-sensitive phosphoprotein in Paramecium. J Biol Chem. 1987 Nov 15;262(32):15734–15739. [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Cummings D. J. Paramecium mitochondrial DNA sequences and RNA transcripts for cytochrome oxidase subunit I, URF1, and three ORFs adjacent to the replication origin. Gene. 1986;44(2-3):243–253. doi: 10.1016/0378-1119(86)90188-5. [DOI] [PubMed] [Google Scholar]
- Quick C. B., Fisher R. A., Harris H. A kinetic study of the isozymes determined by the three human phosphoglucomutase loci PGM1, PGM2, and PGM3. Eur J Biochem. 1974 Mar 1;42(2):511–517. doi: 10.1111/j.1432-1033.1974.tb03366.x. [DOI] [PubMed] [Google Scholar]
- Ray W. J., Jr, Hermodson M. A., Puvathingal J. M., Mahoney W. C. The complete amino acid sequence of rabbit muscle phosphoglucomutase. J Biol Chem. 1983 Aug 10;258(15):9166–9174. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir B. H., Busch G., Vuoso A., Murtaugh T. J. Aspects of signal transduction in stimulus exocytosis-coupling in Paramecium. J Cell Biochem. 1988 Apr;36(4):429–443. doi: 10.1002/jcb.240360411. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Hamasaki T., Reichman M., Murtaugh T. J. Species distribution of a phosphoprotein (parafusin) involved in exocytosis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):930–932. doi: 10.1073/pnas.86.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir B. H., Srisomsap C., Reichman M., Marchase R. B. Parafusin, an exocytic-sensitive phosphoprotein, is the primary acceptor for the glucosylphosphotransferase in Paramecium tetraurelia and rat liver. J Cell Biol. 1990 Sep;111(3):901–907. doi: 10.1083/jcb.111.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Green M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993 Jan 15;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Subramanian S. V., Satir B. H. Carbohydrate cycling in signal transduction: parafusin, a phosphoglycoprotein and possible Ca(2+)-dependent transducer molecule in exocytosis in Paramecium. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11297–11301. doi: 10.1073/pnas.89.23.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse D. B., Putt W., Lovegrove J. U., Morrison K., Hollyoake M., Fox M. F., Hopkinson D. A., Edwards Y. H. Phosphoglucomutase 1: complete human and rabbit mRNA sequences and direct mapping of this highly polymorphic marker on human chromosome 1. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):411–415. doi: 10.1073/pnas.89.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]