Abstract
Purpose
Panobinostat is a novel oral pan-deacetylase inhibitor with promising anti-cancer activity. The study aimed to determine the influence of food on the oral bioavailability of panobinostat.
Methods
This multicenter study consisted of a randomized, three-way crossover, food-effect study period (cycle 1) followed by single-agent panobinostat continual treatment phase in patients with advanced cancer. Patients received panobinostat 20 mg twice weekly, and panobinostat pharmacokinetics was investigated on days 1, 8, and 15 with a randomly assigned sequence of three prandial states (fasting, high-fat, and normal breakfast).
Results
Thirty-six patients were assessed for the food effect on pharmacokinetics and safety in cycle 1, after which 29 patients continued treatment, receiving singleagent panobinostat. Safety and antitumor activity were assessed during the extension period. Panobinostat systemic exposure was marginally reduced (14–16%) following food [geometric mean ratio (GMR) of the AUC0–∞/high-fat breakfast/fasting, 0.84 (90% confidence interval {CI}, 0.74–0.96); normal breakfast/fasting, 0.86 (90% CI, 0.75–1.00)], and interpatient variability (coefficient of variation, 59%) remained essentially unchanged with or without food. Panobinostat Cmax was reduced by 44% (high-fat) and 36% (normal) with median Tmax prolonged by 1–1.5 h following food. Panobinostat was well tolerated, with thrombocytopenia, fatigue, nausea, and vomiting as common adverse events, and demonstrated antitumor activity with one patient with a partial response and six patients with stable disease as best response.
Conclusions
Food produced minor changes in oral panobinostat exposure; thus, panobinostat can be given without regard to food intake in future clinical studies.
Keywords: Panobinostat, Pharmacokinetics, Histone deacetylase inhibitor, Food
Introduction
Panobinostat (LBH589), a hydroxamic acid derivative, is a potent, orally active class I/II/IV pan-deacetylase inhibitor (DACi) with antitumor activity within low nanomolar range. Increased deacetylase (DAC) activity is associated with the survival of malignant cells in vitro, partly through reduced expression of pro-apoptotic genes and upregulated transcription of anti-apoptotic genes [1, 2]. Through the inhibition of DACs, panobinostat causes sustained effects on proteins involved in cell-cycle regulation (p21, p53), gene transcription (histones and transcription factors), angiogenesis (HIF-1α), cytoskeleton (α-tubulin), and protein stabilization (Hsp90) [3]. Together, these effects lead to the inhibition of tumor cell growth and increased tumor cell death [2]. Preclinical studies of panobinostat have demonstrated its potent antitumor activity against multiple cancer cell lines, and xenografts derived from human hematologic malignancies and solid tumors, such as multiple myeloma, Hodgkin lymphoma, acute myeloid leukemia, breast, and prostate cancer [4–9]. Additionally, phase I and II clinical trials have demonstrated clinical activity in various cancers, particularly in Hodgkin lymphoma and multiple myeloma [10–13]. Currently, panobinostat is being studied in a phase III trial in combination with bortezomib and dexamethasone in patients with multiple myeloma (ClinicalTrials.gov Identifier: NCT01023308) [14].
The current study was designed to investigate the effect of food on the disposition of panobinostat. Prior to this study, panobinostat was administered 2 h before or 2 h after food, based on empirical clinical practice. Preliminary results from a pilot food-effect study performed as a component of a phase I clinical trial of panobinostat in a subset of cancer patients (n = 11) suggested that a high-fat meal delayed the time to maximum concentration (Tmax delay ≈ 2 h), with a 45% reduction in maximum concentration of drug [Cmax]; and with only a minimal effect in systemic exposure (Margaret Woo, Novartis Oncology, personal communication). Based on these observations, the current study was designed to formally assess the effect of food on the oral bioavailability of panobinostat with a three-way crossover, randomized trial design in patients with advanced cancer. The study aim was to determine the oral panobinostat absorption profile in the fasted state and compare with panobinostat absorption following a high-fat and a normal breakfast. In addition, the safety, tolerability, and antitumor activity of panobinostat were assessed in patients continuing with single-agent panobinostat treatment after the food-effect assessment.
Patients and methods
This study was conducted according to the ethical principles of the Declaration of Helsinki. The study protocol and amendments were reviewed by the Institutional Review Board, Independent Ethics Committee, or Research Ethics Board at each study center.
Eligibility criteria
Adult patients with histologically or cytologically confirmed advanced solid tumors that were refractory to standard therapy or for which no standard therapy existed were eligible for enrollment. Patients were required to have an ECOG status ≤2 and adequate physiological organ function [hemoglobin ≥9 g/dL, absolute neutrophil count ≥1.5 × 109/L, platelet count ≥100 × 109/L, serum creatinine ≤1.5 × upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤2.5 × ULN, serum bilirubin ≤1.5 × ULN]. Patients were not eligible for enrollment if they had active central nervous system disease or brain metastases, impaired cardiac function, acute or chronic liver or renal disease, impairment of gastrointestinal (GI) function or GI disease that could affect drug absorption, other concurrent severe and/or uncontrolled medical conditions or if they were pregnant or breastfeeding. Patients who were currently receiving medications that inhibit or induce CYP3A enzyme activities or valproic acid for any medical condition were excluded. In addition, patients who received chemotherapy ≤3 weeks prior to randomization, biologic immunotherapy ≤4 weeks prior to randomization, or major surgery ≤2 weeks prior to randomization were not eligible. Written informed consent was obtained prior to any studyspecific screening procedures.
Study design and treatment scheme
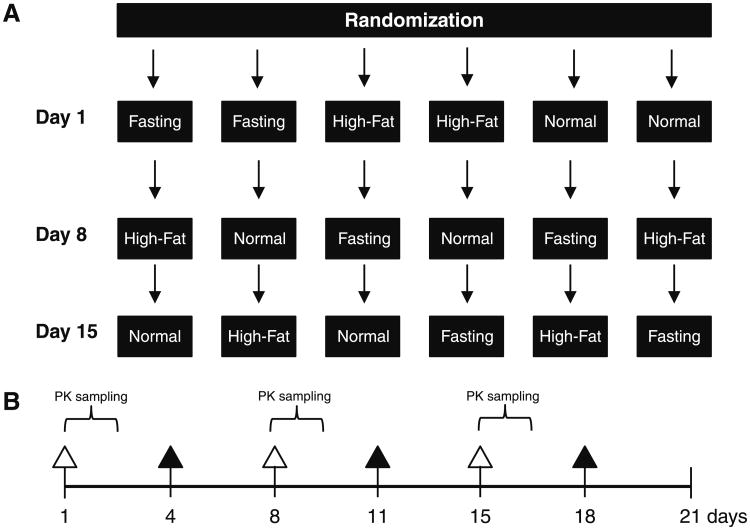
This was an open-label, multicenter, randomized, threeway crossover design to evaluate the effect of food on the oral bioavailability of panobinostat in patients with advanced cancer. Patients were randomized to one of six possible sequences of three prandial conditions on days 1, 8, and 15 (Fig. 1a). The three prandial states were defined as follows: overnight fast (10 h of fasting prior to panobinostat administration and 4 h fasting after), normal breakfast (panobinostat administration within 60 min after starting a normal breakfast), high-fat breakfast (panobinostat administration within 30 min after starting a high-fat breakfast). The normal and high-fat breakfasts contained approximately 500 and 1,000 calories (±10%), respectively, with 35 and 50% of the calories from fat, respectively. Serial venous blood samples were obtained on days 1, 8, and 15 for panobinostat pharmacokinetics (PK) assessment (Fig. 1b). On days 4, 11, and 18, when PK assessment was not scheduled, patients fasted 2 h before and 2 h after dosing with panobinostat. Safety data were collected during cycle 1 and during subsequent treatment cycles for patients who continued in the study. Tumor evaluations were performed prior to treatment and after every two cycles in patients who continued into the continual treatment phase.
Fig. 1.
Design and treatment scheme of the food-effect phase. a Six possible randomly assigned prandial sequence with three prandial states on days 1, 8, and 15. Patients were randomized to receive panobinostat according to one of six sequences of three different prandial states: fasting (10-h overnight fast with continued fasting after 4-h post-panibinostat dosing), high fat and normal breakfast. To ensure the dose was administered on a full stomach in the latter two states, patients were dosed within 30 min of starting the high-fat or 60 min of starting the normal breakfast. High-fat breakfast consisted of 1,000 calories (≈50% fat content), and normal breakfast consisted of 500 calories (≈35% fat content). b Dosing regimen and pharmacokinetic (PK) sampling. Panobinostat was administered orally twice weekly at a dose of 20 mg during a 21-day cycle. Closed triangles indicate days on which panobinostat was dosed without assigned prandial condition and without PK assessment. Open triangles indicate days on which panobinostat was dosed according to a randomly assigned prandial state. During the PK sampling, whole-blood samples were collected predose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 24, and 48 h post-dose
In the food-effect phase (cycle 1), panobinostat 20 mg was administered orally twice weekly on days 1, 4, 8, 11, 15, and 18 of a 21-day cycle (Fig. 1b). This dose and schedule were chosen because of the potential safety risks if food led to a significant increase in panobinostat exposure. In addition, because panobinostat did not accumulate when administered on days 1 and 4 of a weekly schedule [15], the schedule mimicked a single dose and allowed for intrapatient comparison of PK profiles under the three prandial conditions.
During the continual treatment phase, panobinostat 45 mg was administered on days 1, 4, 8, 11, 15, and 18 in 21-day cycles with therapeutic intent. Patients were instructed to fast 2 h before and 2 h after dosing during this portion of the study.
Pharmacokinetic sampling
During cycle 1, serial blood samples (4 mL) were collected via a forearm vein into tubes containing sodium heparin immediately before panobinostat dosing and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 24, and 48 h post-dosing. Blood samples were centrifuged within 30 min of collection at 800 × g for 15 min at 4°C to yield plasma; two aliquots were stored below – 60°C until analysis for panobinostat concentrations.
Panobinostat assay
Panobinostat concentrations were determined in human plasma samples using a specific liquid chromatography–tandem mass spectrometry assay (LC–MS/MS) method with the lower limit of quantification (LLOQ) of 0.5 ng/mL using 0.1 mL sample volume. A semi-automated protein precipitation extraction of human plasma samples was used to separate panobinostat from human plasma protein. The obtained sample extracts were evaporated to dryness, reconstituted with 10% aqueous acetonitrile (containing 0.2% formic acid), and analyzed by LC–MS/MS on a Sciex API3000 or API4000 tandem mass spectrometer (AB Sciex, Foster City, CA, USA). A Waters XBridge C8 column (2.5 μm particle size, 50 × 2.1 mm, Waters, Milford, MA, USA) was employed in reversed-phase chromatography with gradient elution using 10% acetonitrile in water (containing 0.1% formic acid and 0.1% acetic acid, mobile phase A) and 90% acetonitrile in water (containing 0.25% formic acid and 0.25% acetic acid, mobile phase B) at a flow rate of 0.3 mL/min. The assay was linear from 0.5 to 500 ng/mL with the bias and coefficient of variation (CV) values of the quality control sample results, ranging from –0.7–0.7% to 2.3–11.6%, respectively, based on the within-study validation during the sample analysis.
Pharmacokinetic analysis
The panobinostat plasma concentration versus time data following each dose was used to calculate PK parameters including Cmax, Tmax, and AUC0–∞. PK parameters were obtained using the noncompartmental Model 200 of Win-Nonlin Pro software (Version 5.01, Pharsight Corporation, Mountain View, CA, USA). Cmax and Tmax were obtained by visual inspection of the plasma concentration–time curve. AUC0–∞ was calculated using the linear trapezoidal method extrapolated to infinity. The apparent terminal halflife (t1/2) was estimated using the best-fit variables of a single exponential to the log-linear portion of the plasma concentration–time curve using nonweighted linear regression. Mean and CV were calculated for AUC, Cmax, and t1/2, and median, and range was presented for Tmax.
Statistical analysis
A linear mixed effect model was fit using log-transformed AUC and Cmax values to evaluate the food effect. The model included sequence, period, and meal condition as a fixed effect, and patient nested within sequence as a random effect. The sequence has six levels (1–6), the period has three levels (1–3), and the prandial condition has three levels (fasting, normal breakfast, high-fat breakfast). For the food-effect analysis, normal and high-fat breakfasts were considered as the test, and fasting was considered as the reference. Two-sided 90% confidence intervals (CIs) for the true mean differences (normal/fasting, high-fat/fasting) were calculated using the least square mean differences and standard errors, which were derived from intrapatient variance. The point estimates, and lower and upper bounds were then exponentiated to obtain the estimated geometric mean ratios (normal/fasting, high-fat/fasting) and its 90% CI. No model analysis was performed for Tmax. The difference in Tmax (normal breakfast/fasting, high-fat breakfast/fasting) was calculated for each patient, then the median and range of the difference from all patients were defined.
Safety and antitumor effect assessments
All patients who received at least one dose of panobinostat were included in the safety assessment. Laboratory evaluations included hematology, biochemistry, and urinalysis performed at baseline and predose on dosing days in cycle 1 and predose on days 1, 8, and 15 of subsequent cycles. Cardiac assessments included 12-lead electrocardiograms (ECGs) with measurement of QTc (QTcF) interval and were performed at baseline (predose) and postpanobinostat dosing on days 1, 2, 8, 9, 11, 15, and 16 during cycle 1, on days 1, 8, and 15 during subsequent cycles, and at the end of treatment. The incidence of adverse events (AEs) during treatment was assessed and categorized by system organ class, preferred term, severity (based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0), and relationship to the study drug. Serious AEs (SAEs) and AEs that caused discontinuation of treatment, dose adjustment or interruption, or death were recorded. Tumor response was assessed by RECIST (Response Evaluation Criteria in Solid Tumors), version 1.0 guidelines [16].
Results
Patient characteristics
Thirty-six patients (21 male, 15 female) were enrolled and evaluated in this study. The median age was 63 years (range 30–83). The major tumor types were colon (seven patients), lung (four patients), prostate (four patients), and ovarian (four patients); 17 patients had various other tumor types. Twenty-six patients (72%) had received more than three prior chemotherapeutic regimens, including two patients (5.6%) who had received eight prior chemotherapy regimens.
Panobinostat pharmacokinetics
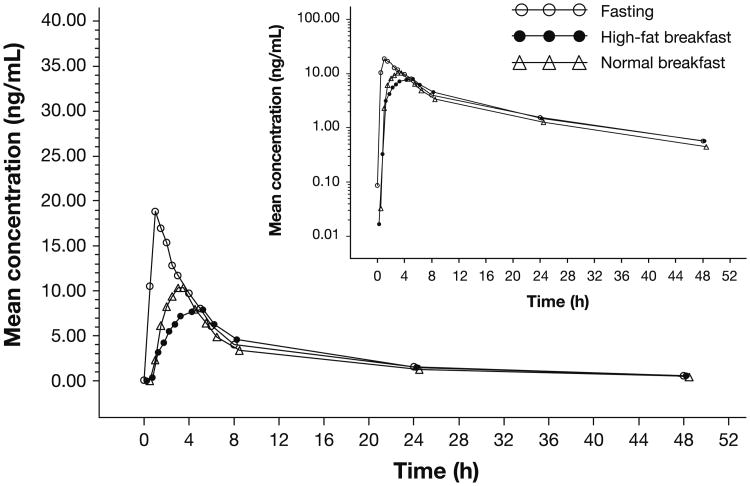
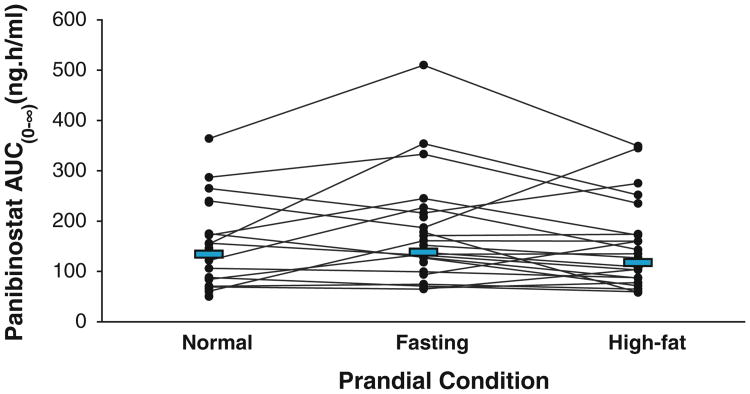
Of the 36 patients, 33 patients were evaluable for PK after an overnight fast, 34 patients after a high-fat breakfast, and 31 patients after a normal breakfast. A graphical display of the mean concentration–time profiles of panobinostat by treatment group is shown in Fig. 2, demonstrating that breakfast decreased the Cmax and delayed the Tmax of panobinostat without changing the elimination phase. PK parameters of panobinostat by prandial state are summarized in Table 1. A decrease in the mean Cmax of panobinostat was observed when patients received a high-fat or normal breakfast versus fasting, although food led to only a marginal decrease in panobinostat AUC0–∞. A graphical representation of individual patient values according to each prandial state is shown in Fig. 3. The mean elimination half-life of panobinostat was not altered by prandial state. The interpatient variability of panobinostat exposure was 59% with or without breakfast. The geometric mean ratios (GMR) using a linear mixed effect model comparing transformed Cmax and AUC0–∞, as well as median differences of Tmax values following the three prandial states are detailed in Table 1.
Fig. 2.
Panobinostat plasma concentration–time profiles following fasting and food consumption. Linear scale (inset semilogarithmic scale) of mean panobinostat plasma concentration versus time following the administration of a 20-mg dose after overnight fasting (open circle), a high-fat breakfast (closed circle), or a normal breakfast (open triangle)
Table 1. Pharmacokinetic parameters of panobinostat under three prandial states.
| PK parameters [unit] | Fasting (n = 33) | High-fat breakfast (n = 34) | Normal breakfast (n = 31) |
|---|---|---|---|
| Prandial state | |||
| AUC0–∞ [ng.h/mL], mean (CV, %) | 176 (59) | 144 (59) | 153 (59) |
| Cmax [ng/mL], mean (CV, %) | 23 (86) | 12 (63) | 14 (65) |
| Tmax [h], median (range) | 1.5 (0.5–6.0) | 4.0 (1.0–8.0) | 2.5 (0.5–6.0) |
| t1/2 [h], mean (CV, %) | 14.5 (32) | 13.7 (36) | 15.7 (49) |
|
| |||
| PK parameter comparison in ratio or median difference | High-fat breakfast/fasting | Normal breakfast/fasting | |
|
| |||
| Treatment comparison | |||
| AUC0–∞, GMR (90% CI) | 0.84 (0.74–0.96) | 0.86 (0.75–1.00) | |
| Cmax, GMR (90% CI) | 0.56 (0.45–0.70) | 0.64 (0.50–0.81) | |
| Tmax [h], median difference (range) | 2.48 (–2.00 to 7.02) | 1.45 (–2.50 to 2.02) | |
AUC0–∞ area under the curve from zero extrapolated to infinity, CI confidence interval, Cmax maximum concentration of drug, CV coefficient of variation, GMR geometric mean ratio, Tmax time for maximum concentration
Fig. 3.
Area under the curve (AUC) changes in individual patients. Individual patient AUC0–∞ values of panobinostat are represented by lines following a normal breakfast, fasting, or high-fat breakfast. Solid blue bars represent the median AUC0–∞ for each prandial condition
Toxicity and adverse effects
In the food-effect phase, the most common AEs of any grade were fatigue (50%), nausea (41.7%), and vomiting (27.8%) (Table 2). There were eight reported grade 3 or 4 AEs, with fatigue, nausea, and vomiting being the most common. Thrombocytopenia was reported in 13.9 % of patients but did not reach grade 3 or 4 severity. Of note, no QTcF >480 ms or >60 ms increase from baseline was observed in patients treated in the food-effect phase of the study. One patient died during the food-effect phase due to progression of disease, and one patient discontinued due to grade 3 fatigue and nausea/vomiting following a normal breakfast. SAEs were reported in a total of seven patients during the food-effect phase; two were study-drug related (grade 3 fatigue and grade 3 vomiting). The other SAEs, reported in five patients, were deemed unrelated to study drug by the investigators and included fatigue and dyspnea; wound hemorrhage and wound infection; hypotension and prostatitis; fever, dehydration, and pneumonia; and dysphagia.
Table 2. Adverse events during food-effect phase (20 mg) and continual treatment phase (45 mg)a.
| Adverse event | Food-effect phase (N = 36) n (%) | Continual treatment phase (N = 29) n (%) | ||
|---|---|---|---|---|
|
|
|
|||
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Total | 34 (94.4) | 8 (22.2) | 26 (89.7) | 19 (65.5) |
| Fatigue | 18 (50.0) | 3 (8.3) | 14 (48.3) | 5 (17.2) |
| Nausea | 15 (41.7) | 2 (5.6) | 11 (37.9) | 1 (3.4) |
| Vomiting | 10 (27.8) | 2 (5.6) | 9 (31.0) | 2 (6.9) |
| Diarrhea | 7 (19.4) | 1 (2.8) | 8 (27.6) | 1 (3.4) |
| Constipation | 7 (19.4) | 0 (0) | 3 (10.3) | 0 (0.0) |
| Headache | 6 (16.7) | 0 (0) | 5 (17.2) | 0 (0.0) |
| Pyrexia | 5 (13.9) | 0 (0) | 3 (10.3) | 0 (0.0) |
| Thrombocytopenia | 5 (13.9) | 0 (0) | 15 (51.7) | 11 (37.9) |
| Abdominal pain | 5 (13.9) | 0 (0) | 6 (20.7) | 1 (3.4) |
| Hypokalemia | 4 (11.1) | 0 (0) | ||
| Insomnia | 4 (11.1) | 0 (0) | ||
| Anorexia | 10 (34.5) | 0 (0.0) | ||
| Dyspnea | 7 (24.1) | 0 (0.0) | ||
| Decreased platelet count | 6 (20.7) | 3 (10.3) | ||
| Anemia | 5 (17.2) | 1 (3.4) | ||
| Cough | 4 (13.8) | 0 (0.0) | ||
| Peripheral edema | 4 (13.8) | 1 (3.4) | ||
Adverse events regardless of study drug relationship occurring in at least 10% of patients based on a data cutoff of January 31, 2009
A summary of drug-related AEs of any grade, including the relationship to prandial state, is shown in Table 3. The occurrence of fatigue appeared less frequent following an overnight fast (2.9%) compared with a high-fat (13.9%) or normal (29.4%) breakfast. Nausea, vomiting, and diarrhea appeared less common when a high-fat breakfast was consumed before dosing (8.3, 2.8, and 0%, respectively), compared with a normal breakfast (23.5, 14.7, and 8.8%, respectively) and with fasting (14.7, 14.7, and 5.9%, respectively).
Table 3. Effect of prandial state on panobinostat-related fatigue and gastrointestinal adverse effects.
| Panobinostat-related adverse event (≥10%) | Overnight fast (n = 34) n (%) | High-fat breakfast (n = 36) n (%) | Normal breakfast (n = 34) n (%) | All patients (n = 36) n (%) |
|---|---|---|---|---|
| Fatigue | 1 (2.9) | 5 (13.9) | 10 (29.4) | 16 (44.4) |
| Nausea | 5 (14.7) | 3 (8.3) | 8 (23.5) | 11 (30.6) |
| Vomiting | 5 (14.7) | 1 (2.8) | 5 (14.7) | 7 (19.4) |
| Diarrhea | 2 (5.9) | 0 (0) | 3 (8.8) | 4 (11.1) |
The frequency of grade 3 and 4 AEs (65.5%) observed in the continual treatment phase, during which the dose of oral panobinostat was increased to 45 mg and treatment duration was longer (mean duration of exposure, 1.5 months), was higher than the frequency observed during the food-effect phase (Table 2). For patients treated in the continual treatment phase, thrombocytopenia was the most common AE of any grade (51.7%) and of grade 3/4 (37.9%). The overall frequency of fatigue (48.3%), nausea (37%), and vomiting (31.0%) in the continual treatment phase was similar to that observed in the food-effect phase (Table 2). In the continual treatment phase, the most common grade 3/4 newly occurring or worsened hematologic laboratory abnormality was decreased platelet count (44.8%) and the most common grade 3/4 biochemical abnormality was hypophosphatemia (10.3%). In addition, QTc-interval abnormalities were observed in two patients in the continual treatment phase. One patient had two absolute QTcF >500 ms, and a measured QTcF interval increase of >60 ms from baseline. The other patient had a single instance of increased QTcF interval >60 ms from baseline.
Antitumor effects
In this patient population (N = 36), the antitumor activity of panobinostat was not determined for eight patients (22.2%) because of early discontinuation of the drug. A partial response was observed in one patient (2.8%) with stage IV clear cell renal carcinoma in cycle 3 and was confirmed in cycle 7. Stable disease was observed in six patients (16.7%) with the following tumors: neuroendocrine, soft tissue sarcoma, colon, head and neck, thyroid, and adenoid cystic sarcoma. The patient with clear cell renal carcinoma and the patient with adenoid cystic sarcoma continued on panobinostat for more than 2 years. Twenty-one patients (58.3%) demonstrated progressive disease.
Discussion
It is well known that food can affect the bioavailability of oral medications [17, 18]. This study represents a comprehensive evaluation of the effect of food on oral bio-availability of panobinostat. Pharmacokinetic parameters obtained in this study under fasting conditions (Table 1) were comparable with PK parameters obtained in two other phase 1 studies, which reported a mean AUC0–∞ of 183 ng h/mL (CV, 56%) and mean plasma Cmax of 23 ng/mL (CV, 57%) in the fasting state [19]. In the current study, the overall oral bioavailability and interpatient variability in systemic exposure (CV, 59%) remained essentially unchanged with or without food, whereas Cmax was reduced and Tmax was slightly prolonged following a high-fat or normal breakfast. The mean half-life of panobinostat was unaltered by breakfast prandial state, suggesting that food mainly influenced the rate (Tmax and Cmax), but not the extent, of panobinostat absorption. Since the oral bioavailability of panobinostat (AUC) was not significantly altered by prandial state and panobinostat Cmax has not been associated with clinical toxicity or efficacy [19], food is unlikely to significantly impact safety and efficacy of panobinostat or contribute to interindividual variability of panobinostat disposition in patients with cancer. The lack of significant food effect is in keeping with the physico-chemical properties of panibinostat, a Biopharmaceutics Classification System Class I like compound. Such compounds are highly soluble, highly permeable, and often their bioavailability is not significantly altered by food ingestion [20]. Furthermore, this study confirms the initial preliminary data on the effect of food on the pharmacokinetics of panobinostat (Margaret Woo, Novartis Oncology, personal communication).
There are caveats and limitations to our study. The study was conducted with a carefully administered breakfast with specified fat and caloric nutritional components; however, these are intended to represent the breadth of food intake at breakfast by patients. The study was conducted on a single “breakfast” occasion, and thus extrapolation to the ambulatory cancer patient “day-in-and-day-out” food/meal intake may not be perfectly reflected from these data. Our study represented the worst-case scenario (i.e., maximum impact of food on panobinostat oral bioavailability). However, not withstanding these caveats, the effect of food on panobinostat AUC is contained within inter-individual variability of systemic exposure as a single agent, thus unlikely to be clinically significant, and the effect of food on panobinostat Cmax is mitigated by the absence of a major effect on its AUC.
A previous study of the effect of food on the oral bioavailability of another oral DACi, vorinostat, demonstrated a statistically significant increase in the systemic exposure [AUC0–∞ (μmol/L h)] by food: GMR high-fat breakfast/fasting, 1.38 (P < 0.001) and a delay in Tmax by 2.5 h [high-fat breakfast, 4 h (2.0–10.0); fasting, 1.5 h (0.5–1.52); P < 0.001]. Since food improved the oral bioavailability of vorinostat with a short half-life of 2 h, the US Food and Drug Administration (FDA) approved dosing labeling of vorinostat states it should be taken once daily with food [21].
In patients who continued with single-agent panobinostat treatment following the completion of the food-effect phase, the most common AEs were fatigue- and gastrointestinal-related events (e.g., nausea, diarrhea, and vomiting). These AEs have been reported with similar frequencies and severities in other clinical studies of panobinostat [11–13]. Thrombocytopenia was reported less frequently in the food-effect phase compared with the continual treatment phase. This would be expected based on the increase in the panobinostat dose and treatment duration in the latter study phase. The overall safety profile of panobinostat does not appear to be affected by concomitant food intake, although fatigue appeared more common following a normal breakfast compared with a high-fat breakfast or fasting. The apparent differences in the incidence of fatigue may be related to food content or the timing of the food rather than due to specific effects of the food on panobinostat disposition. Nausea and vomiting appeared less common in patients following a high-fat meal compared with the other two prandial conditions.
Patients with various solid tumors experienced some clinical benefit in our study, with one patient (2.8%) achieving a partial response and six patients (16.7%) with disease stabilization. This is consistent with activity observed in other phase 1 studies.
In summary, based on the similarity in interindividual PK variability and the prandial state effects on panobinostat AUC, there are unlikely to be substantial differences in panobinostat exposure between patients who fast for 2 h pre- and post-panobinostat administration and those who do not do so in ongoing clinical trials. The clinical importance of these study results is that patients should be offered the flexibility of taking panobinostat either on an empty stomach or with food.
Acknowledgments
We are grateful to the participating patients and their families. William Fazzone, PhD and Peter Simon, PhD provided medical editorial assistance for this manuscript. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Wenkui Li, PhD, Jennifer Gallagher, and the study teams at our respective institutions for contributions to the conduct and analysis of the trial.
Contributor Information
Geoffrey I. Shapiro, Early Drug Development Center, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA
Richard Frank, Whittingham Cancer Center at Norwalk Hospital, Norwalk, CT, USA.
Uday B. Dandamudi, Section of Clinical Pharmacology, Department of Medicine, Dartmouth-Hitchcock Medical Center and Dartmouth Medical School, Lebanon, NH 03756, USA
Thomas Hengelage, Novartis Pharma AG, Basel, Switzerland.
Lily Zhao, Novartis Oncology, East Hanover, NJ, USA.
Lucien Gazi, Novartis Pharma AG, Basel, Switzerland.
Maria Grazia Porro, Novartis Pharma AG, Basel, Switzerland.
Margaret M. Woo, Novartis Oncology, East Hanover, NJ, USA
Lionel D. Lewis, Email: Lionel.D.Lewis@Dartmouth.edu, Section of Clinical Pharmacology, Department of Medicine, Dartmouth-Hitchcock Medical Center and Dartmouth Medical, School, Lebanon, NH 03756, USA.
References
- 1.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 2.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Prince HM, Bishton MJ, Johnstone RW. Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol. 2009;5:601–612. doi: 10.2217/fon.09.36. [DOI] [PubMed] [Google Scholar]
- 4.Catley L, Weisberg E, Kiziltepe T, Tai YT, Hideshima T, Neri P, Tassone P, Atadja P, Chauhan D, Munshi NC, Anderson KC. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocio EM, Vilanova D, Atadja P, Maiso P, Crusoe E, Fernandez-Lazaro D, Garayoa M, San-Segundo L, Hernandez-Iglesias T, de Alava E, Shao W, Yao YM, Pandiella A, San-Miguel JF. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica. 2010;95:794–803. doi: 10.3324/haematol.2009.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiso P, Colado E, Ocio EM, Garayoa M, Martin J, Atadja P, Pandiella A, San-Miguel JF. The synergy of panobinostat plus doxorubicin in acute myeloid leukemia suggests a role for HDAC inhibitors in the control of DNA repair. Leukemia. 2009;23:2265–2274. doi: 10.1038/leu.2009.182. [DOI] [PubMed] [Google Scholar]
- 7.Lemoine M, Buglio D, Jona A, Derenzini E, Medeiros LJ, Berry DA, Younes A. The pan-deacetylase inhibitor panobinostat downregulates HIF-1{alpha} and VEGF and, synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2010;116:2851. doi: 10.1182/blood-2011-01-331421. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N, Sawyers CL. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res. 2009;69:958–966. doi: 10.1158/0008-5472.CAN-08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao R, Nalluri S, Kolhe R, Yang Y, Fiskus W, Chen J, Ha K, Buckley KM, Balusu R, Coothankandaswamy V, Joshi A, Atadja P, Bhalla KN. Treatment with panobinostat induces glucose-regulated protein 78 acetylation and endoplasmic reticulum stress in breast cancer cells. Mol Cancer Ther. 2010;9:942–952. doi: 10.1158/1535-7163.MCT-09-0988. [DOI] [PubMed] [Google Scholar]
- 10.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 11.Mateos M, Spencer A, Taylor K, Lonial S, De La Rubia J, Facon T, Bengoudifa B, Hazell K, Bourquelot PM, San-Miguel JF. Phase Ib study of oral panobinostat (LBH589) plus lenalidomide (LEN) plus dexamethasone (DEX) in patients (Pts) with relapsed (Rel) or Rel and refractory (Ref) multiple myeloma (MM) J Clin Oncol. 2010;28(15S):8030. abstract. [Google Scholar]
- 12.San-Miguel JF, Richardson PGG, Sezer O, Guenther A, Siegel DSD, Blade J, LeBlanc R, Sutherland HJ, Mateos M, Gramatzki M, Hazell KM, Bengoudifa P, Bourquelot PM, Anderson KC. A phase lb study of oral panobinostat and IV bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2010;28(15S):8001. doi: 10.1200/JCO.2012.46.7068. abstract. [DOI] [PubMed] [Google Scholar]
- 13.Sureda A, Younes A, Ben-Yehuda D, Ong T, Kaufman JL, Le Corre CL, Gallagher J, Shen A, Engert A. Final analysis: phase II study of oral panobinostat in relapsed/refractory Hodgkin lymphoma patients following autologous hematopoietic stem cell transplant. Blood. 2010;116:419. abstract. [Google Scholar]
- 14.San Miguel JF, Lonial S, Hungria V, Moreau P, Einsele H, Lee JH, Yoon S, Corradini P, Jedrzejczak WW, Tan DC, Yong K, Guenther A, Wroclawsk-Swacha MM, Weber HJ, Bourquelot PM, Richardson PGG. PANORAMA1: a randomized, double-blind, placebo controlled phase III study of panobinostat in combination with bortezomib and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2011;29(15S):TPS227. [Google Scholar]
- 15.Prince HM, George D, Patnaik A, Mita M, Dugan M, Butterfoss D, Masson E, Culver KW, Burris HA, III, Beck J. Phase I study of oral LBH589, a novel deacetylase (DAC) inhibitor in advanced solid tumors and non-Hodgkin's lymphoma. J Clin Oncol. 2007;25(18S):3500. abstract. [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Jones HM, Parrott N, Ohlenbusch G, Lave T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin Pharmacokinet. 2006;45:1213–1226. doi: 10.2165/00003088-200645120-00006. [DOI] [PubMed] [Google Scholar]
- 18.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43:1127–1156. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Woo MM, Culver K, Li W, Liu A, Scott J, Parker K, Jalaluddin M, Laird G, Cooper MR, Schran HF. Panobinostat (LBH589) pharmacokinetics (PK): implication for clinical safety and efficacy. Ann Oncol. 2008;19(suppl 8):viii161. abstract. Abstract 478P. [Google Scholar]
- 20.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 21.Rubin EH, Agrawal NG, Friedman EJ, Scott P, Mazina KE, Sun L, Du L, Ricker JL, Frankel SR, Gottesdiener KM, Wagner JA, Iwamoto M. A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res. 2006;12:7039–7045. doi: 10.1158/1078-0432.CCR-06-1802. [DOI] [PubMed] [Google Scholar]