Abstract
Staphylococcus aureus (SA) colonization and infection is common, and may promote allergic or inflammatory airway diseases, such as asthma, cystic fibrosis, and chronic rhinosinusitis by interacting with airway epithelial cells. Airway epithelial cells not only comprise a physical barrier, but also play key roles in immune, inflammatory, repair, and remodeling responses upon encounters with pathogens. To elucidate the impact of SA on epithelial-mediated remodeling of allergic airways, we tested the hypothesis that SA can enhance the remodeling process. Normal human bronchial epithelial (NHBE) cells were stimulated with heat-killed SA (HKSA) or transforming growth factor (TGF) α. Cell extracts were collected to measure mRNA (real-time RT-PCR) and signaling molecules (Western blot); supernatants were collected to measure protein (ELISA) after 24 hours of stimulation. Epidermal growth factor receptor (EGFR) signaling inhibition experiments were performed using a specific EGFR kinase inhibitor (AG1478) and TGF-α was blocked with an anti–TGF-α antibody. HKSA induced both mRNA and protein for TGF-α and matrix metalloproteinase (MMP) 1 from NHBE cells by a Toll-like receptor 2–dependent mechanism. Recombinant human TGF-α also induced mRNA and protein for MMP-1 from NHBE cells; anti–TGF-α antibody inhibited HKSA-induced MMP-1, suggesting that endogenous TGF-α mediates the MMP-1 induction by HKSA. HKSA-induced MMP-1 expression was suppressed when a specific EGFR kinase inhibitor was added, suggesting that EGFR signaling was mediating the HKSA-induced MMP-1 release. Exposure or colonization by SA in the airway may enhance the remodeling of tissue through a TGF-α–dependent induction of MMP-1 expression, and may thereby promote remodeling in airway diseases in which SA is implicated, such as asthma and chronic rhinosinusitis.
Keywords: Staphylococcus aureus, remodeling, Toll-like receptor 2, epidermal growth factor receptor, matrix metalloproteinase 1
Clinical Relevance
Exposure or colonization by Staphylococcus aureus in the airway may enhance the remodeling of tissue through a transforming growth factor-α–dependent induction of matrix metalloproteinase 1 expression and may, thereby, promote remodeling in airway diseases in which Staphylococcus aureus is implicated, such as asthma and chronic rhinosinusitis.
Allergic asthma affects roughly 300 million people worldwide (1). It is a chronic inflammatory disease of the airways characterized by infiltration of inflammatory cells, such as eosinophils, as well as T helper (Th) type 2 and Th17 lymphocytes. Structural cells, such as epithelial cells, fibroblasts, and smooth muscle cells, play a role in initiating or exacerbating the disease after encounters with aeroallergens and exacerbation triggers, including inhaled pathogens, such as viruses and bacteria. Epithelial cells are at the mucosal interface, and express multiple Toll-like receptors (TLRs), such as TLR2, -3, and -4, to recognize products of inhaled pathogens (2). Upon TLR activation, epithelial cells generate significant quantities of proinflammatory cytokines, chemokines, and growth factors to coordinate the host response to danger signals (3). Among TLRs, TLR2 recognizes bacterial lipoproteins, peptidoglycan, lipoteichoic acid, and zymosan from fungi, and bacteria (4). TLR2 ligands have been shown to promote Th2 responses (5) and aggravate experimental asthma (6). In addition, TLR2 participates in the immune response to Mycoplasma pneumonia (7), Chlamydophila pneumonia (8), and Staphylococcus aureus (SA) (9), and it has been shown that these TLR2 ligand–expressing pathogens are associated with acute exacerbations of asthma (10). Expression of TLR2 was shown to be up-regulated in asthmatic epithelial cells and in nasal epithelial cells from patients with chronic rhinosinusitis (CRS) (11–13).
Airway tissue from patients with severe asthma undergoes tissue remodeling, including increased collagen deposition, hyperplasia of smooth muscle and submucosal glands, and fibrosis, among other important histological findings (14). Even with adequate treatment, airway tissue remodeling was found in patients with chronic asthma, and is accompanied by decline of lung function (15). At the molecular level, an imbalance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) was one of the key findings in advanced remodeling tissue of patients with asthma (16). Among 25 subtypes of MMPs, MMP-1, -2, -9, and -10 were all found to be elevated in patients with asthma compared with normal subjects (17–20), and elevations of MMP-1 and -9 correlated with asthma severity (15, 21). In a mouse model of allergic inflammation, a broad-spectrum MMP inhibitor produced a decrease in inflammatory cells in bronchoalveolar lavage fluids (22) and lung parenchyma (23), and also decreased airway hyperresponsiveness (24). Cells producing MMPs in the lung were identified as epithelial cells (25), fibroblasts (26), and monocytes (27).
Recent reports indicate that bacterial colonization is present in the upper and lower airway mucosa of patients with asthma (28, 29). Associations between bacterial colonization of neonatal airway and subsequent development of asthma (30), evidence of bronchial infection by intracellular bacteria (31), and efficacy of benefit of long-term macrolide therapy in patients with asthma (32) all provide evidence implicating a connection between bacterial colonization and asthma development. Among the genetically isolated bacterial colonies, SA was found in the lower airways, suggesting the existence of SA in both upper and lower airways (33). Because bronchial epithelial cells express TLRs, we speculated that products of SA may chronically activate TLR2 on epithelial cells in patients colonized by this bacterium. Epithelial TLR2 activation would be expected to activate inflammatory, immune, and perhaps remodeling responses, worsening asthma status.
The mechanisms by which SA-induced TLR activation could contribute to airway remodeling are largely unknown. We therefore focused on the possible influence of SA on epithelial expression of genes known to be involved in the airway remodeling response resulting from activation of epithelial TLR.
Materials and Methods
Reagents
Heat-killed SA (HKSA) was purchased from Invitrogen (Carlsbad, CA) and transforming growth factor (TGF)-α was purchased from R&D Systems (Minneapolis, MN). Dexamethasone (DEX), cycloheximide (CHX), AG1478, a specific epidermal growth factor (EGF) receptor (EGFR) kinase inhibitor, and DMSO were purchased from Sigma-Aldrich (St. Louis, MO). The small interfering RNA (siRNA) against TLR2 and the no-effect control were from Santa Cruz Biotechnology (Santa Cruz, CA), and HiPerFect transfection reagent was from Qiagen (Valencia, CA). Anti–TGF-α antibody and rabbit isotype control were purchased from Abcam (Cambridge, MA).
Cell Culture, Treatments, and Transfection
Primary normal human bronchial epithelial (NHBE; Lonza, Walkersville, MD) cells, from at least three different donors, were plated in 12-well culture plates and either grown in submerged or in air–liquid interface conditions, as previously described (34). NHBE cells were deprived of hydrocortisone and growth factors from the media for 24 hours before stimulation. NHBE cells were treated with HKSA (5 × 108 particles/ml) and TGF-α (100 ng/ml) for up to 24 hours. For knockdown studies, NHBE cells were transfected with siRNA (10 nM) against nonspecific control or TLR2 at 50% confluence using HiPerFect transfection reagent (Qiagen). For inhibition studies, cells were treated with DEX (100 nM), CHX (1 or 10 μg/ml), or AG1478 (100 nM) 1 hour before the stimulation. DEX was kept in a stock of 0.1 M in DMSO, so an appropriate dilution of DMSO was used as a control. Anti–TGF-α or rabbit isotype control antibody (5 μg/ml) was applied 1 hour before stimulation.
Real-Time RT-PCR
Total RNA was isolated from the cells using NucleoSpin RNA II Isolation Kit (Macherey-Nagel, Bethlehem, PA) and cDNA was synthesized from RNA using SuperScriptII reverse transcriptase (Invitrogen). Real-time RT-PCR was performed with the TaqMan method using an Applied Biosystems 7,500 sequence detection system (Life Technologies, Grand Island, NY) as described previously (34). The levels of expression of mRNA were normalized to the housekeeping gene, β-actin, and results were analyzed by quantifying the absolute amount of mRNA. The primers and probe sets for detection of TGF-α, epiregulin (EREG), amphiregulin (AREG), EGF, neuregulin (NRG) -1, TLR2, MMP-1, -2, -9, and -10, and TIMP-1 were purchased from Life Technologies.
ELISA
Levels of MMP-1 and TGF-α in the supernatants of cultured cells were determined with commercially available ELISA kits following the manufacturer’s instructions (R&D Systems).
Western Blot
After stimulation, cells were washed with cold PBS and lysed using M-PER (Thermo Fisher Scientific, Waltham, MA) supplemented with protease and phosphatase inhibitor cocktail (Sigma-Aldrich). Cell lysates were centrifuged at 14,000 × g for 5 minutes. Clarified supernatants were analyzed for total protein using BCA assay (Thermo Fisher Scientific). Protein concentration–matched lysates were used for Western blot analysis. We then subjected whole-cell extracts to 4–12% Bis-Tris gradient gel electrophoresis (Life Technologies) with 3-(N-morpholino) propansulfonic acid SDS running buffer (130 V for 2 h). The gel was transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA) using a semidry transfer method (20 V for 1 h). The blots were blocked with blocking buffer (Rockland Immunochemicals, Gilbertsville, PA) for 1 hour. After blocking, blots were washed in TBST (50 mM Tris, 0.15M NaCl, and 0.05% Tween 20) and incubated with diluted (1:1,000:10,000) primary antibodies overnight. Blots were washed in TBST and incubated with secondary antibody for 1 hour. After washing off secondary antibodies with TBST, blots were exposed in an Odyssey Scanner (LI-COR Biosciences, Lincoln, NE). Densitometry was employed to analyze the intensity of the bands. Blots were stripped using stripping buffer (LI-COR Biosciences) and analyzed again with different primary antibodies. Primary antibody against actin was purchased from MP Biomedicals (Solon, OH) and others were from Cell Signaling Technology (Danvers, MA).
Statistical Analysis
All data are presented as the mean (± SEM), unless otherwise mentioned. Data were normally distributed, differences between groups were analyzed using the paired Student’s t test, and a P value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) software.
Results
HKSA Induced Epithelial Expression of MMPs and EGFR Ligands in a TLR2-Dependent Manner
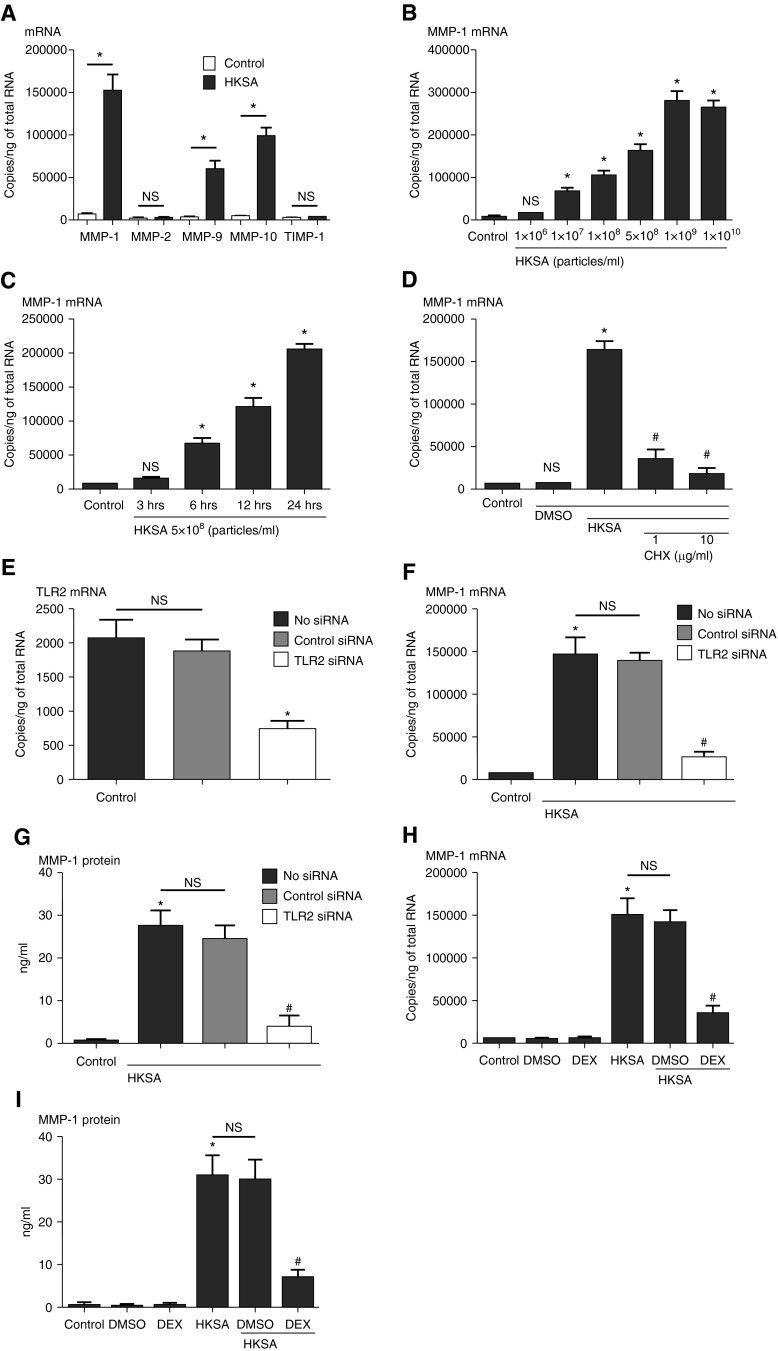
NHBE cells were incubated with HKSA for 3–24 hours, cell lysates were collected to assess expression of mRNA by RT-PCR, and supernatants were collected for protein assay by ELISA. HKSA induced expression of mRNA for MMP-1 (20.1-fold), MMP-9 (13.5-fold), and MMP-10 (17.0-fold) (n = 3, P < 0.01, compared with media-only control), and did not induce MMP-2 or TIMP-1 (Figure 1A). Consistent with RT-PCR data, MMP-1 protein was also detected by ELISA when cells were stimulated with HKSA (Figures 1G and 1I). Because MMP-1 was the most highly induced among the MMPs that we evaluated, we further focused on the mechanism of MMP-1 expression. From the dose–response curve (Figure 1B), we selected 5 × 108 particles/ml as a working concentration to stimulate the epithelial cells in subsequent experiments. From the time course curve (Figure 1C), the 24-hour time point showed a strong induction and was therefore selected for further studies. The addition of the protein synthesis inhibitor, CHX, inhibited the induction of MMP-1 mRNA by HKSA, indicating that MMP-1 gene transcription may require expression of intermediate proteins to be induced by HKSA (Figure 1D). Because HKSA is known to activate TLR2, we tested the influence of knockdown of TLR2. Data in Figure 1E show that siRNA against TLR2 down-regulated TLR2 and inhibited the expression of HKSA-induced MMP-1 at both the mRNA and protein level (Figures 1E–1G). HKSA induction of MMP-1 mRNA and protein was inhibited by the glucocorticoid DEX (Figures 1H and 1I) (75.1 and 78.9% reduction, respectively, compared with HKSA-stimulated cells; n = 3; P < 0.01).
Figure 1.
Submerged normal human bronchial epithelial (NHBE) cells were treated with heat-killed Staphylococcus aureus (HKSA; 5 × 108 particles/ml, except dose dependency) for 24 hours. Cell lysates were collected to assess expression of (A) matrix metalloproteinase (MMP) -1, -2, -9, and -10 and tissue inhibitors of metalloproteinase (TIMP) -1 mRNA by real-time RT-PCR. (B) The concentration dependency and (C) time course of expression of HKSA-induced MMP-1 mRNA were analyzed by RT-PCR. (D) Cycloheximide (CHX; 1 or 10 μg/ml) was applied 1 hour before HKSA stimulation for 24 hours, and expression of MMP-1 mRNA was analyzed by RT-PCR. NHBE cells were transfected with 10 nM small interfering RNA (siRNA) against nonspecific control RNA or Toll-like receptor (TLR) 2. (E) Knockdown efficiency of TLR2 was analyzed by RT-PCR. After transfection with siRNA against control RNA or TLR2, cells were incubated with HKSA for 24 hours, cell lysates were collected to assess (F) MMP-1 mRNA by RT-PCR, and supernatants were collected to assess the expression of (G) MMP-1 protein by ELISA. NHBE cells were pretreated with dexamethasone (DEX; 100 nM) 1 hour before HKSA stimulation. Cell lysates were collected to assess expression of (H) MMP-1 mRNA by RT-PCR, and supernatants were collected for (I) MMP-1 protein detection by ELISA. Data represent mean ± SEM of three independent experiments in three donors. *P < 0.01 by t test when compared with media only or dimethyl sulfoxide (DMSO) control; #P < 0.01 by t test when compared with HKSA-stimulated cells; NS, not significant by t test when compared with media-only control or HKSA-stimulated cells.
Induction of MMP-1 by HKSA Was Dependent on TGF-α Activation of the EGFR Pathway
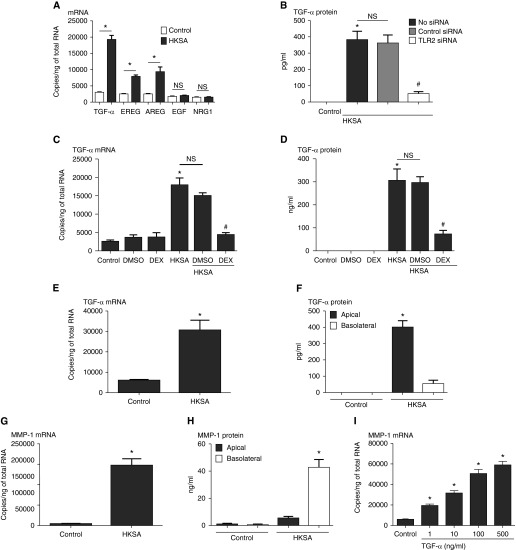
The experiments with CHX, and also the time-dependency experiments, suggested that an intermediate may be produced that is responsible for activating MMP-1 expression. Because EGFR ligands are known to induce MMP-1 expression (35), we screened HKSA-activated cells and found, among the family including TGF-α, EREG, AREG, EGF, and NRG1, that TGF-α was strongly induced (6.8-fold) by HKSA, whereas EREG and AREG were modestly, but significantly, induced (3.9- and 3.2-fold, respectively) (Figure 2A). HKSA-induced TGF-α protein was blocked by siRNA against TLR2 (Figure 2B). Pretreatment with DEX inhibited the induction of TGF-α by HKSA (Figures 2C and 2D). To test if fully differentiated airway epithelial cells express TGF-α, air–liquid interface–cultured NHBE cells were stimulated with HKSA. HKSA induced both TGF-α mRNA and TGF-α protein, and the protein was mainly detected in the supernatants from the apical side of cultured cells (Figures 2E and 2F), whereas MMP-1 protein was detected mainly in the supernatants from the basolateral side of the cultured cells (Figure 2H). We next evaluated whether TGF-α can induce the expression of MMP-1 mRNA, and found a clear concentration-dependent response (Figure 2I); we chose 100 ng/ml as a concentration to use in further experiments.
Figure 2.
Submerged NHBE cells were treated with HKSA (5 × 108 particles/ml) for 24 hours. (A) Cell lysates were collected to assess expression of HKSA-induced epidermal growth factor (EGF) receptor ligand (transforming growth factor [TGF] -α, epiregulin [EREG], amphiregulin [AREG], EGF, and neuregulin [NRG] -1) mRNA by RT-PCR. (B) After transfection with siRNA against control RNA or TLR2, cells were incubated with HKSA for 24 hours and cell lysates were collected to assess TGF-α by ELISA. NHBE cells were pretreated with DEX (100 nM) 1 hour before HKSA stimulation. Cell lysates were collected to assess expression of (C) TGF-α mRNA by RT-PCR, and supernatants were collected for (D) TGF-α protein detection by ELISA. NHBE cells were fully differentiated using an air–liquid interface culture system. Cells were treated with HKSA, and cell lysates were collected to analyze (E) TGF-α and (G) MMP-1 mRNA by RT-PCR, and (F) TGF-α and (H) MMP-1 protein in the basolateral and apical supernatants by ELISA. (I) The concentration response curve of TGF-α–induced MMP-1 mRNA at 24 hours was analyzed by RT-PCR. Data represent mean ± SEM of three independent experiments. *P < 0.01 by t test when compared with media-only control; #P < 0.01 by t test when compared with HKSA- and DMSO-stimulated cells or no siRNA or control siRNA transfected cells; NS, not significant.
Induction of MMP-1 by HKSA Was Dependent on TGF-α Activation of the EGFR Pathway
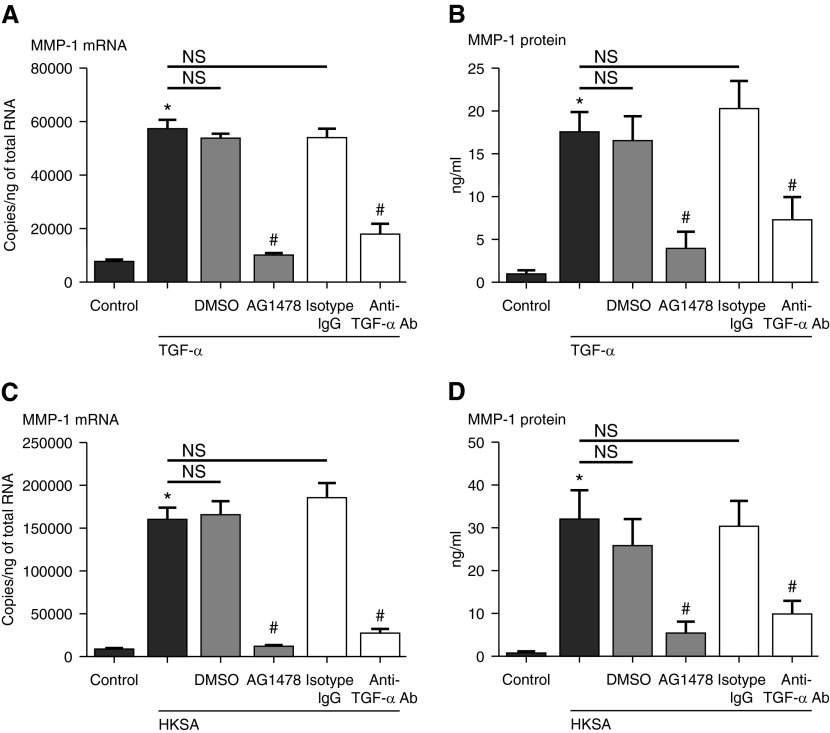
To test whether HKSA activates MMP-1 release by inducing TGF-α that, in turn, activates the EGFR, we stimulated cells with HKSA in the presence of AG1478, an EGFR-specific inhibitor, or a specific antibody against TGF-α before stimulation with either HKSA or TGF-α (Figure 3). As expected, using TGF-α as the stimulus, AG1478 and the antibody inhibited the induction of MMP-1 mRNA (81.6 and 67.1% reduction, respectively, compared with TGF-α–stimulated cells without inhibitor; n = 3; P < 0.01; Figure 3A). Importantly, AG1478 and anti–TGF-α antibody inhibited the HKSA-induced MMP-1 mRNA from NHBE cells (92.4 and 85.0% reduction, respectively, compared with HKSA-stimulated cells; n = 3; P < 0.01; Figure 3C), suggesting that TGF-α and EGFR signaling are involved in the response. A similar pattern of inhibition was obtained when we evaluated MMP-1 protein expression (Figures 3B and 3D).
Figure 3.
Submerged NHBE cells were pretreated with AG1478 (100 nM) or DMSO (0.01%), anti–TGF-α antibody (Ab) (5 μg/ml) or rabbit isotype IgG (5 μg/ml) for 1 hour before stimulation with (A and B) TGF-α (100 ng/ml) or (C and D) HKSA (5 × 108 particles/ml). Cell lysates were collected to assess expression of (A and C) MMP-1 mRNA by RT-PCR, and supernatants were collected for (B and D) MMP-1 protein detection by ELISA. Data represent mean ± SEM of three independent experiments; *P < 0.01 by t test when compared with media-only control; #P < 0.01 by t test when compared with HKSA- and DMSO- or isotype IgG–stimulated cells; NS, not significant.
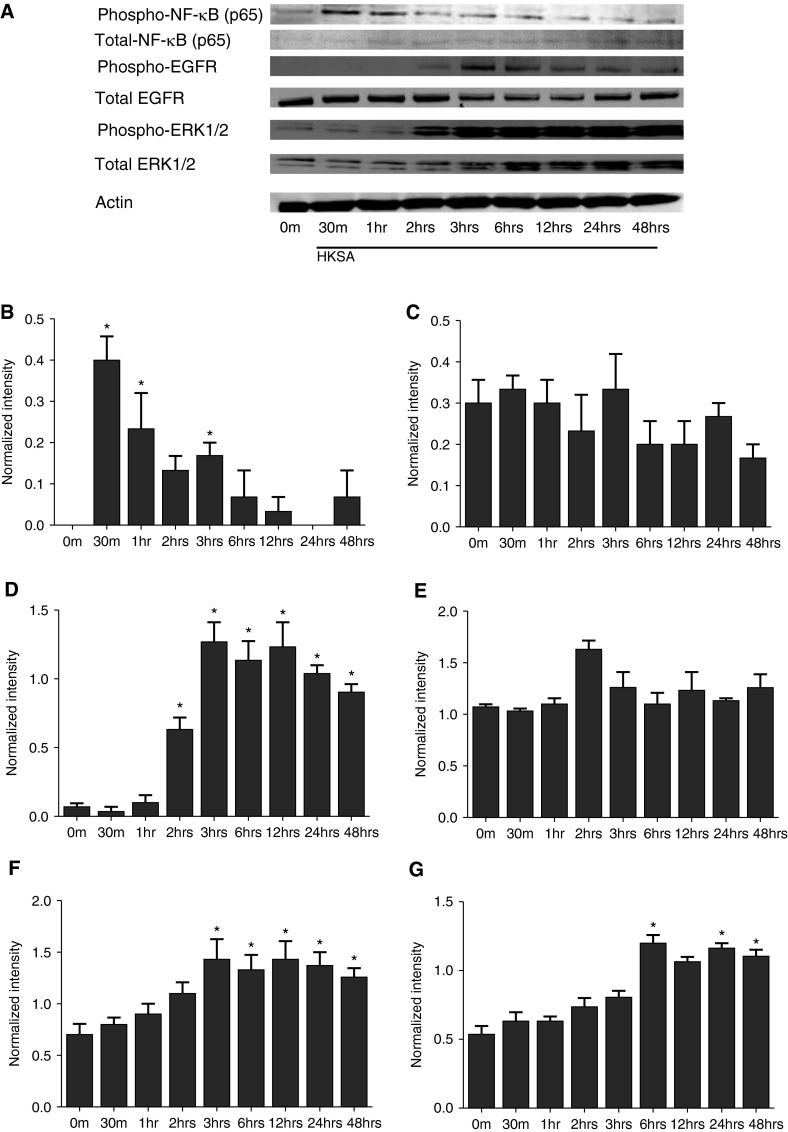
HKSA Induced Early Activation of NF-κB and Delayed Activation of EGFR and Extracellular Signal–Regulated Kinase 1/2
Our results so far indicate that HKSA activates TGF-α release via TLR2 activation and then TGF-α, working through the EGFR, induces MMP-1 expression. TLR2 and EGFR stimulation typically involves the activation of NF-κB and extracellular signal–regulated kinase (ERK) 1/2, respectively. We next evaluated the state of activation of NF-κB and ERK proteins. HKSA stimulation led to activation of NF-κB (p65) as early as 30 minutes after stimulation. Activation of EGFR and downstream ERK1/2 were observed as early as 2 hours after stimulation with HKSA (Figure 4), whereas activation of EGFR and ERK1/2 were observed more rapidly when the NHBE cells were stimulated with TGF-α (see Figure E2A in the online supplement). In addition, an EGFR inhibitor, AG1478, blocked the activation of EGFR by HKSA (Figure E1). This type of delay in ERK activation after HKSA stimulation would be expected if the production of TGF-α was required to occur first.
Figure 4.
Submerged NHBE cells were treated with HKSA (5 × 108 particles/ml) for up to 48 hours and cell lysates were collected at the indicated time points. (A) Cellular protein was collected for Western blot. Expressed bands for (B and C) phospho– and total NF-κB, (D and E) phospho– and total EGF receptor (EFGR), and (F and G) phospho– and total ERK1/2 were quantified using Image J software (NIH, Bethesda, MD) and normalized to internal control actin. Data in B–G represent mean ± SEM of three independent experiments, and images of Western blot are representative of three independent experiments. *P < 0.01 by t test when compared with media-only control.
Discussion
In the present study, we have demonstrated that HKSA activates molecular events associated with remodeling of airway tissue, including induction of MMP-1 and other MMPs. This response occurred in both submerged and fully differentiated primary airway epithelial cells, suggesting that it may be relevant to surface exposure of airway epithelium in vivo, where differentiated cells form tight junctions and possess mucociliary defense mechanisms. Mechanistically, our results suggest that HKSA directly induced epithelial release of TGF-α on the apical surface, and that the TGF-α subsequently induced MMP-1 on the basolateral surface by activating EGFR signaling. Live SA-stimulated NHBE cells also induced both MMP-1 and TGF-α mRNA (20.8- and 8.3-fold, respectively; n = 3; P < 0.01; data not shown), but the live SA–induced MMP-1 mRNA was much more variable than HKSA-induced MMP-1 mRNA from NHBE cells. We assume that growth rate of the live SA may significantly differ from well to well while stimulating the NHBE cells. HKSA induction of TGF-α was mediated by activation of TLR2, based upon knockdown experiments using siRNA targeting TLR2. HKSA-induced MMP-1 was significantly inhibited by pretreatment with anti–TGF-α antibody, suggesting that TGF-α is an essential intermediary inducer of the response. Furthermore, a specific EGFR kinase inhibitor also inhibited the response, suggesting that TGF-α may be functioning through a traditional receptor kinase mechanism. The glucocorticoid, DEX, also inhibited HKSA-induced TGF-α and MMP-1, suggesting that the ability of glucocorticoids to reverse or prevent some types of airway remodeling may reflect a disturbance of this epithelial component of the response.
Recent reports have suggested that colonization with bacteria, including SA, is found in both upper and lower airways of patients with asthma, as assessed by specific bacterial 13S-rRNA screening (28, 29). In addition to SA, investigators have detected approximately 100 bacterial taxa present in patients with asthma, raising the speculation that recognition of colonizing bacteria by pattern recognition receptors, such as TLRs, may play a role in epithelial activation, a key event in the pathogenesis of allergic asthma. Within this line of evidence, Zhang and colleagues (36) reported that bacterial colonization increased airway wall thickness. In the upper airway, colonization of SA has been detected at a higher rate among patients with CRS with nasal polyps and aspirin-sensitive respiratory disorders (37). Collectively, these studies suggest that SA could play a role in remodeling of allergic disease in both upper and lower respiratory airways, and our current study implicates activation of epithelium by SA as an event that enhances the remodeling process.
TLR2 has been shown to be particularly involved in signal transduction of cellular responses to gram-positive bacteria, lipoproteins, and mycobacterial wall constituents (38). Expression of TLR2 was reported to be highly elevated in patients with asthma compared with control subjects (11). In atopic dermatitis, the skin of patients is frequently colonized by SA and other TLR2-activating bacteria, associated with Th2-biased immune activation (39). Moreover, SA colonization and infections are believed to precipitate atopic dermatitis flares in which antibiotic therapies are often effective. From our results, SA failed to induce MMP-1 when TLR2 was knocked down by siRNA on airway epithelial cells, suggesting that TLR2 was the main pathogen recognition receptor in the signaling pathway for SA-induced MMP-1 (Figure 2). In a mouse model, several groups have shown that SA-derived proteins were recognized by TLR2 and that MMP-1 was induced by TLR2 activation (40, 41). Taken together, our results suggest that colonization by SA could initiate a remodeling process by activating TLR2 expressed on airway epithelial cells to induce MMPs that mediate elements of tissue matrix remodeling.
Activation of TLR2 by HKSA resulted in induction of the expression of TGF-α, EREG, and AREG mRNA among the EGFR ligand family, and also activated EGFR signaling, suggesting that one of these ligands was involved in an autocrine activation event (Figures 1 and 2). Puddicombe and colleagues (42) have shown that airway epithelial cells expressed EGFR, and that expression was much greater in asthmatic epithelium than in cells derived from normal subjects. They have also shown that areas of epithelial damage exhibited a strong EGFR immunoreactivity, and suggested that EGFR activation plays an important role in the epithelial damage/repair process in asthma (42). However, it is unlikely that epithelial damage was the only reason for increased EGFR expression in asthmatic epithelium, because EGFR staining was also increased in morphologically intact epithelial cells (42, 43). In addition to ligand-dependent activation, the EGFR system has a ligand-independent mechanism that has been reported, in which transactivation of EGFR by activated G protein–coupled receptors occurs in the absence of soluble EGFR ligands (43). Although Evdonin and colleagues (44) showed that transactivation of EGFR by TLR2 and TLR4 was mediated by heat shock protein 70, our current neutralization experiments suggested that the EGFR activation observed was not independent of ligand, but rather was primarily due to HKSA induction of EGFR ligands, TGF-α in particular. Autocrine or paracrine activation of EGFR by secreted EGFR ligands has also been reported when airway epithelial cells were stimulated with virus, Aspergillus fumigatus, cigarette smoke extract, and bacterial components (45–48). Although the mechanism of the EGFR activation in our studies mainly involved EGFR ligands, transactivation could still be present in our model, because the inhibitory effect of neutralization of TGF-α on the response was incomplete, whereas EGFR inhibition by AG1478 was almost complete. In addition, these results suggest that EREG and AREG may cooperatively activate EGFR with TGF-α. Further study will be needed to determine the role, if any, of transactivation and the role of other EGFR ligands in the epithelial response to HKSA. Because multiple TLR ligands, such as TLR2 and -3, induced expression of EGFR ligands (49), we speculate that EGFR ligand–dependent EGFR pathway activation may be a consistent phenomenon in pathogen recognition receptor–activated epithelial cells.
EGFR ligands are found to be highly expressed in various chronic airway disorders, such as chronic obstructive pulmonary disease and asthma, as a consequence of the need for repair of damaged epithelium (42, 50, 51). Among the EGFR ligand family that we tested (TGF-α, EREG, AREG, EGF, and NRG1), HKSA was most effective in inducing mRNA of TGF-α. As regards the secondary induction of MMP-1 by EGFR family members, in addition to TGF-α, EREG also induced MMP-1 mRNA, and EREG enhanced the expression of TGF-α–induced MMP-1 from NHBE cells (data not shown). Therefore, it is possible, or even likely, that induced EGFR ligands cooperatively activate the EGFR signaling to induce MMP-1 in our model system. Although TGF-α appears to play a central role, it is clear that more experimentation will be necessary to address the role of other family members.
The mechanisms of the anti-inflammatory effects of glucocorticoids have been extensively studied (52, 53). Others have shown that the activated glucocorticoid receptor homodimerizes and interacts with transcription factors, such as NF-κB, and represses the genes activated by these transcription factors (54). In addition, glucocorticoids exert post-transcriptional control on gene expression by decreasing the stability of mRNA for inflammatory genes (55). Our previous studies have suggested that NF-κB is not a major target of glucocorticoids in human airway epithelial cells (52). The mechanisms of the suppressive effect of glucocorticoids on the HKSA- or TGF-α–induced MMP-1 are not yet clear. Future studies will be required to determine the specific mechanism of inhibition of HKSA-induced genes by DEX in airway epithelial cells.
MMP-1 was found to be highly elevated in patients with asthma, and was shown to be induced from airway epithelial cells by cigarette smoke extract, poly I:C, and respiratory syncytial virus (56–58). One of the main properties of MMP-1 is a proteolytic ability to degrade fibrillar collagen. In addition, MMP-1 has the potential to alter local innate immunity by cleaving proinflammatory chemokines, such as CCL2, -7, and -8 and CXCL1, -8, -12 (59). MMP-1 also plays an important part in disrupting tight junctions and, thereby, altering the epithelial barrier (56). Airway epithelial cells are the primary airway mucosal barrier against particulate stimuli from the environment. It is known that asthmatic epithelial cells are defective in barrier function, with incomplete formation of tight junctions and, therefore, inadequate ability to prevent tissue entry of inhaled allergens or virulent pathogens (60). Although none of the synthetic MMP inhibitors has been introduced into clinical practice yet, studies have shown positive results both in clinical settings and in experimental models. Currently, several pharmaceutical companies have developed either pan- or specific MMP inhibitors (61). Among them, a broad-spectrum MMP inhibitor, R94138, showed reduction of allergic airway inflammation in mice (62), and another broad-spectrum MMP inhibitor, marimastat, reduced airway hyperresponsiveness in subjects with asthma (24). Recently, van Zele and colleagues (63) showed that the potent MMP inhibitor, doxycycline, reduced polyp size in patients with CRS with nasal polyps. From our results, SA should be considered as a potential inducer of MMP-1 release in the airways and as another potential cause of barrier disruption in asthma. In addition, strategies targeting MMP-1, TLR2, or EGFR signaling might be considered as therapeutic options in patients with asthma and CRS in whom colonization by SA is present.
In summary, we report that HKSA activated TLR2 and, thereby, induced profound expression of MMP-1 in primary airway epithelial cells. Our data suggest that the MMP-1 expression was dependent on autocrine production of growth factors of the EGF family. This study implicates colonization by SA and other TLR2-activating organisms in tissue remodeling, a hypothetical role that is readily testable in preclinical models or in human subjects affected by allergic diseases characterized by colonization with SA and other TLR activators.
Footnotes
This work was supported in part by National Institutes of Health grants R37HL068546, R01HL078860, R01AI104733, and P01AI106683, by the Ernest S. Bazley Foundation, and by a Showa University Research Grant for Young Researchers (Showa University Research Fund).
Author Contributions: conception and design—T.H., A.K., J.E.N., and R.P.S.; analysis and interpretation—T.H., M.S., L.A.S., R.G.C., and R.P.S.; drafting the manuscript for important intellectual content—T.H. and R.P.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0240OC on September 2, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 3.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal–regulated kinase–mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 6.Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 7.Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, Campbell K, Harbeck RJ, Martin RJ. TLR2 signaling is critical for Mycoplasma pneumoniae–induced airway mucin expression. J Immunol. 2005;174:5713–5719. doi: 10.4049/jimmunol.174.9.5713. [DOI] [PubMed] [Google Scholar]
- 8.Netea MG, Kullberg BJ, Galama JM, Stalenhoef AF, Dinarello CA, Van der Meer JW. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through Toll-like receptor 2–dependent pathways. Eur J Immunol. 2002;32:1188–1195. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, Kamijo S, Hiramatsu K, Ikeda S, Ogawa H, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2–Toll-like receptor 6 pathway. J Allergy Clin Immunol. 2010;126:985–993, e1–e3. doi: 10.1016/j.jaci.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med. 2005;172:1078–1089. doi: 10.1164/rccm.200412-1743PP. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira DS, Annoni R, Silva LF, Buttignol M, Santos AB, Medeiros MC, Andrade LN, Yick CY, Sterk PJ, Sampaio JL, et al. Toll-like receptors 2, 3 and 4 and thymic stromal lymphopoietin expression in fatal asthma. Clin Exp Allergy. 2012;42:1459–1471. doi: 10.1111/j.1365-2222.2012.04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Zhou B, Wang C, Huang Q, Zhang Q, Han Y, Dai W, Fan E, Li Y. Biofilm formation and Toll-like receptor 2, Toll-like receptor 4, and NF-kappaB expression in sinus tissues of patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:104–109. doi: 10.2500/ajra.2012.26.3718. [DOI] [PubMed] [Google Scholar]
- 14.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissonnette EY, Madore AM, Chakir J, Laviolette M, Boulet LP, Hamid Q, Bergeron C, Maghni K, Laprise C. Fibroblast growth factor-2 is a sputum remodeling biomarker of severe asthma. J Asthma. 2014;51:119–126. doi: 10.3109/02770903.2013.860164. [DOI] [PubMed] [Google Scholar]
- 16.Ye S, Henney AM. Detecting polymorphisms in MMP genes. Methods Mol Biol. 2001;151:367–375. doi: 10.1385/1-59259-046-2:367. [DOI] [PubMed] [Google Scholar]
- 17.Cataldo D, Munaut C, Noël A, Frankenne F, Bartsch P, Foidart JM, Louis R. MMP-2– and MMP-9–linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000;123:259–267. doi: 10.1159/000024452. [DOI] [PubMed] [Google Scholar]
- 18.Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax. 1999;54:590–596. doi: 10.1136/thx.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mautino G, Henriquet C, Jaffuel D, Bousquet J, Capony F. Tissue inhibitor of metalloproteinase-1 levels in bronchoalveolar lavage fluid from asthmatic subjects. Am J Respir Crit Care Med. 1999;160:324–330. doi: 10.1164/ajrccm.160.1.9808087. [DOI] [PubMed] [Google Scholar]
- 20.Cataldo DD, Gueders M, Munaut C, Rocks N, Bartsch P, Foidart JM, Noël A, Louis R. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases mRNA transcripts in the bronchial secretions of asthmatics. Lab Invest. 2004;84:418–424. doi: 10.1038/labinvest.3700063. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel SE, Balzar S, Cundall M, Chu HW. Subepithelial basement membrane immunoreactivity for matrix metalloproteinase 9: association with asthma severity, neutrophilic inflammation, and wound repair. J Allergy Clin Immunol. 2003;111:1345–1352. doi: 10.1067/mai.2003.1464. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Song CH, Lee HB, Oh JL, Rhee YK, Park HS, Koh GY. A murine model of toluene diisocyanate–induced asthma can be treated with matrix metalloproteinase inhibitor. J Allergy Clin Immunol. 2001;108:1021–1026. doi: 10.1067/mai.2001.120132. [DOI] [PubMed] [Google Scholar]
- 23.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce C, Thomas PS. The effect of marimastat, a metalloprotease inhibitor, on allergen-induced asthmatic hyper-reactivity. Toxicol Appl Pharmacol. 2005;205:126–132. doi: 10.1016/j.taap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D’Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001;163:786–791. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- 26.Asano K, Shikama Y, Shoji N, Hirano K, Suzaki H, Nakajima H. Tiotropium bromide inhibits TGF-β–induced MMP production from lung fibroblasts by interfering with Smad and MAPK pathways in vitro. Int J Chron Obstruct Pulmon Dis. 2010;5:277–286. doi: 10.2147/copd.s11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chizzolini C, Rezzonico R, De Luca C, Burger D, Dayer JM. Th2 cell membrane factors in association with IL-4 enhance matrix metalloproteinase-1 (MMP-1) while decreasing MMP-9 production by granulocyte–macrophage colony–stimulating factor–differentiated human monocytes. J Immunol. 2000;164:5952–5960. doi: 10.4049/jimmunol.164.11.5952. [DOI] [PubMed] [Google Scholar]
- 28.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.e1–e3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham AF, Johnston SL, Julious SA, Lampe FC, Ward ME. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11:345–349. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 32.Amayasu H, Yoshida S, Ebana S, Yamamoto Y, Nishikawa T, Shoji T, Nakagawa H, Hasegawa H, Nakabayashi M, Ishizaki Y. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann Allergy Asthma Immunol. 2000;84:594–598. doi: 10.1016/S1081-1206(10)62409-X. [DOI] [PubMed] [Google Scholar]
- 33.Følsgaard NV, Schjørring S, Chawes BL, Rasmussen MA, Krogfelt KA, Brix S, Bisgaard H. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med. 2013;187:589–595. doi: 10.1164/rccm.201207-1297OC. [DOI] [PubMed] [Google Scholar]
- 34.Chustz RT, Nagarkar DR, Poposki JA, Favoreto S, Jr, Avila PC, Schleimer RP, Kato A. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ancha HR, Kurella RR, Stewart CA, Damera G, Ceresa BP, Harty RF. Histamine stimulation of MMP-1(collagenase-1) secretion and gene expression in gastric epithelial cells: role of EGFR transactivation and the MAP kinase pathway. Int J Biochem Cell Biol. 2007;39:2143–2152. doi: 10.1016/j.biocel.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Illing R, Hui CK, Downey K, Carr D, Stearn M, Alshafi K, Menzies-Gow A, Zhong N, Fan Chung K. Bacteria in sputum of stable severe asthma and increased airway wall thickness. Respir Res. 2012;13:35. doi: 10.1186/1465-9921-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, van Cauwenberge P, Bachert C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–983. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boguniewicz M, Leung DY. Pathophysiologic mechanisms in atopic dermatitis. Semin Cutan Med Surg. 2001;20:217–225. doi: 10.1053/sder.2001.29379. [DOI] [PubMed] [Google Scholar]
- 40.Page K, Ledford JR, Zhou P, Wills-Karp M. A TLR2 agonist in German cockroach frass activates MMP-9 release and is protective against allergic inflammation in mice. J Immunol. 2009;183:3400–3408. doi: 10.4049/jimmunol.0900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo IH, Carpenter-Mendini A, Yoshida T, McGirt LY, Ivanov AI, Barnes KC, Gallo RL, Borkowski AW, Yamasaki K, Leung DY, et al. Activation of epidermal Toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol. 2013;133:988–998. doi: 10.1038/jid.2012.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 43.Burgel PR, Nadel JA. Epidermal growth factor receptor–mediated innate immune responses and their roles in airway diseases. Eur Respir J. 2008;32:1068–1081. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 44.Evdonin AL, Guzhova IV, Margulis BA, Medvedeva ND. Extracellular heat shock protein 70 mediates heat stress–induced epidermal growth factor receptor transactivation in A431 carcinoma cells. FEBS Lett. 2006;580:6674–6678. doi: 10.1016/j.febslet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Shiomi T, Tschumperlin DJ, Park JA, Sunnarborg SW, Horiuchi K, Blobel CP, Drazen JM. TNF-α–converting enzyme/A disintegrin and metalloprotease-17 mediates mechanotransduction in murine tracheal epithelial cells. Am J Respir Cell Mol Biol. 2011;45:376–385. doi: 10.1165/rcmb.2010-0234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oguma T, Asano K, Tomomatsu K, Kodama M, Fukunaga K, Shiomi T, Ohmori N, Ueda S, Takihara T, Shiraishi Y, et al. Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J Immunol. 2011;187:999–1005. doi: 10.4049/jimmunol.1002257. [DOI] [PubMed] [Google Scholar]
- 47.Baginski TK, Dabbagh K, Satjawatcharaphong C, Swinney DC. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am J Respir Cell Mol Biol. 2006;35:165–174. doi: 10.1165/rcmb.2005-0259OC. [DOI] [PubMed] [Google Scholar]
- 48.Liu K, Gualano RC, Hibbs ML, Anderson GP, Bozinovski S. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J Biol Chem. 2008;283:9977–9985. doi: 10.1074/jbc.M710257200. [DOI] [PubMed] [Google Scholar]
- 49.Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1068–L1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 50.de Boer WI, Hau CM, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125:184–192. doi: 10.1309/W1AX-KGT7-UA37-X257. [DOI] [PubMed] [Google Scholar]
- 51.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001;163:511–516. doi: 10.1164/ajrccm.163.2.2001038. [DOI] [PubMed] [Google Scholar]
- 52.Matsukura S, Kurokawa M, Homma T, Watanabe S, Suzuki S, Ieki K, Takeuchi H, Notomi K, Schleimer RP, Kawaguchi M, et al. Basic research on virus-induced asthma exacerbation: inhibition of inflammatory chemokine expression by fluticasone propionate. Int Arch Allergy Immunol. 2013;161:84–92. doi: 10.1159/000350455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang N, Truong-Tran QA, Tancowny B, Harris KE, Schleimer RP. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J Immunol. 2007;179:578–589. doi: 10.4049/jimmunol.179.1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor–signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 55.Newton R, Seybold J, Kuitert LM, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998;273:32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- 56.Hirakawa S, Kojima T, Obata K, Okabayashi T, Yokota S, Nomura K, Obonai T, Fuchimoto J, Himi T, Tsutsumi H, et al. Marked induction of matrix metalloproteinase-10 by respiratory syncytial virus infection in human nasal epithelial cells. J Med Virol. 2013;85:2141–2150. doi: 10.1002/jmv.23718. [DOI] [PubMed] [Google Scholar]
- 57.Mathis C, Poussin C, Weisensee D, Gebel S, Hengstermann A, Sewer A, Belcastro V, Xiang Y, Ansari S, Wagner S, et al. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air–liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol. 2013;304:L489–L503. doi: 10.1152/ajplung.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritter M, Mennerich D, Weith A, Seither P. Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. J Inflamm (Lond) 2005;2:16. doi: 10.1186/1476-9255-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mortier A, Gouwy M, Van Damme J, Proost P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp Cell Res. 2011;317:642–654. doi: 10.1016/j.yexcr.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556.e1–e12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 61.Vandenbroucke RE, Dejonckheere E, Libert C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur Respir J. 2011;38:1200–1214. doi: 10.1183/09031936.00027411. [DOI] [PubMed] [Google Scholar]
- 62.Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999;162:4212–4219. [PubMed] [Google Scholar]
- 63.Van Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, Hens G, Hellings P, Ebbens FA, Fokkens W, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069–1076, e4. doi: 10.1016/j.jaci.2010.02.020. [DOI] [PubMed] [Google Scholar]