Abstract
Tissue factor pathway inhibitor (TFPI) is the primary inhibitor of the extrinsic coagulation cascade, and its expression is reported to be relatively stable. Various pathophysiologic agents have been shown to influence TFPI activity by regulating its expression or by modifying the protein. It is not clear how TFPI activity is regulated in normal physiology or in injury. Because thrombin and TFPI are locally elaborated in pleural injury, we sought to determine if thrombin could regulate TFPI in human pleural mesothelial cells (HPMCs). Thrombin significantly decreased TFPI mRNA and protein levels by > 70%. Thrombin-mediated down-regulation of TFPI promoted factor X activation by HPMCs. The ability of thrombin to significantly decrease TFPI mRNA and protein levels was maintained at nanomolar concentrations. Protease-activated receptor (PAR)-1, a mediator of thrombin signaling, is detectable in the mesothelium in human and murine pleural injury. PAR-1 silencing blocked thrombin-mediated decrements of TFPI in HPMCs. Thrombin activates PI3K/Akt and nuclear factor κB (NF-κB) signaling in HPMCs. Inhibition of PI3K (by PX-866) and NF-κB (by SN50) prevented thrombin-mediated TFPI mRNA and protein down-regulation. These are the first studies to demonstrate that thrombin decreases TFPI expression in HPMCs. Our findings demonstrate a novel mechanism by which thrombin regulates TFPI expression in HPMCs and promotes an unrestricted procoagulant response, and suggest that interactions between PI3K and NF-κB signaling pathways are linked in HPMCs and control TFPI expression. These findings raise the possibility that targeting this pathway could limit the ability of the mesothelium to support extravascular fibrin deposition and organization associated with pleural injury.
Keywords: pleural mesothelial cells, thrombin, tissue factor pathway inhibitor, PI3K, NF-κB
Clinical Relevance
This is the first report that shows the procoagulant thrombin down-regulates the extrinsic pathway inhibitor tissue factor pathway inhibitor. This effect was found to be nuclear factor κB and PI3K dependent.
Injury to the mesothelium and subsequent inflammation of the pleura is characterized by the increased presence of inflammatory cells, elevated cytokine levels, and in some cases the development of pleural effusions (1, 2). Exudative effusions may organize to form a transitional fibrin mesh that tethers the parietal and visceral pleura and forms fibrin-laden pockets or locules that sequester pleural fluids. These collections are referred to as pleural loculations (2). Loculated pleural effusions are more commonly associated with complicated parapneumonic effusions and empyema. Pleural loculation can lead to pleural fibrosis or symphysis of the pleural surface, with morbidity including respiratory impairment attributable to lung restriction (3).
Fibrin deposition associated with various forms of pleural injury is initiated by activation of the extrinsic coagulation pathway (4–6). This pathway is regulated by the local expression of tissue factor (TF), which binds to activated factor VII (VIIa) to form a procoagulant activation complex. This complex then activates factor X to form Xa, either directly or via activation of factor IX to factor IXa, which converts factor II (prothrombin) to IIa (thrombin). Thrombin in turn converts soluble fibrinogen to insoluble fibrin. The principle inhibitor of surface TF activity is the TF pathway inhibitor (TFPI), which has been reported to be stably expressed by pleural mesothelial cells (PMCs) (7). TFPI forms an inhibitory complex with the extrinsic coagulation cascade complex of TF/VIIa/Xa on the cell surface and blocks further generation of Xa, with limitation of the procoagulant response.
Although thrombin is a powerful procoagulant, its activity is not limited to the potentiation of hemostasis. Thrombin can initiate cell signaling through activation of protease-activated receptors (PARs). PARs are G-coupled protein receptors that are activated by proteolytic cleavage. Cleavage of their amino terminus exposes a cryptic signaling sequence that binds to and activates the receptor. Of the four PAR subtypes (1–4), thrombin is reported to activate PAR-1, -3, and -4 (8). PAR activation triggers calcium release and numerous signaling pathways, including PI3K/Akt and nuclear factor κB (NF-κB) signaling pathways (9–11). In addition, thrombin and other factors involved in fibrin turnover have recently been shown to induce mesenchymal transition of PMCs (12).
TF expression in the normal quiescent pleura is relatively low (12, 13). However, in the inflammatory environment associated with pleural infection or injury, TF expression is dramatically increased and contributes to local fibrin deposition. Inflammatory cytokines present in pleural effusions, such as TNF-α and IL-1β, are known to induce TF expression and activity (14, 15). Although TFPI mRNA is reported to be relatively stable (16), recent reports show that TFPI expression can be attenuated in endothelial cells by the pathophysiologic agonist TNF-α (17, 18). The mechanism by which TFPI expression is suppressed in these cells remains unclear.
Procoagulant proteases such as thrombin are able to induce TF expression and activity in diverse cell types, including PMCs (12, 19). Thrombin is strongly elaborated in evolving pleural injury (12) and, like TF, has been implicated in the pathogenesis of pleural injury and repair (2, 12). TF and TFPI are elaborated by PMCs (7, 12, 19–23). Select proteases including plasmin and thrombin can degrade TFPI protein directly and are thus able potentiate TF activity (19, 24). However, the role of procoagulants such as thrombin on the expression of TFPI in PMCs has not been investigated. Here we show for the first time that thrombin down-regulates TFPI mRNA and protein expression in human PMCs (HPMCs). Suppression of TFPI in HPMCs by thrombin involves coordinate regulation via the PI3K and NF-κB pathways.
Materials and Methods
Additional methods are described in the online supplement.
Primary Human Tissue Samples
Studies using primary human tissue samples were approved by The University of Texas Health Science Center at Tyler Institutional Review Board committee. Deidentified lung tissues blocks containing the pleura from patients with a clinical diagnosis of nonspecific pleuritis were provided by a UTHSCT staff pathologist.
Carbon Black-Bleomycin Murine Model of Pleural Injury
All experiments involving animals were approved by the Institutional Animal Care and Use Committee at the University Of Texas Health Science Center at Tyler. Wild-type C57Bl/6j mice were treated with carbon black/bleomycin (CBB) for 14 days as previously described (12).
Immunohistochemistry
All tissue sections (human and murine) were deparafinized and subjected to sodium citrate antigen retrieval as previously described (12, 20). PARs were detected in human pleural tissues with select antibodies (PAR-1 ATAP2], PAR-2 [SAM11], PAR-3 [H-103], and PAR-4 [C-19]; Santa Cruz Biotechnology, Santa Cruz CA). Select PAR antibodies were used for mouse pleural tissue staining (PAR-1 [Abbiotec, San Diego, CA], PAR-2 [C17; Santa Cruz Biotechnology], PAR-3 [H-103], and PAR-4 [C-19])). Antigen was visualized using Fast Red chromogen (BioGenex, Freemont, CA).
Cell-Free Factor Xa Conversion Assay
Factor X (FX) activation assays were performed as previously described (25, 26). Briefly, conditioned media (CM) collected from PBS and thrombin treated cells were diluted 1:3 in Buffer B (10 mM HEPES, 150 mM NaCl, 11 mM glucose, and 4 mM KCl at pH 7.4 supplemented with 5 mM Ca2+ and 1 mg/ml BSA). Relipidated TF liposomes were added to the diluted CM. FVIIa (10 nM) was added, and the solution was and incubated for 2 minutes at room temperature. FX (170 nM) (diaPharma, Columbus, OH) was added to the solution and incubated for 30 minutes at room temperature. The solution was removed and placed in a microfuge tube containing 50 μl of 0.5 M EDTA. Each sample (50 μl) was then added in duplicate to a 96-well plate. FXa substrate (25 μl) (S-2765, diaPharma) was added to each well, and the plate was read on a Spectromax spectrophotometer. Absorbance readings at 405 nm were taken every 12 seconds for 2 minutes as previously described (12, 20).
Cell Surface FX Activation Assay
HPMCs were treated with PBS or thrombin (7 nM) for 48 hours. Cells were then washed with Buffer B, and cell surface FX activation assays were performed as previously described (12, 20).
Adenoviral Transduction
HPMCs were adenovirally infected to introduce transgenes for β-galactosidase (LacZ) or dominant negative Akt (DN-Akt) (two multiplicities of infection) for 24 hours. Cells were placed in serum-free medium 24 hours before treatment with PBS or THB.
PAR siRNA Transfection
HPMCs were transfected with PAR-1 small interfering RNAs (siRNAs) (200 nM) using RNAimax (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, HPMCs were transfected with PAR-1 siRNA (5′ AGA UUA GUC UCC AUC AAU 3′) (Eurofins, Huntsville AL) for 6 hours at 37°C in serum-free medium (27). Cells were allowed to recover for 48 hours in LHC8 complete medium 48 hours before serum starvation.
Statistics
All statistics were performed using Student’s t test. A P value of < 0.05 was considered significant.
Results
Thrombin Down-regulates TFPI Expression in HPMCs
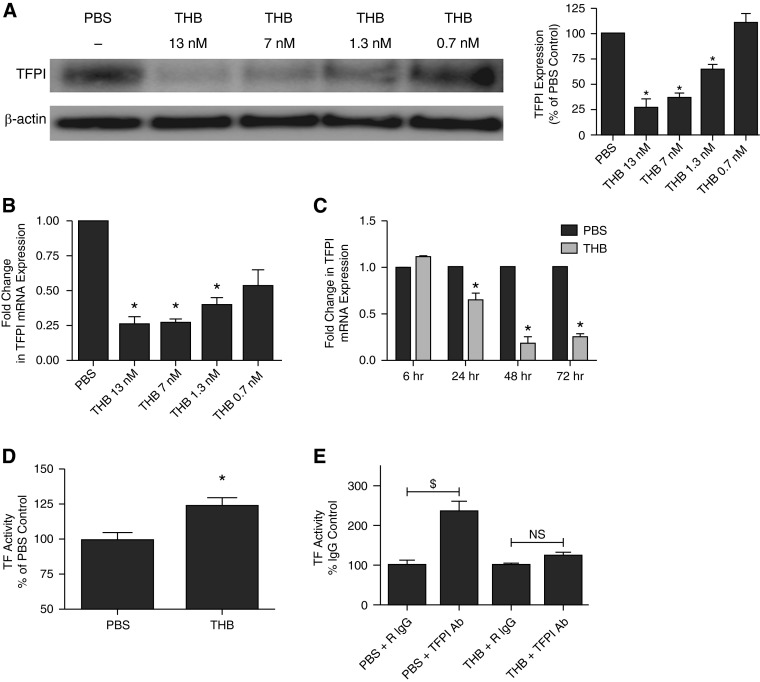
Because HPMCs are known to express TF and its inhibitor TFPI (19) and because thrombin induces TF mRNA and protein expression in HPMCs (19), we sought to determine how thrombin regulates TFPI expression in HPMCs. Serum-starved HPMCs were first treated with PBS or decreasing concentrations of thrombin (13–0.7 nM). The CM- and cell lysates were subjected to SDS-PAGE followed by Western blot analyses. Thrombin treatment (13–1.3 nM) significantly reduced TFPI protein levels by > 60% when compared with PBS-treated controls (P < 0.05) (Figure 1A). The lowest dose of thrombin (0.7 nM) had no effect on TFPI protein levels. These findings were confirmed by parallel, real-time PCR analyses of TFPI mRNA from thrombin-treated HPMCs (Figure 1B). TFPI mRNA levels were likewise significantly reduced (> 70%) in cells treated with thrombin compared with PBS-treated controls (P < 0.05). Both α and β TFPI RNA isoforms were comparably suppressed by thrombin treatment (data not shown).
Figure 1.
Thrombin (THB) decreases tissue factor pathway inhibitor (TFPI) expression in human pleural mesothelial cells (HPMCs). (A and B) Serum-starved HPMCs were treated with decreasing concentrations of THB (13–0.7 nM) for 48 hours. Cell lysates underwent Western blotting for TFPI. β-actin antigen was used as the loading control. Graphed data represent n = 3/treatment. *P < 0.05 when compared with PBS-treated controls. (B) mRNA was isolated from HPMCs treated with decreasing concentrations of THB. Transcribed cDNA was analyzed for TFPI mRNA expression by quantitative PCR (qPCR). Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a normalization control. Graphed data represent n = 3/treatment. *P < 0.05. (C) Serum-starved HPMCs were treated with PBS or THB for 6, 24, 48, and 72 hours. Isolated mRNA from each time point was transcribed into cDNA and analyzed for TFPI mRNA by qPCR. The plotted data represent n = 3/treatment. *P < 0.05 when compared with PBS-treated control. (D) Conditioned media (CM) from PBS- and THB-treated HPMCs were collected and assayed for TFPI activity in a cell-free factor X activation assay. Graphed data represent n = 4/treatment. *P < 0.05. (E) PBS- and THB-treated HPMCs were assayed for surface tissue factor (TF) activity by factor X activation analysis. Data were normalized to PBS/IgG and THB/IgG, respectively. Graphed data represent n = 3/treatment. $P < 0.01 when compared with PBS control. NS, not significant.
Because thrombin significantly reduced TFPI protein and mRNA expression by HPMCs, we sought to determine the optimal treatment interval required for maximal TFPI down-regulation. Serum-starved HPMCs were treated with PBS or thrombin (7 nM) for 6, 24, 48, and 72 hours. RNA was isolated and analyzed by quantitative PCR. Although the reductions in TFPI mRNA levels were significant by the 24-hour time point (30–40%; P < 0.05), maximal thrombin-mediated TFPI down-regulation (> 70%) occurred by 48 hours (Figure 1C). TFPI mRNA levels remained significantly suppressed at the 72-hour time point (P < 0.05).
Because thrombin reduced TFPI mRNA and protein in HPMCs, we sought to determine the functional consequence of reduced TFPI expression by HPMCs. CM collected from PBS- and thrombin-treated cells (48 h) were assayed for their ability to inhibit TF/VIIa-mediated activation of factor X in a cell-free assay. CM from thrombin cells demonstrated a 25% increase in factor X activation compared with the CM of PBS-treated HPMCs (Figure 1D). We next determined cell surface TF activity by thrombin-treated HPMCs by FX activation analyses (Figure 1E). These assays were performed on PBS and thrombin-treated cells in the presence of a TFPI neutralizing antibody or a rabbit isotype–matched control. Whereas TFPI neutralization significantly increased FX activation in PBS-treated cells (P < 0.01), thrombin-treated cells did not demonstrate a significant increase in surface TF activity in the presence of the TFPI neutralizing antibody. These studies show that thrombin-mediated TFPI down-regulation promotes cell surface TF activity in primary HPMCs.
PARs Are Expressed by the Pleural Mesothelium of Human and Murine Lungs
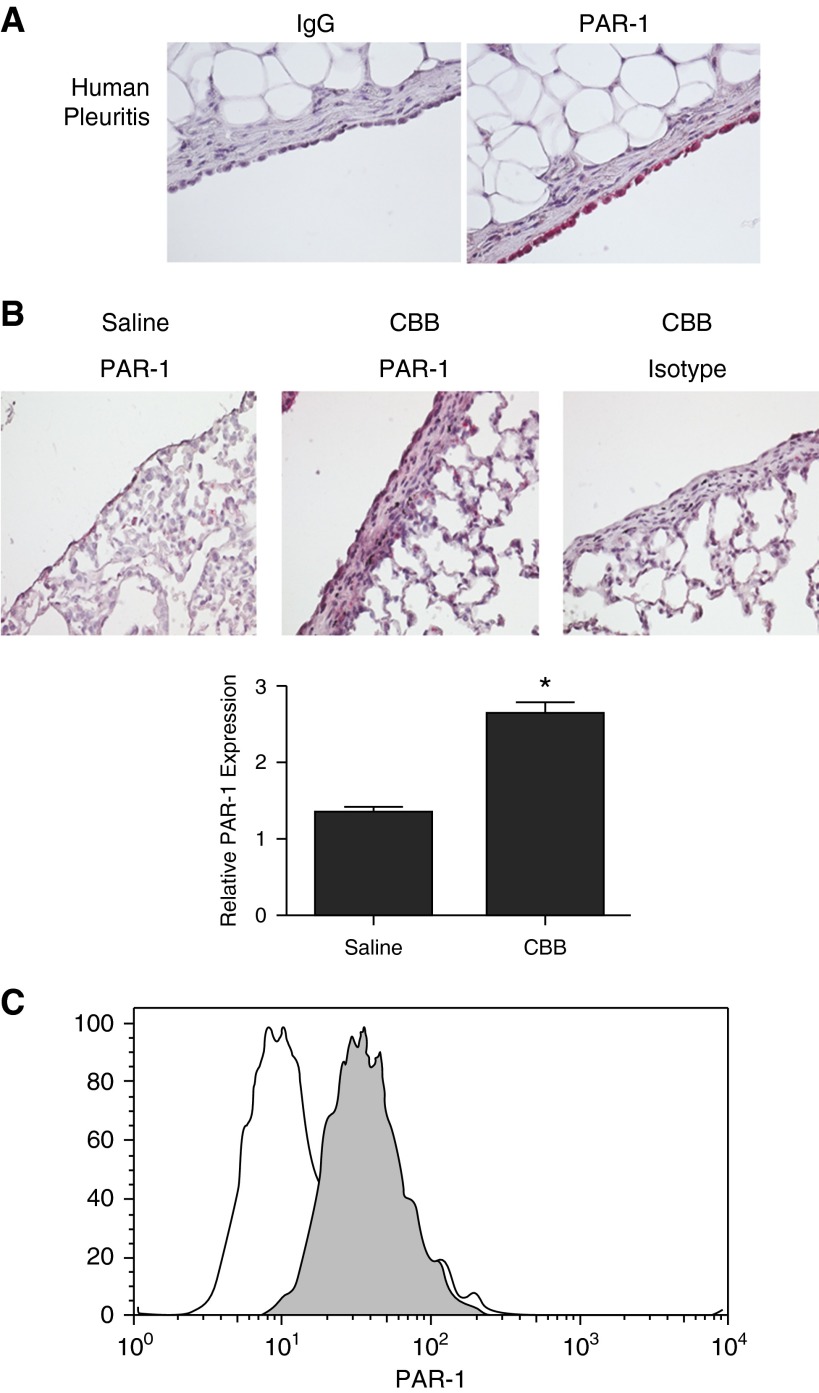
Because thrombin signaling is mediated through proteolytic cleavage of PAR-1, -3, and -4, we sought to determine their expression by the pleural mesothelium in pleuritic lungs sections. In these novel analyses, we found that PAR-1 expression was robust in human pleuritic lung sections (Figure 2A). PAR-1 was detected in the normal human pleura but was relatively lower than levels detected in pleuritic sections (data not shown). PAR-3 and -4 were not detected in normal or pleuritic human pleural lung tissue sections (data not shown). Analyses were next extended to tissue sections from our CBB mouse model of pleural fibrosis (Figure 2B). In these sections, the pleural mesothelium in its entirety was evaluated for PAR expression. PAR-1 expression was detected in the pleural mesothelium in saline control lungs and was significantly increased in CBB injury (Figure 2B). PAR expression was determined in isolated and cultured HPMCs from patients with congestive heart failure (Figure 2C) by FACS analyses. Confirming our immunohistochemistry results, PAR-1 was robustly expressed on the surface of HPMCs. PAR-3 and -4 were below the level of detection in the HPMCs via FACS analyses (data not shown). These data show that PAR-1 is expressed by human and mouse pleural mesothelium and is detectable at the surface of HPMCs.
Figure 2.
Protease activated receptor (PAR)-1 is expressed by the pleural mesothelium in human and murine lung tissue and HPMCs. Tissue sections were prepared from lung tissue sections of patients diagnosed with nonspecific pleuritis (A) and from the lungs of normal mice and mice with carbon black/bleomycin (CBB)-established pleural fibrosis (n = 3/group) (B). The sectioned pleural mesothelium in its entirety was analyzed for PAR-1 expression by immunohistochemical staining. All images were taken at 40× magnification on a Nikon Eclipse Ti inverted microscope. (B) PAR-1 expression was significantly increased in the pleural mesothelium of CBB-injured mice. PAR-1 levels were determined based on the relative intensity of PAR-1 staining (red stain) and evaluated using a four-point scale (30 fields/section for each of n = 3 mice/group). *P < 0.05 when compared with saline control. (C) Primary HPMCs were probed for surface PAR-1 expression by FACs analyses (shaded histogram represents PAR-1 antibody). Illustrated findings are representative of three independent experiments.
Thrombin-Mediated TFPI Down-regulation Is PAR Dependent
Because thrombin signaling is reported to occur through proteolytic activation of PARs, specifically PAR-1, we sought to determine the role of PAR-1 in thrombin-mediated TFPI down-regulation. The effectiveness of PAR-1 targeting siRNA was first evaluated by FACS analyses. siRNA transfection reduced PAR-1 expression to control IgG levels when compared with untransfected and control siRNA-transfected cells (Figure 3A). The role of PAR-1 in thrombin-mediated TFPI down-regulation was determined by quantitative PCR and Western blot analyses. Thrombin treatment reduced TFPI expression in untransfected and control siRNA-transfected cells when compared with PBS-treated controls. PAR-1 siRNA transfection reversed thrombin-mediated TFPI down-regulation when compared with untransfected and control siRNA–transfected cells (Figure 3B). These finding were confirmed by Western blot analyses (Figure 3C). Densitometric analyses of TFPI protein showed that thrombin-mediated TFPI down-regulation was reversed in PAR-1 siRNA–transfected cells. PAR-1 down-regulated cells were next assayed for surface TF activity by FX activation analyses. Although TFPI neutralization significantly enhanced TF activity in PBS-treated control cells (P < 0.05), the same antibody, as expected, failed to enhance TF activity in thrombin-treated cells. Conversely, PAR-1 down-regulation restored TFPI expression and function in thrombin-treated HPMCs (Figure 3D). Specifically, PAR-1 down-regulated cells demonstrated significantly enhanced TF activity in the presence of the TFPI neutralizing antibody when compared with isotype-treated controls (P < 0.05). These data show that thrombin-mediated TFPI down-regulation is PAR-1 dependent.
Figure 3.
THB-mediated TFPI down-regulation is PAR-1 dependent. (A–C) HPMCs were transfected with control or PAR-1–targeting small interfering RNA (siRNA). (A) Untransfected, control, and PAR-1 siRNA–transfected cells were analyzed for PAR-1 expression by FACs analyses (shaded histogram represents PAR-1 antibody). Untransfected, control, and PAR-1 siRNA–transfected cells were treated with PBS or THB and probed for changes in TFPI mRNA (B) and protein (C). Data represent n = 3. *P < 0.05 when compared with PBS control. (D) Control and PAR-1 siRNA–transfected cells were treated with PBS or THB. Cell surface TF activity was determined in the presence of TFPI neutralizing antibody or isotype control. Data represent n = 4/treatment. *P < 0.05 when compared with respective isotype control.
Thrombin-Mediated TFPI Down-regulation Is PI3K Dependent
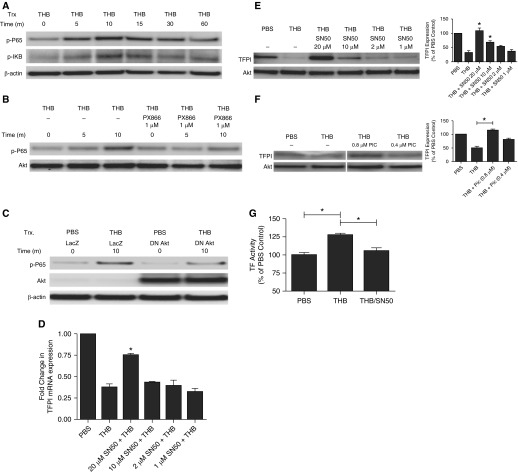
Because we previously showed that thrombin is a potent activator of the PI3K/Akt signaling pathway (12), we sought to determine the role of PI3K signaling in thrombin-mediated TFPI down-regulation. We first confirmed thrombin-mediated activation of the PI3K signaling pathway by Western blot analysis. Untransfected, control, and PAR-1 siRNA–transfected HPMCs were treated with PBS or thrombin and probed for phosphorylated Akt. Thrombin induced robust Akt phosphorylation in untransfected and control siRNA–transfected cells (Figure 4A). Total Akt was used as the loading control. PAR-1 siRNA–transfected cells demonstrated reduced Akt phosphorylation when compared with controls. We next assayed Akt phosphorylation in the presence and absence of the PI3K inhibitor, PX-866 (a generous gift from Scott Peterson, Oncothyreon) (Figure 4B). Akt phosphorylation was detectable 5 minutes after thrombin treatment. As anticipated, PX-866 treatment blocked thrombin-mediated Akt phosphorylation. The role of PI3K in TFPI mRNA down-regulation was next determined. RNA was isolated from thrombin-treated cells (7 nM, 48 h) in the presence of decreasing concentrations of PX-866 (1.0–0.05 µM). Whereas thrombin significantly decreased TFPI mRNA expression by HPMCs, PI3K inhibition (1 µM) significantly blunted thrombin-mediated TFPI down-regulation (P = 0.01) (Figure 4C). Submicromolar concentrations of PX-866 (500 nM) effectively reversed thrombin-mediated TFPI down-regulation (P = 0.02). The mRNA findings were confirmed by Western blot (Figure 4D). PX-866 (1 and 0.5 µM) significantly reversed thrombin-mediated TFPI protein down-regulation (P < 0.05) when compared with thrombin-treated cells. Similar results were found with the pan Akt inhibitor AKT VIII, which inhibits the three isoforms of Akt (data not shown). In functional analyses, PI3K inhibition significantly restored the inhibitory activity of TFPI in the CM of thrombin/PX866-treated cells when compared with thrombin-treated cells (P < 0.05) (Figure 4E). These studies show that PI3K/Akt signaling is required for thrombin-mediated TFPI down-regulation.
Figure 4.

PI3K signaling is required for THB-mediated TFPI down-regulation. (A) HPMCs were transfected with control or PAR-1–targeting siRNA. Untransfected, control, and PAR-1 siRNA–transfected cells were treated with PBS and thrombin, and cell lysates were Western blotted for TFPI. Total Akt antigen was the loading control. (B) HPMCs were treated with THB for 0, 5, and 15 minutes in the presence and absence of PX-866 (1 µM). Cells were lysed, resolved on a SDS-PAGE, and probed for phosphorylated Akt. Akt antigen was used as a loading control. HPMCs were treated with THB in the presence of decreasing concentrations of PX-866 (C and D). (C) Isolated mRNA was transcribed into cDNA and probed for changes in TFPI mRNA. GAPDH mRNA was used as the normalization control. $P = 0.01; *P < 0.05 when compared with THB-treated HPMCs. Data represent n = 3/treatment. (D) Cell lysates and CM from THB-treated cells were probed for TFPI. β-actin antigen was used as the loading control. The figure is representative of three independent experiments. Graphed data represent n = 3/treatment. *P < 0.05 when compared with treatment with THB alone. (E) CM from PBS-, THB-, and THB/PX866-treated HPMCs were collected and assayed for TFPI activity in a cell-free factor X activation assay. The illustrated data represent n = 3/treatment. *P < 0.05.
Thrombin-Mediated TFPI Down-regulation Is NF-κB Dependent
Because thrombin has been reported to activate NF-κB signaling in other cells types (28, 29), we sought to extend the scope of our analyses to the NF-κB pathway in thrombin-mediated TFPI down-regulation in HPMCs. First, we determined that thrombin could initiate NF-κB signaling in HPMCs. Lysates from serum-starved HPMCs were treated with thrombin for 5, 10, 15, 30, and 60 minutes and probed for markers of NF-κB activation, phosphorylated IκB and p65. Thrombin induction of IκB phosphorylation was detectable by 5 minutes and peaked at 10 minutes (Figure 5A). Similarly, p65 phosphorylation was detected by 5 minutes; however, increased phosphorylation was detected throughout the 60-minute time course. NF-κB signaling, as determined by p65 phosphorylation, was reduced in PAR-1 siRNA-transfected cells when compared with control siRNA-transfected cells (data not shown).
Figure 5.
NF-κB blockade reverses THB-mediated TFPI down-regulation. (A) HPMCs were treated with THB for 0, 5, 10, 15, 30, and 60 minutes. Cellular lysates were probed for phosphorylated IκB and p65. β-actin was used as the loading control. (B) HPMCs pretreated with PBS and PX-866 (1 μM) were treated with THB for 0, 5, and 10 minutes. Lysates were probed for phosphorylated p65. Akt was used as the loading control. (C) HPMCs were infected with adenoviral vectors to introduce transgenes for β-galactosidase (LacZ) and dominant negative Akt 1 (DN-Akt). Cells were treated with PBS or thrombin for 10 minutes, and lysates were probed for phosphorylated P65 (p-P65) and total Akt. β-actin was used as the loading control. HPMCs were treated with THB in the presence of decreasing concentrations of SN50 (20–1 μM; D and E). (D) mRNA was isolated and probed for changes in TFPI mRNA levels by qPCR. GAPDH was used as the loading control. The illustrated data represent n = 3/treatment. *P < 0.05 when compared with THB-treated HPMCs. (E) CM were resolved by SDS-PAGE and probed for changes in TFPI protein expression. Illustration is representative of three independent experiments. The data shown represent n = 3/treatment. *P < 0.05 when compared with THB-treated HPMCs. Akt was used as the loading control. (F) HPMCs were treated with THB in the presence of varying concentrations of piceatannol (0.8 and 0.4 μM). CM were resolved by SDS-PAGE and probed for changes in TFPI protein expression. Illustration is representative of three independent experiments. Compiled image was reconstructed from the same gel. The data shown represent n = 3/treatment. *P < 0.05 when compared with THB-treated HPMCs. (G) CM from PBS-, THB-, and THB/SN50-treated HPMCs were collected and assayed for TFPI activity in a cell-free factor X activation assay. The illustrated data represent n = 4/treatment. *P < 0.05 when compared with thrombin treatment alone. Trx, treatment.
Next, we determined if NF-κβ activation was dependent on PI3K activity. Cells treated with PX-866 (1 μM) were treated with thrombin (7 nM) for 5 and 10 minutes, and lysates were assayed by Western blot analyses. PI3K inhibition blunted the ability of thrombin to induce P65 phosphorylation in HPMCs (Figure 5B). These results were confirmed with DN-Akt. Overexpression of DN-Akt reduced thrombin-mediated P65 phosphorylation (Figure 5C). These experiments confirm that thrombin activates NF-κB in HPMCs and that activation of the NF-κB pathway is dependent, at least in part, on PI3K signaling.
Because the NF-κB signaling pathway is activated by thrombin treatment in HPMCs, we studied its role in thrombin-mediated TFPI down-regulation. HPMCs were treated with thrombin in the presence of decreasing concentrations of the NF-κB inhibitor SN50 (20, 10, 2, and 1 μM) (EMD Millipore, Billerica, MA). NF-κB blockade by SN50 (20 μM) significantly reversed thrombin-mediated TFPI mRNA down-regulation (P = 0.002) (Figure 5D). These findings were confirmed by Western blot analyses (Figure 5E). SN50 (20 and 10 μM) significantly reversed thrombin-mediated TFPI protein down-regulation when compared with thrombin-treated cells (P < 0.05). Similar results were found with the NF-κB pathway inhibitor, piceatannol.
Subnanomolar concentrations of piceatannol (0.8 μM) blocked thrombin-mediated TFPI protein down-regulation in HPMCs (Figure 5F). The highest effective concentration of PX-866 (1 μM) and SN50 (20 μM) comparably reversed thrombin-mediated TFPI mRNA and protein down-regulation. In functional analyses, NF-κB inhibition significantly restored the inhibitory activity of TFPI in the CM of thrombin/SN50-treated cells when compared with thrombin-treated cells (P < 0.05) (Figure 5F). These results show that NF-κB signaling in HPMCs is dependent on PI3K activation and that both signaling pathways are required for thrombin-mediated TFPI down-regulation.
Discussion
Our present study documents the novel finding that thrombin attenuates the expression of the principal TF inhibitor, TFPI, in primary HPMCs and promotes TF activity. We found that thrombin-mediated TFPI down-regulation was mediated through PAR-1 and was dependent on PI3K and NF-κB signaling pathways. Our results show that thrombin can potentiate mesothelial cell coagulation through a previously unrecognized mechanism, the down-regulation of TFPI by HPMCs.
Our previous report showed that thrombin induced TF expression and activity in HPMCs (12, 19). In this study, we sought to determine if thrombin could contribute to increased TF activity associated with thrombin treatment. We show that thrombin significantly reduces TFPI mRNA and protein expression in HPMCs. These finding were supported by factor X activation analyses that demonstrated significantly increased factor X activation in CM from thrombin-treated cells when compared with PBS-treated samples. Similar results were obtained with intact cells. Treatment of thrombin-treated cells with anti-TFPI antibodies failed to further increase cell surface TF activity. In contrast, anti-TFPI antibodies increased the TF activity of PBS-treated cells by 2- to 3-fold over the control IgG-treated cells. This supports our conclusion that increased TF activity observed in thrombin-treated cells in the present study comes from thrombin down-regulation of TFPI. It is likely that the short durations of thrombin treatment used in the earlier study could have been the reason for the failure to notice thrombin down-regulation of TFPI in HPMCs (19).
Because thrombin mediates signaling through PAR activation, the profile of PAR expression was determined in pleural tissues. Although thrombin signaling can occur through proteolytic cleavage of PARs 1, 3, and 4, only PAR-1 was detected in human pleural sections, in mouse pleural sections, and on cultured primary HPMCs. These findings allowed us to focus our study of thrombin-mediated signaling events to PAR-1. As expected, down-regulation of PAR-1 in HPMCs completely abrogated the effects of thrombin on TFPI mRNA and protein expression. These findings confirmed that PAR-1 cleavage was critical to thrombin-mediated TFPI down-regulation.
We next determined the signaling events responsible for TFPI down-regulation. Because our previous studies (12) showed robust Akt phosphorylation at the same time point that TFPI was maximally down-regulated (48 h) in thrombin-treated cells, we investigated whether thrombin-induced PI3K signaling plays a role in thrombin down-regulation of TFPI expression in HPMCs. The observation that the PI3K inhibitor PX-866 significantly reversed thrombin-mediated TFPI mRNA and protein down-regulation supports the involvement of the PI3K pathway in thrombin down-regulation of TFPI.
Because the NF-κB signaling pathway has been reported to be downstream of PI3K, we determined the contribution of NF-κB to thrombin-mediated TFPI down-regulation. We found that thrombin potently activated the NF-κB pathway and that blockade of NF-κB abrogated thrombin-dependent TFPI down-regulation. Because PI3K and Akt signaling blockade blunted thrombin-mediated NF-κB activation, we infer that thrombin induction of NF-κB in HPMCs is dependent, in part, on PI3K/Akt. However, the inability to completely attenuate NF-κB activity with the PI3K/Akt antagonists suggests that alternative pathways of NF-κB activation may remain active.
Although the TFPI promoter lacks NF-κB binding sequences (30–32), it does contain several transcription factor binding sites, including an SP1 transcription site. Recent studies show that NF-κB can indirectly regulate SP1-dependent gene expression by modifying SP1/DNA binding (33, 34). These studies showed that NF-κB interferes with SP1 binding through an analogous transcription factor (SP3). Whether a similar mechanism contributes to the thrombin-mediated changes in TFPI in HPMCs remains to be determined. However, any of the numerous genes that are regulated by NF-κB activation could indirectly modify the expression of TFPI by HPMCs. These analyses lie outside the scope of this study.
In summary, we report that thrombin down-regulates TFPI mRNA and protein expression in HPMCs. PI3K and NF-κB signaling are required to mediate the thrombin effect. Although thrombin can potentiate coagulation through TF induction, we show for the first time that thrombin-mediated TFPI down-regulation promotes factor X activation in HPMCs. These findings raise the testable possibility that therapeutics that target signaling cascades such as PI3K and NF-κB and/or PAR-1 signaling could attenuate extravascular fibrin formation and organization associated with fibrosing pleural injuries.
Footnotes
This work was supported by National Institutes of Health grant HL115466 and by the Texas Lung Injury Institute.
Author Contributions: A.J., S.O., K.K., B.Q., and T.A.T. performed experiments presented in the manuscript. U.R.P., V.M.R., S.I., and T.A.T. designed experiments presented in the manuscript. U.R.P., V.M.R., S.I., and T.A.T. prepared and approved manuscript for submission.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0084OC on October 10, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3:75–80. doi: 10.1513/pats.200510-113JH. [DOI] [PubMed] [Google Scholar]
- 2.Idell S. The pathogenesis of pleural space loculation and fibrosis. Curr Opin Pulm Med. 2008;14:310–315. doi: 10.1097/MCP.0b013e3282fd0d9b. [DOI] [PubMed] [Google Scholar]
- 3.Idell S, Jun Na M, Liao H, Gazar AE, Drake W, Lane KB, Koenig K, Komissarov A, Tucker T, Light RW. Single-chain urokinase in empyema induced by pasturella multocida. Exp Lung Res. 2009;35:665–681. doi: 10.3109/01902140902833277. [DOI] [PubMed] [Google Scholar]
- 4.Strange C, Baumann MH, Sahn SA, Idell S. Effects of intrapleural heparin or urokinase on the extent of tetracycline-induced pleural disease. Am J Respir Crit Care Med. 1995;151:508–515. doi: 10.1164/ajrccm.151.2.7842213. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, Pueblitz S, Emri S, Gungen Y, Gray L, Kumar A, Holiday D, Koenig KB, Johnson AR. Regulation of fibrin deposition by malignant mesothelioma. Am J Pathol. 1995;147:1318–1329. [PMC free article] [PubMed] [Google Scholar]
- 6.Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis. 1991;144:187–194. doi: 10.1164/ajrccm/144.1.187. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj MS, Pendurthi U, Koenig K, Pueblitz S, Idell S. Tissue factor pathway inhibitor expression by human pleural mesothelial and mesothelioma cells. Eur Respir J. 2000;15:1069–1078. doi: 10.1034/j.1399-3003.2000.01515.x. [DOI] [PubMed] [Google Scholar]
- 8.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor x-dependent activation of protease-activated receptor 2 by factor viia. Proc Natl Acad Sci USA. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang R, Wang NP, Tanaka KA, Levy JH, Guyton RA, Zhao ZQ, Vinten-Johansen J. Factor xa induces tissue factor expression in endothelial cells by p44/42 mapk and nf-kappab-dependent pathways. J Surg Res. 2011;169:319–327. doi: 10.1016/j.jss.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto D, Hirono Y, Goi T, Katayama K, Matsukawa S, Yamaguchi A. The activation of proteinase-activated receptor-1 (par1) mediates gastric cancer cell proliferation and invasion. BMC Cancer. 2010;10:443. doi: 10.1186/1471-2407-10-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HT, Tsou HK, Tsai CH, Kuo CC, Chiang YK, Chang CH, Fong YC, Tang CH. Thrombin enhanced migration and mmps expression of human chondrosarcoma cells involves par receptor signaling pathway. J Cell Physiol. 2010;223:737–745. doi: 10.1002/jcp.22083. [DOI] [PubMed] [Google Scholar]
- 12.Tucker TA, Jeffers A, Alvarez A, Owens S, Koenig K, Quaid B, Komissarov AA, Florova G, Kothari H, Pendurthi U, et al. Plasminogen activator inhibitor-1 deficiency augments visceral mesothelial organization, intrapleural coagulation, and lung restriction in mice with carbon black/bleomycin-induced pleural injury. Am J Respir Cell Mol Biol. 2014;50:316–327. doi: 10.1165/rcmb.2013-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottles KD, Laszik Z, Morrissey JH, Kinasewitz GT. Tissue factor expression in mesothelial cells: induction both in vivo and in vitro. Am J Respir Cell Mol Biol. 1997;17:164–172. doi: 10.1165/ajrcmb.17.2.2438. [DOI] [PubMed] [Google Scholar]
- 14.Sitter T, Toet K, Fricke H, Schiffl H, Held E, Kooistra T. Modulation of procoagulant and fibrinolytic system components of mesothelial cells by inflammatory mediators. Am J Physiol. 1996;271:R1256–R1263. doi: 10.1152/ajpregu.1996.271.5.R1256. [DOI] [PubMed] [Google Scholar]
- 15.Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR., Jr Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (tf) activity. J Transl Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendurthi UR, Rao LV, Williams JT, Idell S. Regulation of tissue factor pathway inhibitor expression in smooth muscle cells. Blood. 1999;94:579–586. [PubMed] [Google Scholar]
- 17.Grabowski EF, Kushak RI, Liu B, Ingelfinger JR. Shiga toxin downregulates tissue factor pathway inhibitor, modulating an increase in the expression of functional tissue factor on endothelium. Thromb Res. 2013;131:521–528. doi: 10.1016/j.thromres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Jin H, Qiu WB, Mei YF, Wang DM, Li YG, Tan XR. Testosterone alleviates tumor necrosis factor-alpha-mediated tissue factor pathway inhibitor downregulation via suppression of nuclear factor-kappa b in endothelial cells. Asian J Androl. 2009;11:266–271. doi: 10.1038/aja.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kothari H, Kaur G, Sahoo S, Idell S, Rao LV, Pendurthi U. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J Thromb Haemost. 2009;7:121–131. doi: 10.1111/j.1538-7836.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams L, Tucker TA, Koenig K, Allen T, Rao LV, Pendurthi U, Idell S. Tissue factor pathway inhibitor attenuates the progression of malignant pleural mesothelioma in nude mice. Am J Respir Cell Mol Biol. 2012;46:173–179. doi: 10.1165/rcmb.2011-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idell S, Pendurthi U, Pueblitz S, Koenig K, Williams T, Rao LV. Tissue factor pathway inhibitor in tetracycline-induced pleuritis in rabbits. Thromb Haemost. 1998;79:649–655. [PubMed] [Google Scholar]
- 22.Kumar A, Koenig KB, Johnson AR, Idell S. Expression and assembly of procoagulant complexes by human pleural mesothelial cells. Thromb Haemost. 1994;71:587–592. [PubMed] [Google Scholar]
- 23.Idell S, Zwieb C, Kumar A, Koenig KB, Johnson AR. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. Am J Respir Cell Mol Biol. 1992;7:414–426. doi: 10.1165/ajrcmb/7.4.414. [DOI] [PubMed] [Google Scholar]
- 24.Ohkura N, Enjyoji K, Kamikubo Y, Kato H. A novel degradation pathway of tissue factor pathway inhibitor: incorporation into fibrin clot and degradation by thrombin. Blood. 1997;90:1883–1892. [PubMed] [Google Scholar]
- 25.Kothari H, Pendurthi UR, Rao LV. Tissue factor purified from different cellular sources and non-glycosylated tissue factor show similar procoagulant activity. J Thromb Haemost. 2013;11:2066–2068. doi: 10.1111/jth.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh S, Sen P, Pendurthi UR, Rao LV. Activity and regulation of glycopegylated factor viia analogs. J Thromb Haemost. 2008;6:1525–1533. doi: 10.1111/j.1538-7836.2008.03065.x. [DOI] [PubMed] [Google Scholar]
- 27.Mandal SK, Pendurthi UR, Rao LV. Tissue factor trafficking in fibroblasts: involvement of protease-activated receptor-mediated cell signaling. Blood. 2007;110:161–170. doi: 10.1182/blood-2006-10-050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CH, Cheng HW, Ma HP, Wu CH, Hong CY, Chen BC. Thrombin induces nf-kappab activation and il-8/cxcl8 expression in lung epithelial cells by a rac1-dependent pi3k/akt pathway. J Biol Chem. 2011;286:10483–10494. doi: 10.1074/jbc.M110.112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delekta PC, Apel IJ, Gu S, Siu K, Hattori Y, McAllister-Lucas LM, Lucas PC. Thrombin-dependent nf-{kappa}b activation and monocyte/endothelial adhesion are mediated by the carma3.Bcl10.Malt1 signalosome. J Biol Chem. 2010;285:41432–41442. doi: 10.1074/jbc.M110.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petit L, Lesnik P, Dachet C, Hugou I, Moreau M, Chapman J, Rouis M. The promoter of human tissue factor pathway inhibitor gene: identification of potential regulatory elements. Thromb Res. 1999;95:255–262. doi: 10.1016/s0049-3848(99)00040-7. [DOI] [PubMed] [Google Scholar]
- 31.Amini Nekoo A, Iles D. Analysis of a t-287c polymorphism in the tissue factor pathway inhibitor gene and identification of a repressor element in the promoter. Thromb Res. 2008;121:813–819. doi: 10.1016/j.thromres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Tyson DR, Kuppuswamy MN, Broze GJ, Jr, Bajaj SP. Revised DNA sequence of exon 1 and 5′ flanking region of the human tissue factor pathway inhibitor gene. Thromb Res. 1993;70:269–273. doi: 10.1016/0049-3848(93)90134-a. [DOI] [PubMed] [Google Scholar]
- 33.Carver BJ, Plosa EJ, Stinnett AM, Blackwell TS, Prince LS. Interactions between nf-kappab and sp3 connect inflammatory signaling with reduced fgf-10 expression. J Biol Chem. 2013;288:15318–15325. doi: 10.1074/jbc.M112.447318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. Nf-kappab activation limits airway branching through inhibition of sp1-mediated fibroblast growth factor-10 expression. J Immunol. 2010;185:4896–4903. doi: 10.4049/jimmunol.1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]