Abstract
Although alveolar macrophages (AMs) from patients with asthma are known to be functionally different from those of healthy individuals, the mechanism by which this transformation occurs has not been fully elucidated in asthma. The goal of this study was to define the mechanisms that control AM phenotypic and functional transformation in response to acute allergic airway inflammation. The phenotype and functional characteristics of AMs obtained from human subjects with asthma after subsegmental bronchoprovocation with allergen was studied. Using macrophage-depleted mice, the role and trafficking of AM populations was determined using an acute allergic lung inflammation model. We observed that depletion of AMs in a mouse allergic asthma model attenuates Th2-type allergic lung inflammation and its consequent airway remodeling. In both human and mouse, endobronchial challenge with allergen induced a marked increase in monocyte chemotactic proteins (MCPs) in bronchoalveolar fluid, concomitant with the rapid appearance of a monocyte-derived population of AMs. Furthermore, airway allergen challenge of allergic subjects with mild asthma skewed the pattern of AM gene expression toward high levels of the receptor for MCP1 (CCR2/MCP1R) and expression of M2 phenotypic proteins, whereas most proinflammatory genes were highly suppressed. CCL2/MCP-1 gene expression was prominent in bronchial epithelial cells in a mouse allergic asthma model, and in vitro studies indicate that bronchial epithelial cells produced abundant MCP-1 in response to house dust mite allergen. Thus, our study indicates that bronchial allergen challenge induces the recruitment of blood monocytes along a chemotactic gradient generated by allergen-exposed bronchial epithelial cells.
Keywords: macrophages, asthma, allergic inflammation, MCP-1, airway epithelial cells
Clinical Relevance
Although alveolar macrophages from patients with asthma are functionally different from those of healthy individuals, the mechanism by which this transformation occurs has not been studied in asthmatic airway inflammation. In human asthma, allergen challenge alters the population of alveolar macrophages through recruitment of blood monocytes into the luminal airspace. Alveolar trafficking of this monocyte-like macrophage population is directed in part by a chemotactic gradient generated by airway bronchial epithelial cells. Our data indicate that interactions between mucosal epithelial cells and innate immune cells (i.e., macrophages) are pivotal for generating allergic lung inflammation.
Monocyte/macrophages have a remarkable multipotency in response to various inflammatory environments and the ability to infiltrate inflammatory tissues, where they influence the inflammatory process. Blood monocytes are derived from precursors in the bone marrow, adhere to the vascular endothelial surface, and migrate into tissue following chemotactic gradients and inflammatory signals in response to tissue inflammation (1). Monocyte-derived macrophages undergo programmed differentiation into specialized tissue-resident macrophages. In the lung, resident alveolar macrophages (AMs) reside in the alveolar lumen, where they are continuously exposed to a barrage of inhaled inorganic and organic particulates, including allergens, and facilitate the removal of these particles and maintenance of homeostasis of the lung microenvironment. Additionally, macrophages are bathed in the luminal fluid containing locally secreted endogenous mediators that may influence macrophage differentiation and inflammatory phenotype. Unlike circulating monocytes, AMs have intrinsic anti-inflammatory functions capable of preventing excessive inflammation in response to regular exposure to environmental particles (1–3). In response to tissue damage, however, resident macrophages become polarized toward a proinflammatory phenotype. Macrophages have high flexibility in switching from one functional phenotype to another in response to the variation in microenvironmental signals (4). Several studies have indicated that macrophages in the airspace of patients with asthma are functionally different from those from healthy individuals (5, 6). In Th2 cytokine–enriched allergic inflammation associated with exposure to parasites or allergen, alternatively activated M2 macrophages are seen in various animal models, although their role remains controversial (7, 8). A role for macrophages in the cellular and molecular pathogenesis of Th2 inflammation has not been defined in human disease, and it is still unclear how transformation of the macrophage population in the airspace occurs in response to allergen exposure in patients with asthma. Recent reports suggest that mature tissue macrophages undergo massive proliferation at local sites, independent of input from adult hematopoietic stem cells (9, 10). Because locally produced IL-4 induces local proliferation of macrophages in parasite infection, it may play a role in Th2 cytokine–enriched allergic airway inflammation. Although the concept of self-renewal of tissue macrophages may be true under steady state, it remains largely unknown whether resident macrophages respond to tissue stress, which causes rapid turnover of local immune cells, or whether recruitment of new peripheral blood–derived inflammatory macrophages is required in response to tissue inflammation.
Airway epithelial cells (AECs) regulate innate and adaptive immunity in the lung by expressing various molecules that can modulate immune responsive cells (11). AECs potentially regulate macrophages in the airspace in negative or positive ways through direct and indirect mechanisms (12, 13). Here, we report the rapid appearance of a monocyte-derived population of human AMs that are recruited into the airspace upon allergen exposure in response to a chemotactic gradient generated by AECs. This newly recruited population of AMs has a unique profile of gene expression, which is necessary for the generation of allergic airway inflammation and remodeling.
Materials and Methods
Mice and Cells
All of the mice used in this study were bred and housed in a specific pathogen-free barrier facility maintained by the University of Illinois at Chicago (UIC), Biologic Resources Laboratory. All experiments involving mice were conducted with protocols approved by the Institutional Animal Care and Use Committee of the University of Illinois (Chicago, IL). Adult male, 8- to 12-week-old mice with an average weight of 20 to 25 g were used for the experiments. The breeding pairs of MAFIA (macrophage Fas-induced apoptosis) and wild-type control mice (C57BL/6) were purchased from The Jackson Laboratory (Bar Harbor, ME). BEAS-2B cells (CRL-9609) were obtained from ATCC (Manassas, VA).
Subsegmental Bronchoprovocation with Allergen Bronchoscopy Protocol
The subsegmental bronchoprovocation with allergen (SBP-AG) procedure was performed at UIC and at the University of Wisconsin (UW) as described previously (14). Both protocols were approved by the respective Institutional Review Board, and INDs were obtained from the FDA for bronchoscopic administration of allergens to volunteers. In brief, subjects underwent screening for inclusion and exclusion criteria that included skin prick testing to dust mites, short ragweed and cockroach allergens, spirometry with bronchodilator reversibility, and/or methacholine challenge. To obtain the prechallenged bronchial sample, BAL was performed at a subsegmental bronchus before allergen challenge. SBP-AG was performed in a different subsegment. The challenge dose of allergen was calculated by previously defined skin endpoint titration (UIC) or inhalation challenge (UW). At 48 hours after the initial bronchoscopy, BAL was obtained from the challenged segment of the challenged lobe (Experimental site). In the UIC participants, BAL was obtained from the unlavaged segment contralateral to the challenge site (Contralateral site) and from the unchallenged segment adjacent to the challenged lobe (Adjacent site).
DRA Triple-Allergen Asthma Model and Macrophage Depletion
We used the triple-allergen (DRA)-induced allergic asthma model as previously described (14). Briefly, MAFIA mice were sensitized with the DRA allergen mixture on Days 0 and 5 by intraperitoneal injection with alum and then challenged with the DRA mixture at the same concentration used for sensitization on Days 12, 13, and 14 by intranasal delivery. The mice were killed on Day 15, and outcomes were collected as described in Materials and Methods. To deplete macrophages in the DRA-induced asthma model, MAFIA mice were treated with the Fas-ligand cross-linking agent AP20187 (2 mg/kg) on Day 7 by intraperitoneal injection.
Immunohistochemistry and Immunofluorescent Staining
Histologic tissue analyses were performed by UIC Research Histology and Tissue Imaging Core. Briefly, formalin-fixed and paraffin-embedded sections of lung specimens were used for immunohistochemistry (IHC). The primary antibodies used for the studies included anti-mouse monocyte chemoattractant protein (MCP)-1 (ab25124; Abcam, Cambridge, MA). The images were photographed using an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan).
Flow Cytometry
We prepared BAL cells as previously described (14). The cells were fixed using 3.7% paraformaldehyde and blocked with anti-mouse CD16/32 mAb (2.4G2) or IgG from human serum for mouse BAL cells or human BAL and blood mononuclear cells, respectively. Stained cells were collected by CyAn ADP (Beckman Coulter, Inc., Pasadena, CA) and analyzed by Flowjo 7.6.5 software (Tree Star, Inc., Ashland, OR). The antibodies used for mouse experiments were anti-CD11b, CD11c, Siglec-F (BD Pharmingen, San Jose, CA), CD14 (Biolegend, San Diego, CA), MARCO (R&D Systems, Minneapolis, MN), and F4/80 (eBioscience, San Diego, CA). For human samples, anti-CD11b, CD163 (eBioscience), and CD14 (R&D Systems) were used.
Microarray Analysis
BAL samples from subjects with asthma were obtained before and 48 hours after SBP-AG. After BAL fluid was filtered, the mononuclear cell fraction was isolated by Percoll density gradient centrifugation as previously described (14). Alveolar macrophages were further purified from the mononuclear cell fraction by 1 hour of adhesion purification. RNA was extracted from the cells and reverse transcribed (Qiagen, Valencia, CA). Quantitative real-time PCR was used to profile the expression of genes encoding inflammatory chemokines and their receptors as well as innate and adaptive immune responses according to the manufacturer’s instructions (SABiosciences, Valencia, CA). An expression matrix of 137 genes for each sample was normalized to the expression levels of five housekeeping genes. A Heatmap was generated by using Multi Experiment Viewer software (http://www.tm4.org). Raw data and the normalized dataset were deposited in the NCBI Gene Expression Omnibus repository (accession no. GSE54585).
Cytokine Analysis
We analyzed cytokines in BAL fluids and/or lung homogenates using a multiplexed cytokine/chemokine magnetic bead panel (EMD Millipore, Inc., Billerica, MA).
Statistical Analysis
Statistical analyses were performed independently by a professional statistician at the Center for Clinical and Translation Science, Design and Analysis Core at UIC. In brief, normality was examined for all data using the Kolmogorov-Smirnov test and Q-Q plots. For normal distributed data, the two-sample t test was used for two group comparisons with equal variance or unequal variance assumptions. For non-normal distributed data, a nonparametric method (signed rank test) was used for comparisons of two paired samples. All analyses were conducted using the SAS statistical package (version 9.2; SAS Institute, Cary, NC). Statistically significant differences are indicated in the figures as *P < 0.05, **P < 0.01, and ***P < 0.001, unless otherwise indicated.
Results
Macrophages Are Required for the Generation of Allergic Airway Inflammation and Remodeling
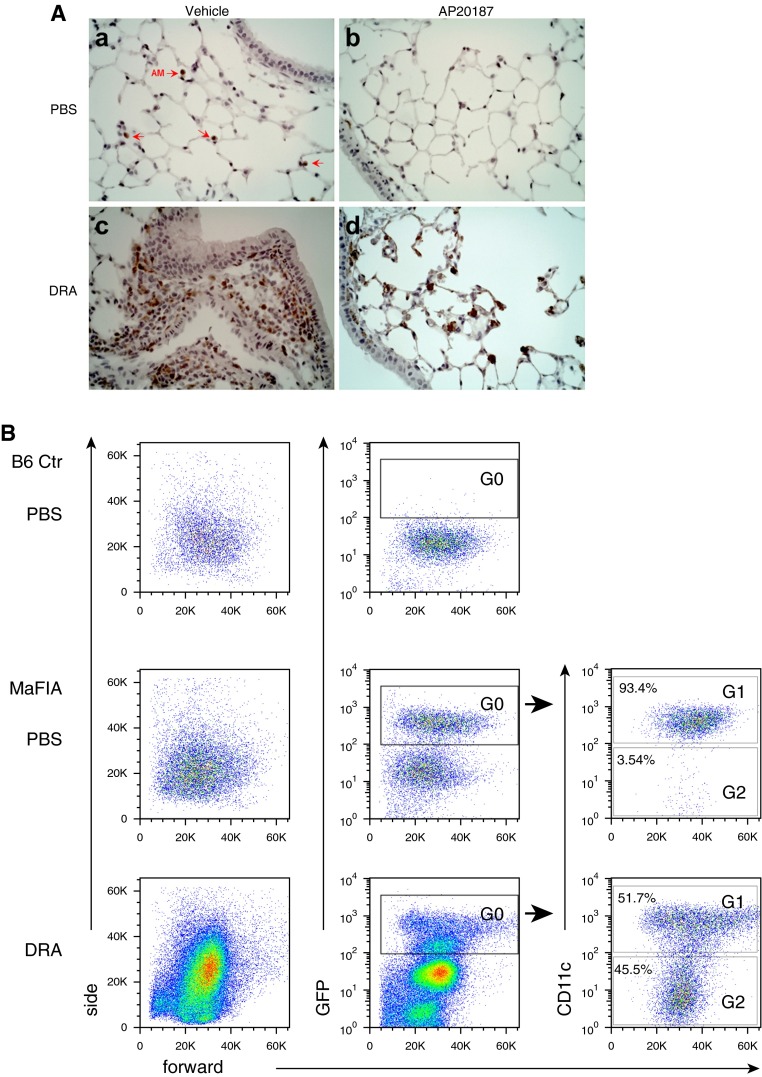
To investigate the role of macrophages in asthma pathogenesis, we used a murine triple allergen model that closely resembles the human asthma protocol for SBP-AG as described elsewhere (14). In this model, mice are sensitized and challenged with three different allergens: dust mite (Dermatophygoides farina), ragweed, and Aspergillus sp. (DRA), which results in intense eosinophilic allergic lung inflammation associated with goblet cell hyperplasia and hypertrophy of airway smooth muscle cells (see Figure E1 in the online supplement) (14). We used MAFIA mice, which transgenically express the macrophage/monocyte-specific colony stimulating factor 1 receptor to drive a promoter-driven suicide gene and a green fluorescent protein (GFP) reporter gene (15). In this model, treatment with dimerizing reagent (AP20187) selectively induces macrophage apoptosis, resulting in macrophage depletion. AP20187 (2 mg/kg) treatment after a period of allergen sensitization had no effect on the development of serum IgE levels (Figures E1 and E2). AP20187 treatment resulted in significant reduction in the blood monocyte population compared with the vehicle-treated group (Figure E3), although there was no significant decrease in the absolute number of alveolar macrophages. AP20187-treated DRA mice showed a marked reduction in eosinophilic infiltration and in IL-4 and IL-5 levels in BAL fluids (Figures 1A and 1B). In lung tissue, these cytokines showed a similar pattern of expression, except the lung tissue IL-4, which showed no change (Figure 1B). We also analyzed the severity of allergic airway inflammation and remodeling. MAFIA mice treated with AP20187 had an attenuated degree of eosinophilic inflammation, goblet cell metaplasia, and smooth muscle hypertrophy when subjected to DRA challenge, compared with MAFIA mice treated with vehicle alone (Figures 1C–1E). These findings indicated a pivotal role for macrophages in generating the asthma phenotype in the DRA mouse model and prompted us to further examine macrophage trafficking and inflammatory phenotype in the murine model and in human subjects with mild intermittent asthma who were subjected to the SBP-AG protocol.
Figure 1.
Macrophages play an important role in asthma pathogenesis and airway remodeling. (A) Total and differential cell counts in bronchoalveolar lavage (BAL) fluid from MAFIA (macrophage Fas-induced apoptosis) mice with and without AP20187 treatment to deplete macrophages (Mac). In the DRA (dust mite [Dermatophygoides farina], ragweed, and Aspergillus sp.) triple-antigen asthma model, allergen challenge induced a huge influx of cells consisting primarily of eosinophils (Eos) and macrophages, which was significantly inhibited by AP20187 treatment. *P < 0.05; **P < 0.01. (B) Concentration of IL-4 and IL-5 in BAL fluids and whole-lung homogenates of the MAFIA mice with or without AP20187 in the DRA triple-allergen asthma model (n = 5 mice per group). *P < 0.05, **P < 0.01, and ***P < 0.0001. (C) Pathologic severity of allergic lung inflammation in MAFIA mice in the DRA triple-allergen asthma model. Lung sections were analyzed by hematoxylin and eosin staining; representative images are shown from three independent experiments. (D) Goblet cell metaplasia in the airway of MAFIA mice in the DRA triple-allergen asthma model as shown by periodic acid–Schiff staining. (E) Airway smooth muscle hyperplasia in MAFIA mice in the DRA triple-allergen asthma model. Immunohistochemistry was performed with anti–α-smooth muscle actin antibody. Bronchial smooth muscle (brown) was visualized along with vascular smooth muscle cells. Lym, lymphocytes.
New Recruitment of Alveolar Macrophages into Allergic Inflammation in the Murine Model of Asthma
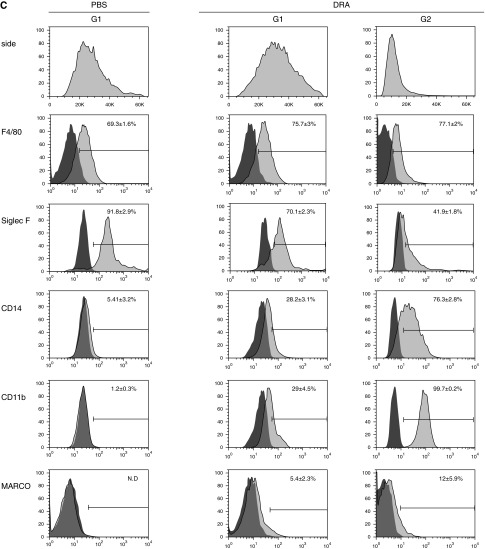
MAFIA mice contain a GFP reporter gene driven by colony stimulating factor 1R promoter, which allows tracking of macrophage populations. Immunohistochemistry staining of GFP in lung tissue showed that DRA allergen challenge induced an extensive macrophage infiltration into the peribronchial submucosal tissue, which was nearly abolished by treatment with AP20187 (Figure 2A). To further investigate the subtype populations of AMs in the luminal airspace, flow cytometry on cells in BAL fluids with and without DRA allergen challenge was performed. In the mouse model, resident AMs expressed low levels of CD11b but had high-level expression of CD11c (16, 17). Before DRA allergen challenge, strongly GFP-positive cells were detected in the BAL fluid of MAFIA mice (G0 in Figure 2B), which were mostly CD11c positive (G1) at baseline, and a few CD11c-negative cells (G2). However, DRA allergen challenge drastically replaced the GFP-positive/CD11c-positive cells with GFP-positive/CD11c-negative cells (G2 in the bottom row of Figure 2B), with the concomitant appearance of a new population of GFP-negative cells that expressed cell surface markers consistent with eosinophils (Figure E4). Further analysis of this newly recruited GFP-positive/CD11c-negative population of cells after DRA allergen challenge (G2 of the bottom row of Figure 2B) revealed that they strongly expressed CD11b and CD14 and were less granular than resident macrophages (right column of Figure 2C).
Figure 2.
Induced recruitment of new alveolar macrophages (AMs) into allergic inflammation in the murine DRA model of allergic asthma. (A) Infiltration of colony stimulating factor 1r promoter–driven green fluorescent protein–positive cells into peribronchial areas in the DRA mouse model of asthma. MAFIA mice were challenged with PBS or DRA with or without AP20187. Immunohistochemical staining was performed with anti-GFP antibody (brown). Red arrows indicate alveolar macrophages. (B) Flow cytometry on BAL cells from C57BL/6 and MAFIA mice. The GFP+ cells in the PBS-challenged BAL fluid of MAFIA mice (G0) were mostly positive for CD11c (G1). In the DRA-challenged MAFIA mice, there was a huge influx of GFP− eosinophils in BAL fluids (see Figure E4 in the online supplement). Further analysis of the GFP+ population from the DRA-challenged MAFIA mice showed a newly recruited GFP+/CD11c− population (G2). (C) Flow cytometric analysis of newly recruited alveolar macrophages in the DRA mouse model of asthma. The newly recruited macrophage population (G2) showed high expression of CD11b and CD14. The numbers indicate mean fluorescence intensity and standard deviation. Ctr, control; MARCO, macrophage receptor with collagenous structure.
Characteristics of a New Population of Alveolar Macrophages Originating from Blood Monocytes in Human Subjects with Asthma Challenged with Allergen
To further investigate this newly recruited population of AMs during human allergic airway inflammation, we used an IRB-approved protocol for SBP-AG, a model of allergic airway inflammation, in human volunteers with mild intermittent asthma as previously described (14). Airway allergen challenge in these subjects with asthma induced a rapid shift in an infiltrating population of cells in BAL fluid by 48 hours, consisting mainly of eosinophils (Figure 3A; Figure E5) (14) and AMs with a different phenotype. As reported previously, a majority of the resident cells in the human BAL fluids were CD163-positive AMs (H1; left column of Figure 3A) (18, 19). In the allergen-challenged experimental site, the newly recruited eosinophils appeared that were CD163 negative (the second column of Figure 3A); these cells were seen to a lesser degree in the adjacent site (the third column of Figure 3A). Further analysis of the CD163-positive population of cells showed that the majority of CD163-positive AMs expressed low levels of CD11b and CD14 before allergen challenge (left lower corner, Figure 3B). However, in the allergen challenge experiments, flow cytometry revealed a remarkable shift in the expression pattern of the CD163-positive cells in the site at 48 hours. These new macrophages were strongly positive for CD11b and accounted for nearly 80% of the entire CD163-positive AMs. Furthermore, these cells exhibited much stronger expression of CD14 (second in the lower row of Figure 3B; Figure E6), consistent with the recruitment of blood monocytes, which are strongly CD14 positive (19). This recruitment was limited to the allergen-challenged site, with minimal spillover to the immediate adjacent bronchopulmonary subsegment (Figures 3A and 3B). CD14-positive blood monocytes from the subjects with asthma also expressed higher CD11b in response to allergen challenge, suggesting the presence of a strong systemic reaction to the local allergen exposure (Figure E7).
Figure 3.
Characteristics of a new population of AMs originating from blood monocytes in acute allergic airway inflammation in human subjects with asthma. (A) Flow cytometric analysis of cells in BAL fluids of subjects with asthma before and after the subsegmental bronchoprovocation with allergen (SBP-AG) protocol. Before allergen challenge, the majority of the cells were CD163+ AMs (H1). After allergen challenge, there was a huge influx of CD163– cells that were mostly eosinophils. (B) A new population of AMs in the allergen-challenged site. The CD163+ AMs (H1) were analyzed further; in the experimental segment, these cells were less granular and smaller than those of the prechallenge or the postchallenge contralateral segment and expressed higher amounts of CD14 and CD11b. The data are representative results obtained from the subject enrolled in the SBP-AG protocol.
Increased Chemotactic Gradient for Macrophages in the Allergic Airspace in Human Subjects with Asthma
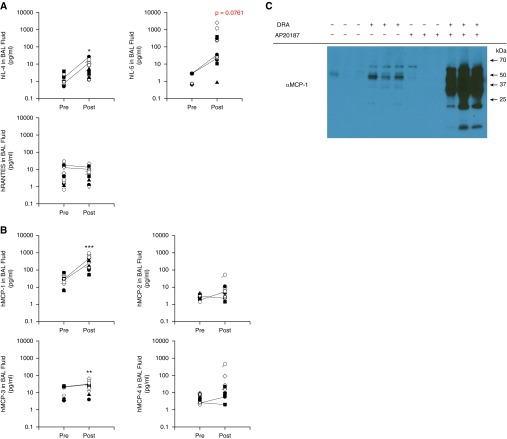
Because a new subtype population of alveolar macrophages was recruited to the site of allergic inflammation in mouse and human subjects, we measured cytokines and chemokines in the alveolar space before and after allergen challenge in human subjects with asthma. We analyzed BAL fluids from two groups of subjects with asthma who underwent the SBP-AG procedure at UIC (n = 5) and UW (n = 9). As anticipated, the level of IL-4 was significantly increased 48 hours after allergen challenge; the level of IL-5 was also increased, approaching statistical significance in the small number of SBP-AG subjects studied. The level of RANTES in BAL fluid was not elevated (Figure 4A), in contrast to increased RANTES seen in the murine model of asthma. The Th2 cytokine release was localized to the challenged site, with minimal spillover into the adjacent bronchopulmonary subsegment (Figure E8). Macrophage chemotactic proteins, MCP-1 and MCP-3, were highly elevated in BAL fluids from the allergen-challenged human subjects with asthma, especially MCP-1, which was markedly increased by ∼14-fold (Figure 4B). Similarly, the DRA mouse asthma model showed an increase in secreted MCP-1 protein in the BAL fluid after DRA challenge (Figure 4C), which paradoxically increased even further in the MAFIA mice treated with AP20187 (left three lanes of Figure 4C).
Figure 4.
Increased chemotactic gradient for macrophages in the airway of allergen-challenged human subjects with asthma and a murine asthma model. (A) Measurement of Th2 cytokines in the BAL fluids of human subjects with asthma undergoing the SBP-AG protocol. Two bronchoscopies, before and after allergen challenge, were performed for each subject. A total of 14 subjects from the University of Illinois at Chicago (UIC) and the University of Wisconsin (UW) cohorts were analyzed. For IL-5, the difference is approaching significance (P = 0.0761). *P < 0.05. (B) Measurement of human monocyte chemoattractant proteins (MCPs) in the BAL fluids of SBP-AG subjects from the UIC and UW cohorts. **P < 0.01, ***P < 0.005. (C) Western immunoblot for MCP-1 in the BAL fluids of pre- and post-DRA challenge mice in the DRA triple-allergen asthma model. AP20187 was administered to induce macrophage depletion in the MAFIA mice. A total of 12 μl of BAL fluid was analyzed in each lane. The predicted size MCP-1 band is present at 11 kD, as are additional (nonspecific) bands identified by the manufacturer (Cat. no. ab7202; Abcam, Cambridge, MA). RANTES, regulated on activation, normal T cell expressed and secreted.
Role of Bronchial Epithelial Cells in the Generation of MCP-1 in the Allergen-Induced Macrophage Chemotactic Milieu
Because MCP-1 was highly elevated in the BAL fluids of human subjects with asthma and mice that underwent the DRA asthma model, we investigated the source of secreted MCP-1 in the milieu of airway inflammation within the alveolar space. Lung tissue from DRA-challenged mice was immunostained for MCP-1 in the airway epithelial cells and other infiltrating inflammatory cells (Figure 5A). Next, a human bronchial epithelial cell line was challenged with dust mite allergen (D. farina) and with preheated house dust mite (HDM) allergen. Treatment with HDM induced abundant secretion of MCP-1 (∼9-fold increase) into the culture media by 12 hours; however, this increase was significantly attenuated by heat denaturation of the HDM allergen (Figure 5B).
Figure 5.
Development of a bronchial epithelial cell–generated macrophage chemotactic milieu in the alveolar microenvironment after airway exposure to allergen. (A) Immunohistochemical staining of lung tissue from the DRA mouse asthma model. Primary rabbit anti–MCP-1 antibody staining was visualized using HighDef red immunohistochemical chromogen. Left panels show specific staining with anti–MCP-1 antibody; right panels show staining with a nonimmune IgG isotype control. (B) House dust mite (HDM)-induced secretion of MCP-1 by BEAS-2B cells. BEAS-2B cells, a normal human bronchial epithelial cell line, was cultured and stimulated with the allergenic extracts of HDM (25 μg/ml) (Greer, Lenoir, NC) for the indicated time periods; the MCP-1 concentration was measured in the culture supernatants. The peak MCP-1 level was induced 12 hours after HDM stimulation, representing an ∼9-fold increase compared with the unstimulated cells. Heat pretreatment to denature the HDM allergen abolished the MCP-1 peak at 12 hours (n = 3). *P < 0.05.
Rapid Shift in the Gene Expression Pattern of Macrophages in the Airspace by Allergic Airway Inflammation
In Figure 1, we had observed that macrophages played an important role in the development of allergic airway inflammation in the mouse allergic asthma model. To identify macrophage genes involved in asthma pathogenesis, we compared the expression of selected genes of alveolar macrophages before and after allergen challenge. AMs from subjects with asthma were purified and total RNA analyzed by quantitative RT-PCR. Most of the proinflammatory genes analyzed, including COX-2, iNOS, TNF-α, and IL-1β, were suppressed in post–allergen challenged AMs compared with prechallenge AMs. However, CCL-17, T helper type 2 cell attracting chemokine, and CCL22 were highly induced in post–allergen challenge human AMs (Figure 6A). In addition, the protein levels of CCL-17 and CCL-22 in BAL fluids of subjects with asthma were markedly increased in the postallergen BAL fluids (>100-fold) (Figure 6B), whereas the level of IL-1β was unresponsive to allergen challenge (Figure E9). MCP-1R, MCP-1, MCP-3, and MCP-4 were also increased in post–allergen challenge AMs, suggesting that, in addition to the bronchial epithelial secretion of MCP-1 (Figure 5A), there is autocrine macrophage activation that may further amplify monocyte recruitment and macrophage differentiation in the airways (Figure 6A).
Figure 6.
AMs rapidly transform their pattern of gene expression in response to allergen-induced airway inflammation. (A) Human AMs were purified from BAL samples of subjects with asthma before and 48 hours after allergen challenge in the SBP-AG protocol. The post–allergen-challenged AMs were obtained from the allergen-challenged site (experimental site). Total RNA was extracted from the cells and reverse transcribed. Quantitative real-time PCR was used to profile the expression of genes encoding inflammatory chemokines and their receptors and those involved in innate and adaptive immune responses. The left panel shows the heatmap (fold increase or decrease) for genes expressed in AMs after allergen challenge compared with before allergen challenge. The right panel indicates the relative expression levels of each gene in the pre– and post–allergen-challenge AMs. Details of these data are available at the NCBI Gene Expression Omnibus repository (accession no. GSE54585). (B) Measurement of CCL-17 and CCL-22 in the BAL fluids of human subjects with asthma undergoing the SBP-AG protocol. Fourteen subjects from the UIC and UW cohorts were analyzed. MDC, macrophage-derived chemokine; TARC, thymus and activation-regulated chemokine. *P < 0.05, ***P < 0.005.
Discussion
Tissue macrophages serve an important role in host immune responses. Despite the well-known fact that AMs are the most prevalent immune-effector cell in the alveolar luminal space and have an important influence on inflammation (13), the role of AMs in allergic asthma has been overlooked. Specifically, the kinetics of AMs has not been fully elucidated in the context of allergic inflammation. Because inflammatory processes usually increase cellular turnover of immune cells, including macrophages (20), it has long been a question how fully differentiated tissue macrophages such as AMs are replenished in response to an inflammatory process. As well, it has been difficult to validate findings from animal model experiments in human subjects because of poor accessibility of macrophages from human tissue samples and difficulties in characterizing macrophage phenotypes. The concept that blood monocytes replenish tissue-resident macrophages has been demonstrated for a variety of tissues under homeostatic and inflammatory conditions (21–23). However, several recent publications have reported that tissue macrophages proliferate and massively expand locally at sites of parasitic infection and atherosclerotic lesions without input from hematopoietic stem cells (9, 10, 24, 25). This self-renewal capacity of tissue macrophages is under the influence of locally produced IL-4 in slowly evolving parasitic infections. This hypothesis has yet to be tested in allergic airway inflammation; however, it seems that this mechanism may not be sufficiently rapid for the development of tissue inflammation in the lung because cell proliferation may not account for the high cellular turnover rate observed in allergic airway inflammation. Under homeostatic conditions, the contribution of bone marrow–derived monocytes to tissue macrophage trafficking might be minimal, whereas monocyte recruitment could become essential during acute infectious conditions or in response to acute airway allergen challenge because of increased turnover of inflammatory cells (26). In contrast to the slowly evolving inflammation seen in parasitic infections, acute allergic airway inflammation occurs rapidly through an IgE-mediated immediate hypersensitivity reaction. After airway allergen challenge in subjects with mild intermittent allergic asthma, we observed immediate bronchial mucosal swelling and massive eosinophilic inflammation in the BAL fluid within 48 hours (14). In the current report, we asked to what extent the recruitment of a bone marrow–derived vascular population of monocytes contributes to the tissue alveolar macrophage population and how this recruitment takes place during acute allergic inflammation. Although we cannot exclude the possibility of proliferation of the local resident macrophage population, our results are consistent with the alveolar recruitment of strong CD14-positive cells originating from blood monocytes within 48 hours of bronchial allergen challenge in human subjects with asthma, a finding recapitulated in the mouse triple antigen DRA model of allergic airway inflammation.
Macrophage recruitment occurs through a chemotactic gradient of monocyte/macrophage-selective chemokines. CCL2 and MCP-1 regulate the migration and infiltration of monocytes and natural killer cells. CCL2 is produced by many cell types, including endothelial cells, fibroblasts, epithelial and smooth muscle cells, monocytes, and microglial cells (27). Increased expression of CCL2 has been reported in asthmatic bronchi (28–30), and blocking the CCL2–CCR2 axis attenuated the asthma phenotype in other animal models of asthma (31). Here, we show that the expression of CCL2 is rapidly induced in the allergic airway of human subjects with asthma by allergen challenge, suggesting that airway epithelial cells are a likely source of luminal CCL2 induced by signaling through their protease-activated receptors because allergen denaturation with heat partially prevents the secretion of CCL2 from epithelial cells (32). Depletion of the macrophage population by treating MAFIA mice with AP20187 enhanced the local production of CCL2 (Figure 4C), which is consistent with the idea that consumption of CCL2 by binding to its receptor, CCR-2, represents an important feedback loop in the maintenance of chemokine homeostasis in lung (33).
It has been debated whether locally recruited monocytes can fully differentiate into resident tissue macrophages. A recent report suggested that extravascular monocytes differentiate only minimally in the steady state, without achieving the full characteristics of resident macrophages (34). Even in acute inflammation, extravascular tissue infiltrating monocytes appear to have more distinct phenotypic and functional characteristics compared with resident macrophages (35). Recruited monocytes express a high level of CCR2, a MCP-1 receptor, in an animal model of encephalitis, which may be required for the entry of these cells. Blockade or genetic deletion of CCR2 prevented severe inflammation, which leads to faster remission (36, 37). In allergic inflammation, we have shown here that, after allergen challenge in a sensitized host, AMs have highly induced CCR2 gene expression compared with prechallenge macrophages, suggesting that CCL2/CCR2 signaling occurs in these cells during human allergic lung inflammation. In addition, our macrophage gene expression profiling indicates that AMs undergo a significant transformation in terms of their inflammatory gene expression in response to allergen challenge; most proinflammatory genes were severely suppressed in post–allergen challenge macrophages, whereas the expression of CCL17 and CCL22 was highly elevated. Because the newly recruited macrophage population was not sorted out from the postchallenge pool, it is unclear to what extent the newly recruited cells contributed to this analysis. It is also possible that the phenotypic transformation of resident macrophages in the Th2-enriched microenvironment in the lung contribute to this pattern of gene expression. The expression levels of CCL17 and CCL22 in post–allergen challenge alveolar macrophages were highly elevated, along with their protein levels in BAL fluids. These genes are thought to play an important role in Th2 inflammation and airway remodeling (38, 39), consistent with our finding that depletion of macrophages in the DRA asthma model attenuated goblet cell metaplasia and smooth muscle hypertrophy (Figure 1). CCL17 and CCL22 are also required for the recruitment of effector T cells to the lung in a model of allergic inflammation (40), which may be related to the concomitant decrease we observed in eosinophil recruitment in the macrophage-depleted mice, although these chemokines are not directly involved in eosinophil chemotaxis. Macrophage depletion resulted in attenuation of allergic inflammation in our experimental setting, and pulmonary macrophages could have either detrimental or beneficial effects in asthma pathogenesis, depending on their phenotypes. Another group has reported opposite results, albeit for a different endpoint, showing that AMs are protective against the development of airway hyperresponsiveness (41). In this report, the investigators used adoptive transfer of resting AMs, which are usually anti-inflammatory, to prevent excessive inflammation.
A limitation of our study is related to the magnitude of macrophage depletion. Whereas blood monocytes were markedly reduced by AP 20187 treatment in the MAFIA mice, resident alveolar macrophages were highly resistant to the treatment, with minimal evidence of depletion. Yet, despite of the ineffective depletion of resident alveolar macrophages, we observed a highly significant attenuation of eosinophilic inflammation in AP20187-treated group. These data indicate that AP20187 treatment sufficiently depletes peripheral blood monocytes so that it blocks the recruitment of blood monocyte–derived macrophages into the airspace, suggesting that newly recruited CD14- and CD11b-positive macrophages, instead of resident macrophages, are essential for generating eosinophilic inflammation. Furthermore, although the MAFIA mouse suicide gene has been reported to be highly specific to mononuclear lineage cells (42), an off-target effect of AP20187 cannot be excluded. Thus, to address the precise role of macrophages in allergic lung inflammation, a highly selective macrophage-specific animal model is needed that selectively depletes individual macrophage populations, such as the resident versus recruited macrophage population.
Our study indicates that newly recruited macrophages in the airway would be a very different phenotype from these resident macrophages and could be proasthmatic. Our study highlights the novel finding that airway luminal macrophages are in a rapidly developing dynamic environment in acute allergic airway inflammation in human asthma and in a mouse model of allergic asthma and are required for generation of the acute allergic response.
Acknowledgments
Acknowledgments
The authors thank the generous subjects with asthma who volunteered for the SBP-AG protocol and the staff of the Clinical Interface Core at the University of Illinois Center for Clinical and Translational Science (UIC-CCTS) for assistance with patient recruitment, screening, and performing bronchoscopy.
Footnotes
This study was supported in part by the National Institutes of Health grants R01 HL075557 and HL103643 (J.W.C.), by a Department of Veterans Affairs merit review grant (G.Y.P. and J.W.C.), and by Professional Development Award NIH-NCATS UL1TR000050 from the UIC-CCTS (G.Y.P.). The SBP-AG protocol at the University of Wisconsin was supported by P01 HL088594, HL056396, M01 RR03186, and UL1 RR025011. Statistical analysis was supported by UIC-CCTS (NIH-NCATS, UL1TR000050).
Author Contributions: Y.G.L. performed the animal experiments and flow cytometric analysis, generated figures, and edited the manuscript. J.J.J. performed bronchial epithelial cell experiments and measured chemokines and cytokines of bronchoalveolar (BAL) fluids. S.N. wrote and maintained the subsegmental bronchoprovocation with allergen (SBP-AG) Institutional Review Board and Investigational New Drug protocol and led SBP-AG patient recruitment efforts. E.B. provided valuable scientific input to the animal experiments. S.C., R.R., M.K., F.Q., and J.D. assisted with the DRA triple-allergen animal model and cytokine measurements. N.N.J. and E.A.B.K. supervised the SBP-AG protocol and provided BAL fluids from the Wisconsin cohort. S.J.A. managed the cell purification core lab for the SBP-AG protocol, wrote the SBP-AG Institutional Review Board protocol, wrote and maintained the mouse DRA asthma model Animal Care and Use protocol, edited the manuscript, and coordinated regular asthma research meetings. V.N. provided valuable scientific input. J.W.C. established the SBP-AG protocol, supervised mouse experiments and performance of the human SBP-AG protocol, designed experiments, and interpreted and analyzed data. G.Y.P. performed the SBP-AG protocol, designed animal experiments, analyzed data, generated figures, and drafted and edited the manuscript. All authors contributed to data discussion and editing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0255OC on October 31, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 5.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–979. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121:1372–1378, 1378 e1371–1373. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, Iwakura Y, van Rooijen N, Gibson GA, St Croix CM, et al. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS One. 2011;6:e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 12.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 13.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 14.Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med. 2013;188:928–940. doi: 10.1164/rccm.201306-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang MK, Raggatt L-J, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 16.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol. 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunz LI, Lapperre TS, Snoeck-Stroband JB, Budulac SE, Timens W, van Wijngaarden S, Schrumpf JA, Rabe KF, Postma DS, Sterk PJ, et al. Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study Group. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir Res. 2011;12:34. doi: 10.1186/1465-9921-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chana KK, Fenwick PS, Nicholson AG, Barnes PJ, Donnelly LE. Identification of a distinct glucocorticosteroid-insensitive pulmonary macrophage phenotype in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2014;133:207–216 e211. doi: 10.1016/j.jaci.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson J, van Furth R. The effect of glucocorticosteroids on the kinetics of mononuclear phagocytes. J Exp Med. 1970;131:429–442. doi: 10.1084/jem.131.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 27.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sousa AR, Lane SJ, Nakhosteen JA, Yoshimura T, Lee TH, Poston RN. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994;10:142–147. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- 29.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153:1398–1404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- 30.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–1383. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 31.Mellado M, Martín de Ana A, Gómez L, Martínez C, Rodríguez-Frade JM. Chemokine receptor 2 blockade prevents asthma in a cynomolgus monkey model. J Pharmacol Exp Ther. 2008;324:769–775. doi: 10.1124/jpet.107.128538. [DOI] [PubMed] [Google Scholar]
- 32.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 33.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, et al. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol. 2005;288:L350–L358. doi: 10.1152/ajplung.00061.2004. [DOI] [PubMed] [Google Scholar]
- 34.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 36.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanovic R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of ccl17. J Allergy Clin Immunol. 2012;130:1404–1412 e1407. doi: 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–27. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 42.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]