Abstract
Significant advances in the treatment of pulmonary arterial hypertension (PAH) over the last two decades have led to the introduction of multiple classes of oral therapy, but the disease remains devastating for many patients. Disease progression, in spite of oral monotherapy, is a major problem, and alternative therapy, such as infusion of prostacyclins, is cumbersome and carries considerable potential morbidity. Use of combination oral therapy, including drugs from both the endothelin receptor antagonist and phosphodiesterase-5 inhibitor classes, has increased, and there is some evidence to support this approach. Given the multiple options now available in pulmonary hypertension (PH) therapy, biomarkers to guide treatment decisions could be helpful. Here, we review the evidence for and against the clinical use of molecular biomarkers relevant to PH pathogenesis, emphasizing assayable markers that may also inform more rational selection of agents that influence pathways targeted by treatment. We emphasize the interactive nature of changes in mediators and messengers, such as endothelin-1, prostacyclin, brain natriuretic peptide (which has demonstrated biomarker utility), nitric oxide derivatives, and cyclic guanosine monophosphate, which play important roles in processes central to progression of PAH, such as vascular remodeling, vasoconstriction, and maladaptive right ventricular changes, and are relevant to its therapy. Accordingly, we propose that the identification and use of a molecular biomarker panel that assays these molecules in parallel and serially might, if validated, better inform unique patient phenotypes, prognosis, and the rational selection and titration of combination oral and other therapy in individual patients with PH/PAH.
Keywords: right ventricle, endothelin, nitric oxide, biomarker
Clinical Relevance
Multiple classes of therapy exist now for pulmonary hypertension, but outcomes remain disappointing. Prospective identification of biomarkers that can help guide decision making are needed. We review treatment-related candidate biomarkers and their interactions in light of the emerging adoption of combination therapy.
Pulmonary hypertension (PH) is a progressive, fatal disease characterized by elevated pulmonary arterial pressure and secondary right ventricular (RV) dysfunction. PH may arise idiopathically (known as idiopathic pulmonary arterial hypertension [iPAH]) or due to certain associated conditions (such as connective tissue disease, anorexigen use, human immunodeficiency virus [HIV], et cetera; collectively, group I). PAH affects between 5 and 15 people per 1 million adults in the general population (1, 2). Far more commonly, PH can arise in the setting of chronic heart, lung, or thromboembolic disease, representing PH categories II–IV, respectively, as classified by consensus and referred to as World Health Organization (WHO) groups (3, 4). The pathogenesis of PAH is highly complex and characterized by pulmonary endothelial cell (EC) dysfunction, imbalance of vasoactive (and vasoproliferative) mediators, oxidative stress, metabolic dysregulation, fibrosis, and inflammation (5). Mutations in bone morphogenetic protein receptor II have been estimated to underlie a subset of familial and idiopathic cases of PAH (6, 7). However, these mutations and exposures account for only a fraction of cases, and the disease process remains poorly understood. There is a pressing need to better characterize these patients to formulate prognosis, develop better and novel treatments, and to guide selection among existing therapies (3). Generation of biomarker knowledge from clinical trial data has been limited, in part because of lack of standardization of trial design, small study sizes, and limited longitudinal follow up.
PAH and Its Treatment
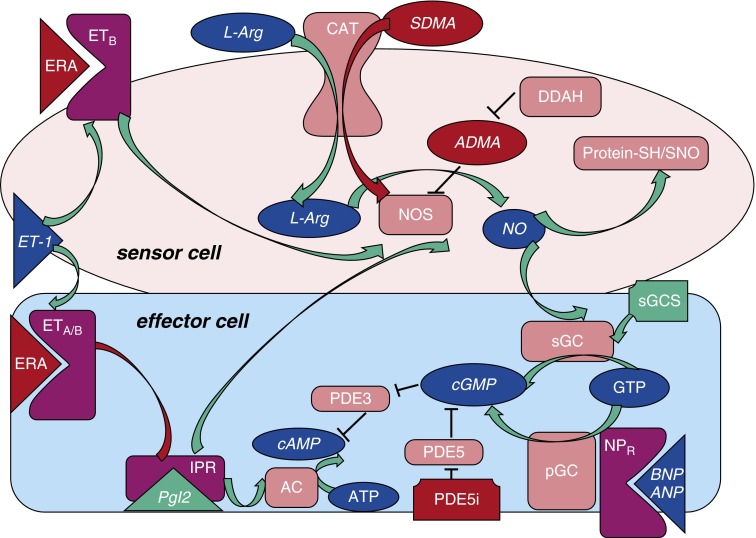
Dysregulation of multiple vasodilator/antiproliferative and vasoconstrictor/vasoproliferative mediators has been closely associated with the disease. Knowledge of these regulators has given rise to four main therapeutic classes specific to the treatment of PAH (Figure 1): prostacyclin agonists (epoprostenol, treprostinil, beraprost, and iloprost), endothelin (ET) receptor antagonists (ERAs; bosentan, ambrisentan, and macitentan), phosphodiesterase (PDE)-5 inhibitors (PDE5is; sildenafil or tadalafil), and soluble guanylate cyclase (sGC) stimulators (riociguat). In randomized, controlled trials, these agents promote improvements in symptoms, exercise tolerance, and hemodynamics, and retard disease progression (8–14). However, the response to any given treatment is not predictable, and outcomes in PAH remain suboptimal. For example, 3-year survival rates are only roughly 70%, despite the availability of these multiple therapies (15). Because the literature lacks comparative clinical trials, an evidence-based approach to drug class selection has not been developed beyond a preference for oral agents in early to moderate disease and parenteral therapies in advanced disease (4). Therefore, treatment is typically selected empirically, and is influenced, as in other diseases, in part by an individual physician’s perception of medication efficacy and side effects, and by the costs to the patient and payor coverage.
Figure 1.
Vasoregulatory mediators and/or biomarkers relevant to current pulmonary arterial hypertension (PAH) therapy, and their interactions. Endogenous mediators (blue ovals), enzymes or other protein targets (both shown as pink rectangles), mediator-relevant drugs (green [stimulatory] or red [inhibitory]), and PAH-associated derangements are interconnected (also red). Mediators produced by a sensor cell may influence an adjacent or distant effector cell. Classically, this involves signaling by endothelial cells (ECs; “sensor cells”) to influence “effector cells” (e.g., vascular smooth muscle cells [SMCs]), but, in PAH, many cell types are involved. Relevant mediators may act in an autocrine fashion also. The vasoconstrictor and mitogenic peptide, endothelin (ET)-1, acting at ETA or ETB receptors, is antagonized by ET-1 receptor antagonists (ERA). ECs also produce nitric oxide (NO) from the precursor amino acid, L-arginine (L-Arg); asymmetric dimethyl arginine (ADMA) and symmetric dimethyl arginine (SDMA) (red) may antagonize this. Levels of ADMA depend, in part, on its catabolism by dimethylarginine dimethylaminohydrolase (DDAH). Antiproliferative and pulmonary vasodilator actions of NO produced by lung ECs can be mediated by the activation of soluble guanylate cyclase (sGC) in, for example, vascular SMCs, or by more durable S-nitroso (SNO) modification (S-nitrosylation) of regulatory thiols (SH) on key proteins. Cyclic guanosine monophosphate (cGMP) formation from GTP is the result when sGC is stimulated by NO. Notably, brain natriuretic peptide (BNP; or atrial natriuretic peptide [ANP]) can also elicit the formation of cGMP from GTP via a membrane-bound GC enzyme (particulate GC), possibly contributing to the (paradoxically) elevated cGMP levels typical of advanced PAH. Other mediator interactions include prostacyclin receptor- and endothelin receptor (ETR)-triggered NO synthesis, and cyclic adenosine monophosphate (cAMP)/cGMP cross-talk via phosphodiesterases (PDEs). For example, PDE3 can catabolize either, while PDE5 is selective for cGMP. Mediators that are also potential or proven biomarkers are in italics. AC, adenylate cyclase; CAT, cationic amino acid transporter; IPR, prostacyclin receptor; NOS, nitric oxide synthase; NPR, natriuretic peptide receptor; pGC, particulate guanylate cyclase; Pgl2, prostacyclin.
PAH in the Era of Combination Oral Therapy
As newer therapies for PAH emerge (i.e., riociguat, macitentan, oral treprostinil, and selexipag) (16), the number of options for monotherapy and combination strategies is increasing. Both monotherapy strategies followed by add-on therapy (9, 16–20), as well as initial (“up-front”) double-therapy (21–23), have yielded mixed results compared with monotherapy in terms of the benefits of various combinations and/or agent-sequencing strategies. In the midst of ambivalent data and the absence of clear treatment guidelines, it is important to identify more precise and rapidly available predictors of treatment response and prognosis. Objective biomarkers that indicate the disease stage (in addition to disease severity, as reflected by invasive hemodynamics) and response to therapeutic intervention would be useful tools for the management of PAH. Currently, a strong acute pulmonary vasodilator response to inhaled nitric oxide (NO) or prostacyclin remains the only established prognostic variable of treatment success leading to clear guidance: to select a high-dose calcium channel blocker as the treatment of choice. This small subset of patients with PAH can be more precisely phenotyped as “vasoreactive” early in the recommended algorithm, resulting in favorable long-term outcomes, but only about 10% of the PAH population shows vasoreactivity (24, 25). In other diseases, such as cancer and HIV/acquired immune deficiency syndrome, biomarkers have been used to better characterize the genotypic and/or phenotypic characteristics of a patient, which can, in turn, lead to more optimized treatment tailored to that individual patient (e.g., antiretroviral treatment resistance testing in HIV, human epidermal growth factor receptor 2 (HER2) in breast cancer) (26). Here, we discuss the potential for candidate biomarkers to play a similar role in PAH, with a special emphasis on treatment decision making.
Paucity of Existing Biomarkers in PAH
The U.S. Food and Drug Administration defines a biomarker as a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or biological responses to a therapeutic intervention (27). In addition, assays for a useful biomarker should be reproducible and cost-effective. Given the complexity of PAH and our incomplete understanding of its pathogenesis, numerous markers have been investigated for potential as biomarkers. Almost none of these, however, has been identified and validated as clinically relevant. This is likely due, in part, to small study sizes and lack of adequate power. In addition, PAH is a heterogeneous disease, and long-term data are limited. The only biomarker recommended by current guidelines for risk stratification is either brain natriuretic peptide (BNP) or the N-terminal fragment (NT) of pro-BNP (NT-proBNP) (28). Similarly, only BNP is cited in guidelines addressing PAH treatment endpoints: a “normal” BNP is suggested as a treatment goal (29).
PAH Biomarkers Relevant to Therapy
Although the development and characterization of PAH therapeutics have largely focused on only three discrete pathways, there is, in fact, rich cross-talk among these axes (as partially reflected in Figure 1), yielding multifactorial pathways and interrelated mediators worthy of targeting. For instance, preclinical evidence indicates a role for ET-1 itself in the response to PDE5is, sGCSs, ERAs, and prostacyclin agonists and their combinations (30, 31). One of the key features of PAH is endothelial dysfunction characterized by decreased endothelial NO production, and the centrality of NO deficiency in PAH has been discussed (32, 33). It is possible that biomarkers in the NO/sGC/cyclic guanosine monophosphate (cGMP) or related ET or prostanoid/cyclic adenosine monophosphate (cAMP) axes may help characterize, more objectively, the stage or severity of PAH and predict optimal therapy for maximal treatment response, given that the currently available oral therapies for PAH act directly upon these same targets. General reviews discussing biomarkers in pulmonary diseases have been published previously (34–39). This review focuses on the potential utility of ET-1–related, NO/sGC/cGMP–related, and other serologic biomarkers in decision making in the treatment of PAH. The role of these biomarkers in prognosis or correlation with important clinical endpoints is addressed secondarily; however, their role in screening and detection are beyond the scope of this review. Much of our understanding of these mediators (and potential biomarkers) has focused on the abnormal ECs and smooth muscle cells (SMCs) of the vascular wall in PAH. However, these molecules can also transduce intercellular and intracellular signaling involving a wide variety of cell types that may contribute to the development and progression of PAH and its consequences, including the pericyte, lymphocyte, macrophage, fibroblast and myofibroblast, endothelial progenitor cell, and of course, the cardiomyocyte (40–43).
NO
NO is an important endogenous vasodilator and antiproliferative agent, and acts, in part, through the activation of sGC to generate cGMP. NO is synthesized from L-arginine and oxygen (O2) via a multistep reaction catalyzed by endothelial NO synthases (eNOSs). Recently, it has been discovered that NO can alternatively be produced via the reduction of the anion nitrite (NO2−) by multiple proteins, including hemoglobin (Hb), myoglobin, and xanthine oxidase (44, 45). NO2− reduction is favored under hypoxia, which is coincidentally when endogenous formation of NO from NOS is impaired (45, 46). The reduction of eNOS expression in the lungs of patients with PAH supports the concept of deficient NO as a pathogenic factor in PAH (32). However, others have observed a regional distribution of eNOS enzyme notable for high levels in the plexiform lesions of patients with PAH (47).
Asymmetric and Symmetric Dimethylarginines
Asymmetric dimethyl arginine (ADMA) and symmetric dimethyl arginine (SDMA) are methylarginines that inhibit NO synthesis. ADMA blunts NO synthesis by competing with L-arginine for eNOS binding sites, whereas SDMA interferes with intracellular arginine uptake. Both ADMA and SDMA are known to be elevated in the serum of patients with group I PAH compared with control subjects (48, 49). This may be related to a decrease in the enzyme that degrades ADMA, dimethylarginine dimethylaminohydrolase, in patients with PAH (48). In a study of 57 patients with group I PAH, ADMA correlated (Table 1) with right atrial pressure, mixed venous O2 saturation, and cardiac index (49). Furthermore, the study found that ADMA levels independently predicted survival in patients with idiopathic PAH (49). ADMA has also been shown to have prognostic significance in other disease processes. In patients with chronic thromboembolic PH (CTEPH), ADMA levels were elevated compared with healthy control subjects and correlated with mixed venous O2 saturation, right atrial pressure, and cardiac index (50). ADMA decreased in patients with CTEPH 12 months after pulmonary endarterectomy (50). Other groups have found that ADMA levels predict cardiovascular and overall mortality in patients with end-stage renal disease and coronary artery disease (51, 52). Future clinical trials to assess the relationship of ADMA levels to PAH treatment may be of value.
Table 1.
Biomarker Correlations with Clinical Endpoints and Prognosis in Pulmonary Hypertension
| Biomarker | Population (WHO Group) | Patients (n) | Clinical Endpoint | Correlation | Reference |
|---|---|---|---|---|---|
| eNO | I–IV | 41 | PAP | Yes | (59) |
| CI | |||||
| RVSP (1 yr later) | |||||
| SNO | I | 14 | PAP | Yes | (65) |
| PVR | |||||
| ADMA | I | 57 | MVO2 | Yes | (49) |
| RAP | |||||
| CI | |||||
| Mortality | |||||
| ADMA | IV | 135 | Svo2 | Yes | (50) |
| RAP | |||||
| CI | |||||
| Urine cGMP | I | 19 | Svo2 | Yes | (68) |
| CI | |||||
| Plasma cGMP | I–IV | 13 | PVR | Yes | (72) |
| CI | |||||
| PCWP | |||||
| ANP | I | 9 | PCWP | No | (76) |
| CI | |||||
| ANP | I, III–V | 18 | Mean PAP | No | (69) |
| PCWP | |||||
| PVR | Yes | ||||
| RAP | |||||
| Svo2 | |||||
| BNP | IV | 34 | PVR | Yes | (78) |
| BNP | I | 394 | 6MWD | Yes | (14) |
| Clinical worsening | |||||
| WHO functional class | |||||
| Borg dyspnea score | |||||
| NT-proBNP | I (scleroderma-associated PAH) | 68 | Mean PAP | Yes | (79) |
| PVR | |||||
| 6MWD | |||||
| Mortality | |||||
| NT-proBNP | I, III–V | 118 | Mean PAP | Yes | (80) |
| PVR | |||||
| RAP | |||||
| CO | |||||
| CI | |||||
| Mortality | |||||
| NT-proBNP | I | 198 | Mortality | Yes | (83) |
| 6MWD | |||||
| WHO functional class | |||||
| ET-1 | I, III | 7 | Mean PAP during hypoxia | Yes | (88) |
| ET-1 | I | 21 | RAP | Yes | (91) |
| PA oxygen saturation | |||||
| ET-1 | I, V | 33 | RAP | Yes | (92) |
| Indexed PVR | |||||
| CI | |||||
| Svo2 | |||||
| 6MWD | No | ||||
| NYHA functional class | |||||
| Time to transplant or death | |||||
| ET-1 | I | 16 | PVR | Yes | (93) |
| Mean PAP | |||||
| CO | |||||
| CI | |||||
| 6MWD | |||||
| RDW | I–V | 162 | Mortality | Yes | (97) |
| Mean RAP | No | ||||
| Mean PAP | |||||
| CI | |||||
| PCWP | |||||
| PVR | |||||
| RDW | I | 139 | 6MWD | Yes | (98) |
| WHO functional class | |||||
| Mean RAP | |||||
| CI | |||||
| Survival |
Definition of abbreviations: 6MWD, 6-minute-walk distance; ADMA, asymmetric dimethyl arginine; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CI, cardiac index; CO, cardiac output; cGMP, cyclic guanosine monophosphate; eNO, exhaled nitric oxide; ET, endothelin; MVO2, maximal oxygen consumption; NT-proNBP, N-terminal fragment of pro-BNP; NYHA, New York Heart Association; PA, pulmonary arterial; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RDW, red blood cell distribution width; RVSP, right ventricular systolic pressure (estimated by echocardiography); SNO, S-nitrosothiol; Svo2, mixed venous hemoglobin oxygen saturation; WHO, World Health Organization.
NO and Exhaled NO in PAH
The half-life of NO itself in biological fluids is variable, but typically between 0.1 and 5 seconds, thus limiting its use as a potential biomarker. Previous animal studies have suggested that end-exhaled NO represents NO production from the pulmonary circulation (53). Whereas some have demonstrated local underproduction of NO in exhaled airway gas from patients with PAH (54–56), others have demonstrated normal values (57). A study of nine patients with PAH secondary to left-to-right intracardiac shunts found similar baseline exhaled NO (eNO) values in patients and control subjects, but 18 hours after inhaled epoprostenol, eNO values were increased in patients compared with control subjects (58). Another group found that treatment with IV epoprostenol increased eNO levels in patients with both primary and secondary PAH after 24 hours (59). This did not reliably correlate with immediate hemodynamic changes (Table 2), although most patients on epoprostenol had a decreased RV systolic pressure after 12 months of therapy (59). Girgis and colleagues (60) found that eNO levels were decreased in 10 patients with WHO group I PAH compared with control subjects. After treatment for 3 months with the ERA, bosentan, eNO levels increased and were similar to those of control subjects (60).
Table 2.
Treatment-Related Biomarker Changes in Pulmonary Hypertension
| Therapy | Population WHO Group (n) | Biomarker (Change) | Assay Method | Clinical Endpoint | Ref. |
|---|---|---|---|---|---|
| Prostanoid: epoprostenol | I–V (9) | eNO (increased) | Chemiluminescence | RVSP: acute decrease | (59) |
| Prostanoid: iloprost | I, III, IV | cGMP (decreased) | Radioimmunoassay | PVR: acute decrease | (69) |
| mPAP: acute decrease | |||||
| Prostanoid: iloprost | I, III, IV (18) | ANP (decreased) | Radioimmunoassay | PVR: acute decrease | (69) |
| mPAP: acute decrease | |||||
| Prostanoid: inhaled treprostinil in addition to bosentan or sildenafil | I (235) | NT-proBNP (decrease) | Not specified | 6MWD: increase | (18) |
| ERA: bosentan | I (10) | eNO and urine NOx (both increased) | Urine NOx: chemiluminescence | 6MWD: increase at 3 mos. | (60) |
| ERA: ambristentan | I (192) | BNP (decrease) | 6MWD: increase | (14) | |
| Time to clinical worsening: improve | |||||
| Borg dyspnea score: improvement | |||||
| WHO functional class: improvement | |||||
| sGC stimulator: riociguat | I (443) | NT-proBNP (decrease) | Not specified | 6MWD: increase | (16) |
| PVR: acute decrease | |||||
| WHO functional class: improvement | |||||
| Time to clinical worsening: improvement | |||||
| sGC stimulator: riociguat | IV (261) | NT-proBNP (decrease) | Not specified | 6MWD: increase | (85) |
| PVR: acute decrease | |||||
| WHO functional class: improvement | |||||
| NO/SNO donor: ethyl nitrite | I (9) | RBC SNO | Photolysis–chemiluminescence | PVR: acute decrease | (65) |
| SaO2: acute increase | |||||
| PDE5 inhibitor: sildenafil | I (13) | cGMP (increased) | ELISA | PVR: decrease cardiac index: increase | (72) |
| Pulmonary thromboendarterectomy | IV (135) | ADMA (decrease) | HPLC | Survival after pulmonary thromboend-arterectomy | (50) |
| Pulmonary thromboendarterectomy | IV (34) | BNP (decrease) | Radioimmunoassay | PVR: decrease | (78) |
Definition of abbreviations: ERA, ET-1 receptor antagonist; mPAP, mean pulmonary arterial pressure; NO, nitric oxide; NOx, NO metabolites; PDE, phosphodiesterase; RBC, red blood cell; SaO2, arterial oxygen saturation; sGC, soluble guanylate cyclase.
NO Metabolites: NO2− and Nitrate
NO rapidly oxidizes into NO2− and nitrate (NO3−), which are collectively referred to as NO metabolites (NOx). There has been interest in the measurement of NOx as a surrogate of in vivo NO activity (61). However, these metabolites are significantly affected by dietary factors (62) and glomerular filtration (63). Reported plasma NOx levels in patients with PAH have been variable (41, 57, 60, 64), but urinary NOx was found to be lower in patients with PAH on an NO3−/NO2−–restricted diet (60). The group also found an increase in urinary NOx with bosentan therapy (60). However, current studies unfortunately do not link either NOx or exhaled NO to clinical outcomes or serve in a predictive fashion to help guide therapeutic decision making. Further investigation must be performed to characterize the potential role of these biomarkers in assessing severity and response to treatment in PAH.
Red Blood Cell S-Nitrosothiols
NO binds reversibly to a reactive thiol sulfur of the Cysβ93 cysteine residue of the Hb β globin chain, forming an S-nitrosothiol (SNO)-Hb (SNO-Hb). The SNO side group, a durable and bioactive derivative of NO, is effectively released and exported from the red blood cell (RBC) in small amounts to effect intercellular signaling when deoxygenation triggers the conformational switch in Hb from the oxygenated “R” structure to the deoxygenated “T” structure, in which NO/SNO binding is no longer favored. In this way, the RBC Hb is able to couple the demand for regional increases in blood flow with the O2 needs of the tissue (46). Patients with advanced PAH had decreased RBC SNO (65). Furthermore, administration of ethyl NO2− to patients with PAH restored RBC SNO-Hb levels to the normal range along with immediate improvements in RBC-dependent vasoactivity, pulmonary vascular resistance (PVR), and blood O2 uptake by the lung (65). The concentrations of SNO-Hb and NOx increased after inhaled NO therapy in infants with persistent PH of the newborn (64). This supports the concept that SNO-Hb is a vehicle for extrapulmonary actions of inhaled NO (66). Larger studies are needed to determine the utility of RBC SNO as a PAH biomarker that predicts disease severity and/or response to treatment.
cGMP
cGMP is the product of GC, which is present intracellularly in two forms: a cytosolic form activated by NO, and a membrane-bound form activated by members of the natriuretic peptide family. It is an intracellular second messenger in platelets, vascular smooth muscle, and other cells that can also be measured in the circulation. Several groups have found that plasma levels of cGMP correlate with levels of natriuretic peptides in patients with heart failure (67). Urinary cGMP levels are higher in patients with PAH compared with healthy control subjects or patients with asthma, and correlated inversely with cardiac index (68). Another study found elevated plasma cGMP levels in patients with PAH compared with control subjects, which also correlated with atrial natriuretic peptide (ANP) levels (69). The elevated cGMP in group I PAH presumably reflects GC activation by BNP and/or ANP, rather than by bioactive NO and its derivatives, which may be depressed in PAH (70). Indeed, the natriuretic peptide receptors couple to particulate (membrane-bound) GC (see Figure 1), and cGMP elevations via particulate GC stimulation may therefore act quite differently from sGC-induced cGMP elevations. The PDE5is (e.g., sildenafil) act by inhibiting the degradation of cGMP (Table 3), prolonging its bioavailability, thereby promoting vasodilation and blunting vascular remodeling. PDE5 expression is increased in PAH, and its expression/activity is up-regulated in the abnormal RV cardiomyocyte as compared with healthy individuals (71). A study of 13 patients with PAH demonstrated an increase in plasma cGMP levels immediately after treatment with sildenafil and inhaled NO (72). This correlated with a decrease in PVR and increase in cardiac index (72). A study of 18 patients with PAH identified an immediate decrease in cGMP levels after iloprost inhalation, along with reductions in PVR and pulmonary artery pressure (PAP) (69). Thus, cGMP may be sensitive not only to NO and its derivatives, but also to prostanoids, with negative feedback, for example. Further investigation and longer follow up are necessary to better understand the role and responses of cGMP and its potential to individualize treatment decisions.
Table 3.
Current Therapeutic Classes in Pulmonary Hypertension and Relation to Selected Molecular Biomarkers
| Therapeutic Class | Targeted PH Dysfunction | Desired Mechanistic Result | |
|---|---|---|---|
| Stimulators | Prostacyclin analogs | Decreased adenylate cyclase stimulation by upstream prostacyclin (PgI2) | Restore cAMP signaling |
| sGC stimulators | Decreased sGC stimulation by upstream NO | Restore cGMP signaling by stimulating its production | |
| Inhibitors | ET-1 receptor antagonists | Increased expression and/or activity of ET-1 | Blocks harmful effects of ET-1 |
| PDE5 inhibitors | Increased expression and/or activity of PDE5, with degradation of cGMP | Prevent harmful effects of PDE5, by preserving cGMP |
Definition of abbreviations: cAMP, cyclic adenosine monophosphate; PH, pulmonary hypertension.
cAMP
In response to binding of prostacyclin to its receptor, adenylate cyclase is activated, leading to increased production of the second messenger cAMP in various cell types, including those responsible for the modulation of vascular remodeling in PAH. For example, intracellular cAMP in human pulmonary arterial SMCs is elevated by the approved prostacyclin analogs, iloprost and treprostinil (73). Similarly, platelet cAMP was doubled by infusion of iloprost in healthy male volunteers (74). The role of cAMP and the case for its potential utility in guiding therapeutic decision making in PAH, therefore, have substantial homology to that of cGMP. In fact, a case can be made for examining the two mediators in concert, because of the potential for cross-talk between cAMP and cGMP, in that certain enzymes responsible for their degradation (PDEs) are shared.
NT-ProBNP and the N Terminal of ANP
Natriuretic peptides were among the first biomarkers identified in patients with PAH. BNP is a natriuretic hormone released primarily from the heart (particularly the ventricles) due to myocardial strain (75). The main action of BNP is natriuresis and vasodilation via the renin/angiotensin pathway. The prohormone, proBNP, is split into BNP and the inactive metabolite, NT-proBNP. ANP is a peptide hormone secreted from cardiac atria in response to increased atrial pressure, as is the NT of ANP (NT-ANP).
ANP levels were elevated in a small group of patients with PAH compared with healthy control subjects, but did not change immediately after prostacyclin infusion (76). Wiedemann and colleagues (69) identified significantly elevated baseline ANP levels in patients with PAH compared with control subjects. Levels correlated with baseline right atrial pressure, PVR, and mixed venous O2 saturation of Hb (69). Moreover, treatment of patients with PAH with inhaled iloprost acutely decreased ANP levels, which correlated with decreases in mean PAP and PVR (69).
Compared with ANP, BNP is much more stable and is less sensitive to temperature changes, making it a more practical test (77). NT-proBNP has an advantage over BNP in that its metabolic clearance is slower. Similar to ANP, BNP and NT-proBNP correlate with hemodynamic parameters (including mean PAP and PVR) and the severity of PH (69, 78–80). Baseline levels of both BNP and NT-proBNP also predict mortality in patients with PAH (79–82). Furthermore, serial decreases in BNP or NT-proBNP predict survival, and serial increases predict mortality (83). In adult patients with sickle cell disease and PAH, NT-proBNP is an independent predictor of mortality (84).
BNP and NT-proBNP levels have also been assessed as reporters of the response to therapy. Addition of inhaled trepostinil to bosentan or sildenafil for 12 weeks decreased NT-proBNP levels and increased 6-minute walk distance (6MWD) (18). BNP levels also decreased after 12 weeks of therapy with ambrisentan (14). Furthermore, in patients with CTEPH, BNP decreased significantly 1 month after pulmonary thromboendarterectomy, a change that correlated with the change in total PVR (78). Treatment with the sGC stimulator, riociguat, led to significant reductions in NT-proBNP versus placebo in studies of patients with PAH (16) and those with inoperable/residual CTEPH (85).
In summary, the natriuretic peptides are important biomarkers in PAH. BNP’s value as a biomarker in PAH may be primarily as an indicator of right heart failure rather than underlying PAH pathogenesis. Indeed, because RV function is a central determinant of PAH outcome, there is a pressing need to advance the understanding of the determinants of adaptive versus maladaptive right heart remodeling in PAH, and related biomarkers may help identify these patients to tailor therapy. BNP and ANP stimulate the production of cGMP (a likely contributor to the high baseline cGMP in patients with severe PAH) (70), and the elevated cGMP may help to protect the right heart (86). Prospective studies are necessary to help determine the clinical significance of thresholds for BNP, assayed alone or in concert with cGMP, in individual patients.
ET-1 and C-Terminal–proET-1
ET-1 is a potent vasoconstrictor and stimulates proliferation of pulmonary artery SMCs. It is produced by the endothelium through cleavage of the biologically inactive precursor, big ET-1, by ET-converting enzyme. Big ET-1 might be a more reliable biomarker, because of its longer circulating half-life (87). Two subtypes of ET receptor, ETA and ETB, mediate the biological actions of ET-1. ETA receptors are expressed on SMCs and cardiac myocytes, whereas ETB receptors are expressed on SMCs and vascular ECs. Activation of ETA and ETB receptors on SMCs cause vasoconstriction, cell proliferation, and hypertrophy, whereas activation of ETB receptors on ECs promote clearance of ET-1 from the circulation and results in the release of vasodilators with antiproliferative properties, such as NO and prostacyclin. This is significant, because, whereas bosentan antagonizes both ETA and ETB (Table 3), ambrisentan selectively antagonizes ETA. Although this distinction has not translated into dramatic differences in clinical responses to ETA-specific (versus nonspecific) monotherapy, it is possible that, in the setting of combination therapy, particularly with a PDE5i or sGCS, the clinical benefits of sparing the ETB receptor may be unmasked (23).
Plasma ET-1 is elevated in patients with PAH and correlated with gas exchange and hemodynamic indices (88–92). Levels of both ET-1 and its precursor, big ET-1, were correlated with PVR, mean PAP, cardiac output, cardiac index, and 6MWD (93). Levels of ET-1 also correlated with right atrial pressure, indexed PVR, cardiac index, and mixed venous O2 saturation, but not with New York Heart Association (NYHA) functional class or time to transplant or death (92). In an exploratory analysis, the ET-1 precursor, C-terminal (CT)-proET-1, provided prognostic data independent of that from other commonly used biomarkers (94).
Interactions of ET-1 with NO or Prostanoids
The ability of ET-1 to stimulate NO production may have particular impact when ERAs are administered. In the presence of ETA receptor inhibition, ET-1 increases, reflecting a feedback regulation loop. The greater circulating ET-1 may then elicit increased NO production via the unblocked ETB receptors without influencing the (inhibited) ETA receptors (95). In rat pulmonary arteries preconstricted by ET-1, vasorelaxation induced by varying (ETA-specific and nonspecific) ERAs was similar (31). However, the relaxation in response to ambrisentan was synergistic with that in response to the sGC stimulator, riociguat, whereas the sGC stimulator did not synergize with the nonspecific ERAs, consistent with an important role for ETB-mediated NO production by ET-1. The vasodilatory effect induced by ETA receptor blockade was essentially abolished by infusion of the NOS inhibitor, l-NAME, whereas prostacyclin inhibition did not impact the response in healthy humans (96). The antiproliferative effects of therapeutics targeting the prostacyclin, ET-1, and NO/cGMP pathways may also interact. Patel and colleagues (30) found that the proliferation of distal pulmonary artery SMCs obtained from patients with PAH was inhibited by the prostacyclin analog, treprostinil, and the effect was additive with that elicited by a PDE5i or a sGCS (i.e., cGMP-elevating agents). In contrast, the antiproliferative effect of treprostinil was actually attenuated in the presence of dual-receptor ERAs, bosentan or macitentan. Given the network of potential interactions among these therapy-related biomarkers, it is rational to posit that simultaneous assay of players in the prostacyclin, ET-1, and L-arginine/NO/cGMP pathway may better inform the selection of therapy in appropriate patients.
RBC Distribution Width
RBC distribution width (RDW) indicates the variability in the size of RBCs, and is routinely reported in automated complete blood counts. RDW was shown retrospectively to independently predict survival in PAH, and even outperformed NT-proBNP in one cohort (97). RDW also predicted survival independently of 6MWD and NT-proBNP (98), and predicted survival in left heart failure, coronary artery disease, and renal disease (99).
Other Molecular Biomarkers in PAH
See the online supplement for a current review of additional circulating biomarkers of potential utility in PAH prognosis or decision making, but not necessarily directly related to treatment pathways.
Conclusions and Future Directions
Numerous circulating biomarkers have shown at least promise for informing clinical prognosis, guiding therapeutic decisions, and/or providing other unique information relevant to patients with PH. Candidate biomarkers, even when intimately related to the disease process and its treatment, may prove to be useless clinically when tested. Several criteria should be met before a candidate biomarker, or biomarker panel, is considered clinically useful and worthy of a change in practice, or even inclusion as a study endpoint. These criteria include general and interlaboratory reproducibility, relevance to the disease process (and ideally to its therapy too, in this case), cost effectiveness, and validation, preferably with good external validity as well. In the case of the serologic biomarkers discussed here, existing assays are largely feasible and measurements reproducible in clinical and research laboratories. Notably, some endothelium-derived species, such as NO derivatives (NO2−, SNOs) and ET-1, require special handling (acquisition in NO2−-free plasticware) and short time to assay, respectively. Cost effectiveness has not been specifically studied, and is best assessed in larger studies validating their utility in, for example, phenotyping patients likely to respond to agent A (or A + B) rather than agent C.
Recent advances in PAH treatment include the addition of new therapeutic drug classes available for oral or inhaled administration, and the finding that up-front combination oral therapy—at least when the combination of tadalafil and ambrisentan is used—may be superior to initiation of either agent alone (23). In this new treatment era, it is rational to examine whether a panel of vascular biomarkers can prospectively identify those patients most likely to benefit from a given (upfront) dual, sequential (add-on), or single-agent therapy regimen. Illustrating the web of related mediators, BNP elevation leads to increases in cGMP that may be adaptive; selective ERAs can preserve or even promote the generation of bioactive NO species. We therefore suggest a new approach in the quest for a clinically useful biomarker. In this era, a useful biomarker or biomarker panel needs to recognize the interactions among candidate mediators/potential serologic biomarkers, the sensitivity of these signals to therapy, and the emerging practice of upfront and other combination therapy. Accordingly, we are investigating, prospectively, the clinical utility of a panel of biomarkers that reflect or interact with the biological pathways targeted by prevalent therapies. Specifically, we are examining longitudinal changes in ET-1, NT-proBNP, cGMP, and the NO derivatives, NO2−, NO3−, and SNO in patients with PH (from various WHO groups) on oral monotherapy or combination therapy, and in relationship to changes in clinical variables, such as 6MWD and hemodynamics. The goal is to identify biomarker signatures that might predict favorable responses to each of multiple therapies (or their combination), and subsequently test resulting hypotheses in future, larger studies. Indeed, interactions among those biomarkers associated with therapeutic targets are numerous and complex, suggesting that assaying them in a coordinated and longitudinal fashion is optimal. Because PAH is a rare disease, and given the substantial morbidity and mortality in spite of these advances in therapy, the identification of such biomarker tools is urgently needed to help optimize and individualize therapy.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge Dr. Kishan Parikh for critically reviewing the manuscript, Dr. Victor Tapson for constructive discussions, and Mr. Casey Herbert for assistance with the illustration.
Footnotes
This work was supported by Bayer HealthCare, National Institutes of Health grant HL107608, and Department of Veterans Affairs grant BX-000281.
A.C.S. designed the literature search, wrote the paper, and revised critically. A.C.D. conceived the work, wrote the paper, and revised critically. T.J.M. conceived the work, supervised the literature search, wrote the paper, revised critically, and is accountable for the accuracy and integrity of the paper. All authors gave approval of the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0438TR on January 22, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 2.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 3.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, Hill NS, Humbert M, Kawut SM, Krowka M, et al. ATS Committee on Pulmonary Hypertension Phenotypes. An official American Thoracic Society statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med. 2014;189:345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, Palevsky HI, Rich S, Sood N, Rosenzweig EB, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449–475. doi: 10.1378/chest.14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 6.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 7.Deng Z, Haghighi F, Helleby L, Vanterpool K, Horn EM, Barst RJ, Hodge SE, Morse JH, Knowles JA. Fine mapping of PPH1, a gene for familial primary pulmonary hypertension, to a 3-cM region on chromosome 2q33. Am J Respir Crit Care Med. 2000;161:1055–1059. doi: 10.1164/ajrccm.161.3.9906051. [DOI] [PubMed] [Google Scholar]
- 8.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–1871. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, et al. Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil therapy for pulmonary arterial hypertension Circulation 20091192894–2903..[Published erratum appears in Circulation 124:e279.] [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 11.Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, et al. Aerosolized Iloprost Randomized Study Group. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 12.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, et al. Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 13.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 14.Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, et al. Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 15.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 16.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, et al. PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 17.Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, et al. PACES Study Group. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, Badesch DB, Frost AE, Shapiro SM, Laliberte K, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142:1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 20.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, et al. SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Barst RJ, Robbins IM, Channick RN, Galiè N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin V, Channick R, Ghofrani H-A, LeMarie J-C, Naeije R, Packer M, Souza R, Tapson V, Tolson J, Hoeper M. Effect of bosentan and sildenafil combination therapy on morbidity and mortality in pulmonary arterial hypertension (PAH): results from the COMPASS-2 study [abstract] Chest. 2014;146:860A. [Google Scholar]
- 23.Galiè N, Barbera JA, Frost A. AMBITION: a randomised, multicentre study of first-line ambrisentan and tadalafil combination therapy in subjects with pulmonary arterial hypertension (PAH) Eur Respir J. 2014;44(Suppl 58):2916. [Google Scholar]
- 24.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 25.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 26.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. Development & approval process (drugs): glossary. 2014[updated 2014 Jan 28; accessed 2015 Feb 10]. Available from: http://www.fda.gov/drugs/developmentapprovalprocess/drugdevelopmenttoolsqualificationprogram/ucm284395.htm
- 28.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, Palazzini M, Park MH, Tapson VF, Sitbon O. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D73–D81. doi: 10.1016/j.jacc.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Patel JAAD, Nelsen AC, Silverstein AM, Clapp LH. Comparison of current therapies to inhibit endothelin-induced growth of pulmonary artery smooth muscle cells (PAMSCs) derived from patients with pulmonary arteiral hypertension. Eur Resp Society. 2014;44:2355. [Google Scholar]
- 31.Liang F, Yang S, Fan P, Jiang Z, Gillies HC, Yao L, Belardinelli L. Ambrisentan and riociguat synergistically relax endothelin-induced contraction of rat pulmonary arteries [abstract] Am J Respir Crit Care Med. 2014;189:A3324. [Google Scholar]
- 32.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 33.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–646. doi: 10.1164/rccm.201304-0686PP. [DOI] [PubMed] [Google Scholar]
- 34.Cracowski JL, Leuchte HH. The potential of biomarkers in pulmonary arterial hypertension. Am J Cardiol. 2012;110(6 suppl):32S–38S. doi: 10.1016/j.amjcard.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: what do we know? Chest. 2013;144:274–283. doi: 10.1378/chest.12-1246. [DOI] [PubMed] [Google Scholar]
- 36.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32:503–512. doi: 10.1183/09031936.00160307. [DOI] [PubMed] [Google Scholar]
- 37.Rafeq S, Shah AM, Preston IR. Biomarkers in pulmonary arterial hypertension. Int J Clin Pract Suppl. 2009;162:36–41. doi: 10.1111/j.1742-1241.2009.02117.x. [DOI] [PubMed] [Google Scholar]
- 38.Heresi GA. Clinical perspective: biomarkers in pulmonary arterial hypertension. Int J Clin Pract Suppl. 2011;169:5–7. doi: 10.1111/j.1742-1241.2010.02598.x. [DOI] [PubMed] [Google Scholar]
- 39.Kramer F, Dusek A. Biomarkers of lung tissue remodeling in pulmonary diseases: implications for clinical practice and research. Curr Respir Med Rev. 2012;8:100–107. [Google Scholar]
- 40.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007;175:1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bédard E, Gibbs JS, Bauersachs J, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 42.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 45.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci USA. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonveaux P, Lobysheva II, Feron O, McMahon TJ. Transport and peripheral bioactivities of nitrogen oxides carried by red blood cell hemoglobin: role in oxygen delivery. Physiology (Bethesda) 2007;22:97–112. doi: 10.1152/physiol.00042.2006. [DOI] [PubMed] [Google Scholar]
- 47.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol. 1998;185:313–318. doi: 10.1002/(SICI)1096-9896(199807)185:3<313::AID-PATH93>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, Aigner C, Fink L, Muyal JP, Weissmann N, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J. 2005;19:1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 49.Kielstein JT, Bode-Böger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, Hoeper MM. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–1418. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 50.Skoro-Sajer N, Mittermayer F, Panzenboeck A, Bonderman D, Sadushi R, Hitsch R, Jakowitsch J, Klepetko W, Kneussl MP, Wolzt M, et al. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2007;176:1154–1160. doi: 10.1164/rccm.200702-278OC. [DOI] [PubMed] [Google Scholar]
- 51.Valkonen VP, Päivä H, Salonen JT, Lakka TA, Lehtimäki T, Laakso J, Laaksonen R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–2128. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 52.Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 53.Cremona G, Higenbottam T, Takao M, Hall L, Bower EA. Exhaled nitric oxide in isolated pig lungs. J Appl Physiol (1985) 1995;78:59–63. doi: 10.1152/jappl.1995.78.1.59. [DOI] [PubMed] [Google Scholar]
- 54.Riley MS, Pórszász J, Miranda J, Engelen MP, Brundage B, Wasserman K. Exhaled nitric oxide during exercise in primary pulmonary hypertension and pulmonary fibrosis. Chest. 1997;111:44–50. doi: 10.1378/chest.111.1.44. [DOI] [PubMed] [Google Scholar]
- 55.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:917–923. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 56.Kharitonov SA, Cailes JB, Black CM, du Bois RM, Barnes PJ. Decreased nitric oxide in the exhaled air of patients with systemic sclerosis with pulmonary hypertension. Thorax. 1997;52:1051–1055. doi: 10.1136/thx.52.12.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Archer SL, Djaballah K, Humbert M, Weir KE, Fartoukh M, Dall’ava-Santucci J, Mercier JC, Simonneau G, Dinh-Xuan AT. Nitric oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:1061–1067. doi: 10.1164/ajrccm.158.4.9802113. [DOI] [PubMed] [Google Scholar]
- 58.Forrest IA, Small T, Corris PA. Effect of nebulized epoprostenol (prostacyclin) on exhaled nitric oxide in patients with pulmonary hypertension due to congenital heart disease and in normal controls. Clin Sci (Lond) 1999;97:99–102. [PubMed] [Google Scholar]
- 59.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung. 2001;179:233–243. doi: 10.1007/s004080000064. [DOI] [PubMed] [Google Scholar]
- 60.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med. 2005;172:352–357. doi: 10.1164/rccm.200412-1684OC. [DOI] [PubMed] [Google Scholar]
- 61.Rhodes P, Leone AM, Francis PL, Struthers AD, Moncada S, Rhodes PM.The L-arginine:nitric oxide pathway is the major source of plasma nitrite in fasted humans Biochem Biophys Res Commun 1995209590–596.. [Published erratum appears in Biochem Biophys Res Commun 211:705.] [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Brown MA, Tam SH, Chan MC, Whitworth JA. Effects of diet on measurement of nitric oxide metabolites. Clin Exp Pharmacol Physiol. 1997;24:418–420. doi: 10.1111/j.1440-1681.1997.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 63.Wennmalm A, Benthin G, Edlund A, Kieler-Jensen N, Lundin S, Petersson AS, Waagstein F. Nitric oxide synthesis and metabolism in man. Ann N Y Acad Sci. 1994;714:158–164. doi: 10.1111/j.1749-6632.1994.tb12040.x. [DOI] [PubMed] [Google Scholar]
- 64.Ibrahim YI, Ninnis JR, Hopper AO, Deming DD, Zhang AX, Herring JL, Sowers LC, McMahon TJ, Power GG, Blood AB. Inhaled nitric oxide therapy increases blood nitrite, nitrate, and S-nitrosohemoglobin concentrations in infants with pulmonary hypertension. J Pediatr. 2012;160:245–251. doi: 10.1016/j.jpeds.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, Nudelman R, Yan Y, Krichman AD, Bashore TM, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci USA. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–160. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirata Y, Ishii M, Matsuoka H, Sugimoto T, Iizuka M, Uchida Y, Serizawa T, Sato H, Kohmoto O, Mochizuki T, et al. Plasma concentrations of alpha-human atrial natriuretic polypeptide and cyclic GMP in patients with heart disease. Am Heart J. 1987;113:1463–1469. doi: 10.1016/0002-8703(87)90663-6. [DOI] [PubMed] [Google Scholar]
- 68.Bogdan M, Humbert M, Francoual J, Claise C, Duroux P, Simonneau G, Lindenbaum A. Urinary cGMP concentrations in severe primary pulmonary hypertension. Thorax. 1998;53:1059–1062. doi: 10.1136/thx.53.12.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiedemann R, Ghofrani HA, Weissmann N, Schermuly R, Quanz K, Grimminger F, Seeger W, Olschewski H. Atrial natriuretic peptide in severe primary and nonprimary pulmonary hypertension: response to iloprost inhalation. J Am Coll Cardiol. 2001;38:1130–1136. doi: 10.1016/s0735-1097(01)01490-5. [DOI] [PubMed] [Google Scholar]
- 70.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate–dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 71.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 72.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- 73.Clapp LH, Finney P, Turcato S, Tran S, Rubin LJ, Tinker A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol. 2002;26:194–201. doi: 10.1165/ajrcmb.26.2.4695. [DOI] [PubMed] [Google Scholar]
- 74.Krause W, Krais T. Pharmacokinetics and pharmacodynamics of the prostacyclin analogue iloprost in man. Eur J Clin Pharmacol. 1986;30:61–68. doi: 10.1007/BF00614197. [DOI] [PubMed] [Google Scholar]
- 75.Tonelli AR, Haserodt S, Aytekin M, Dweik RA. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ. 2013;3:20–30. doi: 10.4103/2045-8932.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morice AH, Pepke-Zaba J, Brown MJ, Thomas PS, Higenbottam TW. Atrial natriuretic peptide in primary pulmonary hypertension. Eur Respir J. 1990;3:910–913. [PubMed] [Google Scholar]
- 77.Buckley MG, Marcus NJ, Yacoub MH, Singer DR. Prolonged stability of brain natriuretic peptide: importance for non-invasive assessment of cardiac function in clinical practice. Clin Sci (Lond) 1998;95:235–239. [PubMed] [Google Scholar]
- 78.Nagaya N, Ando M, Oya H, Ohkita Y, Kyotani S, Sakamaki F, Nakanishi N. Plasma brain natriuretic peptide as a noninvasive marker for efficacy of pulmonary thromboendarterectomy. Ann Thorac Surg. 2002;74:180–184, discussion 184. doi: 10.1016/s0003-4975(02)03654-8. [DOI] [PubMed] [Google Scholar]
- 79.Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, Nair D, Denton CP, Black CM, Coghlan JG. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 80.Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, Vogeser M, Muehling O, Behr J. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest. 2007;131:402–409. doi: 10.1378/chest.06-1758. [DOI] [PubMed] [Google Scholar]
- 81.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 82.Sztrymf B, Souza R, Bertoletti L, Jaïs X, Sitbon O, Price LC, Simonneau G, Humbert M. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010;35:1286–1293. doi: 10.1183/09031936.00070209. [DOI] [PubMed] [Google Scholar]
- 83.Mauritz GJ, Rizopoulos D, Groepenhoff H, Tiede H, Felix J, Eilers P, Bosboom J, Postmus PE, Westerhof N, Vonk-Noordegraaf A. Usefulness of serial N-terminal pro-B–type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2011;108:1645–1650. doi: 10.1016/j.amjcard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 84.Klings ES, Machado RF, Barst RJ, Morris CR, Mubarak KK, Gordeuk VR, Kato GJ, Ataga KI, Gibbs JS, Castro O, et al. American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189:727–740. doi: 10.1164/rccm.201401-0065ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, et al. CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 86.Lee DI, Kass DA. Phosphodiesterases and cyclic GMP regulation in heart muscle. Physiology (Bethesda) 2012;27:248–258. doi: 10.1152/physiol.00011.2012. [DOI] [PubMed] [Google Scholar]
- 87.Hemsén A, Ahlborg G, Ottosson-Seeberger A, Lundberg JM. Metabolism of Big endothelin-1 (1–38) and (22–38) in the human circulation in relation to production of endothelin-1 (1–21) Regul Pept. 1995;55:287–297. doi: 10.1016/0167-0115(94)00119-i. [DOI] [PubMed] [Google Scholar]
- 88.Allen SW, Chatfield BA, Koppenhafer SA, Schaffer MS, Wolfe RR, Abman SH. Circulating immunoreactive endothelin-1 in children with pulmonary hypertension: association with acute hypoxic pulmonary vasoreactivity. Am Rev Respir Dis. 1993;148:519–522. doi: 10.1164/ajrccm/148.2.519. [DOI] [PubMed] [Google Scholar]
- 89.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 90.Vincent JA, Ross RD, Kassab J, Hsu JM, Pinsky WW. Relation of elevated plasma endothelin in congenital heart disease to increased pulmonary blood flow. Am J Cardiol. 1993;71:1204–1207. doi: 10.1016/0002-9149(93)90646-t. [DOI] [PubMed] [Google Scholar]
- 91.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, Rich S. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26:1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 92.Montani D, Souza R, Binkert C, Fischli W, Simonneau G, Clozel M, Humbert M. Endothelin-1/endothelin-3 ratio: a potential prognostic factor of pulmonary arterial hypertension. Chest. 2007;131:101–108. doi: 10.1378/chest.06-0682. [DOI] [PubMed] [Google Scholar]
- 93.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 94.Silva Marques J, Martins SR, Calisto C, Gonçalves S, Almeida AG, de Sousa JC, Pinto FJ, Diogo AN. An exploratory panel of biomarkers for risk prediction in pulmonary hypertension: emerging role of CT-proET-1. J Heart Lung Transplant. 2013;32:1214–1221. doi: 10.1016/j.healun.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 95.Hocher B, Schwarz A, Slowinski T, Bachmann S, Pfeilschifter J, Neumayer HH, Bauer C. In-vivo interaction of nitric oxide and endothelin. J Hypertens. 2004;22:111–119. doi: 10.1097/00004872-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 96.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist–mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 97.Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868–872. doi: 10.1016/j.amjcard.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 98.Rhodes CJ, Wharton J, Howard LS, Gibbs JS, Wilkins MR. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart. 2011;97:1054–1060. doi: 10.1136/hrt.2011.224857. [DOI] [PubMed] [Google Scholar]
- 99.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]