Abstract
Acute cellular rejection is a known risk factor for the development of obliterative bronchiolitis, which limits the long-term survival of lung transplant recipients. However, the T cell effector mechanisms in both of these processes remain incompletely understood. Using the mouse orthotopic lung transplant model, we investigated whether C57BL/6 T-bet−/− recipients of major histocompatibility complex (MHC)-mismatched BALB/c lung grafts develop rejection pathology and allospecific cytokine responses that differ from wild-type mice. T-bet−/− recipients demonstrated vigorous allograft rejection at 10 days, characterized by neutrophilic inflammation and predominantly CD8+ T cells producing allospecific IL-17 and/or IFN-γ, in contrast to IFN-γ–dominant responses in WT mice. CD4+ T cells produced IL-17 but not IFN-γ responses in T-bet−/− recipients, in contrast to WT controls. Costimulation blockade using anti-CD154 Ab significantly reduced allospecific CD8+IFN-γ+ responses in both T-bet−/− and WT mice but had no attenuating effect on lung rejection pathology in T-bet−/− recipients or on the development of obliterative airway inflammation that occurred only in T-bet−/− recipients. However, neutralization of IL-17A significantly attenuated costimulation blockade–resistant rejection pathology and airway inflammation in T-bet−/− recipients. In addition, CXCL1 (neutrophil chemokine) was increased in T-bet−/− allografts, and IL-17 induced CXCL1 from mouse lung epithelial cells in vitro. Taken together, our data show that T-bet–deficient recipients of complete MHC-mismatched lung allografts develop costimulation blockade–resistant rejection characterized by neutrophilia and obliterative airway inflammation that is predominantly mediated by CD8+IL-17+ T cells. Our data support T-bet–deficient mouse recipients of lung allografts as a viable animal model to study the immunopathogenesis of small airway injury in lung transplantation.
Keywords: mouse orthotopic lung transplant, acute rejection, T-bet, IL-17, neutrophils
Clinical Relevance
Our results suggest a potential role for targeting the development or activity of IL-17 as a therapy in lung transplantation, although these approaches would have to be carefully considered in balance with potential host defense implications. Our results also support using T-bet–deficient mice in mouse orthotopic lung transplantation to further study the pathogenesis of murine airway inflammation and injury after lung transplantation.
Lung transplantation is the final therapeutic option for select patients with end-stage lung disease. However, its long-term success is limited by chronic allograft rejection or by the bronchiolitis obliterans syndrome (BOS), resulting in decreased survival compared with other solid organ transplant recipients (1, 2). Acute cellular rejection (ACR) occurs in 50% of lung transplant recipients (LTRs) and has been shown to increase the risk of BOS. Although T cells are widely believed to play a central role in the immunopathogenesis of ACR and obliterative bronchiolitis (OB), the cellular mechanisms driving these processes are incompletely understood. Specifically, the T cell subsets and effector cytokines involved and the interactions with innate immune cells that eventually result in airway obliteration, the hallmark of OB, remain to be elucidated.
The development of the mouse orthotopic lung transplant animal model is a significant advance to study T cell immune responses in conjunction with lung rejection pathology (3). An early study in this model showed that wild-type (WT) recipients of major histocompatibility complex (MHC) class I/II-mismatched allografts maintained intact airway epithelium despite fulminate lung rejection (4). In this setting, type 1–mediated allograft rejection appears predominant, with marked allograft preservation in response to costimulation blockade (5–7). In contrast, a recent study in this model showed obliterative airway lesions in minor histocompatibility mismatched transplants that were significantly reduced after neutralization of IL-17A (8). Also notable was a recent report of similar airway lesions in fully MHC-mismatched transplants treated with conventional immunosuppression, including cyclosporine A and corticosteroids, although cytokine responses were not evaluated in this study (9). Although IFN-γ–mediated type 1 responses have been demonstrated in ACR in multiple experimental transplant models, including the mouse orthotopic lung transplant model, these responses have also been shown to be dispensable for rejection. In fact, IFN-γ expression is essential for establishing durable allograft acceptance in several studies (10–12).
The transcription factor Tbx 21 (T-bet) has been shown to be critical for type 1 lineage commitment in CD4+ T cells and also is expressed in CD8+ T cells (13, 14). T-bet has also been shown to repress IL-17 immunity via regulation of the transcription factor runx-1 (15). Two studies in the mouse heterotopic heart transplant model evaluated T-bet–deficient recipients and showed predominant IL-17 responses driving ACR that were costimulation blockade resistant (16, 17). We hypothesized that acute lung allograft rejection would differ in T-bet−/− recipients and would be predominantly mediated by IL-17–producing T cells. Herein, we show that B6 T-bet−/− recipients of BALB/c lung allografts demonstrate mixed cellular rejection comprised of type 1 and type 17 CD8+ T cell–predominant inflammation, with the development of obliterative, neutrophilic airway inflammation. Treatment with anti-CD154 therapy significantly reduced CD8+ type 1 responses but did not affect ACR or airway inflammation, whereas anti–IL-17A therapy significantly attenuated both. These findings demonstrate an important role for IL-17–dependent lung rejection and neutrophilic airway inflammation in the setting of T-bet deficiency.
Materials and Methods
Mice
The Johns Hopkins University and University of Pittsburgh Institutional Animal Care and Use Committees approved all animal protocols. C57BL/6 (I-ab, H-2b), B6.129S6-Tbx21tm1Glm/J (B6 T-bet−/−), and BALB/c (I-ad, H-2d) mice were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were housed in the Johns Hopkins University or the University of Pittsburgh animal facilities under specific pathogen-free conditions.
Orthotopic Lung Transplant
Allogeneic transplantations were performed in the BALB/c → B6 WT or BALB/c → B6 T-bet−/− strain combinations. Donor mice were sedated with etomidate (1 mg, intraperitoneally), intubated, and maintained on inhaled isoflurane until they were killed. Recipients were both initially sedated and maintained on inhaled isoflurane. Transplantation was performed using a cuffed technique as previously described (18). Mice received subcutaneous buprenorphine (0.03–0.05 mg/kg) before extubation and every 6 hours thereafter as needed. Animals were killed for analysis at 10 days after transplant.
Medium and Reagents
Cell culture medium RPMI 1640 (Biofluids, Rockville, MD) was supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM glutamine, 1 mM sodium pyruvate, 1% NEAA, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM β-mercaptoethanol, and 25 mM HEPES (Biofluids, Rockville, MD). Mice received 500 μg anti-CD154 (clone MR-1; BioXcell, West Lebanon, NH) intraperitoneally on Days 0 and 2 after transplant. Mice received 200 μg anti–IL-17A (clone 17F3; BioXcell) intravenously via tail vein on Days 0, 2, 4, and 6 after transplant.
Cell Preparations, Stimulation, and Cytokine Detection
Spleen, draining lymph nodes, and lungs were harvested from mice on Day 10 after transplant. Tissue was processed, and mononuclear cells were isolated as previously described (6). Isolated responder cells from tissue were cultured for 5 hours in medium alone or with BALB/c splenocytes (1:1) with brefeldin A (10 μg/ml) (Sigma-Aldrich) present for the final 2 hours of stimulation.
Flow Cytometry
The following antibodies were purchased from BD PharMingen (San Diego, CA): phycoerythrin (PE)-labeled anti–IL-17A; allophycocyanin (APC)-labeled anti–IFN-γ; FITC-labeled anti–TNF-α; peridinin-chlorophyll-protein complex (PerCP) Cy5.5-labeled anti-CD8; Alexa-700–labeled anti-CD4; and all respective isotype antibodies. Biotinylated anti-H2Dd was purchased from Biolegend (San Diego, CA), and Pacific Blue–labeled streptavidin was purchased from Invitrogen (Grand Island, NY). Surface antibody staining and intracellular cytokine staining was performed as previously described (19, 20). Flow cytometry analysis was performed using a FACSAria instrument and Flowjo software for analysis (Tree Star Inc., San Carlos, CA).
Histopathology and Acute Rejection Pathology Scoring
Grafts were fixed in 10% formalin, embedded in paraffin, sectioned, and stained using hematoxylin and eosin. Stained sections of grafts were scored by three blinded observers using a point system developed to grade the severity of rejection pathology. Points (0–4) were given for the presence and severity of each of the following characteristics (1): peribronchial/perivascular inflammatory cell infiltrate, (2) interstitial inflammatory cell infiltrate, (3) alveolar inflammatory cell infiltrate, and (4) intraluminal airway inflammation. The score is the mean of the points for the four characteristics (range, 0–4). For quantification of neutrophils, a previously published method was used (21). Antimyeloperoxidase was purchased from Abcam (Cambridge, MA).
Details regarding determination of CXCL1 levels are provided in the online supplement.
Statistical Analysis
Variables were compared by Student’s t test unless otherwise specified using Graphpad software. P < 0.05 was considered statistically significant.
Results
T-bet Deficiency in MHC-Mismatched Mouse Orthotopic Lung Transplant Is Associated with Severe Acute Rejection Pathology Characterized by Polymorphonuclear Cell Infiltration, Obliterative Airway Inflammation, and Low Graft CD4:CD8 Ratio
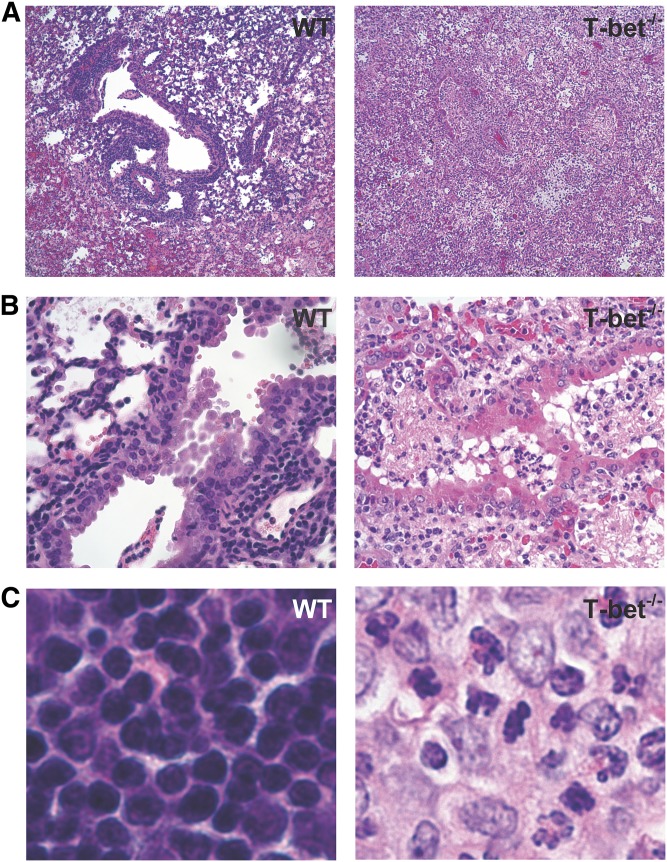
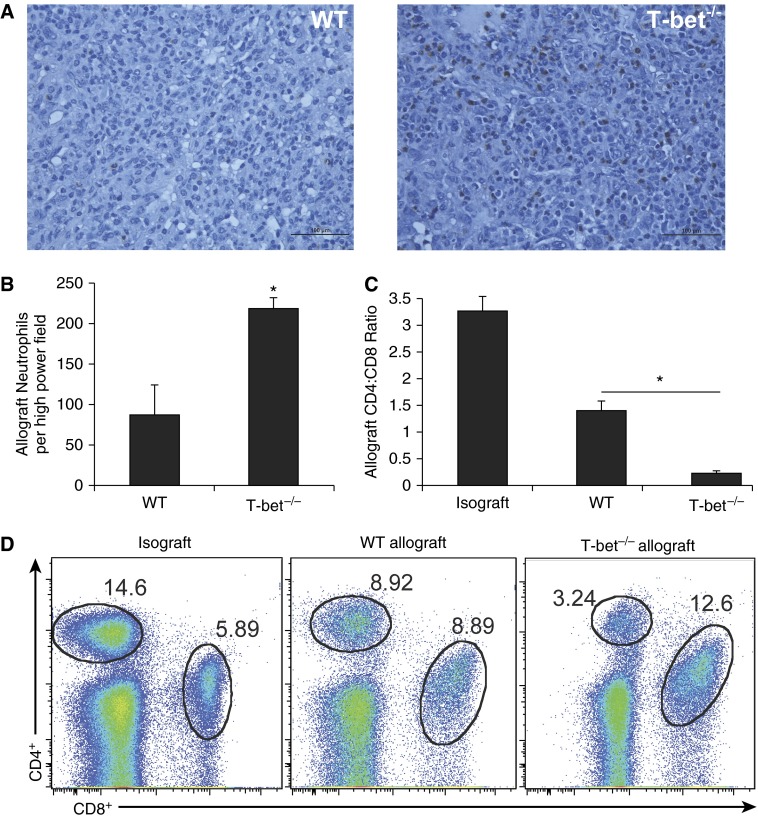
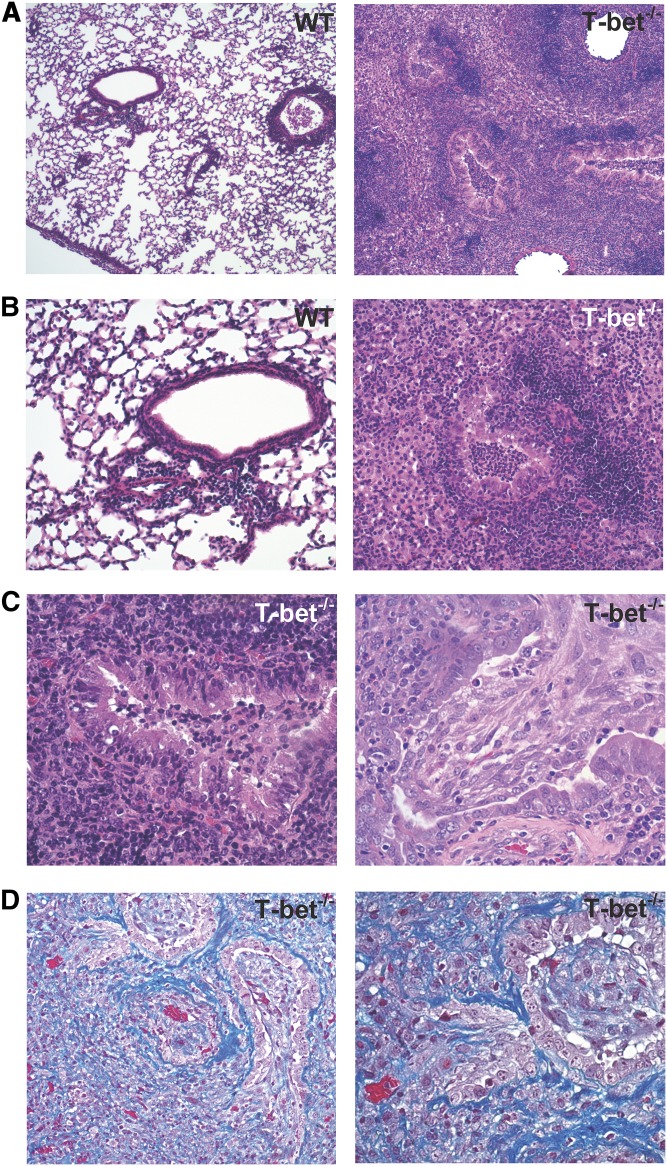
To characterize the acute rejection pathology that develops in the absence of T-bet, we compared allografts from T-bet−/− recipients (C57BL/6 background) of left orthotopic lung transplants from BALB/c donors to allografts from C57BL/6 WT recipients. At Day 10, histologic examination demonstrated that lung allografts from T-bet−/− recipients have marked peribronchial and perivascular inflammatory cellular infiltrate, as seen in WT recipients (Figure 1A), as well as inflammation and injury in the lung allograft parenchyma. However, we also observed a mixed cellular inflammatory infiltrate plugging the airway lumens of T-bet−/− recipients resulting in luminal obliteration not seen in allografts from WT recipients (Figure 1B). In addition, the mixed cellular infiltrate present in T-bet−/− recipients is comprised largely of polymorphonuclear cells, in contrast to the lymphocyte-predominant inflammation seen in allografts from WT recipients (Figure 1C). Indeed, allografts from T-bet−/− recipients had significantly higher numbers of neutrophils compared with allografts from WT recipients (Figures 2A and 2B). Analysis of the graft-infiltrating lymphocytes in T-bet−/− recipients revealed a markedly decreased CD4:CD8 ratio compared with WT allograft and WT isograft recipients (Figures 2C and 2D). Together, these data show qualitative differences in lung allograft acute rejection pathology in mice with T-bet deficiency compared with WT animals.
Figure 1.
Lung allografts from T-bet−/− recipients develop severe rejection pathology marked by polymorphonuclear inflammation and intraluminal airway inflammation. (A) Hematoxylin and eosin (H&E)–stained sections (original magnification: ×4) of lung allografts from wild-type (WT) and T-bet−/− recipients at Day 10. (B) H&E-stained sections (original magnification: ×20) of lung allografts demonstrating the appearance of small airways. (C) H&E-stained sections (original magnification: ×40) of lung allografts from WT and T-bet−/− recipients at Day 10, showing the presence of polymorphonuclear cells in allografts from T-bet−/− recipients.
Figure 2.
Severe rejection pathology in T-bet−/− recipients is characterized by significantly higher allograft neutrophil count and lower allograft CD4:CD8 ratio compared with WT. (A) Histologic sections of lung allografts from WT and T-bet−/− recipients at Day 10 showing neutrophil staining with antimyeloperoxidase. (B) Total number of neutrophils per high-power field on histological sections of lung allografts stained with antimyeloperoxidase (10 different high-power fields per allograft counted; n = 3 mice per group; P = 0.034; * denotes statistical significance). (C) Lung allograft CD4:CD8 ratios at Day 10 (n = 3–8 mice per group; * denotes statistical significance). (D) Representative flow plots of lung allograft mononuclear cells at Day 10 showing frequencies of CD4+ and CD8+ cells.
Allospecific T Cell Responses from T-bet−/− Recipients during Acute Rejection Are Marked by Robust IL-17 and IFN-γ Production from CD8+ T Cells
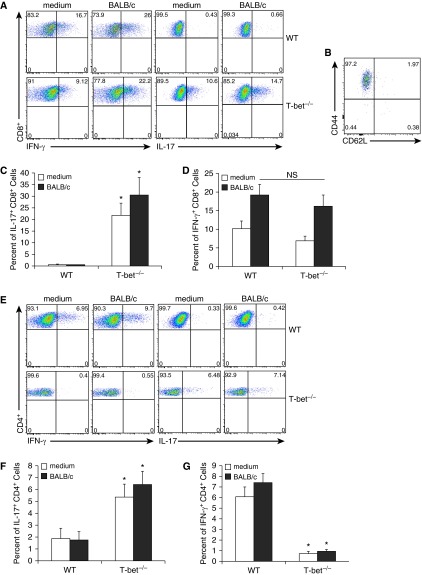
To elucidate any differences in allospecific T cell effector function occurring in the setting of T-bet deficiency, we evaluated T cell cytokine responses in mononuclear cells recovered from the lung allografts of T-bet−/− and WT recipients at Day 10 after culture in medium alone and after coculture with BALB/c splenocytes. As shown in a representative plot in Figure 3A, CD8+ T cells from T-bet−/− recipient allografts have high frequencies of constitutive IL-17+ cells that increased in response to in vitro restimulation with BALB/c splenocytes, in striking contrast to WT recipients (Figures 3A and 3C). These CD8+IL-17+ cells are characterized by high expression of CD44 and low expression of CD62L, consistent with an effector phenotype (Figure 3B). However, T-bet−/− mice demonstrated similar lung allograft allospecific CD8+IFN-γ+ responses to those observed in WT mice (Figures 3A and 3D). In addition, we evaluated other effector responses, including TNF-α, IL-4, and IL-22, and only detected low frequencies of TNF-α+ CD8+ cells in WT and T-bet−/− recipients (< 2%; data not shown). We also evaluated CD4+ T cell responses in the lung allografts from T-bet−/− mice and found increased IL-17 compared with WT mice, although with significantly impaired IFN-γ production (Figures 3E–3G). In summary, these data indicate that there are marked differences in alloeffector cytokine production between WT and T-bet–deficient recipients of MHC-mismatched mouse orthotopic lung transplantation that occur during acute lung rejection.
Figure 3.
Lung allografts from T-bet−/− recipients demonstrate robust allospecific CD8+ IL-17 responses in addition to IFN-γ responses. (A) Representative flow plots of lung allograft mononuclear cells after culture in medium alone and after coculture with BALB/c splenocytes, gating on CD8+ cells. (B) CD62L and CD44 expression in CD8+ IL-17+ cells from T-bet allograft after coculture with Balb/c splenocytes, gating on CD8+ IL-17+ cells. (C) Percentage of lung allograft CD8+ cells positive for IL-17 in WT and T-bet−/− recipients after culture in medium alone and after coculture with BALB/c splenocytes (n = 3–5 mice per group; P = 0.0007 and 0.002; * denotes statistical significance). (D) Percentage of lung allograft CD8+ cells positive for IFN-γ in WT and T-bet−/− recipients after culture in medium alone and after coculture with BALB/c splenocytes (n = 3–6 mice per group; P = 0.56). NS, not significant. (E) Representative flow plots of lung allograft mononuclear cells after culture in medium alone and after coculture with BALB/c splenocytes, gating on CD4+ cells. (F) Percentage of lung graft CD4+ cells positive for IL-17 in WT and T-bet−/− recipients after culture in medium alone and after coculture with BALB/c splenocytes (n = 3 mice per group; P = 0.04 and 0.02; * denotes statistical significance). (G) Percentage of lung allograft CD4+ cells positive for IFN-γ in WT and T-bet−/− recipients after culture in medium alone and after coculture with BALB/c splenocytes (n = 3–6 mice per group; P = 0.004 and 0.002; * denotes statistical significance).
CD154/CD40 Costimulation Blockade Skews T Cell Responses from T-bet–Deficient Mice to an IL-17 Predominance
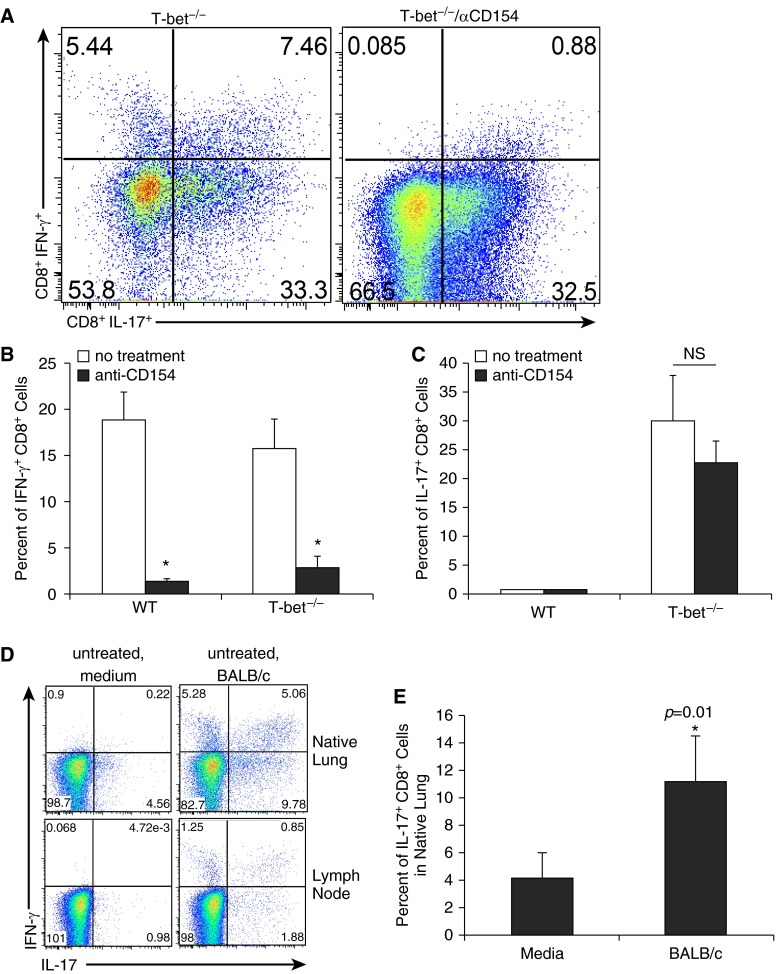
We and others have shown that anti-CD154 Ab therapy markedly reduces allospecific IFN-γ+ responses from T cells and attenuates acute rejection pathology after mouse orthotopic lung transplant (5, 6). We therefore evaluated T cell effector responses present in the lung allografts of T-bet−/− recipients treated with anti-CD154 Ab to determine the effects of CD154/CD40 costimulation blockade on these responses. Allospecific CD8+IL-17+ responses remained robust despite anti-CD154 treatment and were not significantly different when compared with untreated T-bet−/− recipients (Figures 4A and 4C). In addition, CD4+IL-17+ responses were not altered in the presence of anti-CD154 treatment (data not shown). In contrast, allospecific CD8+IFN-γ+ responses to BALB/c antigen restimulation were markedly attenuated after CD154/CD40 blockade, as in WT recipients (Figures 4A and 4B). The highest frequencies of residual IFN-γ+ cells were CD8+ T cells that coexpressed IL-17a (Figure 4A). These CD8+ cells coexpressing IFN-γ and IL-17 were also found in the native lung, spleen, and draining lymph nodes of T-bet−/− recipients (Figure 4D; spleen data not shown). In the lymph node and native lung (in contrast to the allograft), it is unlikely that significant alloantigen is present in culture in medium alone, and there are also much less constitutive IL-17 responses present after culture in medium alone. Therefore, the increase in CD8+IL-17+ responses in the native lung and lymph node with addition of BALB/c splenocytes to cultures more clearly demonstrates the allospecificity of these immune responses (Figure 4D). There is a significant increase in IL-17 responses in the CD8+ cells from the native lung of T-bet−/− recipients after coculture with BALB/c splenocytes compared with culture in medium alone (Figure 4E). Together, these data show that CD154/CD40 costimulation blockade differentially affects CD8+ allospecific responses in the setting of T-bet deficiency, effectively skewing T cell responses toward an IL-17–predominant response.
Figure 4.
CD8+ IL-17 responses in T-bet−/− recipients remain robust despite anti-CD154 therapy, which effectively abrogates CD8+ IFN-γ responses. (A) Representative flow plots of lung allograft mononuclear cells, gating on CD8+ cells, from an untreated T-bet−/− recipient and a T-bet−/− recipient treated with anti-CD154. (B) Percentage of lung allograft CD8+ cells positive for IFN-γ in WT and T-bet−/− recipients receiving no treatment and receiving anti-CD154 treatment (n = 3-6 mice per group, P = 0.002 and 0.008; * denotes statistical significance). (C) Percentage of lung allograft CD8+ cells positive for IL-17 in WT and T-bet−/− recipients receiving no treatment and receiving anti-CD154 treatment. (D) Representative flow plots of native lung and thoracic lymph node mononuclear cells, gating on CD8+ cells, from an untreated T-bet−/− recipient after culture in medium alone, and after coculture with BALB/c splenocytes. (E) Percentage of native lung CD8+ cells positive for IL-17 in T-bet−/− recipients receiving no treatment after culture in medium alone and after coculture with BALB/c splenocytes (n = 5 mice per group; * denotes statistical significance). NS, not significant.
CD154/CD40 Costimulation Blockade in T-bet−/− Recipients Is Marked by Severe Acute Lung Rejection and Obliterative Airway Inflammation That Is IL-17 Dependent
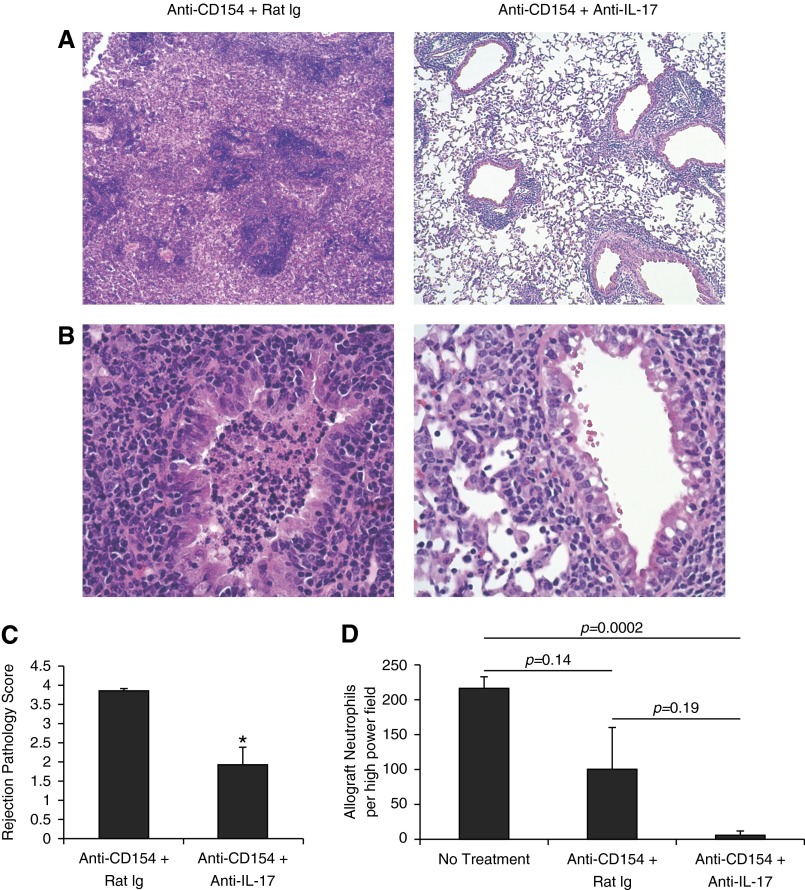
We evaluated the effect of anti-CD154 therapy on acute lung rejection pathology in T-bet−/− recipients. Unlike lung allografts from WT recipients, allografts from anti-CD154–treated T-bet−/− recipients demonstrate severe acute rejection pathology at Day 10 (Figures 5A and 5B). We observed high-grade inflammatory cell infiltrate around blood vessels and airways along with massive infiltration throughout the lung parenchyma, marked by increased interstitial and alveolar inflammation (Figures 5A and 5B). In addition, we again observed an organizing mixed cellular inflammatory infiltrate plugging the airway lumens of anti-CD154–treated T-bet−/− recipients resulting in luminal obliteration, as seen in allografts from untreated T-bet−/− recipients but not WT recipients (Figures 5A–5C). These airway lesions varied from luminal plugs in which mononuclear cells and neutrophils could be distinguished versus lesions that showed more organized fibrosis (Figures 5C and 5D). Given the lack of improvement in acute rejection pathology in T-bet−/− recipients treated with anti-CD154, we did verify that CD4+ cells from T-bet−/− mice express CD154 when activated in vitro (data not shown). Next, we examined the role of IL-17 in acute rejection pathology and obliterative airway inflammation. The coadministration of anti–IL-17 Ab in anti-CD154–treated T-bet−/− mice significantly reduced acute rejection pathology and strikingly reduced intraluminal airway inflammation in these mice compared with an anti–IL-17 isotype control Ab (Figures 6A–6C). The rejection pathology in lung allografts from mice treated with anti–IL-17 Ab in addition to anti-CD154 was also characterized by a significant reduction in neutrophil counts (Figure 6D). Our studies demonstrate an IL-17–dependent mechanism for acute lung rejection pathology and obliterative airway inflammation in anti-CD154–treated T-bet−/− recipients.
Figure 5.
Severe rejection pathology and airway obliteration in lung allografts from T-bet−/− recipients is resistant to anti-CD154. (A, B) H&E-stained sections (original magnification: ×4, ×10) of lung allografts from WT and T-bet−/− recipients treated with anti-CD154. (C) H&E-stained sections (original magnification: ×20) of lung allografts from T-bet−/− recipients treated with anti-CD154 demonstrating a range of airway pathology. (D) Trichrome-stained section (original magnification: ×10, ×20) of lung allograft from T-bet−/− recipient treated with anti-CD154.
Figure 6.
Anti–IL-17 therapy significantly improves lung allograft rejection pathology, including airway inflammation/obliteration and graft neutrophilia in T-bet−/− recipients treated with anti-CD154. (A, B) H&E-stained sections (original magnification: ×4, ×20) of lung allografts from T-bet−/− recipients treated with anti-CD154 alone and treated with anti-CD154 + anti–IL-17. (C) Rejection pathology scores of lung allografts from T-bet−/− recipients treated with anti-CD154 alone and treated with anti-CD154 + anti–IL-17 (n = 4 mice per group; P = 0.005; * denotes statistical significance). (D) Total number of neutrophils per high-power field on histological sections of lung allografts stained with antimyeloperoxidase (10 different high-power fields per allograft counted; n = 3 mice per group).
IL-17 Induces CXCL1 in Mouse Lung Epithelial Cells and Is Associated with Increased Lung Allograft CXCL1 Levels in T-bet−/− LTRs
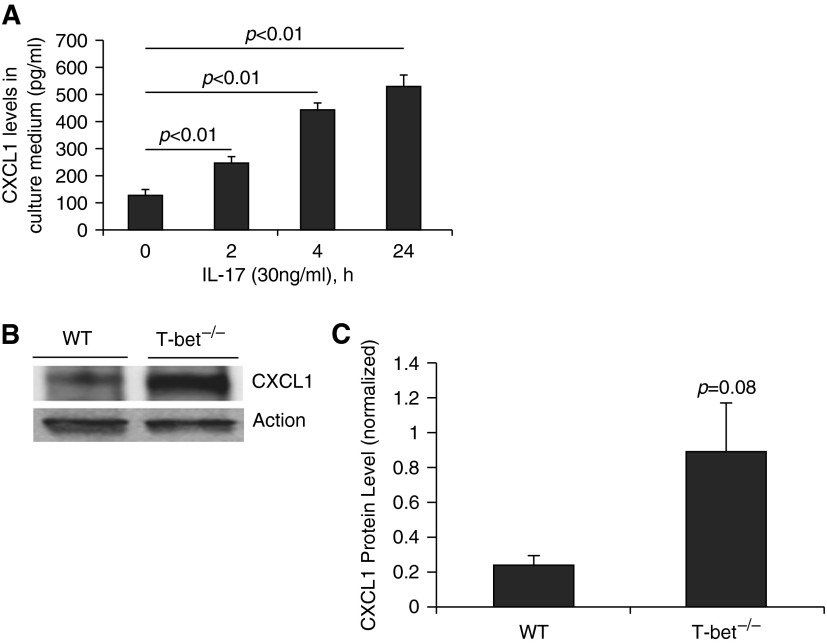
It has previously been shown that intratracheal LPS and IL-17 induce CXCL1, a neutrophil chemokine, in the bronchoalveolar space in mice (22, 23). In addition, Sharma and colleagues have recently demonstrated that IL-17 induces CXCL1 expression in mouse epithelial cells (24). We also treated mouse epithelial cells with murine IL-17 and found increased CXCL1 in the media at 2 hours. CXCL1 levels were increased 5-fold by 24 hours (Figure 7A). This direct effect of IL-17 on mouse epithelial cells provides a potential mechanism for neutrophil influx mediated by epithelial cells in our transplant model. Having shown robust IL-17 responses in T-bet−/− recipients, we next sought to determine whether CXCL1 levels were increased in lung allografts from these mice compared with allografts from WT recipients. We found a 3-fold increase in CXCL1 levels in lung allograft homogenates from T-bet−/− recipients compared with WT recipients (Figures 7B and 7C). These data demonstrate one plausible mechanism for IL-17–induced neutrophilic inflammation in mouse recipients of lung transplantation.
Figure 7.
IL-17A induces CXCL1 in mouse epithelial cells. (A) CXCL1 levels in culture medium from mouse lung epithelial cells incubated with murine IL-17 at 2, 4, and 24 hours. (B) Representative Western blot for CXCL1 in homogenates of lung allografts from a WT and T-bet−/− recipient. (C) CXCL1 protein levels in homogenates of lung allografts from WT and T-bet−/− recipients.
Discussion
The role of allospecific T cell effector responses in lung allograft rejection remains incompletely understood. Herein, our studies in a fully MHC-mismatched mouse orthotopic lung transplant model show that T-bet deficiency results in lung allograft inflammation mediated predominantly by CD8+ T cells and marked by high IL-17 and IFN-γ production, in contrast to only type 1 inflammation in WT recipients. In addition, we demonstrate that T-bet–deficient recipients develop airway inflammation that obliterates airway lumens. Unexpectedly, treating T-bet−/− recipients with anti-CD154 resulted in persistent obliterative airway inflammation characterized by increased organization/fibrosis and skewing of CD8+ T cells to an IL-17–predominant alloeffector response. The coadministration of anti–IL-17Ab and anti-CD154 Ab significantly attenuated lung allograft rejection pathology and markedly reduced acute rejection pathology, allograft neutrophilia, and airway inflammation. Collectively, our findings demonstrate an IL-17–dependent T cell mechanism for lung allograft rejection and obliterative airway inflammation in the absence of T-bet–mediated repression.
Our findings are consistent with a previous report of CD8+IL-17–mediated costimulation-blockade resistant allograft rejection in a MHC class I and II mismatched BALB/c → B6 murine heterotopic cardiac transplantation model (17). That study demonstrated the detection of CD8+IFN-γ+IL-17+ responses in the allograft but not in the spleen and that neutralization of IL-17 or depletion of CD8+ T cells reversed costimulation blockade–resistant rejection. Although our studies show a distribution of these double-positive cells in the lung allograft and in secondary lymphoid tissue, we too demonstrate attenuation of rejection with neutralization of IL-17. There are two studies investigating the effects of T-bet deficiency on T cell responses in other experimental transplant models (a murine pulmonary graft-versus-host disease model and a murine heterotopic cardiac transplant model) using partially MHC mismatched donor/recipient strain combinations (16, 25). In both of these studies, IL-17 responses were demonstrated in CD4+ T cells but not in CD8+ T cells, suggesting that the degree of MHC mismatching may play a role in resultant allospecific T cell effector responses.
In our mouse model of lung transplantation, we demonstrate the intact ability of T-bet−/−CD8+ T cells to produce IFN-γ. However, detection of CD8+IFN-γ+ T cells in T-bet−/− allograft recipients differs from the findings in experimental autoimmune myocarditis, in which myocardial-infiltrating CD8+ T cells were incapable of producing IFN-γ after restimulation with anti-CD3/anti-CD28, in contrast to cells isolated in the periphery (26). In another study, T-bet was shown to be essential for the development of CD8+IFN-γ+ lymphocyte–dependent autoimmune diabetes using the rat insulin promoter–lymphocytic choriomeningitis virus transgenic model (27). Thus, the ability of CD8+ T cells from T-bet−/− mice to produce IFN-γ appears to vary in different experimental systems.
It is well established that T-bet plays a crucial role in Th1 lineage development (13, 14, 28). Therefore, it was not surprising that we were unable to detect significant IFN-γ from CD4+ T cells from T-bet−/− recipients, unlike in WT mice. However, our studies also demonstrated CD4+ T cells from T-bet−/− recipients as a source of IL-17, albeit less than CD8+ T cells, in contrast to WT controls. The induction of antigen-specific CD4+IL-17+ T cells (TH17 cells) has previously been shown to be negatively regulated by IFN-γ and IL-4 and to be important for the induction of experimental autoimmune encephalitis (29). Our results suggest that impaired CD4+ T cell production of IFN-γ in the absence of T-bet is sufficient to enable the induction of TH17 cells in the setting of solid organ transplantation. However, it is also plausible that other CD4+ T cell subsets, such as regulatory T cells, are insufficient in numbers and/or impaired in the absence of T-bet. Regulatory T cells have been previously shown to be low in a T-bet–deficient mouse model of pulmonary graft-versus-host disease (25). However, in models of autoimmune colitis, regulatory T cells from T-bet−/− mice have been shown to have equal or superior suppressive function in vivo (30, 31). Thus, questions remain regarding the role of low CD4+ T cell numbers and function in lung allograft rejection under conditions of T-bet deficiency.
Several lines of evidence suggest IL-17 plays an important role in BOS in human LTRs. Using a trans vivo delayed type hypersensitivity assay to measure foot pad swelling after injection of collagen V and peripheral blood mononuclear cells from LTRs, Burlingham and colleagues showed that blockade of IL-17 and TNF-α, along with depletion of CD4+ or CD14+ monocytes, significantly reduced collagen V–specific trans vivo delayed type hypersensitivity assay responses and that increased responses significantly correlated with BOS (32). A subsequent cross-sectional study of bronchoalveolar lavage (BAL) cell pellets and supernatants showed increased levels of IL-17/IL-23 mRNA along with increased IL-8 mRNA and protein in samples from LTRs with BOS (33). A recent study in the mouse orthotopic lung transplant model demonstrated that neutralization of IL-17 prevented the development of OB lesions that occurred in ∼ 50% of mice undergoing a partially MHC-mismatched transplant (C57BL/10 → C57BL/6), although this study did not address the cellular source(s) of IL-17 (8). In the current study, our findings show that CD8+ > CD4+ T cells are sources of IL-17 and play an important role in inflammatory intraluminal airway lesions that develop in the setting of acute rejection in a complete MHC mismatch. Although we show IL-17 responses in T cells, it is also possible that other cell populations play a role in IL-17–mediated injury in this model, as previously demonstrated in ischemic-reperfusion injury (34). A recent study in LTRs showed IL-17 involvement in lymphocytic bronchiolitis lesions in LTRs, with CD8+ T cells identified as a major source of IL-17 on immunohistochemical staining (35). Together, these studies provide broad lines of evidence suggesting an important role for IL-17 in lung allograft rejection and potentially obliterative airway disease.
We have demonstrated these robust, allospecific IL-17 responses in genetically manipulated T-bet−/− mice. However, the effect of pharmacologic immunosuppression on T-bet expression in WT mouse recipients and in human recipients of lung transplantation is unknown. Therefore, this molecular state could be relevant to the clinical field of lung transplant. In fact, a recent study showed the development of OB lesions in mouse recipients of orthotopic lung transplant that were treated with calcineurin inhibitors and steroids, whereas these lesions have not been previously described in the untreated, fully MHC-mismatched mouse model (9), suggesting a possible role of immunosuppression effects in the development of these lesions. Moreover, we believe that understanding the downstream, pathologic effects of the CD8+ IL-17+ responses we show is essential given the above evidence linking IL-17 to lung allograft rejection and potentially OB.
Our studies also reveal an unexpected persistence of significant intraluminal airway inflammation under conditions of anti-CD154 Ab therapy despite significantly limited allospecific IFN-γ production from allografts infiltrating CD8+ T cells in T-bet−/− recipients. This therapy effectively established a state of “unopposed IL-17” in response to alloantigen. Although we cannot rule out concomitant responses to autoantigens, we show that this skewing of the alloimmune response toward an IL-17–predominant response was associated with persistent airway neutrophilia and airway luminal fibrosis in some lesions. IL-17 has been implicated in other models of fibrosis, and bleomycin-induced pulmonary fibrosis has recently been demonstrated to be dependent on IL-17A (36). The mechanisms by which IL-17 leads to fibrosis will require more investigation, but they are most likely multifold and involve many cell types.
The potential role of neutrophils in the pathogenesis of murine obliterative airways disease also requires further study because several earlier studies in lung transplant recipients have shown BAL neutrophilia to be correlated with BOS (37–39). Here, we show increased allograft neutrophilia in T-bet–deficient recipients of lung transplant that is abolished with anti-CD154 and anti–IL-17 therapy, suggesting that this effect is mediated by CD8+ IL-17 responses. Although we do see a nonsignificant decrease in allograft neutrophil counts in T-bet–deficient recipients treated with anti-CD154 therapy alone, there are studies showing an additive effect of IFN-γ on neutrophil recruitment mediated by IL-17 and other pathways (40, 41). We also demonstrate in vitro CXCL1 induction from mouse lung epithelial cells in response to IL-17, as recently shown by another group (24), which provides a plausible in vivo mechanism for neutrophil recruitment during rejection and diminution in the setting of IL-17 neutralization. Moreover, we have detected increased levels of CXCL1 in T-bet−/− recipients of lung transplant, which are characterized by significant IL-17 effector responses, thereby linking the presence of IL-17 to CXCL1, a potent neutrophil chemokine, in this particular model. Although there are likely other mechanisms by which IL-17 effects neutrophilic inflammation, we have identified the induction of CXCL1 as a pathway active in this model. The effects of IL-17 on lung epithelial cells during allograft rejection have not been fully described but may provide insight into the pathogenesis of OB. Further studies are needed to identify the cellular source of CXCL1 in this model of lung allograft rejection and to elucidate the cellular pathways involved linking IL-17 to CXCL1 and neutrophilia.
In addition to the presence of IL-17 in lung allografts, limited amounts of intragraft IFN-γ may significantly contribute to the severe rejection pathology and obliterative airway inflammation observed in T-bet−/− recipients. Indeed, earlier studies in several experimental systems have demonstrated a critical role for IFN-γ in the establishment of allograft tolerance under conditions of costimulation blockade (10, 11). More recently, Coley and colleagues showed that not only were IFN-γR1−/− mice protected from breakthrough CD8+ alloeffector cells associated with graft rejection but that neutralization of IFN-γ in these protected mice resulted in allograft loss, indicating that IFN-γ signaling in the graft itself was required for acceptance (12). Specifically, the lack of CD4+ T cell–derived IFN-γ in rejecting T-bet−/− recipients may be integral to their resistance to anti-CD154 therapy, as an earlier study found CD4+ T cells and IFN-γ to be independent factors required for costimulation blockade–induced tolerance (42).
There are several caveats to our studies. Although our studies demonstrate the development of airway inflammatory lesions in T-bet−/− allograft recipients that bear some similarities to human OB pathology, one should exercise caution in extrapolating these findings to human OB. For example, the preservation of the airway epithelia in some of the lesions we show appears to differ from human OB histology. Another notable difference is the rapid kinetics of the development of the airway pathology we show in mouse orthotopic lung transplant, in contrast to the usual tempo observed in LTRs, although accelerated BOS is known to occur (2). It remains to be seen how the airway lesions we show would progress with more time. Nonetheless, our findings provide plausible cellular and molecular mechanisms that corroborate previous human studies of BOS in LTRs.
We observed incomplete resolution of lung allograft rejection despite IL-17 blockade, suggesting that other cytokines and/or cells contribute to pathology in T-bet−/− mice. Another possibility contributing to some persistent pathology is incomplete neutralization of IL-17, despite our frequent dosing schedule. In addition, although we did not examine the function of innate cells in this study, it has become clear that T-bet plays a critical role in innate immunity and the interaction of innate cells with cells of the adaptive immune system (43). It is therefore possible that the absence of T-bet in innate cells in our model contributes in a meaningful way to the allograft rejection pathology and T cell effector responses we see in these T-bet−/− recipients. Finally, in our studies we did not treat allograft recipients with conventional immunosuppression to evaluate the effects of more targeted immunomodulation on allograft rejection and allo-specific responses; however, a recent study using this approach raises interesting and unanswered questions regarding the role of these therapies in obliterative airways disease development (9).
In summary, our studies provide new insights into the role of differential cytokine production and lung allograft rejection, identifying CD8+ T cells as a predominant source of allo-specific IL-17 production, but also CD4+ T cells, in the absence of T-bet. Moreover, neutrophilic inflammation was significantly increased in T-bet−/− recipient lung allografts and observed in inflammatory airway lesions. We report that after mouse orthotopic lung transplantation in T-bet−/− mice, the addition of IL-17 neutralization therapy to anti-CD154 therapy is effective in attenuating neutrophil-predominant allograft rejection pathology and obliterative airway inflammation and that IL-17 directly enhances CXCL1 production from lung epithelia in vitro and is associated with markedly increased CXCL1 levels in our model. These results suggest a potential role for targeting the development or activity of IL-17 as a therapy in lung transplantation, although these approaches would have to be carefully considered in balance with potential host defense implications. Finally, our results support using T-bet–deficient mice in mouse orthotopic lung transplantation to further study the pathogenesis of murine airway inflammation and injury after lung transplantation.
Footnotes
This work was supported in part by National Institutes of Health grant AI079175 (J.F.M.).
Author Contributions: Concept and design: E.A.L., J.M.D., C.P.O.'D., and J.F.M. Data acquisition: E.A.L., J.M.D., T.A.C., H.L.M., S.G., I.P., N.C., M.L., N.M.W., J.Z., and Y.Z. Analysis and interpretation: E.A.L., J.M.D., T.A.C., J.Z., Y.Z., and J.F.M. Manuscript preparation and editing: E.A.L. and J.F.M.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0059OC on October 6, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The registry of the international society for heart and lung transplantation: twenty-seventh official adult lung and heart-lung transplant report–2010. J Heart Lung Transplant. 2010;29:1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., III Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7:1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, Richardson SB, Lai J, Garbow JR, Patterson GA, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007;37:625–630. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okazaki M, Sugimoto S, Lai J, Kornfeld CG, Hotchkiss RS, Richardson SB, Li W, Kreisel FH, Huang HJ, Patterson GA, et al. Costimulatory blockade-mediated lung allograft acceptance is abrogated by overexpression of bcl-2 in the recipient. Transplant Proc. 2009;41:385–387. doi: 10.1016/j.transproceed.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 6.Dodd-o JM, Lendermon EA, Miller HL, Zhong Q, John ER, Jungraithmayr WM, D'Alessio FR, McDyer JF. Cd154 blockade abrogates allospecific responses and enhances cd4(+) regulatory t-cells in mouse orthotopic lung transplant. Am J Transplant. 2011;11:1815–1824. doi: 10.1111/j.1600-6143.2011.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, Sugimoto S, Tietjens JR, Patterson GA, Krupnick AS, et al. Cd4+ t lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180:4754–4762. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, et al. Neutralizing il-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vleeschauwer S, Jungraithmayr W, Wauters S, Willems S, Rinaldi M, Vaneylen A, Verleden S, Willems-Widyastuti A, Bracke K, Brusselle G, et al. Chronic rejection pathology after orthotopic lung transplantation in mice: the development of a murine bos model and its drawbacks. PLoS ONE. 2012;7:e29802. doi: 10.1371/journal.pone.0029802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. Ifn-gamma is critical for long-term allograft survival induced by blocking the cd28 and cd40 ligand t cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 11.Hassan AT, Dai Z, Konieczny BT, Ring GH, Baddoura FK, Abou-Dahab LH, El-Sayed AA, Lakkis FG. Regulation of alloantigen-mediated t-cell proliferation by endogenous interferon-gamma: implications for long-term allograft acceptance. Transplantation. 1999;68:124–129. doi: 10.1097/00007890-199907150-00023. [DOI] [PubMed] [Google Scholar]
- 12.Coley SM, Ford ML, Hanna SC, Wagener ME, Kirk AD, Larsen CP. Ifn-gamma dictates allograft fate via opposing effects on the graft and on recipient cd8 t cell responses. J Immunol. 2009;182:225–233. doi: 10.4049/jimmunol.182.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, t-bet, directs th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 14.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of t-bet in th1 lineage commitment and ifn-gamma production in cd4 and cd8 t cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 15.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses t(h)17 differentiation by preventing runx1-mediated activation of the gene encoding rorgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, et al. A novel role of cd4 th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrell BE, Csencsits K, Lu G, Grabauskiene S, Bishop DK. Cd8+ th17 mediate costimulation blockade-resistant allograft rejection in t-bet-deficient mice. J Immunol. 2008;181:3906–3914. doi: 10.4049/jimmunol.181.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jungraithmayr WM, Korom S, Hillinger S, Weder W. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg. 2009;137:486–491. doi: 10.1016/j.jtcvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 20.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 22.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. Il-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: Il-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 23.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am J Respir Cell Mol Biol. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AK, Mulloy DP, Le LT, Laubach VE. Nadph oxidase mediates synergistic effects of il-17 and tnf-alpha on cxcl1 expression by epithelial cells after lung ischemia-reperfusion. Am J Physiol Lung Cell Mol Physiol. 2014;306:L69–L79. doi: 10.1152/ajplung.00205.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gowdy KM, Nugent JL, Martinu T, Potts E, Snyder LD, Foster WM, Palmer SM. Protective role of t-bet and th1 cytokines in pulmonary graft-versus-host disease and peribronchiolar fibrosis. Am J Respir Cell Mol Biol. 2012;46:249–256. doi: 10.1165/rcmb.2011-0131OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juedes AE, Rodrigo E, Togher L, Glimcher LH, von Herrath MG. T-bet controls autoaggressive cd8 lymphocyte responses in type 1 diabetes. J Exp Med. 2004;199:1153–1162. doi: 10.1084/jem.20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, et al. Role of t-bet in commitment of th1 cells before il-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 29.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of cd4 t cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by t-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor t-bet regulates mucosal t cell activation in experimental colitis and crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. Il-17-dependent cellular immunity to collagen type v predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, Wuyts WA, Van Raemdonck DE, Dupont LJ, Verleden GM. The role of the il23/il17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer t cell-derived il-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verleden SE, Vos R, Vandermeulen E, Ruttens D, Vaneylen A, Dupont LJ, Verbeken EK, Verleden GM, Van Raemdonck DE, Vanaudenaerde BM. Involvement of interleukin-17 during lymphocytic bronchiolitis in lung transplant patients. J Heart Lung Transplant. 2013;32:447–453. doi: 10.1016/j.healun.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and il-1beta-mediated pulmonary fibrosis is il-17a dependent. J Exp Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng L, Walters EH, Ward C, Wang N, Orsida B, Whitford H, Williams TJ, Kotsimbos T, Snell GI. Airway neutrophilia in stable and bronchiolitis obliterans syndrome patients following lung transplantation. Thorax. 2000;55:53–59. doi: 10.1136/thorax.55.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neurohr C, Huppmann P, Samweber B, Leuschner S, Zimmermann G, Leuchte H, Baumgartner R, Hatz R, Frey L, Ueberfuhr P, et al. Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant. 2009;28:468–474. doi: 10.1016/j.healun.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 39.DiGiovine B, Lynch JP, III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of il-8. J Immunol. 1996;157:4194–4202. [PubMed] [Google Scholar]
- 40.Kawaguchi M, Onuchic LF, Li XD, Essayan DM, Schroeder J, Xiao HQ, Liu MC, Krishnaswamy G, Germino G, Huang SK. Identification of a novel cytokine, ml-1, and its expression in subjects with asthma. J Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 41.Bonville CA, Percopo CM, Dyer KD, Gao J, Prussin C, Foster B, Rosenberg HF, Domachowske JB. Interferon-gamma coordinates ccl3-mediated neutrophil recruitment in vivo. BMC Immunol. 2009;10:14. doi: 10.1186/1471-2172-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-cd154 antibody in thymectomized mice requires cd4(+) t cells, interferon-gamma, and ctla4. J Clin Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]