Abstract
Three billion people are exposed to household air pollution from biomass fuel use. Exposure is associated with higher incidence of pneumonia, and possibly tuberculosis. Understanding mechanisms underlying these defects would improve preventive strategies. We used human alveolar macrophages obtained from healthy Malawian adults exposed naturally to household air pollution and compared them with human monocyte-derived macrophages exposed in vitro to respirable-sized particulates. Cellular inflammatory response was assessed by IL-6 and IL-8 production in response to particulate challenge; phagosomal function was tested by uptake and oxidation of fluorescence-labeled beads; ingestion and killing of Streptococcus pneumoniae and Mycobacterium tuberculosis were measured by microscopy and quantitative culture. Particulate ingestion was quantified by digital image analysis. We were able to reproduce the carbon loading of naturally exposed alveolar macrophages by in vitro exposure of monocyte-derived macrophages. Fine carbon black induced IL-8 release from monocyte-derived and alveolar macrophages (P < 0.05) with similar magnitude responses (log10 increases of 0.93 [SEM = 0.2] versus 0.74 [SEM = 0.19], respectively). Phagocytosis of pneumococci and mycobacteria was impaired with higher particulate loading. High particulate loading corresponded with a lower oxidative burst capacity (P = 0.0015). There was no overall effect on killing of M. tuberculosis. Alveolar macrophage function is altered by particulate loading. Our macrophage model is comparable morphologically to the in vivo uptake of particulates. Wood smoke–exposed cells demonstrate reduced phagocytosis, but unaffected mycobacterial killing, suggesting defects related to chronic wood smoke inhalation limited to specific innate immune functions.
Keywords: indoor air pollution, alveolar macrophages, innate immunity, Streptococcus pneumoniae, Mycobacterium tuberculosis
Clinical Relevance
Half of the world’s population is exposed daily to biomass fuel smoke, and this exposure is associated with increased risk of pneumonia, particularly in children. Smoke exposure is also associated with chronic lung disease and cardiovascular death. The mechanisms underlying smoke-related susceptibility to pulmonary infection and chronic disease are not known, but such knowledge would be invaluable in improving preventive measures. The uptake of particulate smoke by lung cells is strongly dose and time dependent, making intracellular particulate load a useful biomarker of exposure. Particulates from several sources cause a common dose-dependent inflammatory response in lung cells and macrophages. Particulates cause inhibition of phagocytosis and impairment of oxidative burst that is consistent with impaired defense against infection.
Household air pollution (HAP) related to biomass fuel use is a major risk factor for poor health (1–3), particularly respiratory infection in adults and children (4, 5). In low- and middle-income countries, where bacterial pneumonia is the biggest cause of infant mortality (6, 7), the odds ratio of pneumonia in HAP-exposed children is 1.78 compared with unexposed individuals, equating to 1 million excess childhood deaths per year (8). Rates of severe pneumonia in children can be reduced by interventions that reduce wood smoke exposure through the use of “clean” cook stoves with improved emissions characteristics (9).
In countries where biomass is the predominant form of household energy, the most common reason for acute admission to hospital is pneumonia (10, 11). The etiology in over one-half of cases is Streptococcus pneumoniae (12). Populations with high biomass fuel use also have a high incidence of tuberculosis (TB) (13). The prevalence of TB is increased in individuals exposed to household smoke (14–16), but these case–control studies are limited by confounding factors (17, 18). Despite epidemiological insights, the mechanism by which HAP might impair lung defense against infection are unclear.
As the main pulmonary phagocyte, human alveolar macrophages (HAMs), ingest inhaled smoke particulates (19). HAMs from people exposed to biomass smoke are heavily loaded with carbonaceous particulates; uptake varies according to fuel usage (20, 21). The extent of macrophage carbon loading can act as a biomarker of exposure, and may be determined from bronchoalveolar lavage (BAL) or sputum specimens (20, 22).
Acute smoke exposure has been studied in firefighters, where inhalation of wood smoke is characterized by proinflammatory cytokine production (23, 24). In wood smoke acute exposure models, dampening of macrophage activation in response to bacterial challenge is noted (25). Chronic exposure is likely to generate different responses (26). It is likely that the interactions between particulate matter (PM) and the HAMs in part define the observed increased susceptibility to infection (27).
We exposed alveolar and monocyte-derived macrophages (MDMs) to respirable-sized smoke particulates to assess: (1) the reliability of the MDM model when compared with HAM responses; (2) the functional effects of particulate on HAMs, including the effect on cytokine production, phagocytosis, oxidative burst, and pathogen killing; and (3) the relative effects of different wood and particle types.
Materials and Methods
Methods are described in detail in the online supplement.
Cell Culture
BAL fluid was obtained from healthy volunteers (28), and HAMs used at Days 1–3. Monocytes were obtained from Buffy coats by density gradient centrifugation (Lymphoprep, Axis-Shield; Manchester, UK) before enrichment using with CD14 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturers’ protocols. Cells were used at 7–10 days of culture on borosilicate glass. Nonadherent cells were removed by gentle washing. All cells were cultured at 37°C in 5% CO2.
PM Challenge
Fine carbon black (FCB; Haeffner, Chepstow, UK) was prepared by repeated sonication and vortexing in saline. Wood smoke particles were obtained by controlled combustion (see Materials and Methods in the online supplement). Cells were cultured for 5 hours in sonicated particulate suspensions. Culture medium was stored at −80°C for cytokine ELISA (BD OptEIA; Becton-Dickinson, East Rutherford, NJ).
Exposure time and doses were optimized such that in vitro experiments approximated the microscopic appearance of samples obtained from HAP-exposed Malawian participants (20) (Figure 1). At least 100 Fields B–stained microscope fields were analyzed using digital image analysis (Image SXM; Dr. S. Barrett, Liverpool, UK, http://www.liv.ac.uk/∼sdb/ImageSXM/), as previously described (20). Briefly, cytoplasm was identified by automated threshold analysis of Fields B stain (red coloration). Particulate was similarly quantified by its lack of staining and low light transmission. After analysis, images were excluded if visual inspection revealed debris or artifact.
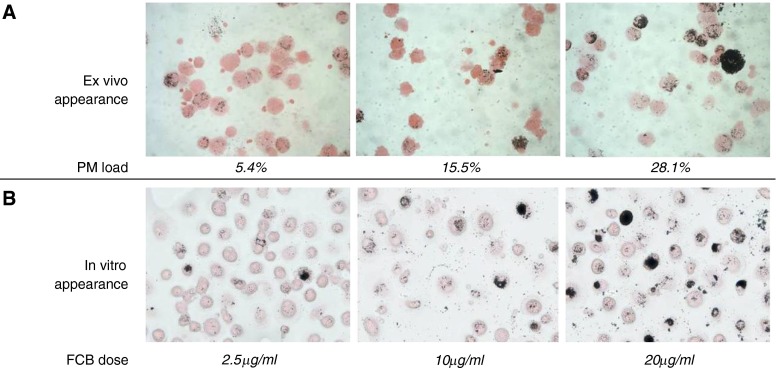
Figure 1.
In vivo and ex vivo exposure of human alveolar macrophages (HAMs) to particulate household air pollutants results in similar cytoplasmic particulate loading. HAMs from three Malawian volunteers domestically exposed to low, moderate, or high concentrations of indoor air pollution are shown in (A) (left, middle, and right panels, respectively). The panel shows sample images taken from bronchoalveolar lavage cytospins stained with eosin, with PM appearing as black deposits that are heterogeneously distributed among the cells. The percentage of cytoplasmic load of PM calculated by Image SXM software is indicated above each image. (B) HAMs from healthy volunteers from the United Kingdom who had no prior exposure to wood smoke or severe outdoor air pollution. In the images shown, macrophages have been exposed ex vivo to fine carbon black (FCB) to match the particulate loading seen in naturally exposed Malawian subjects. The dose of particulate material added in suspension to adherent macrophages in culture to match the appearance in (A) is shown for each image. The heterogeneity of natural exposure is reproduced in this experimental design. PM, particulate matter.
FITC-Labeled Bead Challenge
Macrophages were incubated with particulate suspension, washed, and incubated for 2 hours with FITC-labeled 2-μm silica beads (ratio of 5:1 macrophage; FLUKA, Neu-Ulm, Germany), washed, and counted by fluorescence microscopy.
Bacterial Challenge
Heat killed S. pneumoniae serotype 1 was incubated with carboxyfluorescein-succinimidyl ester for 30 minutes in the dark, washed, opsonized with 20% human serum, and diluted to 4 × 107 CFU/ml, as assessed by dilution plating (29).
Particulate-loaded macrophages were exposed to labeled bacteria at a ratio of 1:10 for 16 hours. After washing and Fields staining, a minimum of 200 cells per group was assessed for bacterial association at 40× magnification.
Mycobacterium tuberculosis (H37Rv) was diluted to 2.5 × 106 CFU/ml (30). Macrophages were incubated with particulate for 8 hours, washed, then challenged with mycobacterial suspension at an estimated ratio of 10 bacteria per macrophage. After 16 hours, phagocytosis was assessed by auramine staining. At least 200 macrophages were counted for bacterial internalization. Unstained cells were lysed at 16 hours or 7 days. Semiquantitative cultures of supernatants and cell lysates were performed (Mycobacteria Growth Indicator Tube [MGIT]; Becton-Dickinson), and positivity checked by fluorescence microscopy (31).
Macrophage Function Reporter Bead Assays
Intraphagosomal oxidative burst was measured as detailed previously (32). Briefly, silica beads coupled to IgG to facilitate Fc receptor–mediated uptake were labeled with oxidation-sensitive dichlorodihydrofluorescein-diacetate-succidimyl ester and Alexa 633-SE (Invitrogen, Carlsbad, CA). Beads were incubated with alveolar macrophages for 60 minutes and fixed in 4% paraformaldehyde. Flow cytometry quantified the oxidation-dependent increase in fluorescein emission compared with calibrator (“activity index”; FACSCalibur [Becton-Dickinson]; FlowJo [Tree Star, Ashland, OR]).
Statistical Analysis
Cytokine release, oxidative burst, and internalization by particulate exposure category were compared by one-way ANOVA with Tukey test (P < 0.05). HAM and MDM particulates were compared using Student’s t test. Bacillary numbers and mean time to MGIT positivity (nonparametric) were compared using Kruskal-Wallis tests. GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) was used for analysis.
Results
Macrophage Uptake of PM
A total of 82 healthy adults from Malawi (median age, 33 yr; range, 21–67 yr) and 12 from Liverpool (median age, 26 yr; range, 18–65 yr) underwent bronchoscopy with no serious adverse events. All Malawi volunteers reported HAP exposure. Ex vivo, HAMs demonstrated considerable heterogeneity with respect to the density of particulate loading (Figure 1A). We reproduced this feature in both HAMs and MDMs from healthy adult volunteers (Liverpool, UK) by in vitro exposure to sonicated particulate suspensions (Figure 1B). Cytoplasmic area occupied by particulates in ex vivo HAMs from Malawi ranged from 0.1 to 28.1% (median, 1.3%).
Dose- and time-dependent responses to particulates in human alveolar and MAMs.
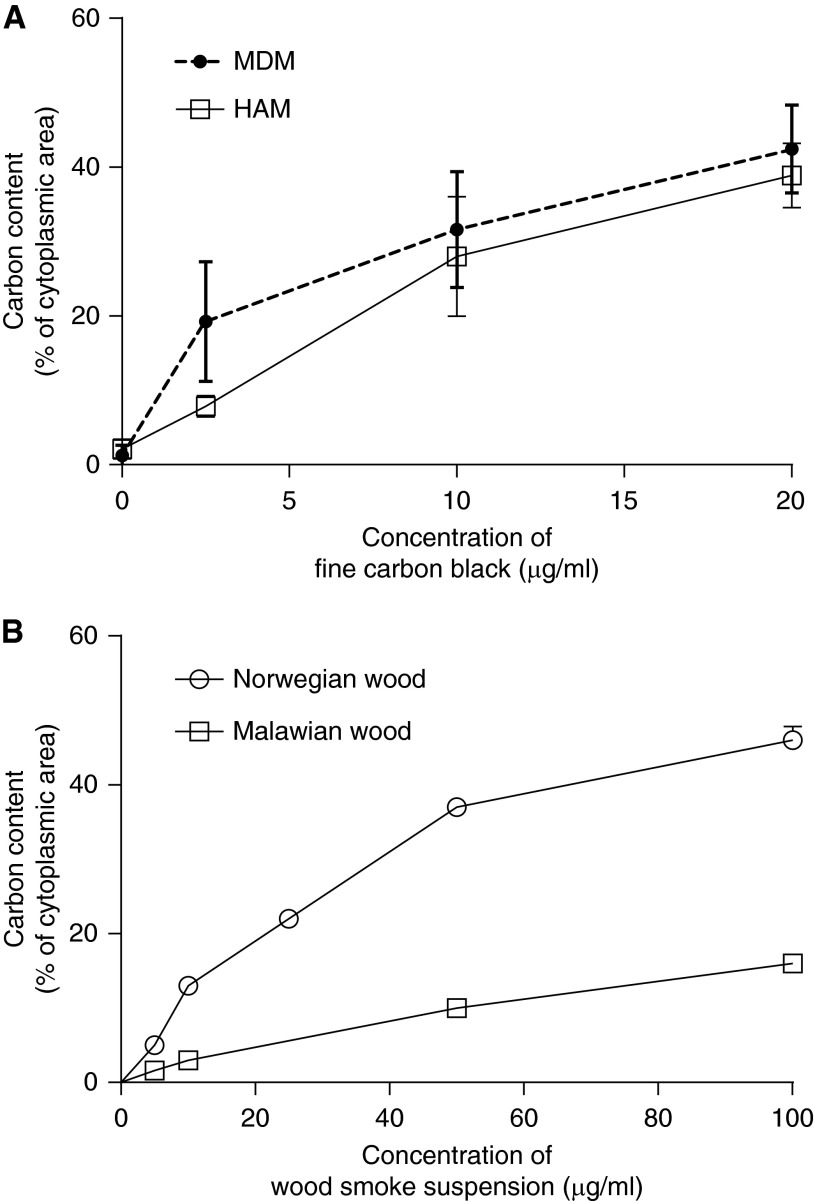
HAMs were isolated from five volunteers. Dose-dependent uptake of particulate was demonstrated in both cell types. The median particulate load in Liverpool HAMs challenged in vitro for 5 hours with 2.5 μg/ml to 20 μg/ml of FCB was 7.25% (range, 7–10%) and 37.8% (range, 35–45%), respectively; for MDMs, 19% (range, 11–28%) and 43% (range, 35–49%). There was no significant difference between particulate uptake by HAMs and MDMs (P = 0.794; Figure 2).
Figure 2.
Similar dose-dependent response after exposure of HAMs and monocyte-derived macrophages (MDMs) to FCB. Macrophage appearance varies by type of wood smoke particulate applied. (A) The percentage of cytoplasmic area occupied by PM by dose of FCB added in suspension to human macrophages. HAMs and MDMs show a similar dose dependency (n = 5 in each experiment). (B) The study design repeated to compare wood smoke from Norwegian birch trees with that obtained from Malawi mopane woodland. The dose-dependent response is maintained, but mopane wood produces a less opaque cytoplasmic appearance compared with birch. Error bars represent mean with 95% confidence interval (CI).
The time-dependent uptake of FCB by MDMs is shown in Figure E1 in the online supplement. The PM load scores obtained after the addition of 20 μg/ml FCB showed an initial exponential increase from 1.95% at 40 minutes to 43.4% at 5 hours. The time–carbon burden relationship appeared to plateau between 5 and 24 hours.
The dose-dependent responses to smoke particulates derived from Malawian cooking wood, particulates from Norwegian birch wood, and FCB were different in HAMs and MDMs. Equivalent exposures generated greater cytoplasmic particulate load with Norwegian wood compared with Malawi wood or FCB. HAM data are shown in Figure 2B.
Inflammatory Cytokine Response to Particulate Challenge in HAMs and MDMs
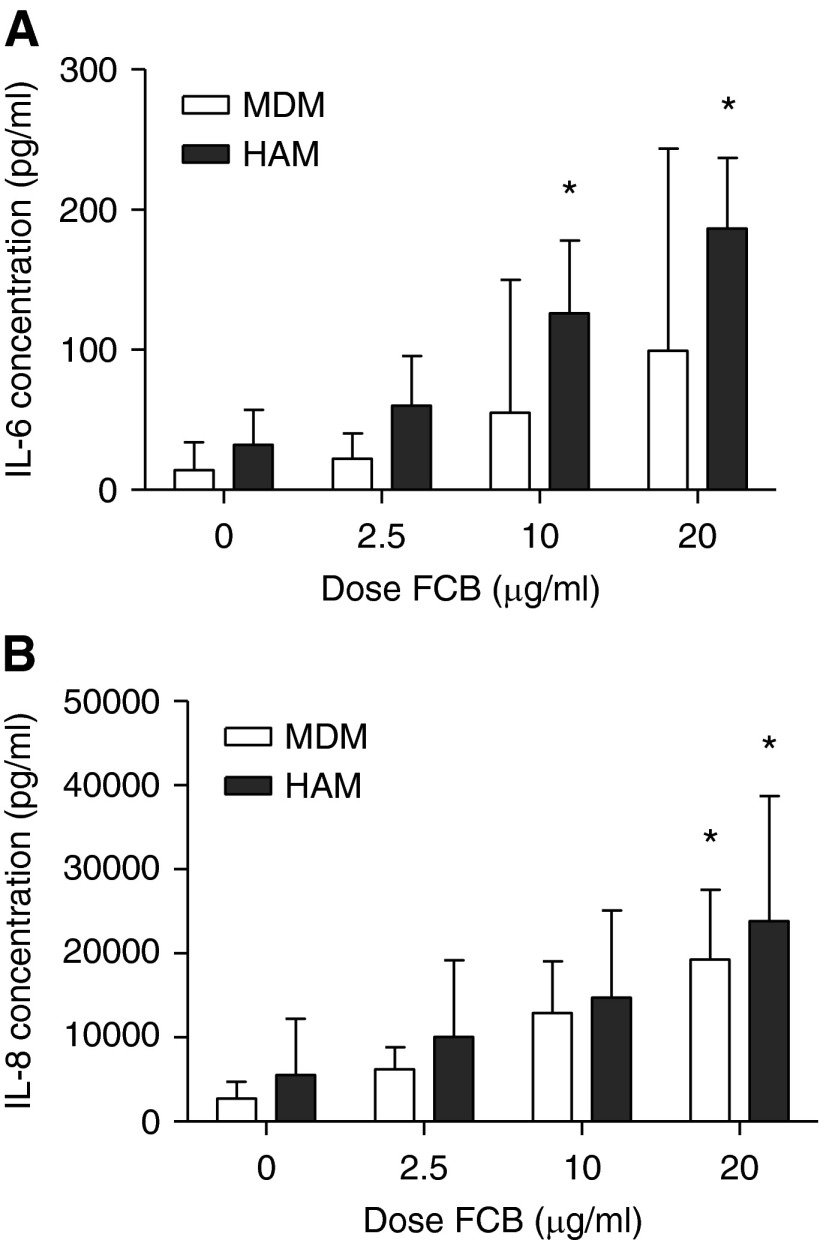
A dose-dependent increase in both IL-6 and IL-8 was seen using 5-hour FCB challenge with both HAMs and MDMs from healthy UK volunteers (Figure 3). Baseline cytokine production was not significantly different in HAMs and MDMs for IL-6 and IL-8 (P = 0.23 and P = 0.45, respectively). There were significant increases in IL-8 production between baseline and 20 μg/ml FCB challenge for MDMs (P < 0.05) and HAMs (P < 0.05). The magnitudes of response were similar, with log10 increases of 0.93 (SEM = 0.21) and 0.74 (SEM = 0.19), respectively, being observed.
Figure 3.
Particulate exposures result in dose-dependent cytokine release from both HAMs and MDMs. FCB added to either HAMs or MDMs (n = 5 in each experiment) resulted in dose-dependent increases in the concentration of IL-6 (A) and IL-8 (B) at 5 hours of incubation. There was a higher concentration of both cytokines produced by HAMs compared with MDMs at all doses. Error bars represent 95% CI. *P < 0.05 compared with no particulate challenge by one-way ANOVA with Tukey’s test.
Reduced Association of both IgG-Coated Beads and S. pneumoniae in Particulate-Loaded MDMs
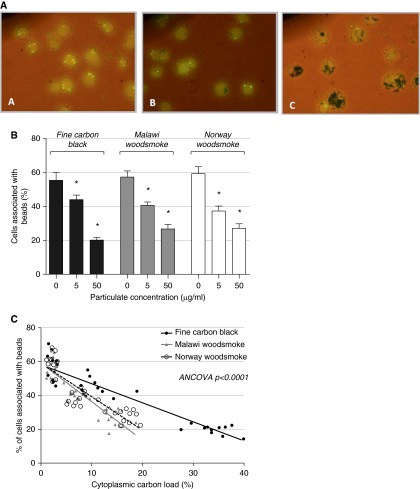
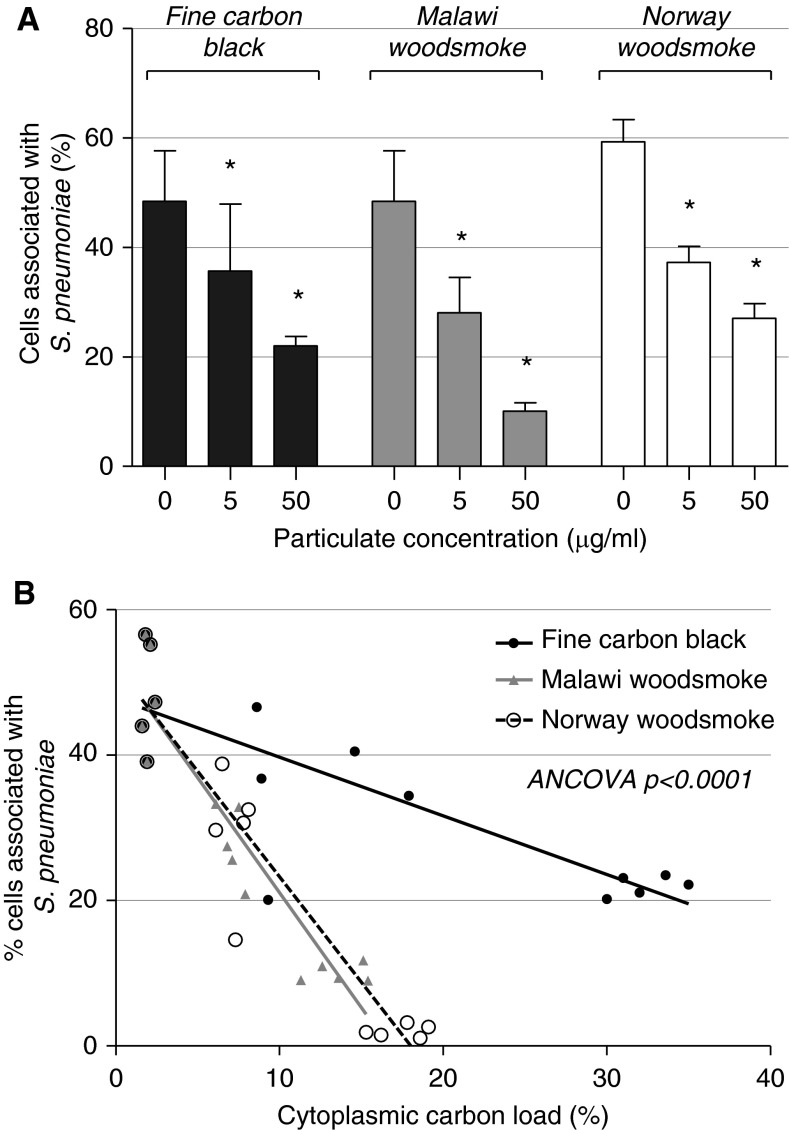
Fluorescent bead ingestion by particulate-loaded MDMs was reduced in a dose-dependent manner (Figures 4A and 4B); 57% (SD = 6.2) of control cells were associated with beads. At 5 μg/ml, the percentage of cells associated with beads was reduced to 43% (SD = 3.50; P = 0.002) with FCB, 40% (SD = 3.02; P < 0.001) with Malawian wood, and 37% (SD = 4.38; P < 0.001) with Norwegian wood.
Figure 4.
Particulate exposure results in a dose-dependent reduction in fluorescent bead association by MDMs. MDMs were exposed to FCB, Malawi wood smoke particulates (MW), or Norwegian wood smoke particulates (NW). Phagocytosis was assessed by association or uptake of fluorescent beads. (A) Representative images obtained by fluorescence microscopy. Phagocytosis rates are shown in (B). Error bars indicate 95% CI. *P < 0.01 compared with no particulate challenge by one-way ANOVA with Tukey’s test. (C) The association between cytoplasmic particulate density and bead association, with linear model fitting for each particulate type. The model gradients are different by analysis of covariance (ANCOVA) testing (P < 0.001).
Within each condition, the macrophage particulate load varied by individual. We therefore examined the relationship between macrophage particulate content and bead uptake (see Figure 4C). With linear regression models, there was a negative correlation between particulate content and inferred phagocytosis rate, and the magnitude of the effect varied by particulate type. Malawi and Norwegian wood smoke treatments had a larger effect on bead association than FCB according to this measure (analysis of covariance P < 0.001).
Similar results were seen with S. pneumoniae (Figure 5A): the percentage of MDMs associated with carboxyfluorescein-succinimidyl ester–labeled S. pneumoniae was dependent on the dose of FCB and Malawian and Norwegian wood smoke; 48% (SD = 7.43%) of control cells were associated with S. pneumoniae. After treatment with 5 μg/ml PM, this proportion was reduced by FCB to 34% (SD = 5.86%; P = 0.047), by Malawian wood smoke to 27% (SD = 5.20%; P = 0.009), and by Norwegian wood smoke to 27% (SD = 4.08%; P = 0.006). At 50 μg/ml, bead association in macrophages was further reduced. Malawian wood had the strongest inhibitory effect, which was significant compared with FCB (P < 0.001; Figure 5B).
Figure 5.
Particulate exposure results in a dose-dependent reduction in the phagocytosis of fluorescent Streptococcus pneumoniae (S. pneumoniae) by MDMs. MDMs were exposed to increasing doses of FCB, MW, or NW. MDMs that had been pretreated with particulates for 8 hours were washed and carboxyfluorescein succinimidyl ester–labeled pneumococci (opsonized serotype 1) presented at a ratio of 10 bacteria:1 cell for 2 hours. (A) Comparison of the percentage of cells associated with pneumococci in each condition. Error bars indicate 95% CI. *P < 0.01 compared with no particulate challenge by one-way ANOVA with Tukey’s test. (B) The association between cytoplasmic particulate density and bead association, with linear model fitting for each particulate type. The model gradients are different by ANCOVA testing (P < 0.001): Malawi wood shows the most marked dose-dependent effect, followed by Norwegian wood, followed by FCB.
MDM Phagocytosis and Killing of M. tuberculosis
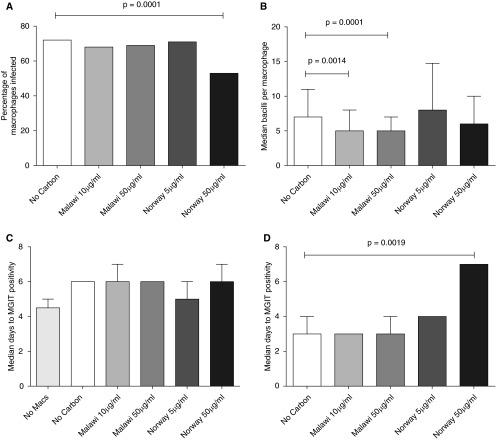
Pre-exposure of MDMs to 50 μg/ml Norwegian wood smoke was associated with a significant decrease in the proportion of M. tuberculosis–infected MDMs compared with particulate-unexposed control subjects (53 and 72%, respectively; P = 0.0001; Figure 6A).
Figure 6.
Particulate wood smoke exposure alters phagocytosis, but not killing, of Mycobacterium tuberculosis by MDMs. A single batch of MDMs was exposed to Malawi wood smoke (Malawi) or Norwegian wood smoke (Norway), and then challenged with M. tuberculosis. Phagocytosis was assessed at 16 hours by auramine staining. Both culture supernatant (at Day 1) and cell lysate (at Day 7) underwent liquid culture. Phagocytosis: the proportion of macrophages (Macs) with associated M. tuberculosis ([A] minimum 200 macrophages counted per condition) and the number of mycobacteria in each macrophage ([B] minimum 100 macrophages counted per condition) are shown. Killing: median time to culture positivity is shown for supernatants at Day 1 (C) (n = 7 replicate wells) and cell lysates at Day 7 (D) (n = 9 and n = 4 replicate wells for Malawi and Norwegian wood, respectively). Error bars represent interquartile ranges. Statistical tests are nonparametric: Kruskal-Wallis (A and D) and Wilcoxon tests (B). MGIT, Mycobacteria Growth Indicator Tube.
Pre-exposure to Malawian wood smoke at two different concentrations resulted in no difference in the proportion of M. tuberculosis–infected MDMs, but was associated with a significantly reduced median number of mycobacteria seen per cell compared with control MDMs (five bacilli/cell and seven bacilli/cell, respectively; P < 0.0001). This was not observed in MDMs exposed to Norwegian wood smoke (Figure 6B).
There were no significant differences between groups in 16-hour supernatant mycobacterial load (MGIT days to culture positivity; Figure 6C), and no significant differences in 16-hour cell lysate quantitative culture (data not shown). There were also no differences between quantitative MGIT culture of Day 7 lysates from TB-infected MDMs exposed to Malawian wood smoke, or the lower concentration of Norwegian wood smoke (Figure 6D). Exposure to the higher concentration of Norwegian wood smoke (50 μg/ml) was associated with significantly increased median days to MGIT positivity for Day-7 lysates (7 d compared with 3 d for controls; P < 0.05), implying a reduced mycobacterial load (Figure 6D). This may be due to reduced number of viable MDMs in cell culture.
Reporter Bead Oxidative Burst Results
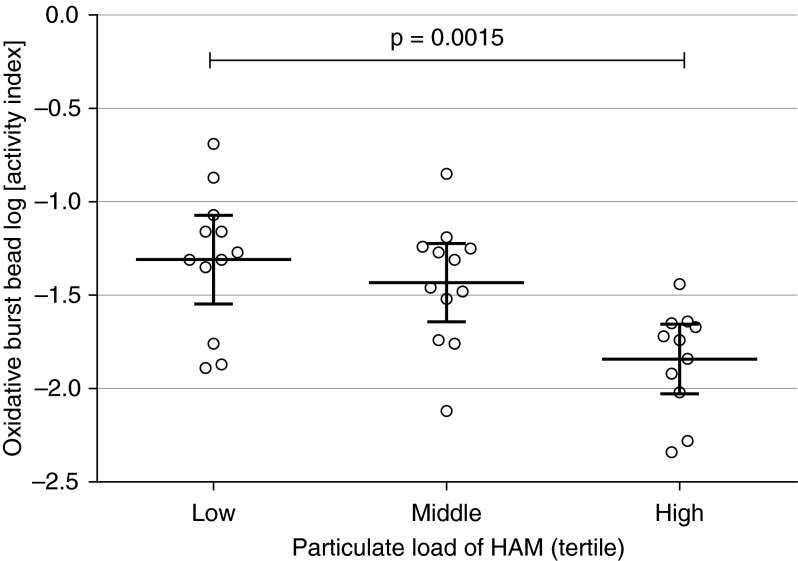
BAL samples from 38 Malawian participants naturally exposed to wood smoke were tested for the macrophage capacity for oxidative burst. Oxidative burst activity was significantly reduced in HAMs that had a higher PM load (ANOVA P < 0.01; Figure 7).
Figure 7.
The association of PM load in HAMs (ex vivo) with oxidative burst function. HAMs derived from naturally smoke-exposed Malawian adults were examined using flow cytometric macrophage functional assays. There was a significant difference in the oxidative burst activity between the lower (n = 12), middle (n = 12), and upper (n = 11) tercile of PM loading in HAMs. Significance testing by one-way ANOVA (P = 0.001). Error bars are indicated by thick horizontal lines, with 95% CI on each side.
Discussion
HAP causes almost 4 million deaths per year due to respiratory infections, chronic lung disease, cancer, and cardiovascular effects (3, 33). Pulmonary TB has also been associated with HAP, but epidemiological evidence is of low quality (34). In low- and middle-income countries, HAP exposure results mostly from the combustion of biomass fuels for cooking, heating, and lighting (2). In this study, we present data linking relevant carbon and wood smoke particulates to human pulmonary infection in the context of this global problem. First, in vitro modeling can reproduce in vivo exposures for alveolar macrophages. The appearance of HAMs exposed in vivo to inhaled indoor air pollution can be reproduced by in vitro exposure to respirable-sized combustion products of HAP-relevant fuel sources. Using HAMs and MDMs derived from healthy volunteers, cytokine production in response to in vitro FCB challenge was consistent with an acute inflammatory response. Second, wood smoke exposure has the potential to reduce internalization of relevant respiratory pathogens. Phagocytosis of beads, S. pneumoniae, and M. tuberculosis appeared impaired, but there was no effect on killing of M. tuberculosis. Finally, in vivo alveolar macrophage particulate loading is inversely related to capacity to produce an effective antibacterial response.
Cytokine data presented here are consistent with in vitro and in vivo studies. In murine models, BAL concentrations of proinflammatory mediators, including IL-6, rise in acute carbon particle exposures (35) and chronic wood smoke exposures (36), which is associated with monocyte influx to the lung. Coculture models of airway epithelium have demonstrated the additional inflammatory potential of wood smoke over washed particles (37). Human data from fire fighters occupationally exposed to forest fires also show systemic up-regulation of IL-6 and IL-8 (23). Our fine carbon data suggest that MDMs and HAMs share similar dose–response profiles with respect to IL-6 and IL-8 production.
The phagocytosis results are consistent with murine models in which PM from outdoor air pollution increases the binding of S. pneumoniae in vitro and reduces internalization (38). In the same study, the rate of killing of internalized bacteria was similar, but overall killing was reduced due to decreased internalization. Our study offers human evidence that phagocytic association and internalization of pathogenic bacteria are disrupted. We observed a greater effect on phagocytosis per particulate mass of wood smoke than with FCB. Wood smoke exposure is a complex insult, inducing a number of effects, including increased cytosolic reactive oxygen species (39). Compounds, such as transition metals, endotoxin (40), and other volatile compounds, may be adsorbed onto the core of carbon particles. This substantially alters the toxicity profile of wood smoke compared with carbon alone (22).
After bacterial ingestion by the alveolar macrophage, an intact oxidative burst response is key to antibacterial function in the lung. Oxidative mechanisms are important in the killing of extracellular organisms, such as S. pneumoniae, and the production of reactive oxygen intermediates is part of the host response to M. tuberculosis (41). There is evidence of the detrimental impact of particulates on alveolar macrophage phagocytosis both in vitro and in animal studies (26). Experimental human studies have not addressed phagocytic function, and could not reflect the chronicity and intensity of real-world HAP exposures (42, 43). We demonstrated a functional deficit in alveolar macrophage function in the context of everyday human biomass smoke exposure: macrophages containing higher levels of particulates showed a reduction in superoxide generation. Proinflammatory effects do not explain this finding, as this would be expected to increase respiratory burst (44). Anergy resulting from protracted innate immune activation might explain the finding, and is the focus of further work.
Taken together, our observations of reduced phagocytosis and oxidative burst capacity are consistent with the clinical and epidemiological trials that associate respiratory tract infections and HAP. In the Randomized Exposure Study of Pollution Indoors and Respiratory Effects (RESPIRE) trial, cases of severe hypoxic respiratory syncytial virus–negative pneumonia occurred more commonly in children with greater wood smoke exposure (9). Our findings suggest an explanation, that specific defects in the pulmonary innate immune system are responsible, and that these reduce the effectiveness of alveolar macrophage–mediated defense against common lower respiratory tract pathogens.
Our data for M. tuberculosis are not conclusive. Although Malawian wood smoke exposure was associated with a reduced number of mycobacteria per macrophage, high-dose Norwegian smoke exposure resulted in a reduced proportion of infected macrophages and appeared toxic to cells. There was no overall effect on killing of M. tuberculosis by macrophages. A previous study did not show alterations in phagocytosis or phagosomal maturation with in vivo particulate exposure (45). However, mice deficient in the reduced nicotinamide adenine dinucleotide phosphate oxidase show only a transient increase in sensitivity to M. tuberculosis infection, indicating that the superoxide burst is not key to the control of TB infection (46), which likely involves other microbiocidal mechanisms not addressed in this current study.
We examined two wood types to assess if dose equivalents from different sources might have disparate effects. Differential effects of burning (47) were minimized by using products of consistent and controlled combustion. Despite the predominance of black carbon in all particulate types, particulate loading, as quantified by digital image analysis, varied, and did not necessarily correlate with effect. For example, although Malawi wood smoke showed less particulate loading per mass exposure, it had a greater effect on phagocytosis of beads, pneumococci, and M. tuberculosis than the comparators. HAP fuel use is likely to change considerably in the context of intervention studies, exposure reduction campaigns (48), poverty reduction, and improvements in technology (49). We recommend, in this context, when studying HAP in different regions, or making comparisons between populations with varying fuel use, that particulate load should not be used in isolation to quantify exposure or to estimate effect.
There is considerable heterogeneity in lung macrophages particle uptake between cells in the same microplate well. These appearances are similar to those of HAMs naturally exposed to biomass smoke. As laboratory cells were exposed in a consistent manner, these data imply that particle uptake by lung macrophages is determined by the individual cell propensity or capacity for association and phagocytosis, and not simply because of the duration of exposure. Differential states of activation or polarization might explain these differences, but we were unable to test this. Neither is it possible to clearly establish if phagocytosis of bacteria was completed, or if this effect lies in a reduced association or adhesion to the cell.
In conclusion, our study demonstrates that human macrophages challenged with smoke ex vivo are similar to HAP-exposed macrophages in vivo. We show dose-related increases in cytokine production and decreases in phagocytosis and oxidative burst function in response to smoke exposure. We propose that particulate loading of alveolar macrophages offers a marker of macrophage functional impairment, and a link between household particulate exposure and respiratory infection.
Acknowledgments
Acknowledgments
The authors thank the volunteers at Queen Elizabeth Central Hospital, Blantyre and staff at Malawi-Liverpool-Wellcome Clinical Research Laboratories, especially Dr. K. Jambo, Dr. H. Mwandumba, Rose Malamba, and Mr. A. Kankwatira for their help with bronchoscopy, sample processing, and laboratory work. They are greatly indebted to all the work of Dr. H. Tolmie at Liverpool School of Tropical Medicine. They also thank Dr. A. Warman for his help with tuberculosis culture and N. Swann for her help with macrophage carbon loading development. The authors acknowledge the National Institute for Health Research Biomedical Research Centre in Microbial Diseases and the North-West Development Authority for infrastructural support, and Dr. A. Kochbach (Norwegian Institute of Public Health), Dr. S. Semple, Prof. K. Donaldson, and Dr. R. Duffin for helpfully providing particulate material for use in the experiments.
Footnotes
This work was supported by Wellcome Trust grant 080,065 (S.B.G.), Wellcome Trust Project Grant 083,606/A/07/Z (R.S.H.), Malawi-Liverpool-Wellcome Trust Clinical Research Program 084,679/Z/08/Z (R.S.H.), and National Institutes of Health grants HL100928 and AI095519 (D.G.R.). Ethical permission was obtained from Sefton Research Ethics Committee, United Kingdom (06/Q1501/192) and College of Medicine Research Ethics Committee, Malawi (P05.06.460).
Author Contributions: literature review, laboratory work, data analysis, and manuscript drafting—J.R., A.N.A., and J.S.; ethics, laboratory work, participant recruitment, literature review, data analysis, and manuscript drafting and writing—D.G.F.; laboratory work, data analysis, and manuscript drafting—D.M., A.K.A.W., D.G.W., K.B., and A.K.; software design, data analysis, and manuscript drafting—S.B.; laboratory work, data analysis, and manuscript drafting—S.J.G.; literature review, data analysis, and manuscript drafting—D.G.R.: assay development, laboratory work, and manuscript drafting—K.M.; study design, ethics, data analysis, and manuscript drafting—R.S.H.; principal investigator, study design, grant writing, ethics, data analysis, and manuscript drafting and writing—S.B.G.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0188OC on September 25, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor air pollution from household use of solid fuels. comparative quantification of health risks, global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization; 2004. pp. 1435–1494. [Google Scholar]
- 2.Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzati M, Viegi G. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in kenya: an exposure–response study. Lancet. 2001;358:619–624. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- 5.Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55:518–532. doi: 10.1136/thorax.55.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet. 2006;368:1048–1050. doi: 10.1016/S0140-6736(06)69334-3. [DOI] [PubMed] [Google Scholar]
- 7.Mulholland K. Childhood pneumonia mortality—a permanent global emergency. Lancet. 2007;370:285–289. doi: 10.1016/S0140-6736(07)61130-1. [DOI] [PubMed] [Google Scholar]
- 8.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. Effect of reduction in household air pollution on childhood pneumonia in guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 10.Reid A, Dedicoat M, Lalloo D, Gilks CF. Trends in adult medical admissions in a rural South African hospital between 1991 and 2002. J Acquir Immune Defic Syndr. 2005;40:53–56. doi: 10.1097/01.qai.0000174251.35398.72. [DOI] [PubMed] [Google Scholar]
- 11.Ogun SA, Adelowo OO, Familoni OB, Jaiyesimi AE, Fakoya EA. Pattern and outcome of medical admissions at the ogun state university teaching hospital, Sagamu—a three year review. West Afr J Med. 2000;19:304–308. [PubMed] [Google Scholar]
- 12.Scott JA, Hall AJ, Muyodi C, Lowe B, Ross M, Chohan B, Mandaliya K, Getambu E, Gleeson F, Drobniewski F, et al. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in kenya. Lancet. 2000;355:1225–1230. doi: 10.1016/s0140-6736(00)02089-4. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Global tuberculosis report 2012. Geneva: WHO; 2012. [Google Scholar]
- 14.Kolappan C, Subramani R. Association between biomass fuel and pulmonary tuberculosis: a nested case–control study. Thorax. 2009;64:705–708. doi: 10.1136/thx.2008.109405. [DOI] [PubMed] [Google Scholar]
- 15.Braun JS, Hoffmann O, Schickhaus M, Freyer D, Dagand E, Bermpohl D, Mitchell TJ, Bechmann I, Weber JR. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun. 2007;75:4245–4254. doi: 10.1128/IAI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pokhrel AK, Bates MN, Verma SC, Joshi HS, Sreeramareddy CT, Smith KR. Tuberculosis and indoor biomass and kerosene use in Nepal: a case–control study. Environ Health Perspect. 2010;118:558–564. doi: 10.1289/ehp.0901032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kan X, Chiang CY, Enarson DA, Chen W, Yang J, Chen G. Indoor solid fuel use and tuberculosis in china: a matched case–control study. BMC Public Health. 2011;11:498. doi: 10.1186/1471-2458-11-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gninafon M, Ade G, Ait-Khaled N, Enarson DA, Chiang CY. Exposure to combustion of solid fuel and tuberculosis: a matched case–control study. Eur Respir J. 2011;38:132–138. doi: 10.1183/09031936.00104610. [DOI] [PubMed] [Google Scholar]
- 19.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 20.Fullerton DG, Jere K, Jambo K, Kulkarni NS, Zijlstra EE, Grigg J, French N, Molyneux ME, Gordon SB. Domestic smoke exposure is associated with alveolar macrophage particulate load. Trop Med Int Health. 2009;14:349–354. doi: 10.1111/j.1365-3156.2009.02230.x. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni NS, Prudon B, Panditi SL, Abebe Y, Grigg J. Carbon loading of alveolar macrophages in adults and children exposed to biomass smoke particles. Sci Total Environ. 2005;345:23–30. doi: 10.1016/j.scitotenv.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni N, Pierse N, Rushton L, Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355:21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
- 23.Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32:129–138. doi: 10.1183/09031936.00097707. [DOI] [PubMed] [Google Scholar]
- 24.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 25.Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. Adverse effects of wood smoke PM(2.5) exposure on macrophage functions. Inhal Toxicol. 2013;25:67–76. doi: 10.3109/08958378.2012.756086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 27.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 28.Collins AM, Rylance J, Wootton DG, Wright AD, Wright AKA, Fullerton DG, Gordon SB. Bronchoalveolar lavage (BAL) for research; obtaining adequate sample yield. J Vis Exp. 2014;(85):e4345. doi: 10.3791/4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg (Lond) 1938;38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFarland J. The nephalometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc. 1907;XLIX(14):1176–1178. [Google Scholar]
- 31.Pheiffer C, Carroll NM, Beyers N, Donald P, Duncan K, Uys P, van Helden P. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis. 2008;12:792–798. [PubMed] [Google Scholar]
- 32.Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol. 2009;9:594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortimer K, Gordon SB, Jindal SK, Accinelli RA, Balmes J, Martin WJ. Household air pollution is a major avoidable risk factor for cardiorespiratory disease. Chest. 2012;142:1308–1315. doi: 10.1378/chest.12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HH, Suk CW, Lo HL, Huang RY, Enarson DA, Chiang CY. Indoor air pollution from solid fuel and tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18:613–621. doi: 10.5588/ijtld.13.0765. [DOI] [PubMed] [Google Scholar]
- 35.Goto Y, Ishii H, Hogg JC, Shih CH, Yatera K, Vincent R, van Eeden SF. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med. 2004;170:891–897. doi: 10.1164/rccm.200402-235OC. [DOI] [PubMed] [Google Scholar]
- 36.Tesfaigzi Y, McDonald JD, Reed MD, Singh SP, De Sanctis GT, Eynott PR, Hahn FF, Campen MJ, Mauderly JL. Low-level subchronic exposure to wood smoke exacerbates inflammatory responses in allergic rats. Toxicol Sci. 2005;88:505–513. doi: 10.1093/toxsci/kfi317. [DOI] [PubMed] [Google Scholar]
- 37.Kocbach A, Herseth JI, Lag M, Refsnes M, Schwarze PE. Particles from wood smoke and traffic induce differential pro-inflammatory response patterns in co-cultures. Toxicol Appl Pharmacol. 2008;232:317–326. doi: 10.1016/j.taap.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Kobzik L. Effect of concentrated ambient particles on macrophage phagocytosis and killing of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2007;36:460–465. doi: 10.1165/rcmb.2006-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu PL, Chen YL, Chen YH, Lin SJ, Kou YR. Wood smoke extract induces oxidative stress–mediated caspase-independent apoptosis in human lung endothelial cells: role of AIF and ENDOG. Am J Physiol Lung Cell Mol Physiol. 2005;289:L739–L749. doi: 10.1152/ajplung.00099.2005. [DOI] [PubMed] [Google Scholar]
- 40.Semple S, Devakumar D, Fullerton DG, Thorne PS, Metwali N, Costello A, Gordon SB, Manandhar DS, Ayres JG. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118:988–991. doi: 10.1289/ehp.0901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to mycobacterium tuberculosis. Adv Exp Med Biol. 2003;531:241–247. doi: 10.1007/978-1-4615-0059-9_20. [DOI] [PubMed] [Google Scholar]
- 42.Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, Olin AC. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65:319–324. doi: 10.1136/oem.2006.032458. [DOI] [PubMed] [Google Scholar]
- 43.Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, Rodes CE, Vette AF, Balbus JM. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect. 2013;121:1120–1128. doi: 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.VanderVen BC, Yates RM, Russell DG. Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic. 2009;10:372–378. doi: 10.1111/j.1600-0854.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, Molyneux ME, Squire SB. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol. 2004;172:4592–4598. doi: 10.4049/jimmunol.172.7.4592. [DOI] [PubMed] [Google Scholar]
- 46.Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47(phox−/−) mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallsten G, Gustafson P, Johansson L, Johannesson S, Molnar P, Strandberg B, Tullin C, Barregard L. Experimental wood smoke exposure in humans. Inhal Toxicol. 2006;18:855–864. doi: 10.1080/08958370600822391. [DOI] [PubMed] [Google Scholar]
- 48.Romieu I, Riojas-Rodriguez H, Marron-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180:649–656. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- 49.Pomory CM. Color development time of the Lowry protein assay. Anal Biochem. 2008;378:216–217. doi: 10.1016/j.ab.2008.04.015. [DOI] [PubMed] [Google Scholar]