Abstract
Fighting pathogens and maintaining tissue homeostasis are prerequisites for survival. Both of these functions are upheld by the immune system, though the latter is often overlooked in the context of the CNS. The mere presence of immune cells in the CNS was long considered a hallmark of pathology, but this view has been recently challenged by studies demonstrating that immunological signaling can confer pivotal neuroprotective effects on the injured CNS. In this review we describe the temporal sequence of immunological events that follow CNS injury. Beginning with immediate changes at the injury site including death of neural cells and release of damage-associated molecular patterns (DAMPs), and progressing through innate and adaptive immune responses, we describe the cascade of inflammatory mediators and the implications of their post-injury effects. We conclude by proposing a revised interpretation of immune privilege in the brain, which takes beneficial neuro-immune communications into account.
Neuro-immune Communication
The immune system exists pervasively throughout the body, defending against invaders, supporting tissue healing and maintaining homeostasis. Though clearly important in the periphery, its role in the central nervous system (CNS) is complicated by several unique mechanisms. The unperturbed CNS is separated from the periphery by the blood–brain barrier (BBB), a selectively permeable barrier that prevents immune cell infiltration and free passage of blood-borne molecules into the healthy brain (Broadwell and Sofroniew, 1993; Bush et al., 1999; Habgood et al., 2007; Muldoon et al., 2013). Furthermore, early experiments by Peter Medawar and others demonstrated that foreign tissue grafted into the CNS elicits a delayed immune response (Medawar, 1948). These observations paved the way for the concept of CNS “immune privilege”, that the brain was privileged from normal immune surveillance. Indeed, in the healthy state no peripheral immune cells are detectable in the CNS parenchyma, although resident microglia are found throughout the brain and the meningeal spaces are highly populated by various immune cells (Derecki et al., 2010; Kivisakk et al., 2009; Shechter et al., 2013).
The nature of neuroimmune interactions is controversial, with various factors, including the concept of immune privilege, the existence of the blood–brain barrier, and the observation that excessive autoimmune CNS inflammation drives pathology in multiple sclerosis (Ousman et al., 2007; Steinman, 2014) contributing to the notion that the activity of the peripheral immune system is harmful to the CNS and does not support its function. This was the prevailing dogma for decades, but over the last few years it has been increasingly challenged. Emerging data suggest that the peripheral immune system indeed participates in the maintenance of homeostatic brain functions, with reports showing key neuroimmune interactions regulating adult neurogenesis, learning behavior, the ability to cope with psychological stress, and other brain functions (reviewed in (Kipnis et al., 2012)). Persuasive evidence indicates, moreover, that the immune system also supports the injured CNS (Raposo and Schwartz, 2014; Walsh et al., 2014b), offers protection against CNS infections (Norose et al., 2011), and plays a beneficial role in pathological states such as Alzheimer's disease (Hickman and El Khoury, 2010), glaucoma (Schwartz and London, 2009), and several other neurodegenerative conditions (Derecki et al., 2012; Frenkel et al., 2003; Yong and Rivest, 2009).
The role of the immune system in the context of CNS injury has been particularly well studied. Injury to the CNS elicits a distinct inflammatory cascade that begins with cell death and progresses through multiple molecular and cellular phases (Figure 1). This is similar to the inflammatory cascade described in injuries to peripheral tissues, where the immune system, if well controlled, is generally thought to support healing. However, the consequences of the immune response to CNS injury remain controversial, with some groups reporting aspects of it to be beneficial (Huang et al., 1999; Kurimoto et al., 2013; Shechter et al., 2009; Walsh et al., 2015; Yin et al., 2006) while others describe it as destructive (Evans et al., 2014; Gonzalez et al., 2007; Kroner et al., 2014; Popovich et al., 1999; Yawata et al., 2008).
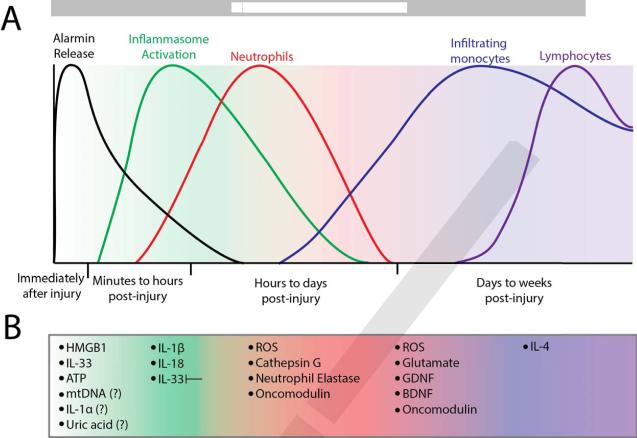
Figure 1. Kinetics of the molecular and cellular immune response to CNS injury.
(A) The phases of molecular and cellular inflammation after CNS injury. Danger-associated molecular patterns (DAMPs) such as IL-33, HMGB1 and ATP are released immediately following CNS injury. The inflammasome is activated soon after and produces IL-1β and IL-18. Neutrophils arrive hours after injury and stay for several days, while monocytes begin infiltrating within the first day and remain present. Lymphocytes begin to arrive days to weeks post-injury. (B) Specific inflammatory molecules active at each time post injury are listed.
DAMPs, PAMPs, and Alarmins—Dialing 911 for Tissue Injury
The immune system has evolved to respond not only to pathogens but also to virtually any insult that threatens homeostasis, including trauma, cellular and metabolic dysfunction, ischemia-reperfusion injury, or environmental irritants. Inflammation occurs readily after sterile insults (Chen and Nunez, 2010) and generally entails a familiar cascade of recruitment of neutrophils, monocytes, and lymphocytes to the site of injury and their activation there. This recruitment of peripheral immune cells is preceded by an immediate response from local cells that sense danger and “sound the alarm” by producing chemokines and cytokines. Immune activation in general is a tightly controlled process, and with good reason; an inappropriate response can result in devastating damage to CNS tissue and the development of autoimmune disease, while the inability to mount a proper immune response when needed can have fatal consequences.
How do cells discern between health and injury—sounding the alarm and initiating an immune response only when necessary? A classical explanation is that the immune system discriminates between “self” and “non-self” (Janeway, 1992), using specialized T- and B- cell receptors that respond to foreign antigens and only mounting a response to anything deemed “non-self”. This explanation is clearly not satisfactory, as in the context of sterile inflammation there would be no “non-self” antigens to trigger a response. An alternate model for how the immune system chooses whether or not to respond, coined by Matzinger as the “danger theory”, states that rather than responding to self vs. non-self, the immune system initially responds to danger or damage signals that can be either pathogen or self-derived (Matzinger, 2001, 2002). Thus, damage associated molecular patterns (DAMPs) can be either endogenously derived “alarmins” or exogenous “pathogen associated molecular patterns” (PAMPs) (Bianchi, 2007). PAMPs and alarmins represent the presence of pathogen or tissue damage, respectively, and are detected by pattern recognition receptors (PRRs) to initiate and amplify an immune response (Bianchi, 2007). Many types of DAMPs are expressed in the healthy CNS, and are released after injury to initiate inflammation.
DAMPs encompass an extremely diverse class of molecules, ranging from bacterial lipids or peptides to endogenous proteins, nucleic acids and metabolites. Though diverse, all DAMPs share the characteristic of promoting immune activation in response to damage. A well-characterized group of DAMPs consists of intracellular proteins that are expressed at a basal level within a cell and are released after cell injury. Examples of endogenous protein DAMPs are interleukin (IL)-1α (Eigenbrod et al., 2008), IL-33 (Schmitz et al., 2005), high-mobility group protein B1 (HMGB1) (Scaffidi et al., 2002), and the S100 class of proteins (Zitvogel et al., 2010), all of which bind specific receptors and promote initiation of inflammation after injury. Interestingly, many endogenous protein DAMPs (including IL-33, HMGB1 and IL-1α) are concentrated in the nucleus, where they presumably perform non-alarmin functions, though these are generally not fully understood.
A second class of DAMPs comprises nucleic acids and nucleotide derivatives. Among the best described of these is mitochondrial DNA (mtDNA). While eukaryotic DNA has a characteristic methylation pattern that renders it non-immunogenic, mtDNA more closely resembles prokaryotic DNA (Zhang et al., 2010) and is capable of stimulating some of the same pathways as those evolved for sensing pathogenic bacteria. mtDNA becomes more immunogenic when oxidized, a condition that occurs under severe cell stress and endows it with greater specificity for damaged cells (Ding et al., 2013). ATP and uric acid are two purine nucleotide derivatives that also have well-studied alarmin properties. ATP is a multifunctional molecule, well known for storing energy and as a signaling molecule that can mediate diverse effects. However, abundant ATP in the extracellular space is also detected by the immune system as a DAMP. Yet another nucleotide derived DAMP is uric acid, a metabolite that is soluble intracellularly, but upon exposure to the extracellular environment precipitates and forms crystals of monosodium urate. Both uric acid and ATP can activate the inflammasome cascade (described in detail below), and through it promote production of several inflammatory chemokines and cytokines (Kim et al., 2015; Lee et al., 2012).
DAMPs alert the immune response to tissue damage, and although sterile inflammation can promote wound healing it can also potentiate disease. In recent years, non-communicable chronic diseases that are potentiated by dysregulated sterile inflammation have replaced infectious diseases as the preeminent threat to human health (Mortality and Causes of Death, 2015). Persisting sterile inflammation that results from aberrant tissue damage indeed plays pivotal roles in the pathogenesis of various prevalent human diseases including atherosclerosis, type 2 diabetes, obesity, and some neurodegenerative diseases (Lukens et al., 2012b).
Pattern Recognition Receptors Alert the Immune System to Pathogens and Cell Damage
PRRs represent a large group of receptors with an even larger repertoire of potential ligands. There are numerous PRR families, including the Toll-like receptors (TLRs), NOD-like receptors (NLRs), AIM2-like receptors (ALRs), RIG-I-like receptors (RLRs), and C-type lectin receptors (CLRs). Each of these categories has multiple representatives and species differences; for example, humans have 10 TLRs and 22 NLRs, whereas mice have 12 TLRs and 34 NLRs (Bryant and Monie, 2012). Many of these PRRs in turn have multiple ligands, often a combination of PAMPs and DAMPs. One example is the Toll-like receptor 4 (TLR4), well known for binding to lipopolysaccharide (LPS) found on the outer core of Gram-negative bacteria, which also binds to a wide array of endogenous DAMPs such as HMGB1 (Erridge, 2010).
Despite their diversity of receptors and ligands, specific classes of PRRs tend to induce similar intracellular signaling cascades. For example, TLRs are found on intra- or extracellular membranes and typically signal through MyD88 and/or TRIF in response to protein or nucleic acid DAMPs (Erridge, 2010). NLRs are cytoplasmic PRRs and often signal through the inflammasome protein complex (Latz et al., 2013). RLRs, cytoplasmic PRRs that recognize double-stranded RNA, play an important role in host defense against viruses by recruiting the adaptor protein IPS1 and signaling through IRF family and NF-κB transcription factors (Loo and Gale, 2011). ALRs are also found in the cytosol and initiate inflammasome activation following recognition of cytosolic double-stranded DNA (Ratsimandresy et al., 2013). A final PRR is the receptor for advanced glycation endproducts (RAGE). This receptor, found on the extracellular surface, was originally described as a receptor that recognizes glycosylated proteins and lipids, but was later found also to detect other proteins such as S100B, amyloid-beta fibrils, and the alarmin HMGB1 (Xie et al., 2013). RAGE couples with mDia1 and Src to activate a myriad of signaling pathways that affect cell survival, autophagy, cell motility, and inflammation (through NF-κB) (reviewed in (Xie et al., 2013)).
CNS Injury
Millions of people suffer traumatic CNS injuries every year (Rutland-Brown et al., 2006). Although the results can be quite variable depending on the location and severity of trauma, CNS injuries fail to regenerate over time and often lead to permanent disability (Ruffolo et al., 1999). Traumatic spinal cord injury (SCI), for example, results in at least some degree of paralysis on discharge in over 99% of cases (Center, 2014). Coupled with this poor prognosis is a dire lack of effective therapies. The only pharmacological treatment currently approved for improvement of neurologic recovery after SCI is methylprednisone, a glucocorticoid with immunosuppressive properties. Though the standard care for many years, evidence in support of its beneficial effects is limited (Bracken, 2012) and its use is currently under debate (Lammertse, 2013).
Why does the CNS not recover after injury? Neuroscientists have been mystified by this question for decades, and as yet no single explanation can fully provide the answer. In general, obstacles to recovery can be divided into two categories: barriers to regeneration, either neuron-intrinsic (Liu et al., 2011) or neuron-extrinsic (Fawcett et al., 2012); and secondary death—the persistence, in the days and weeks after injury, of ongoing progressive neuronal degeneration beyond the primary lesion (Algattas and Huang, 2014; Yoles and Schwartz, 1998). Untreated or inadequately treated, these two major phenomena are targeted by neuroregenerative and neuroprotective agents, respectively, and represent the bulk of efforts in the quest for effective therapies for CNS injury.
Cell Death after CNS Injury
As touched upon above, injury to the CNS is characterized by two distinct phases of cell death. First, mechanical trauma at the time of injury causes direct damage to neurons, glia, vasculature, and meningeal cells, inducing necrotic death of neurons and glia (Grossman et al., 2001). Then, over the following days and weeks an ongoing process of secondary death leads to increased lesion size and worsened outcome, primarily owing to apoptotic cell death (Dusart and Schwab, 1994; Liu et al., 1997; Lytle and Wrathall, 2007). The kinetics and relative contribution of primary versus secondary death to total neuron loss varies between injuries and depends, in particular, on the severity of injury (Yoles and Schwartz, 1998). In the case of crush injury to the spinal cord, most of the motor neurons that suffered mechanical damage are lost in minutes to hours after the injury (Fehlings et al., 2012). Similarly, the trauma is rapidly followed by a primary wave of glial degeneration (Fehlings et al., 2012), where astrocytes undergo maximal loss within 24 hours (Lytle and Wrathall, 2007) and CC1+ oligodendrocytes degenerate over the first post-injury week (Casha et al., 2001). Secondary death of both neurons and glia is caused by the combined effects of noxious stimuli from free radicals generated after injury (Algattas and Huang, 2014; Carrico et al., 2009), excitotoxicity owing to the release of glutamate from neurons, microglia and macrophages (Yawata et al., 2008), swelling of injured tissue causing its further crushing within limited space (Bareyre et al., 1997), hypoxia and metabolic dysfunction that results from impaired blood flow (a switch from aerobic to anaerobic glycolysis among other things) (Algattas and Huang, 2014), and aspects of inflammation (Loane and Byrnes, 2010).
Inflammation begins soon after injury and remains a prominent force throughout its progression (Shechter and Schwartz, 2013). In subsequent sections we will discuss the molecular and cellular inflammatory cascades of events that occur in the minutes, days, and weeks after CNS injury. Particular attention will be focused on the initiation of CNS inflammation and the implications of immune-cell activity for injury outcome, with the goal of outlining our current understanding of CNS inflammation and its beneficial and detrimental effects.
Immediately after Injury: Alarmin Release and Glial Activation
As discussed earlier, injury to peripheral tissues results in cell death and the release of alarmins with subsequent induction of inflammation (Chen and Nunez, 2010). Which alarmins, if any, initiate inflammation after CNS injury? Below we discuss three alarmins—ATP, HMGB1, and IL-33—that have been shown to act in the CNS (Figure 2).
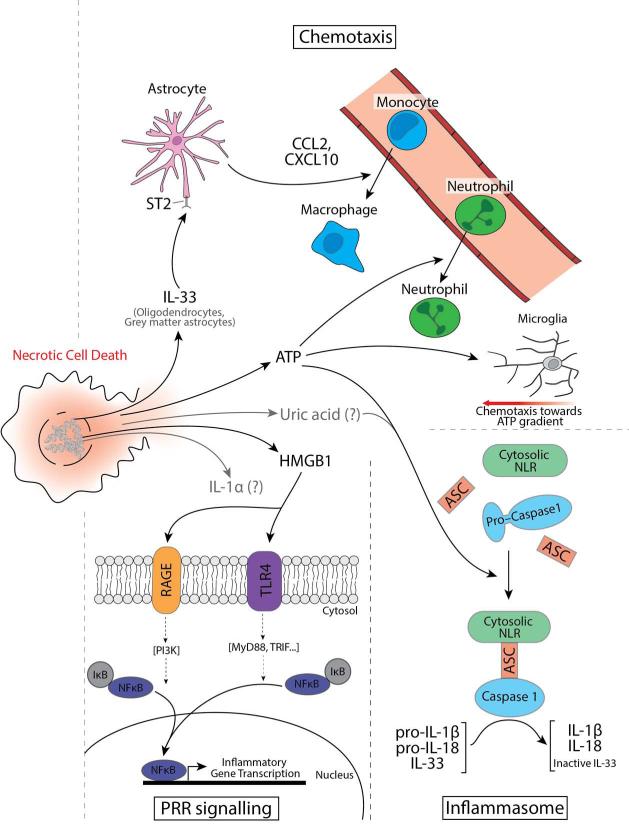
Figure 2. Necrotic cell death causes the release alarmins into the extracellular space.
Necrotic cell death releases peptide and nucleic acid derivative alarmins that initiate inflammation. IL-33 plays an important role in bringing monocyte-derived macrophages into the CNS through upregulation of astrocytic chemokine expression. ATP promotes chemotaxis of neutrophils (through its activation of the inflammasome), and is directly chemotactic to microglial processes. ATP and uric acid also activates the inflammasome, stimulating the assembly of the cytosolic NLR, ASC, and pro-caspase 1. Pro-caspase 1 is auto-cleaved to mature caspase 1, which cleaves pro-IL-1β and pro-IL-18 to active forms and IL-33 to an inactive form. HMGB1 acts as on TLR4 and RAGE receptors and directly promotes inflammatory cytokine and chemokine production. An important transcription factor downstream of both receptors is NF-κB, important in enhancing inflammation and cellular infiltration, but the RAGE receptor has several other downstream signaling pathways (not shown here). IL-1α and uric acid (gray arrows), are important inducers of immune responses in response to tissue damage in the periphery, however their roles in response to CNS injury remained poorly defined.
ATP
ATP participates in many signaling events both in physiological and in pathological contexts (reviewed in (Franke et al., 2012)), and its presence in the extracellular space does not always imply cell damage. However, when released abundantly and in conjunction with other DAMPs, ATP plays an important role in initiating the immune response to CNS trauma. Release of ATP generally promotes inflammation by inciting the inflammasome activity of NLRP3, a cytoplasmic NLR (Di Virgilio, 2007). This activation results in production of the inflammatory cytokines IL-1β and IL-18, which exert broad proinflammatory effects including induction of chemokine production (Lukens et al., 2012b). ATP is a potent stimulator of several chemokines, including CCL3 produced by microglia (Kataoka et al., 2009) and CCL2 produced by astrocytes (Panenka et al., 2001). ATP signaling is particularly critical for neutrophil recruitment, as transcranial delivery (through diffusion across a thinned skull) of a purine receptor antagonist can suppress neutrophil responses following traumatic brain injury (TBI) (Roth et al., 2014). Although the chemotactic effect of ATP on neutrophils can be direct (Chen et al., 2006), the results in TBI model were consistent with inflammasome inhibition (thus resulting in decreased neutrophil chemotaxis) (McDonald et al., 2010). Microglial processes are also highly sensitive to ATP, showing dramatic chemotaxis to ATP gradients (Davalos et al., 2005). Two-photon in-vivo imaging disclosed that within minutes microglia respond in an ATP-dependent manner to cortical injury (Davalos et al., 2005; Nimmerjahn et al., 2005). Interestingly, high extracellular ATP can induce further release of ATP from astrocytes, and such ATP-induced ATP release is an important mechanism for amplifying its alarmin effects in both neutrophil and microglial chemotaxis (Davalos et al., 2005; Roth et al., 2014).
High-mobility Group Protein B1
HMGB1 is a ubiquitous nuclear protein that is expressed in virtually all cells of the CNS (Daston and Ratner, 1994; Enokido et al., 2008; Gao et al., 2011; Tenenbaum et al., 2006). HMGB1 has been shown to play pleiotropic roles in the CNS in both health and disease. It is important, for instance, in zebrafish brain development (Zhao et al., 2011), and continues to serve an architectural and maintenance role in the nucleus of adult cells (Lange and Vasquez, 2009). After injury, however, extracellular HMGB1 serves as a potent activator of inflammation (Chen et al., 2011; Scaffidi et al., 2002). Once released, HMGB1 can signal via both TLR4 and RAGE receptors to potentiate the migration, proliferation, and differentiation of immune cells (Degryse et al., 2001).
In the CNS, HMGB1 strongly upregulates several chemokines in astrocytes, including neutrophil chemoattractants such as CXCL1, CXCL2 and CCL3, and T-cell chemoattractants such as CX3CL1, CCL2, CCL5 and CCL20 (Pedrazzi et al., 2007). HMGB1 has also been shown to potentiate post-traumatic CNS damage that correlates with increased infiltration of neutrophils. Inhibition of HMGB1 by antibody-mediated neutralization or by pharmacological blockers was found to lead to improved recovery from both SCI (Zhai et al., 2012) and TBI (Gu et al., 2014; Okuma et al., 2012). Furthermore, genetic ablation of the RAGE receptor was reported to improve outcome in a mouse model of SCI (Guo et al., 2014). Interestingly, HMGB1 has also been found beneficial in promoting CNS repair in non-mammalian organisms. For example, a gecko paralog of HMGB1 was shown to signal via RAGE to oligodendrocytes to coordinate myelination after CNS injury (Dong et al., 2013) and to neurons to promote neurite outgrowth (Saleh et al., 2013). HMGB1 also contributes to axonal regeneration and recovery after spinal cord transection in a zebrafish model (Fang et al., 2014). The opposing roles of HMGB1 described in different organisms may be less puzzling, however given a recent study demonstrating that paralogs of gecko HMGB1 have other functions, unrelated to inflammation, that directly support remyelination and CNS regeneration in these animals (Dong et al., 2013).
Interleukin-33
Like HMGB1, IL-33 is a nuclear alarmin that is widely expressed in many tissues, with the highest expression observed in the skin, lung, brain and spinal cord (Schmitz et al., 2005). In the nucleus IL-33 appears to participate in gene silencing through its association with heterochromatin (Carriere et al., 2007), but this notion has been contested by high-resolution imaging methodologies showing that it actually associates with euchromatin (Kakkar et al., 2012). The precise role of IL-33 in the nuclei of healthy cells remains poorly characterized. Its function are clearer, however, after cellular injury. IL-33 is released after cellular necrosis and acts through its specific receptor (IL-33R), a heterodimer of ST2 and the IL-1 receptor-associated protein (IL-1RAP), to initiate inflammation in multiple cell types.
The IL-33R signals through MyD88, the adaptor protein also used by most TLRs and the IL-1 receptor, and similarly results in activation of NFκB and MAP kinase signaling. Specific expression of the IL-33R by certain immune cells links IL-33 to type 2 immune responses. These responses, typically found in asthma, allergy, and anti-parasite immunity, are associated with mast cells, type 2 innate lymphocytes (ILC2s), and type 2 helper T cells (Th2), all of which exhibit enriched IL-33R expression.
High levels of IL-33 expression in the CNS were originally reported some 10 years ago (Schmitz et al., 2005), but its cellular expression pattern, its function as an alarmin, and its actions on local cells there remains an active area of investigation. Early studies yielded somewhat conflicting reports on IL-33 regulation and localization in the CNS. In-vitro cultured astrocytes express IL-33, and this expression was greatly potentiated by treatment with other DAMPs (Hudson et al., 2008; Yasuoka et al., 2011). Another group, studying IL-33 in the experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis, reported IL-33 expression in healthy astrocytes and neurons with little change after EAE induction (Jiang et al., 2012). In yet another study of the regulation of IL-33 expression in the developing CNS, IL-33 was produced in both developing astrocytes and oligodendrocyte precursor cells (OPCs) (Wicher et al., 2013). However, this group did not detect IL-33 expression in adult animals.
We recently readdressed the question of IL-33 expression in the healthy adult CNS by first measuring IL-33 mRNA in several different tissues and brain regions (Gadani et al., 2015). A strong correlation was identified between myelination and IL-33 content among brain regions, which ultimately reflected enriched expression of IL-33 in CC1+ oligodendrocytes. IL-33 was also generally expressed by astrocytes in gray matter but not in white matter. In contrast, IL-33 expression was not detectable in microglia, neurons, or OPCs. Flow cytometric analysis of whole brain showed that approximately 30% of all brain cells express IL-33 (Gadani et al., 2015). Furthermore, IL-33 was released by CNS tissue after injury, becoming detectable in the cerebrospinal fluid after spinal cord contusion and in the supernatant of in vitro-damaged spinal cords. Secretion of IL-33 following tissue damage in vitro was not associated with enhanced IL-33 expression, suggesting that its release after CNS damage is probably not a result of increased IL-33 transcription but rather the release of endogenously expressed protein (Gadani et al., 2015).
IL-33 is known to influence inflammation in many peripheral disease models (Oboki et al., 2010), in sepsis (Jiang et al., 2012) and in EAE (Jiang et al., 2012; Oboki et al., 2010), but its role in the post-injury regulation of CNS repair is only now beginning to be elucidated. IL-33 can stimulate CCL2 production by mixed glia in vitro (Gadani et al., 2015; Kempuraj et al., 2013), and IL-33–/– mice show significantly reduced production of several chemokines at the injury site after SCI (Gadani et al., 2015). This defect in chemokine expression was coupled with reduced recruitment of peripheral monocytes, impaired recovery, and increased lesion volume after SCI, and with decreased neuronal survival after optic nerve crush (Gadani et al., 2015). Furthermore, exogenous administration of CCL2 into the site of SCI in IL-33–/– mice was found to promote recovery, suggesting that delayed monocyte recruitment underlies increased pathology in these animals (Gadani et al., 2015). Interestingly, IL-33 signaling can also be potentiated to improve outcomes in wild-type mice, as intraperitoneal injection of exogenous IL-33 was found to improve locomotor recovery and reduce lesion size after SCI (Pomeshchik et al., 2015).
It should be noted that many of the alarmins and DAMPs—including IL-1α, mtDNA, and uric acid—shown to coordinate immune responses to peripheral tissue injury have not been adequately studied in the context of in vivo injury to the CNS. Future studies will be needed in order to delineate the involvement of these molecules in the regulation of responses to CNS injury.
Minutes to Hours after CNS Injury: Inflammasome Activation, Cytokine Production and Neutrophil Recruitment
Cytokine Secretion and the Inflammasome
DAMPs released immediately after injury initiate a cascade of cellular and molecular immune mediators to amplify inflammation. Early molecular players in this cascade include cytokines such as TNF, IL-6, or IL-1β, which are upregulated rapidly by local and infiltrating immune cells in response to DAMPs (Ransohoff and Brown, 2012). These molecules are critical amplifiers of the innate immune response to CNS injury, and represent potential therapeutic targets. For example, TNF has been identified as a detrimental cytokine, expressed early after injury by red blood cell-engulfing macrophages (Kroner et al., 2014). Furthermore, studies blocking early TNF signaling in vivo show promise in rodent models and humans to improve outcome after CNS trauma (Esposito and Cuzzocrea, 2011). Similarly blocking IL-6 signaling promotes recovery in rodent spinal cord injury (Mukaino et al., 2010; Okada et al., 2004).
One of the most potent mechanisms by which DAMPs can provoke inflammatory signaling is through the activation of inflammasome platforms and release of cytokines like IL-1β. Inflammasomes are multiprotein complexes that generally consist of three main components: a cytosolic PRR, the enzyme caspase-1, and the adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) (Lukens and Kanneganti, 2014). Recognition of PAMPs and DAMPs by inflammasome-associated PRRs promotes the assembly of inflammasome components into a multiprotein complex and culminates in secretion of active IL-1β and IL-18. The generation of mature IL-1β and IL-18 is a coordinated multi-step process, where the first step requires transcription and translation of pro-IL-1β and pro-IL-18, and is often initiated by tumor necrosis factor (TNF), IL-1α or TLR-induced activation of NF-κB signaling (Bauernfeind et al., 2009). The second step requires assembly of the intracellular PPR, the adaptor protein ASC, and pro-caspase-1. These proteins form the inflammasome complex, which is needed to orient pro-caspase-1 for autocleavage and activation. Once activated, caspase-1 cleaves pro-IL-1β and pro-IL-18, providing the final step required for their secretion and inflammatory signaling (Figure 2).
Members of the NLR and ALR families, as well as the protein Pyrin, have all been shown to function as cytoplasmic PRRs that coordinate inflammasome formation and activation. To date a number of NLRs (NLRP1, NLRP3, NLRP6, NLRP7, NLRP12 and NLRC4) and ALRs (AIM2 and IFI16) have been reported to induce inflammasome activation (Latz et al., 2013; Lukens and Kanneganti, 2014). These intracellular PPRs can orchestrate inflammasome signaling in response to a variety of diverse pathogen- or danger-associated triggers. For example, the ALRs AIM2 and IFI16 activate the inflammasome in response to the presence of cytosolic DNA (Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Roberts et al., 2009), whereas, NLRC4 induces inflammasome signaling following recognition of bacterial flagellin or type III secretion system-associated bacterial proteins (Wang et al., 2011). NLRP3, on the other hand, can trigger inflammasome formation and activation in response to both pathogenic and endogenous danger signals (Strowig et al., 2012). Examples of NLRP3 inflammasome triggers that are released in response to CNS trauma include ATP, uric acid, reactive oxygen species, and necrotic cells. Interestingly, NLRP3 inflammasome-dependent cytokine production was recently reported to contribute to the pathogenesis of multiple neuroinflammatory and neurodegenerative disorders including multiple sclerosis, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS) (Halle et al., 2008; Lukens et al., 2012a; Meissner et al., 2010). Inflammasome-associated proteins are highly expressed in the CNS, and inflammasomes have been reported to assemble in glial cells and neurons (Liu et al., 2013; Tomura et al., 2012; Walsh et al., 2014a).
Recent studies suggest critical roles for inflammasomes in promoting CNS tissue damage in models of TBI and SCI (de Rivero Vaccari et al., 2012). In both cases, neutralization of the NLRP1 and NLRP3 inflammasomes with anti-ASC antibodies significantly decreased the post-injury pathology (ASC is an intracellular target, and although that study showed that anti-ASC antibodies reduce inflammasome activity and enter neurons, it is unclear conceptually how this happens) (de Rivero Vaccari et al., 2009; de Rivero Vaccari et al., 2008). While those authors focused on and attributed the observed effects to neuronal inflammasomes, treatment with anti-ASC antibodies should globally disrupt inflammasome mobilization in all CNS cells and would also in theory affect glial inflammasomes. It therefore remains unclear whether the most potent inflammasome activity/IL-1β production after injury is exhibited by microglia, neurons, or astrocytes. Those few initial works imply harmful roles for inflammasome activation in models of CNS injury. Additional studies are needed, however, to establish how specific inflammasome-derived cytokines and downstream events, such as pyroptosis, contribute to overall disease pathogenesis.
Inflammasome activation also has implications for the alarmin IL-33. This alarmin is a member of the IL-1 cytokine family along with IL-1β, IL-1α, and IL-18. Cleavage by caspase-1, in contrast to it effect on IL-1β and IL-18, inactivates IL-33 (Cayrol and Girard, 2009). Certain other cleavage forms of IL-33, however, are more potent than the native form, particularly the products of neutrophil elastase and cathepsin G cleavage (Lefrancais et al., 2012). This makes sense, given the alarmin nature of IL-33. IL-1β is produced by effector cells and requires multiple signals to become fully active. IL-33, on the other hand, carries a rapid message from necrotic cells and therefore logically requires no processing to elicit its activity. Furthermore, neutrophils generally degranulate at the injury site, secreting enzymes like elastase that can cleave native IL-33 to a more active form and reinforce the effect of its release soon after injury, before it is neutralized by the inflammasome (Lefrancais et al., 2012).
Innate immunity – Neutrophils
After most injuries, the first peripheral cells to arrive on the scene are neutrophils. These are granular cells that are continuously produced in the bone marrow, and after a short life in circulation—about 5 days in humans and 12 hours in mice (Pillay et al., 2010)—are cleared by bone-marrow and liver macrophages (Shi et al., 2001; Summers et al., 2010). Once mobilized to a site of irritant-induced tissue damage, neutrophils can exert potent effector functions that include the rapid release of cytokines, chemokines, lytic enzymes, and growth factors (Summers et al., 2010). As with other injuries, large numbers of neutrophils appear at the site of CNS injury as soon as 4 hours after its occurrence and remain there for 2–3 days (Carlson et al., 1998; Trivedi et al., 2006). Numerous factors can promote neutrophil recruitment, including the chemokines KC and MIP2 (CXCL1 and 2, respectively). A recent study also showed that purinergic receptor-induced signaling, probably via inflammasome activity and increased chemokine production, profoundly influences neutrophil mobilization to sites of CNS injury (Roth et al., 2014).
Neutrophils are specialists in fighting pathogens, secreting key anti-microbial products, but their impact on sterile injury is less clear. Following their extravasation neutrophils release large amounts of effector molecules including proteases, cytokines, and free oxygen radicals to combat any invading pathogens (Amulic et al., 2012). On neuronal cells, however, these efforts have nonspecific and harmful effects. Blocking of the neutrophil-secreted enzyme elastase indeed improved recovery of rats after SCI (Tonai et al., 2001). Prevention of neutrophil migration into injured CNS tissue through inhibition of CXCR2 was found to ameliorate post-injury neuronal loss (Semple et al., 2010), as well as to promote tissue repair and enhance functional recovery (Gorio et al., 2007). An alternative method of preventing neutrophil influx into the injured tissue was based on knockout of C5, a neutrophil chemotactic component of the complement cascade; C5–/– mice were shown to have a deficit in neutrophil infiltration into the site of cryoinjury that correlated with a decrease in lesion size (Sewell et al., 2004). A number of other studies have linked beneficial pharmacological treatment to a decrease in initial neutrophil recruitment (Naruo et al., 2003; Ozevren et al., 2014).
Despite the wealth of supporting evidence, however, it might be premature to conclude that neutrophils are universally detrimental in CNS injury. In one study the authors sought to directly address the role of neutrophils in SCI by depleting mice of neutrophils using a depleting antibody that targeted the neutrophil surface protein Ly6G/GR-1. These mice showed worse functional hindlimb recovery and delayed astrocyte reactivity, suggesting that neutrophils have a positive effect on the local glial response (Stirling et al., 2009). An important caveat acknowledged by those authors, however, was the nonspecific nature of the cell depletion. Neutrophils in the antibody-treated mice were severely reduced, but both circulating monocytes and lymphocytes were also lowered, albeit to a lesser extent (Stirling et al., 2009). Another study of neutrophil depletion after crush injury of the mouse optic nerve (this time using a more neutrophil-specific Ly6G clone) also demonstrated a beneficial role for neutrophils, and showed that improved outcome of CNS injury was associated with their production of the atypical growth factor oncomodulin (Kurimoto et al., 2013). Given the existing data, therefore, it remains unclear whether the overall effect of neutrophils is beneficial or detrimental, and isolated aspects of their function can have dichotomous effects on outcome (Figure 3).
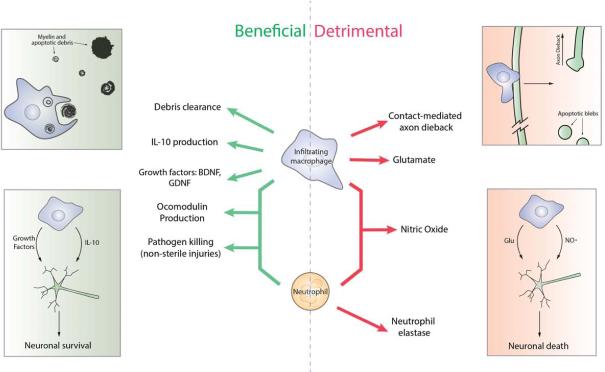
Figure 3. Beneficial and detrimental roles for macrophages and neutrophils in CNS injury.
Macrophages and neutrophils have been described to promote both beneficial and detrimental outcomes following CNS injury. Whether these cells orchestrate CNS repair or exacerbate tissue damage following CNS trauma depends on the specific factors that are generated. Beneficial roles for macrophages (top left) in the CNS include their ability to clear cell debris and produce growth factors and other protective molecules including BDNF, GDNF and IL-10. Detrimental roles for macrophages (top right) include production of glutamate and through contact-mediated axon dieback. Both macrophages and neutrophils beneficially produce the atypical growth factor oncomodulin and clear pathogens in non-sterile injuries (left), and detrimentally produce the free radical nitric oxide (right). Neutrophils additionally secrete the enzyme elastase, which was shown to be detrimental following injury (right).
Hours to Days Post CNS Injury: Infiltration of Monocytes
Innate immunity – Monocyte-derived Macrophages
Blood monocytes can be classified into two types based on their surface molecule profiles and unique functions: Ly6Chi monocytes, which express the chemokine receptor CCR2 and low levels of the chemokine receptor CX3CR1, and Ly6Clo monocytes (blood-resident monocytes), which express high levels of CX3CR1. Ly6Chi monocytes circulate in the blood and are recruited to sites of tissue injury in response to endothelial activation and chemokine gradients. Monocyte-derived macrophages (from now on termed just “macrophages” to distinguish them from microglia and other tissue-resident populations) are prominent cells at injury sites.
Macrophages are generally believed to carry out beneficial functions such as orchestrating wound-healing responses and coordinating the removal of debris in models of peripheral tissue injury. Infiltrating macrophages initially promote inflammation, but later they are necessary for its proper resolution, promotion of angiogenesis, scar formation, and secretion of growth factors (reviewed in (Brancato and Albina, 2011; Koh and DiPietro, 2011; Werner and Grose, 2003)). As expected on the basis of these functions, ablation of macrophages delays normal peripheral wound-healing responses in rodents (Mirza et al., 2009; Ramsebner et al., 2010) and regeneration of limbs in amphibians (Godwin et al., 2013). Macrophages are also important to regeneration after peripheral nerve injury, where they are robustly recruited by Schwann cells along the injured nerve and play a vital role in clearing myelin and apoptotic debris (Brosius Lutz and Barres, 2014; Tofaris et al., 2002; Vargas et al., 2010). The role of macrophages after CNS injury, however, is more controversial, with studies showing them to be either beneficial (Batchelor et al., 1999; Gadani et al., 2015; Kotter et al., 2001; London et al., 2011; Prewitt et al., 1997; Shechter et al., 2009; Shechter et al., 2013; Yin et al., 2006) or harmful (Evans et al., 2014; Horn et al., 2008; McPhail et al., 2004; Popovich et al., 1999), as discussed in detail in the next section.
Beneficial versus Destructive Roles of Macrophages in CNS Injury
After entering the CNS injury site macrophages have several functions, some of them shown to be beneficial and others detrimental (Figure 3). A well-known neurotoxic product of activated microglia and macrophages is glutamate, a primary CNS excitatory neurotransmitter, which is released in large quantities after injury and contributes to both neuronal excitotoxicity and secondary degeneration (Bullock et al., 1995; Doble, 1999; Yawata et al., 2008). Moreover, TNF—a cytokine robustly produced after injury—is a major stimulator of microglial production of glutamate and can further potentiate glutamate-induced killing of neurons (Leonoudakis et al., 2008; Olmos and Llado, 2014). Macrophages also contribute to secondary death through the production of nitric oxide synthase (iNOS) and free radicals. Free oxygen radicals are abundantly produced at the injury site and contribute to neuronal death (Lewen et al., 2000; Roth et al., 2014). Expression of iNOS by macrophages and perivascular cell is upregulated within hours of injury, and subsequent production of nitrogen oxide radicals can provoke neuronal apoptosis (Satake et al., 2000). Studies by Jerry Silver and colleagues have uncovered direct contacts between macrophages and neurons both in vivo and in vitro, which appear to precede and correlate with axon retraction (Evans et al., 2014; Horn et al., 2008). The nature of this interaction, its molecular mediators or purposes, is unclear. However, axon retraction in vivo is markedly reduced by treatment with clodronate liposomes, which kill engulfing phagocytes (Horn et al., 2008).
In addition to the many reports on the detrimental effects of macrophages, evidence from numerous labs suggests that macrophages also contribute to tissue protection and regeneration after CNS injury. Macrophages secrete growth-promoting molecules including neurotrophins and oncomodulin (Dougherty et al., 2000; Hashimoto et al., 2005; Yin et al., 2006), phagocytose inhibitory myelin debris (Ma et al., 2002), and promote pathogen clearance in non-sterile injuries (Shi and Pamer, 2011). One compelling link between inflammation and axon regrowth is oncomodulin, an atypical growth factor mentioned above as a neutrophil product. Oncomodulin is a potent factor in promoting axonal growth and is also produced by inflammatory macrophages after CNS injury (Yin et al., 2006). Stimulation of macrophages with the TLR2 agonist zymosan increases macrophage production of oncomodulin, which promotes axon regrowth in vitro and in vivo through the activation of CaMKII-dependent signaling (Yin et al., 2006). While its effects have been well studied in the eye, less is known about the role of oncomodulin in other CNS injury models such as SCI or TBI.
Schwartz and colleagues in 2009 addressed the overall impact of macrophages on SCI using two methods: by increasing the pool of monocytes via adoptive transfer, and by depleting myeloid cells using the CD11cDTR→WT bone-marrow chimeric mouse (diphtheria toxin receptor (DTR) is expressed only on CD11c+ cells, allowing blood-borne macrophages to be targeted) (Shechter et al., 2009). In both conditions macrophages proved beneficial; the addition of macrophages increased functional recovery, while their ablation with CD11cDTR exacerbated CNS pathology (Shechter et al., 2009). A major function identified in that work and ascribed to macrophages was production of IL-10, a cytokine that both dampens and promotes resolution of inflammation (Shechter et al., 2009). This study supported previously observed beneficial aspects (mostly reported from the same lab) of boosting macrophage numbers after CNS injury (Bomstein et al., 2003; Lazarov-Spiegler et al., 1998; Rapalino et al., 1998; Schwartz, 2010; Schwartz et al., 1999; Schwartz and Yoles, 2006).
In one line of experiments spanning numerous studies over several decades, homologous macrophages were activated in vitro in co-culture with explanted tissue that typically heals well (such as skin or peripheral nerve) before they were delivered directly to the CNS injury site (Schwartz, 2010; Schwartz and Yoles, 2006). The intention was to add phagocytes and encourage debris clearance, exploiting their activation by DAMPs from the skin (or other tissue with efficient healing properties) to instruct macrophages to become better tissue healers. This strategy proved to be beneficial in animal models of SCI (Schwartz, 2010), and was tested in phase I (Knoller et al., 2005) and II clinical trials (Jones et al., 2010; Lammertse et al., 2012). The phase II trials, however, failed to show a significant difference between control and treatment groups (Lammertse et al., 2012).
Macrophage Polarization in CNS Injury – is it Really that Simple?
Macrophages are heterogeneous immune cells that can be activated to fight pathogens and promote tissue regeneration (Epelman et al., 2014). These functions are often attributed to two macrophage phenotypes, M1 and M2. These phenotypes and their respective stimuli have been well described in vitro. M2 macrophages are induced by the cytokines IL-4 or IL-13 and characteristically express the markers arginase 1 (Arg1), CD206, and YM-1, while M1 macrophages are induced by interferon (IFN)-γ and LPS and express iNOS, TNF, and CCL5 as markers (Gordon, 2003; Sica and Mantovani, 2012). As described above, discrete beneficial and detrimental functions after CNS injury have been defined for macrophages (Figure 3). M1/M2 skewing is a convenient paradigm for explaining these divergent macrophage effects, with M1 products being harmful and M2 products beneficial, and numerous studies have been focused on macrophage skewing and the proportions of M1 versus M2 macrophages in the injured CNS (Girard et al., 2013; Kigerl et al., 2009; Kumar et al., 2013; Stirling et al., 2014; Turtzo et al., 2014; Wang et al., 2013). It is becoming increasingly evident, however, that although these paradigms clearly exist under controlled in vitro settings, they are too simplistic to describe macrophage phenotypes in vivo (Martinez and Gordon, 2014; Murray et al., 2014). This is apparent after CNS injury, where M1 and M2 markers do not appear in discrete groups but are often expressed simultaneously by inflammatory macrophages. For example, at early time points after injury (1–3 days), both the M1 marker Nos2 (the gene that encodes iNOS) and the M2 marker Arg1 (the gene encoding Arginase 1) are upregulated at the injury site (Kigerl et al., 2009). In another study by Hsieh and colleagues, Arg1-poitive cells were isolated and analyzed by microarray analysis at 3DPI after mouse TBI. They found, interestingly, that Arg1-positive cells, though assumed to be M2, do not express many other M2 markers and actually express multiple M1 markers (including nos2, consistent with the abovementioned findings of Kigerl et al.) (Hsieh et al., 2013). These findings are not altogether surprising, as it is known that macrophage polarization is not stringently defined in vivo (Martinez and Gordon, 2014). Macrophages can exhibit simultaneous expression of M1 and M2 markers, and may be able, given appropriate stimuli, to switch phenotypes (Pettersen et al., 2011; Sica and Mantovani, 2012; Vogel et al., 2013).
Under normal physiological conditions macrophage subsets in the body are heterogeneous (Ginhoux et al., 2010), and individual characteristics are imparted by tissue-specific signals (Butovsky et al., 2014; Okabe and Medzhitov, 2014). It is likely that similar heterogeneity in the macrophage population also develops after CNS injuries, and that under those conditions the unique infiltrating macrophage phenotype is influenced by the myriad of extracellular and inflammatory signals that ensue as a result of CNS tissue damage.
In summary, M1/M2 classifications as they exist under in vitro conditions do not appear to apply neatly to macrophages after CNS injury, and recent findings highlight the diversity of macrophage phenotypes that can arise from different stimulations (Xue et al., 2014). It thus seems that rather than trying to fit CNS-infiltrating macrophages into categories that are largely based on responses to isolated stimuli in vitro, efforts would be better spent in attempting to understand the unique subset(s) existing at the CNS injury site. These subsets can probably not be defined simply as “inflammatory” (M1) and “anti-inflammatory” (M2), but rather by unique, nuanced sets of functions and markers that are dependent on complex temporal, spatial, and signaling factors.
The ultimate role of macrophages in any sterile injury, and particularly in CNS injury, is an area of active investigation. Macrophages have been reported to perform both beneficial and detrimental functions in response to CNS trauma, but the degree of impairment caused by their depletion suggests that their overall role is protective (Gadani et al., 2015; Shechter et al., 2009). Improved characterization of the discrete macrophage phenotypes that arise in response to CNS injury will help to uncover the specific molecular pathways that promote beneficial macrophage responses during trauma, and can be expected to aid in the development of novel macrophage-based therapies for the treatment of CNS injury.
Days to Weeks Post CNS Injury: Recruitment of Adaptive Immune Cells
As with macrophages and neutrophils, the beneficial or detrimental nature of the adaptive immune system in CNS injury is a hazy picture that is slowly becoming better defined. Partly based on early dogma in the field of CNS injury, adaptive immune responses to the injury were assumed to be largely detrimental by default (Hickey et al., 1991; Popovich et al., 1996). Strikingly, however, secondary degeneration is more extensive in rodents that lack both T and B cells than in their wild-type counterparts (Moalem et al., 1999; Serpe et al., 1999; Yoles et al., 2001), suggesting a previously unknown neuroprotective role for adaptive immune cells in CNS injury. In immunodeficient (SCID or nude) mice, reconstitution of the immune compartment with T cells was found to improve recovery from CNS injury (Kipnis et al., 2002; Serpe et al., 2003), further suggesting that T cells have a role to play in neuroprotection. Interestingly, T cells specific to brain-restricted antigens were found to be particularly potent in promoting neuroprotection (Moalem et al., 1999), and it seemed that their migration to the injured CNS and their accumulation there were most probably governed by their CNS-antigen specificity (Archambault et al., 2005; Ling et al., 2006). Transfer of autoreactive T cells directed against CNS antigens indeed substantially reduced secondary degeneration after nerve injury in rats, and this neuroprotection could be conferred either by active immunization (with spinal cord homogenates or purified myelin proteins and adjuvant) or by passive immunization (via the transfer of pre-activated CNS-specific T cells) (Byram et al., 2004; Hauben et al., 2000; Moalem et al., 1999). However, antigen specificity is not an absolute requirement for T cell-mediated neuroprotection, as our recent studies have shown that T cells can endow appreciable neuroprotection after CNS injury even in the absence of antigen recognition (Walsh et al., 2015),
In the adaptive immune system pattern recognition has been largely overlooked, despite the fact that it plays an important part in the function of lymphocytes not only by affecting cellular migration and activation but also through modulation of their activity (Iwasaki and Medzhitov, 2010; Pasare and Medzhitov, 2004). Studies have identified PRRs on CD4+ T cells and pointed to TLR signaling as an important player in CD4+ T cell-mediated neuroprotection through its activity on regulatory T cells (Pasare and Medzhitov, 2003). One of the major mechanisms underlying PRR-induced activation of immune cells is induction of MyD88-mediated signaling. We recently observed that production of the neuroprotective cytokine IL-4 by CD4+ T cells in response to CNS injury is not dependent on classical T cell-receptor / MHC II engagement, but is critically dependent on MyD88-mediated signaling (Walsh et al., 2015). These findings point to pivotal roles for PRR-triggered activation of MyD88 signaling in the generation of neuroprotective T cell responses, although the specific PRR ligands that mediate this effect still remain to be elucidated. IL-4 production by injury-induced T cells was shown to promote CNS recovery downstream of this activation by acting on neurons directly, not via IL-4 signaling in macrophages (Walsh et al., 2015).
Conclusion
How the immune system responds to CNS injury remains a matter of debate, and there is still no general consensus as to whether it is mainly deleterious or beneficial. The notion that peripheral immunity causes only damage to the CNS is still prevalent, and is supported by the early relative success of the immunosuppressive drug methylprednisone in the treatment of CNS injury and the recognition that inflammation underlies the pathology of the autoimmune disease multiple sclerosis (Steinman et al., 2002). However, as described in this review, multiple lines of evidence from various models of CNS trauma now clearly demonstrate that immune cells can also have beneficial functions in the CNS. Moreover, emerging data suggest that there is extensive crosstalk between the immune system and glial cells even in the absence of overt CNS immunopathology. The concept of immune privilege was derived from early studies demonstrating that rejection of engrafted tissue is delayed in the brain, and should not be interpreted to preclude beneficial neuroimmune interactions, either in the healthy CNS or after injury. Inflammatory cells clearly have both beneficial and detrimental functions after CNS injury, but it appears that the overall effect of immune-cell subtypes on injury, as assessed by depletion or knockout studies, is generally beneficial.
This review surveys the progress of research carried out over the past two decades on the immune response to CNS injury, starting with the seminal discoveries in connection with DAMPs and the initiation of inflammation, and progressing through subsequent cell infiltration events. With regard to the specific events that occur in CNS injury, we note that the response of the immune system to brain injury does not differ substantially from its response to injury of any peripheral tissue.
Countries charge their military with two vital assignments—to destroy hostile groups invading their borders, and to aid their citizens when the need arises to cope, for example, with natural disasters. We view the immune system as functioning in much the same way; it defends the organism against dangerous invading pathogens, while also helping to protect it from the devastating effects of sterile injuries. Deeper understanding of DAMPs in the CNS will shed light on the pathologies associated with its injury, as well as on a host of neurodegenerative syndromes associated with sterile inflammation such as Alzheimer's disease (Rubio-Perez and Morillas-Ruiz, 2012), ALS (Frakes et al., 2014), autism spectrum disorders (Patterson, 2011), and epilepsy (Marchi et al., 2014). Further understanding of the initiation of inflammation will enable us to design more effective therapies for timely beneficial modulation of the immune response.
Acknowledgments
This work was primarily supported by a grant from the National Institute of Neurological Disorders and Stroke, NIH (NS061973 award to J. K).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Sachin P. Gadani – wrote the sections of the manuscript related to innate immune response, designed the manuscript, and created the figures.
James T. Walsh – wrote the sections of the manuscript related to adaptive immune response and participated in manuscript design.
John R. Lukens – wrote sections of the manuscript related to inflammasomes, participated in the design, and proofread the entire manuscript.
Jonathan Kipnis – designed the manuscript, participated in writing and proofreading, and oversaw the entire process of manuscript preparation.
All authors declare no conflict of interests.
References
- Algattas H, Huang JH. Traumatic Brain Injury pathophysiology and treatments: early, intermediate, and late phases post-injury. International journal of molecular sciences. 2014;15:309–341. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annual review of immunology. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. doi: 10.1002/eji.200425864. [DOI] [PubMed] [Google Scholar]
- Bareyre F, Wahl F, McIntosh TK, Stutzmann JM. Time course of cerebral edema after traumatic brain injury in rats: effects of riluzole and mannitol. Journal of neurotrauma. 1997;14:839–849. doi: 10.1089/neu.1997.14.839. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. Journal of immunology. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bomstein Y, Marder JB, Vitner K, Smirnov I, Lisaey G, Butovsky O, Fulga V, Yoles E. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. Journal of neuroimmunology. 2003;142:10–16. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- Bracken MB. Steroids for acute spinal cord injury. The Cochrane database of systematic reviews. 2012;1:CD001046. doi: 10.1002/14651858.CD001046.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. The American journal of pathology. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Experimental neurology. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A, Barres BA. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Developmental cell. 2014;28:7–17. doi: 10.1016/j.devcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Bryant CE, Monie TP. Mice, men and the relatives: cross-species studies underpin innate immunity. Open biology. 2012;2:120015. doi: 10.1098/rsob.120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Myseros JS, Marmarou A, Woodward JJ, Young HF. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Annals of the New York Academy of Sciences. 1995;765:290–297. doi: 10.1111/j.1749-6632.1995.tb16586.x. discussion 298. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Experimental neurology. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Carrico KM, Vaishnav R, Hall ED. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J Neurotrauma. 2009;26:1369–1378. doi: 10.1089/neu.2008-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center NSCIS. Facts and Figures at a Glance. 2014 [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Rodriguez Guerrero A, Kobayashi S, Ma WY, Liu SY, Zhu P, Baba H. High-mobility group box-1 and its receptors contribute to proinflammatory response in the acute phase of spinal cord injury in rats. Spine. 2011;36:2122–2129. doi: 10.1097/BRS.0b013e318203941c. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Daston MM, Ratner N. Amphoterin (P30, HMG-1) and RIP are early markers of oligodendrocytes in the developing rat spinal cord. Journal of neurocytology. 1994;23:323–332. doi: 10.1007/BF01188500. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S. P2X4 receptors influence inflammasome activation after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3058–3066. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. The Journal of experimental medicine. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends in pharmacological sciences. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1077. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacology & therapeutics. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Dong Y, Gu Y, Huan Y, Wang Y, Liu Y, Liu M, Ding F, Gu X, Wang Y. HMGB1 protein does not mediate the inflammatory response in spontaneous spinal cord regeneration: a hint for CNS regeneration. The Journal of biological chemistry. 2013;288:18204–18218. doi: 10.1074/jbc.M113.463810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiology of disease. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. The European journal of neuroscience. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. Journal of immunology. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido Y, Yoshitake A, Ito H, Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochemical and biophysical research communications. 2008;376:128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? Journal of leukocyte biology. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S. Anti-TNF therapy in the injured spinal cord. Trends in pharmacological sciences. 2011;32:107–115. doi: 10.1016/j.tips.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, Silver J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Experimental neurology. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Pan HC, Lin SL, Zhang WQ, Rauvala H, Schachner M, Shen YQ. HMGB1 contributes to regeneration after spinal cord injury in adult zebrafish. Molecular neurobiology. 2014;49:472–483. doi: 10.1007/s12035-013-8533-4. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Schwab ME, Montani L, Brazda N, Muller HW. Defeating inhibition of regeneration by scar and myelin components. Handbook of clinical neurology. 2012;109:503–522. doi: 10.1016/B978-0-444-52137-8.00031-0. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Wilson JR, Frankowski RF, Toups EG, Aarabi B, Harrop JS, Shaffrey CI, Harkema SJ, Guest JD, Tator CH, et al. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. Journal of neurosurgery Spine. 2012;17:151–156. doi: 10.3171/2012.4.AOSPINE1259. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JP, et al. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81:1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. Journal of immunology. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S, Brough D, Lopez-Castejon G, Giles J, Rothwell NJ, Allan SM. Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices. Glia. 2013;61:813–824. doi: 10.1002/glia.22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Hickey MJ, Espinosa JM, Nistor G, Lane TE, Keirstead HS. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regenerative medicine. 2007;2:771–783. doi: 10.2217/17460751.2.5.771. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gorio A, Madaschi L, Zadra G, Marfia G, Cavalieri B, Bertini R, Di Giulio AM. Reparixin, an inhibitor of CXCR2 function, attenuates inflammatory responses and promotes recovery of function after traumatic lesion to the spinal cord. J Pharmacol Exp Ther. 2007;322:973–981. doi: 10.1124/jpet.107.123679. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Experimental neurology. 2001;168:273–282. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- Gu XJ, Xu J, Ma BY, Chen G, Gu PY, Wei D, Hu WX. Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin J Traumatol. 2014;17:1–7. [PubMed] [Google Scholar]
- Guo JD, Li L, Shi YM, Wang HD, Yuan YL, Shi XX, Hou SX. Genetic ablation of receptor for advanced glycation end products promotes functional recovery in mouse model of spinal cord injury. Molecular and cellular biochemistry. 2014;390:215–223. doi: 10.1007/s11010-014-1972-z. [DOI] [PubMed] [Google Scholar]
- Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. The European journal of neuroscience. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16:99–102. doi: 10.1097/00001756-200502080-00004. [DOI] [PubMed] [Google Scholar]
- Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, et al. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. Journal of Neuroscience. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hickman SE, El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer's disease. CNS & neurological disorders drug targets. 2010;9:168–173. doi: 10.2174/187152710791011982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Kim CC, Ryba BE, Niemi EC, Bando JK, Locksley RM, Liu J, Nakamura MC, Seaman WE. Traumatic brain injury induces macrophage subsets in the brain. European journal of immunology. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. Journal of leukocyte biology. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunology today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, Alves-Filho JC, Togbe D, Goodyear CS, Linington C, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. European journal of immunology. 2012;42:1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- Jones LA, Lammertse DP, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Poonian D, Betz RR, Knoller N, Heary RF, et al. A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal cord. 2010;48:798–807. doi: 10.1038/sc.2010.29. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. The Journal of biological chemistry. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108:115–125. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A. Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:643–650. doi: 10.1007/s11481-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Lee SH, Kim YG, Kim SY, Seo JW, Choi YW, Kim DJ, Jeong KH, Lee TW, Ihm CG, et al. Hyperuricemia-induced NLRP3 activation of macrophage contributes to the progression of diabetic nephropathy. American journal of physiology Renal physiology. 2015:ajprenal 00637 02014. doi: 10.1152/ajprenal.00637.2014. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nature reviews Immunology. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: Naturally occurring CD4(+)CD25(+) regulatory T cells suppress the ability to withstand injury to the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Annals of neurology. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. Journal of neurosurgery Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert reviews in molecular medicine. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiology of aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI. Neutrophils express oncomodulin and promote optic nerve regeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14816–14824. doi: 10.1523/JNEUROSCI.5511-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertse DP. Clinical trials in spinal cord injury: lessons learned on the path to translation. The 2011 International Spinal Cord Society Sir Ludwig Guttmann Lecture. Spinal cord. 2013;51:2–9. doi: 10.1038/sc.2012.137. [DOI] [PubMed] [Google Scholar]
- Lammertse DP, Jones LA, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Falci SP, Heary RF, Choudhri TF, Jenkins AL, et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal cord. 2012;50:661–671. doi: 10.1038/sc.2012.39. [DOI] [PubMed] [Google Scholar]
- Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Molecular carcinogenesis. 2009;48:571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature reviews Immunology. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Schwartz M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia. 1998;24:329–337. [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Zhao P, Beattie EC. Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. Journal of neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Ling C, Sandor M, Suresh M, Fabry Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J Neurosci. 2006;26:731–741. doi: 10.1523/JNEUROSCI.3502-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, Zhou ML, Zhu L, Hang CH. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochemical research. 2013;38:2072–2083. doi: 10.1007/s11064-013-1115-z. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annual review of neuroscience. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. The Journal of experimental medicine. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Barr MJ, Chaplin DD, Chi H, Kanneganti TD. Inflammasome-derived IL-1beta regulates the production of GM-CSF by CD4(+) T cells and gammadelta T cells. Journal of immunology. 2012a;188:3107–3115. doi: 10.4049/jimmunol.1103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Gross JM, Kanneganti TD. IL-1 family cytokines trigger sterile inflammatory disease. Frontiers in immunology. 2012b;3:315. doi: 10.3389/fimmu.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Kanneganti TD. Beyond canonical inflammasomes: emerging pathways in IL-1-mediated autoinflammatory disease. Seminars in immunopathology. 2014;36:595–609. doi: 10.1007/s00281-014-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. The European journal of neuroscience. 2007;25:1711–1724. doi: 10.1111/j.1460-9568.2007.05390.x. [DOI] [PubMed] [Google Scholar]
- Ma M, Wei T, Boring L, Charo IF, Ransohoff RM, Jakeman LB. Monocyte recruitment and myelin removal are delayed following spinal cord injury in mice with CCR2 chemokine receptor deletion. Journal of neuroscience research. 2002;68:691–702. doi: 10.1002/jnr.10269. [DOI] [PubMed] [Google Scholar]
- Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends in neurosciences. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Essay 1: the Danger model in its historical context. Scandinavian journal of immunology. 2001;54:4–9. doi: 10.1046/j.1365-3083.2001.00974.x. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- McPhail LT, Stirling DP, Tetzlaff W, Kwiecien JM, Ramer MS. The contribution of activated phagocytes and myelin degeneration to axonal retraction/dieback following spinal cord injury. The European journal of neuroscience. 2004;20:1984–1994. doi: 10.1111/j.1460-9568.2004.03662.x. [DOI] [PubMed] [Google Scholar]
- Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. British journal of experimental pathology. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13046–13050. doi: 10.1073/pnas.1002396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Mortality GBD, Causes of Death C. Global, regional, and national age sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaino M, Nakamura M, Yamada O, Okada S, Morikawa S, Renault-Mihara F, Iwanami A, Ikegami T, Ohsugi Y, Tsuji O, et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Experimental neurology. 2010;224:403–414. doi: 10.1016/j.expneurol.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, Engelhardt B, Hallenbeck JM, Lonser RR, Ohlfest JR, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruo S, Okajima K, Taoka Y, Uchiba M, Nakamura T, Okabe H, Takagi K. Prostaglandin E1 reduces compression trauma-induced spinal cord injury in rats mainly by inhibiting neutrophil activation. Journal of neurotrauma. 2003;20:221–228. doi: 10.1089/08977150360547125. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norose K, Kikumura A, Luster AD, Hunter CA, Harris TH. CXCL10 is required to maintain T-cell populations and to control parasite replication during chronic ocular toxoplasmosis. Investigative ophthalmology & visual science. 2011;52:389–398. doi: 10.1167/iovs.10-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, Ohsugi Y, Iwamoto Y, Yoshizaki K, Kishimoto T, Toyama Y, Okano H. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. Journal of neuroscience research. 2004;76:265–276. doi: 10.1002/jnr.20044. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, Yoshino T, Ohtsuka A, Otani N, Tomura S, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72:373–384. doi: 10.1002/ana.23602. [DOI] [PubMed] [Google Scholar]
- Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators of inflammation. 2014;2014:861231. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Ozevren H, Toklu HZ, Berkman MZ, Turan P, Sirvanci S, Kahveci R. Riluzole alleviates early neutrophil infiltration, brain oedema and lipid peroxidation in the traumatic brain tissue but does not induce neurotoxicity in non-traumatic brain tissue in rats. Injury. 2014 doi: 10.1016/j.injury.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends in molecular medicine. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, Pontremoli S, Melloni E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol. 2007;179:8525–8532. doi: 10.4049/jimmunol.179.12.8525. [DOI] [PubMed] [Google Scholar]
- Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, Pierson KC, Pitts-Kiefer A, Fan L, Belkin DA, Wang CQ, Bhuvanendran S, Johnson-Huang LM, et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. The Journal of investigative dermatology. 2011;131:1322–1330. doi: 10.103/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, Wojciechowski S, Kanninen K, Koistinaho J, Malm T. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain, behavior, and immunity. 2015;44:68–81. doi: 10.1016/j.bbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Experimental neurology. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. Journal of neuroscience research. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Niesman IR, Kane CJ, Houle JD. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Experimental neurology. 1997;148:433–443. doi: 10.1006/exnr.1997.6694. [DOI] [PubMed] [Google Scholar]
- Ramsebner R, Ludwig M, Parzefall T, Lucas T, Baumgartner WD, Bodamer O, Cengiz FB, Schoefer C, Tekin M, Frei K. A FGF3 mutation associated with differential inner ear malformation, microtia, and microdontia. The Laryngoscope. 2010;120:359–364. doi: 10.1002/lary.20689. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. The Journal of clinical investigation. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature medicine. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia. 2014;62:1895–1904. doi: 10.1002/glia.22676. [DOI] [PubMed] [Google Scholar]
- Ratsimandresy RA, Dorfleutner A, Stehlik C. An Update on PYRIN Domain-Containing Pattern Recognition Receptors: From Immunity to Pathology. Frontiers in immunology. 2013;4:440. doi: 10.3389/fimmu.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. TheScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo CF, Friedland JF, Dawson DR, Colantonio A, Lindsay PH. Mild traumatic brain injury from motor vehicle accidents: factors associated with return to work. Arch Phys Med Rehabil. 1999;80:392–398. doi: 10.1016/s0003-9993(99)90275-7. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Saleh A, Smith DR, Tessler L, Mateo AR, Martens C, Schartner E, Van der Ploeg R, Toth C, Zochodne DW, Fernyhough P. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Experimental neurology. 2013;249:149–159. doi: 10.1016/j.expneurol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain research Molecular brain research. 2000;85:114–122. doi: 10.1016/s0169-328x(00)00253-9. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schwartz M. “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: from skin-activated macrophages to infiltrating blood-derived cells? Brain, behavior, and immunity. 2010;24:1054–1057. doi: 10.1016/j.bbi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Lazarov-Spiegler O, Rapalino O, Agranov I, Velan G, Hadani M. Potential repair of rat spinal cord injuries using stimulated homologous macrophages. Neurosurgery. 1999;44:1041–1045. doi: 10.1097/00006123-199905000-00057. discussion 1045-1046. [DOI] [PubMed] [Google Scholar]
- Schwartz M, London A. Immune maintenance in glaucoma: boosting the body's own neuroprotective potential. Journal of ocular biology, diseases, and informatics. 2009;2:73–77. doi: 10.1007/s12177-009-9025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Yoles E. Immune-based therapy for spinal cord repair: autologous macrophages and beyond. Journal of neurotrauma. 2006;23:360–370. doi: 10.1089/neu.2006.23.360. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol Dis. 2010;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:Rc7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, Sandor M, Fabry Z. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J Neuroimmunol. 2004;155:55–63. doi: 10.1016/j.jneuroim.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS medicine. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. CNS sterile injury: just another wound healing? Trends Mol Med. 2013;19:135–143. doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Immunology of relapse and remission in multiple sclerosis. Annual review of immunology. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annual review of neuroscience. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain : a journal of neurology. 2014;137:707–723. doi: 10.1093/brain/awt341. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends in immunology. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum T, Essmann F, Adam R, Seibt A, Janicke RU, Novotny GE, Galla HJ, Schroten H. Cell death, caspase activation, and HMGB1 release of porcine choroid plexus epithelial cells during Streptococcus suis infection in vitro. Brain research. 2006;1100:1–12. doi: 10.1016/j.brainres.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1939–1947. doi: 10.1038/jcbfm.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. Journal of neurochemistry. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clinical neuroscience research. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. Journal of. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11993–11998. doi: 10.1073/pnas.1001948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. Journal of neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nature reviews Neuroscience. 2014a;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, Gadani S, Hechler D, Golz G, Rosenberger K, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. The Journal of clinical investigation. 2015;125:699–714. doi: 10.1172/JCI76210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology. 2014b;141:340–344. doi: 10.1111/imm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Shen J, Xiong N, Zhao H, Chen Y. Protein kinase B is involved in Nogo-66 inhibiting neurite outgrowth in PC12 cells. Neuroreport. 2011;22:733–738. doi: 10.1097/WNR.0b013e32834a58e8. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiological reviews. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wicher G, Husic E, Nilsson G, Forsberg-Nilsson K. Developmental expression of IL-33 in the mouse brain. Neuroscience letters. 2013;555:171–176. doi: 10.1016/j.neulet.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cellular signalling. 2013;25:2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain research. 2011;1385:8–17. doi: 10.1016/j.brainres.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Yawata I, Takeuchi H, Doi Y, Liang J, Mizuno T, Suzumura A. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life sciences. 2008;82:1111–1116. doi: 10.1016/j.lfs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nature neuroscience. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Experimental Neuroloy. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- Yong VW, Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64:55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Zhai CL, Zhang MQ, Zhang Y, Xu HX, Wang JM, An GP, Wang YY, Li L. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin. 2012;33:1477–1487. doi: 10.1038/aps.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Kuja-Panula J, Rouhiainen A, Chen YC, Panula P, Rauvala H. High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. The Journal of biological chemistry. 2011;286:23200–23213. doi: 10.1074/jbc.M111.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]