Abstract
Pluripotent human hepatic stem cells have broad research and clinical applications, which are, however, restricted by both limited resources and technical difficulties with respect to isolation of stem cells from the adult or fetal liver. In this study, we developed a convenient and efficient method involving a two-step in situ collagenase perfusion, gravity sedimentation, and Percoll density gradient centrifugation to enrich and maintain highly proliferative human fetal liver stem cells (hFLSCs). Using this method, the isolated hFLSCs entered into the exponential growth phase within 10 days and maintained sufficient proliferative activity to permit subculture for at least 20 passages without differentiation. Immunocytochemistry, immunofluorescence, and flow cytometry results showed that these cells expressed stem cell markers, such as c-kit, CD44, epithelial cell adhesion molecule (EpCAM), oval cell marker-6 (OV-6), epithelial marker cytokeratin 18 (CK18), biliary ductal marker CK19, and alpha-fetoprotein (AFP). Gene expression analysis showed that these cells had stable mRNA expression of c-Kit, EpCAM, neural cell adhesion molecule (NCAM), CK19, CK18, AFP, and claudin 3 (CLDN-3) throughout each passage while maintaining low levels of ALB, but with complete absence of cytochrome P450 3A4 (C3A4), phosphoenolpyruvate carboxykinase (PEPCK), telomeric repeat binding factor (TRF), and connexin 26 (CX26) expression. When grown in appropriate medium, these isolated liver stem cells could differentiate into hepatocytes, cholangiocytes, osteoblasts, adipocytes, or endothelial cells. Thus, we have demonstrated a more economical and efficient method to isolate hFLSCs than magnetic-activated cell sorting (MACS). This novel approach may provide an excellent tool to isolate highly proliferative hFLSCs for tissue engineering and regenerative therapies.
Introduction
The transplantation of human hepatic stem cells to the liver as an alternative therapy for the treatment of various liver diseases has aroused increasing interest in the field of stem cell therapy.1–4 However, the lack of healthy donor livers, low proliferative ability of cultured hepatocytes, and poor viability of hepatocytes after cryopreservation pose an obstacle to long-term maintenance, sub-culturing, and efficient transplantation.5–7 These problems are likely to be overcome by liver stem cells, which have an excellent pluripotent ability and potential to generate both hepatocytes and biliary epithelial cells.8–10 Therefore, robust in vitro expansion of hepatic stem cells without loss of their developmental potential, as well as establishment of cell differentiation protocols for the generation of functional hepatocytes, is essential to therapeutic cell transplantation.11,12 Only then will they become an invaluable tool for stem cell therapy, liver repopulation, drug development, establishment of a hepatic virus culture model, and bio-artificial liver support systems.9,13
During liver development, the hepatic bud arises from the foregut endoderm, and the number of hepatic stem cells varies with the developmental stage, mostly in fetal and neonatal livers.14–16 In adults, the number of hepatic stem cells is limited, which makes isolation of hepatic stem cells challenging.17 The fetal liver (FL), which has an enriched population of liver stem cells with low cell immunogenicity and strong proliferative ability, is an appealing source for the isolation of liver stem cells.18 In rodents, there is considerable success in isolating precursor cells from the fetal liver and oval cells from the adult liver.19,20 Suzuki et al. isolated murine fetal liver stem cells (c-met+/CD49F+/CD29+/CD45−/CDTER119−) that not only differentiated into hepatocytes and bile duct cells, but also were capable of differentiating into intestinal and pancreatic epithelial cells.21 However, due to strong human immune rejection of xenografts, the stem cells derived from rodents are unlikely to be applied clinically.22,23
The traditional three-dimensional co-culture approach to isolation of human fetal liver stem cells (hFLSCs) is both complicated and time consuming, taking as much as over 3 months for cells to enter into the exponential growth phase.24–26 Fluorescence or magnetic-activated cell sorting (FACS or MACS) based on the immunoselection of negative or positive surface markers (e.g., CD34, c-kit, Thy-1, or epithelial cell adhesion molecule [EpCAM]) on the liver stem cells was developed in the previous decade.12,27–29 However, these cell-surface markers are not specific to liver stem cells because liver stem cells represent a dynamic cellular compartment that keeps changing morphology and phenotypes in correlation with their differentiation, and there is no known specific marker for identification of hFLSCs.30,31 In addition, isolation procedures have been proven difficult and laborious; the special requirements for equipment and skills as well as the high costs limit the broad application of FACS or MACS. Moreover, various digestive enzymes used in different dissociation methods may also target cell-surface proteins non-specifically, consequently affecting the analysis of important cell-surface markers in the liver stem cells.32
To overcome the above limitations, we developed a user-friendly and efficient multi-step method to isolate hFLSCs with highly proliferative capacity, which consisted of a two-step in situ collagenase perfusion followed by gravity sedimentation and Percoll density gradient centrifugation (denoted as CSP method). To assess the efficacy of this method, the cell growth characteristics, immunophenotype, cell-surface markers, gene expression profiles, and pluripotent differentiation function of isolated cells were examined. This CSP method proved to be more user friendly when used to enrich liver stem cells than the MACS method. More importantly, because this method did not require any specific cell-surface markers, which may affect the development of hFLSCs,33,34 it was able to provide a large number of hFLSCs for clinical application and experimental study.
Materials and Methods
Ethics
This work was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The Ethics Committee confirmed that the study had complied with the regulations concerning ethics of scientific research formulated by the Institute of Health and Environmental Medicine and the Peking Union Medical College Hospital. Human fetal liver tissues were obtained from aborted fetuses at 12–20 weeks gestation with informed consent from patients. All of the donors had been screened serologically for syphilis, toxoplasmosis, rubella, hepatitis B and C, human immunodeficiency virus 1, cytomegalovirus, parvovirus, and herpes simplex types 1 and 2.
Cell isolation by the CSP method
After they were aborted, the fetuses were used to separate liver stem cells within 2 hr, and their peritoneal fluid containing peritoneal cells was collected as a supplement to culture medium. Non-hepatic tissue was removed. A single-cell suspension was prepared by the methods of combination of two-step in situ collagenase perfusion, gravity sedimentation, and Percoll density gradient centrifugation according to Mathijs et al. with modifications.35 The liver was perfused after cannulation of the umbilical vein. The heart was cut through, and the thoracic inferior vena cava was occluded with forceps. The organ was washed with Hanks' calcium- and magnesium-free buffer for 3 min. After the liver was free of blood, the calcium-free buffer was replaced by 100 mL of type IV collagenase (1 mg/mL, Sigma, USA) buffer for 15 min. A perfusion rate of 7 mL/min and a temperature around 37°C were maintained during the entire procedure. After the perfusion was terminated, the whole liver was quickly excised from the body cavity and transferred to a sterile Petri dish. The gall bladder and remnants of the diaphragm were removed.
Cells were released by disrupting the liver capsule mechanically and were separated from undigested tissue with a sterile 70-μm mesh nylon filter. After that, cells were washed twice with Dulbecco's modified Eagle medium (DMEM)/F12 (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, USA), and pelleted by centrifugation at 500×g for 5 min at 4°C. The cell pellet was re-suspended in 50 mL of DMEM/F12. Each 10 mL of the cell suspension was left in a vertically positioned tube for 20 min on ice to obtain stem cell sediment by intercellular adhesion and gravity. After sedimentation, different volumes of supernatant, including 3 mL, 5 mL, 7 mL, and 9 mL, were collected and centrifuged at 500×g for 5 min. The cell deposition was re-suspended with 5 mL of DMEM/F12 containing 10% FBS and cultured on plastic dishes coated with collagen type I to observe how many colonies of stem cell were formed. The sediment of stem cells in each tube was re-suspended with 3 mL of DMEM/F12 and underwent Percoll density gradient centrifugation containing a series of different density layers of Percoll (30%, 50%, 70%, and 80%), centrifuged at 12,000×g for 30 min at 4°C. Cells at each interface between the density layers of Percoll were collected and washed twice with DMEM/F12, and then the cells were cultured on plastic dishes coated with collagen type I to observe the monoclonal formation. The viability of the cells was assessed by the 0.2% Trypan Blue dye exclusion test.
Cell isolation by MACS
MACS, one of the most widely used methods, was also used in this study as a standard comparison.29,36 Briefly, after the cell pellet was re-suspended in DMEM/F12 in the CSP method, cell sorting for EpCAM+ cells was performed using MACS according to the manufacturer's instructions (Dynabeads® Biotin Binder Invitrogen™, USA). A total of 5×107 cells were incubated in the dark at 4°C with antibody biotin anti-human EpCAM (Santa Cruz, USA) for 20 min at a concentration of 3 μg/mL in 500 μL of phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA (Sigma, USA). EpCAM+ cells were labeled with magnetic beads using a mixer that provided tilting and rotation of the tubes to prevent the Dynabeads from settling down at the bottom of the tube. After 15 min of incubation, the tube was placed in a magnet for 2 min, and the supernatant was discarded. Finally, the target cells were released by washing the beads twice with 2 mL of the release buffer. All incubation and selection steps were performed on ice or at 4°C with addition of 10% Accutase (Innovative Cell Technologies, San Diego, CA) to prevent aggregation of cells.
Cell culture and cryopreservation
hFLSCs were incubated at 37°C in an atmosphere of about 5% CO2 on type I collagen- (Sigma, USA) coated polystyrene flasks or six-well plates (Corning, USA). Cells were grown in DMEM/F12 medium supplemented with 5% peritoneal fluid, 50 mg/L l-glutamine (Sigma, USA), 50 U/mL penicillin, 50 U/mL streptomycin, 1 mmol/L pyruvic acid sodium, 2% B27 (Gibco, USA), 10% FBS, 10 ng/mL hepatocyte growth factor (HGF; Pepro Tech, USA), 20 ng/mL of epidermal growth factor (EGF; Pepro Tech, USA), 10 ng/mL leukemia inhibitory factor (LIF; Chemicon, USA), and 10 ng/mL stem cell growth factor (SCF; Pepro Tech, USA). Non-adherent cells were removed after 24 hr and subsequently the medium was half-changed every 2 days. For sub-passaging, near-confluent cultures were split 1:3 using TrypLE (Gibco, USA) for 2–3 min at 37°C.
A total of 1×106 cells were re-suspended in 2 mL of freezing mixture (DMEM/F12, FBS, and dimethylsulfoxide at a 7:2:1 ratio [vol/vol], respectively) in cryofreezing containers (Nalge Nunc International, NY). Cells were stored at −80°C for 1 day and transferred to liquid nitrogen. The freezing process was applied to both cultured cells and freshly isolated cells with these two methods.
Immunocytochemistry of cultured cells
The trypsinized cells were collected using PBS. Smears were prepared on glass slides and fixed with 95% alcohol for 10–15 min. Endogenous peroxidases were eliminated in 3% hydrogen peroxidase (H2O2) for 10 min followed by blocking with 5% normal goat serum (Zymed, USA) for 15 min. Afterward, cells were incubated with mouse anti-human alpha-fetoprotein (AFP), oval cell marker-6 (OV-6), cytokeratin 8 (CK8), CK19, and EpCAM (Santa Cruz, USA) diluted 1:100 (in PBS) or control mouse immunoglobulin G (IgG) at 37°C for 3 hr and washed with PBS three times. Smears were then incubated with biotin-conjugated goat anti-mouse IgG (Santa Cruz, USA) at room temperature for 15 min and then washed with PBS three times. Smears were stained with streptavidin-horseradish peroxidase conjugate (SA-HRP; Zymed, USA) at 37°C for 15 min and then washed with PBS three times; the chromogenic reaction was developed with 3,3′-diaminobenzidine (DAB) or 3-amino-9-ethyl-carbazole (AEC) (Boster, China), and some of the smears were counterstained with Hematoxylin.
Fluorescence microscopy imaging of cultured cells
The trypsinized cells were collected by washing with PBS. Smears were prepared on glass slides and fixed with methanol at −20°C for 5 min and acetone at 4°C for 15 sec. The smears were washed with PBS and blocked with 1% BSA (Sigma, USA) in PBS for 1 hr at room temperature, while cells were incubated with mouse anti-human c-Kit (Santa Cruz, USA) diluted 1:100 (in PBS) at 37°C for 3 hr. After washing with PBS three times, smears were stained with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG (Santa Cruz, USA), diluted 1:1000 in PBS at room temperature for 45 min, and protected from light. Blocking solution alone, mouse IgG, and normal rabbit serum were used as negative controls. After incubation, the cells were washed with PBS twice and analyzed under fluorescence and visible light. Nuclear counterstaining was achieved by 5 min of incubation with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, USA).
Analysis of cell-surface markers by fow cytometry
For analysis of cell-surface makers, the hFLSCs suspension was prepared by trypsin/EDTA, and then 5×105 cells were suspended in 1 mL of blocking buffer prior to staining, which consisted of a base buffer of PBS with 0.3% BSA (Sigma, USA). Cells were stained sequentially with phycoerythrin (PE)-conjugated mouse monoclonal anti-human c-kit (Biolegend, USA), FITC-conjugated mouse monoclonal anti-human CD44, EpCAM, OV-6, CK19, CK18 (Abcam, USA), AFP, albumin (ALB), CD90, CD34, or CD81 (R&D, USA) for 60 min at 4°C, and washed twice with PBS. Cells were also stained with 4 μg/mL propidium iodide in PBS before being passed through a FACSCalibur (BD Biosciences, San Jose, USA) flow cytometer. Mouse IgG1κ–FITC, IgG2aκ–FITC (Abcam, USA) were used as isotype controls. The experiments were repeated three times.
Analysis of gene expression
Gene expression was analyzed by quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR). Total RNA from hFLSCs was extracted using TRIzol (Invitrogen, USA) according to the manufacturer's instructions and reverse-transcribed by Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo(dT)(12–18) primer (Gene Link, Hawthorne, NY). cDNA was used as template in conventional PCR with gene-specific primers (for sequences, see Table 1); the forward primer possessed a 5′ overhang for T7-promoter sequence (5′-gac tcg taa tac gac tca cta tag gg). This amplified gene-specific DNA was used for in vitro transcription with T7 RNA polymerase (Takara, In vitro Transcription T7 Kit, DaLian), generating gene-specific RNA (with an additional 5′-ggg included by T7 RNA polymerase) used as standards in real-time RT-qPCR using gene-specific primers without the 5′ overhang; standard ranges were linear from 1 to 108 templates. Primers were selected on different exons wherever possible. Quantitative real-time RT-PCR was performed in a LightCycler instrument using the LightCycler RNA Master SYBR Green I kit (Invitrogen, USA).
Table 1.
List of Primer Sequences Used in the Study
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product length (bp) |
|---|---|---|---|
| ALB | gtgggcagcaaatgttgtaa | tcatcgacttccagagctga | 188 |
| C3A4 | gcctggtgctcctctatcta | ggctgttgaccatcataaaagc | 187 |
| CK19 | ccgcgactacagccactact | gagcctgttccgtctcaaac | 152 |
| CKIT | gatgacgagttggccctaga | caggtagtcgagcgtttcct | 233 |
| CLDN3 | accaccaccaccaacacc | tgaggtttcacagtccatgc | 211 |
| CX26 | actccaccagcattggaaag | cgtagcacacgttcttgcag | 150 |
| CX32 | accaattcttccccatctcc | cctcaagccgtagcattttc | 132 |
| DPP4 | tccatatccaaaggcaggag | tctttcttgtgttgcccatg | 237 |
| EpCAM | ctggccgtaaactgctttgt | agcccatcattgttctggag | 182 |
| NCAM | agggcacttatcgctgtgag | atcctttgtccagctcatgg | 200 |
| PEPCK | ggatgaagtttgacgcacaa | cggccacattggtaaagatt | 144 |
| TRF | ctacacaggcgctttcaggt | taccatcaaggcacagcaac | 152 |
| GAPDH | atgttcgtcatgggtgtgaa | gtcttctgggtggcagtgat | 173 |
ALB, albumin; C3A4, cytochrome P450 3A4; CK19, cytokeratin 19; CKIT, c-kit (v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog); CLDN, claudin; CX, connexin; DPP4, dipeptylpeptidase 4; EpCAM, epithelial cell adhesion molecule; NCAM, neural cell adhesion molecule; PEPCK, phosphoenolpyruvate carboxykinase; TRF, transferrin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
In vitro hepatocyte, osteogenic, adipogenic, and endothelial differentiation
For hepatocyte differentiation, cells were cultured in DMEM/F12 that was supplemented with 10% FBS, 10 ng/mL HGF, and 10 ng/mL fibroblast growth factor (FGF4; Pepro Tech, USA).37 The medium was changed twice per week for 3 weeks. To evaluate the differentiation, cells were fixed with 4% paraformaldehyde for 20 min and stained with Periodic Acid Schiff (PAS; Sigma, USA) for 20 min at room temperature. After 15 days, the secretion of ALB into the supernatant of hFLSCs was evaluated under culture conditions described above. For this purpose, culture medium was changed completely every 2 days. Secretion of ALB was determined by nephelometry (Siemens, Germany), in which case cells were cultured without FBS. The glucose 6-phosphatase (G6Pase) activity was determined as described by Teutsch.38 In addition, we also investigated the Indocyanine Green assay (ICG; Sigma, USA), urea production (Bioassay, USA), and low-density lipoprotein uptake assay (ADI, USA) for liver function assay.39,40
For cholangiocyte differentiation, cells were treated with biliary differentiation medium (DMEM/F12 supplemented with 10−5 M linoleic acid–ALB [Sigma, USA], 5.10−8 M 3, 3,5-triiodo-l-thyronine [Sigma, USA], 0.2 IU insulin, 6.10−4 M vitamin C [Invitrogen, USA], 6.10−5 M human apo-transferrin [Sigma, USA], 1 mM sodium pyruvate [Sigma, USA], 50 ng/mL human growth hormone and 10 ng/mL interleukin-6 [hGH and IL-6; Pepro Tech, USA], 25 ng/mL EGF [Pepro Tech, USA], 10 μM sodium taurocholate hydrate [Sigma, USA]) according to Dianat et al. with modifications.41 To evaluate the differentiation, immunofluorescence was used to identify the expression of CK19 and CK7 in cultured cells.
For osteogenic differentiation, cells were cultured in DMEM supplemented with 10% FBS, 12 mmol/L l-glutamine, 20 mmol/L β-glycerol phosphate, 50 ng/mL thyroxine, 1 nmol/L dexamethasone, and 0.5 μmol/L ascorbate 2-phosphate (all from Sigma, USA).42 The medium was changed twice per week for 3 weeks. To evaluate the differentiation, cells were fixed with 4% paraformaldehyde for 20 min at room temperature and stained with Alizarin Red (pH 4.1) (Sigma, USA) for 20 min at room temperature. Von Kossa stain was used after 21 days of culture to detect the deposition of calcium in the extracellular matrices. Briefly, cell layers were washed with PBS, fixed with 4% paraformaldehyde, immersed in a solution of 1% silver nitrate (AgNO3), and exposed to bright ultraviolet light for 45 min. After being washed with distilled water, black stain, which indicated the presence of phosphate, was detected by developing the samples in a 5% sodium thiosulfate bath for 10 min.
For adipogenic differentiation, cells were cultured in DMEM supplemented with 10% FBS, 12 mmol/L l-glutamine, 5 μg/mL insulin, 50 μmol/L indomethacin, 1×10−6 mol/L dexamethasone, and 0.5 μmol/L 3-isobutyl-1-methylxanthine (all from Sigma, USA).43 The medium was changed twice per week for 3 weeks. To evaluate the differentiation, cells were fixed with 4% paraformaldehyde for 20 min at room temperature and stained with 0.5% Oil Red O (Sigma, USA) in methanol (Sigma, USA) for 20 min at room temperature. The ratio of positive to total cells was calculated as percentages.
Endothelial cell differentiation was obtained by culturing hFLSCs in Endothelial Basal Medium (EBM; Cambrex, USA) with 10 ng/mL vascular endothelial growth factor (VEGF; Sigma, USA) for 10 days as described previously.43 To evaluate the differentiation, immunohistochemistry and immunofluorescence were used to identify the phenotypes, including CD31 and Factor VIII-related antigen of the cultured cells.
Statistical analyses
All experiments were conducted as at least three separate experiments and in duplicate. Data are expressed as mean±standard deviation (SD) and analyzed with SPSS for Windows (v.17.0, SPSS Inc., Chicago, IL). To determine statistical significance, an analysis of variance (ANOVA) was performed. p values less than 0.05 were considered to be statistically significant.
Results
Isolation and characterization of hFLSCs
Although the number of colony-forming units (CFUs) from supernatant after sedimentation gradually increased from 3 to 9 mL, they were all lower than 20 CFUs (Fig. S1; Supplementary Data are available at www.liebertonline.com/rej). We finally decided to remove 9 mL of supernatant in this method.
The single cells, which were acquired between the two Percoll layers from the top to bottom, were seeded on plastic dishes coated with collagen type I. The Trypan Blue dye exclusion test showed that the viability of the newly isolated liver stem cells with the CSP method and MACS method all exceeded 85% (Fig. 1A). The cell culture results showed that plentiful CFUs were found in cells obtained between 50% and 70% Percoll, and the expression of EpCAM, c-kit, CD44, and OV-6 in these cells was above 90% (Fig. S2). Finally, 50% and 70% Percoll at a density ranging from 1.067 to 1.090 g/mL were used for cell isolation. The peritoneal cells, mainly macrophages and lymphocytes that could provide the cell factor for clone formation, were rapidly depleted within 1 week under culture conditions, because they could not proliferate in vitro without the specific factors.44,45 To exclude liver fibroblast contamination, the main cell markers of liver fibroblasts (CD34, CD90, AFP, and ALB) for cells isolated with the CSP and MACS methods were investigated by flow cytometry. The results showed that they all expressed below 5% (Fig. S3). After cells were seeded on plastic dishes, small colonies of flattened blast-like cells were observed with cobblestone-like morphology characteristic of epithelial cells in 5–7 days (Fig. 1B, C). About 10 days later, these cells isolated by the CSP method entered into the exponential growth phase (Fig. 1D, E and Fig. 2). In contrast, cells isolated by MACS took 2 weeks to form colony units and 3 weeks to enter into the exponential growth phase. The monoclonal formulation process can be seen in Fig. S4. Although initial differences existed in cell morphology and properties, the hFLSCs became homogeneous during a series of sub-passaging culture and remained the same morphologically for many passages. It was noteworthy that the cell growth was quite robust, demonstrating an excellent capacity to initiate long-term cultures over several weeks to months. Clones were further derived by serial dilution and maintained for up to 2 months and 20 passages.
FIG. 1.

Morphological aspect of isolated human fetal liver stem cells (hFLSCs). Trypan Blue dye exclusion test of hFLSCs (A, magnification 200×); hFLSCs at first stage (B, magnification 200×; C, 400×); The nuclei are stained blue (DAPI 4,6-diamidino-2-phenylindole) in the DAPT assay. The merge vision represents positive cells. hFLSCs form colonies during proliferation (D, 400×; E, 200×); hFLSCs at confluence (F, 200×). Scale bars, 50 μm (A, B, E, and F); 100 μm (C and D). Color images available online at www.liebertpub.com/rej
FIG. 2.

Cell growth curve of isolated human fetal liver stem cells (hFLSCs). Initially, 300,000 cells were plated in one of the six-well dish and expanded in Dulbecco's modified Eagle medium (DMEM)/F12. Cells were sub-cultured and counted every 3–4 days. The total number of the cells is reported on the graph. (▪) Freshly cultured cells; (●) cryopreserved cells.
Growth characteristics of hFLSCs
The hFLSCs were sub-cultured every 3–4 days and the population doubled for 10–13 times after signs of senescence were exhibited (Fig. 2). Growth characteristics were measured in different colonies with similar results. The population doubling time ranged from 48 to 72 hr. At confluence, hFLSCs exhibited contact inhibition and formed densely packed monolayers (Fig. 1F). Once confluence was reached, cells stopped dividing. Cells maintained high viability in subsequent culture. Even after cryopreservation, most of the cells remained attached to culture dishes soon after thawing and were able to establish long-term cultures. The cryopreserved cells were also passaged for 20 generations. No differences of growth kinetics were observed between cryopreserved cells and fresh cultures (Fig. 2). There was no significant difference in main surface maker expression or gene expression profiles (Table S1 or Table S2).
Immunocytochemistry and immunophenotype of hFLSCs
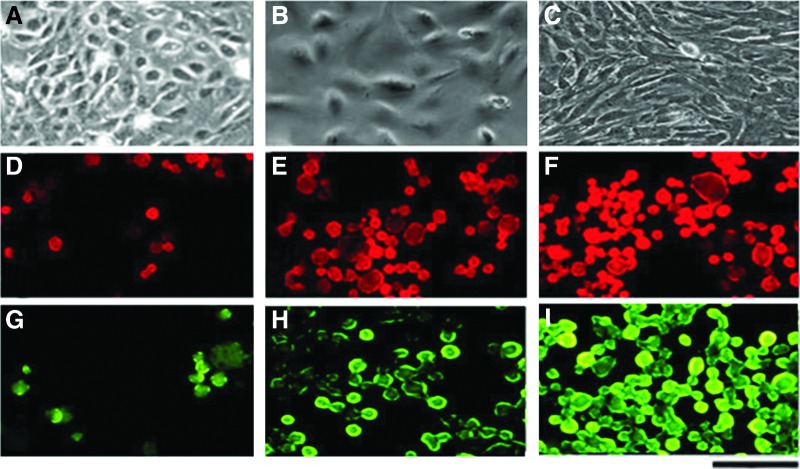
To determine the immunophenotype of hFLSCs, we used immunofluorescent or immunohistochemcal staining. hFLSCs were positive for EpCAM, c-kit, and OV-6 for all passages. These markers were consistent with the stem cell signature reported in mouse liver stem cells.37,46 hFLSCs were also positive for the fetal liver-specific marker AFP and epithelial markers such as CK18 and CK19, which are characteristic markers of hepatocytes and bile duct cells, respectively (Fig. 3).
FIG. 3.
Characterization of human fetal liver stem cells (hFLSCs) by immunohistochemistry (A–F) and immunofluorescence (G, H). hFLSCs are positive for fetal liver-specific marker alpha-fetoprotein (AFP) (A, magnification 400×; brown 3,3′-diaminobenzidine [DAB] stain), stem cell marker OV-6 (B, magnification 400×; counterstained with Hematoxylin), epithelial markers such as CK18 (C, 400×; red 3-amino-9-ethylcarbazole [AEC] stain) and CK19 (D, 200×; red AEC stain), and fetal liver stem cell marker CD326 (E, magnification 200×; red AEC stain). Negative control (F, 200×). They are also positive for stem cell marker c-Kit (G, magnification 400×), nucleus of cells were observed by 4′,6-diamidino-2-phenylindole (DAPI) staining (H, mangification 400×). Scale bars, 50 μm (D, E, and F); 100 μm (A, B, C, G, and H). Color images available online at www.liebertpub.com/rej
Phenotyping profiles of hFLSCs
To determine the expression profiles of hFLSCs, several widely accepted stem cell markers were detected by flow cytometry immediately after separation, including EpCAM, c-kit, CD44, and OV-6, which were not only highly expressed in more than 90% of the freshly isolated cells (passage 1 [P1]) with these two methods, but also in the cultured cells until passage 20 (P 20) (Fig. 4A–D). There was no significant difference between the passages in these main stem cell makers (p>0.05) for cells generated using the CSP method. We also selected one hepatoblast marker for the fetal liver (AFP), non-pathological bile duct epithelium marker CK19, and a hepatic cell marker CK18 to identify cells from all passages. When isolated by CSP or MACS methods, 10%–30% of the cells were positive for AFP, and the positivity remained steady with passages from P1 to P20 (Fig. 4F). There was no significant difference between the passages in cells isolated by these two methods. CK19 and CK18 were positive in about 50%–60% and 20%–40% of hFLSCs (Fig. 4G, H), respectively. The constant expression of AFP, CK19, and CK18 reflected the bi-potential nature to differentiate into hepatic and biliary lineages. Additionally, the mature hepatic marker, ALB, was moderately expressed in the hFLSCs (below 5%, Fig. 4I).
FIG. 4.
Phenotyping profiles of human fetal liver stem cells (hFLSCs) obtained by the collagen perfusion, gravity sedimentation, and Percoll density gradient centrifugation (CSP) method and magnetic-activated cell sorting (MACS) method from passage 1 (P1) to P20. Nine kinds of surface markers including epithelial cell adhesion molecule (EpCAM), c-kit, CD44, OV-6, CD81, alpha-fetoprotein (AFP), CK19, CK18, and albumin (ALB) are represented from A to I. (ART) CSP method; (ART) MACS method. ( ) Significant statistical difference (p<0.05) of the expression of cell-surface markers for these two methods.
) Significant statistical difference (p<0.05) of the expression of cell-surface markers for these two methods.
Gene expression profiles of hFLSCs
In terms of RNAs of hFLSCs, the gene expression profiles were quantitatively verified using qRT-PCR (Table 2). In general, analysis on freshly isolated or cultured stem cells showed similar results. All passages of hFLSCs isolated from the livers showed very similar gene expression profiles, with high expression of c-kit, EpCAM, CK19, neuronal cell adhesion molecule (NCAM), and claudin 3 (CLDN3), but low levels of expression of AFP and little or no expression of ALB or any liver-specific genes (e.g., phosphoenolpyruvate carboxykinase [PEPCK], telomeric repeat binding factor [TRF], connexin 26 (CX26), and cytochrome P450 3A4 [C3A4]). Moreover, mRNA expression profiles including c-kit, EpCAM, AFP, CK19, CK18, and ALB were similar to those of phenotype profiles of hFLSCs from all passages (Fig. 4 and Table 2).
Table 2.
Comparison of Quantitative Gene Expression Between Different Passages of Human Fetal Live Stem Cells with Two Methods
| Ratios of quantitative gene expression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P3 | P5 | P10 | P15 | P20 | |||||||
| CSP | MACS | CSP | MACS | CSP | MACS | CSP | MACS | CSP | MACS | CSP | MACS | |
| ALB | 0.00±0.00 | 0.09±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.01±0.00 | 0.00±0.00 | 0.06±0.02 | 0.46±0.01 | 0.12±0.04 | 0.78±0.15 |
| AFP | 0.09±0.02 | 0.08±0.03 | 0.07±0.01 | 0.10±0.03 | 0.11±0.03 | 0.24±0.13 | 0.06±0.00 | 0.13±0.01 | 0.19±0.14 | 0.56±0.12 | 0.68±0.13 | 1.68±0.48 |
| DPP4 | 1.34±0.98 | 1.16±0.32 | 1.21±0.13 | 1.11±0.09 | 1.03±0.22 | 1.45±0.38 | 0.93±0.21 | 0.89±0.27 | 1.18±0.42 | 1.07±0.29 | 1.49±0.56 | 2.19±0.43 |
| C3A4 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.07±0.00 | 0.01±0.00 | 0.03±0.00 |
| EpCAM | 7.15±1.12 | 6.71±2.31 | 5.38±3.72 | 5.69±2.48 | 7.06±2.99 | 6.28±2.49 | 6.88±2.43 | 5.67±1.45 | 5.73±2.41 | 3.47±1.21 | 5.03±1.78 | 3.00±0.48 |
| CK19 | 57.8±8.40 | 44.5±7.65 | 48.5±6.98 | 42.5±4.76 | 51.2±10.2 | 45.6±9.20 | 36.2±9.34 | 46.7±7.76 | 57.2±9.2 | 53.2±12.8 | 64.7±7.28 | 67.0±9.18 |
| NCAM | 1.77±0.32 | 1.12±0.14 | 1.60±0.53 | 1.47±0.32 | 1.80±0.96 | 1.79±0.46 | 2.19±0.69 | 2.05±0.24 | 3.66±1.01 | 4.93±1.31 | 4.69±1.34 | 5.71±0.91 |
| CKIT | 0.32±0.09 | 0.43±0.01 | 0.54±0.10 | 0.67±0.09 | 0.65±0.12 | 0.78±0.05 | 0.28±0.09 | 0.48±0.15 | 0.45±0.18 | 0.69±0.08 | 0.39±0.08 | 0.31±0.04 |
| PEPCK | 0.01±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.02±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.09±0.01 | 0.00±0.00 | 0.01±0.00 |
| CLDN3 | 0.51±0.01 | 0.42±0.14 | 0.52±0.12 | 0.47±0.10 | 0.34±0.08 | 0.56±0.14 | 0.76±0.14 | 0.84±0.12 | 0.82±0.14 | 0.92±0.04 | 1.12±0.17 | 1.32±0.21 |
| TRF | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.05±0.00 | 0.00±0.00 | 0.00±0.00 |
| CX26 | 0.00±0.00 | 0.00±0.00 | 0.02±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.03±0.00 | 0.07±0.00 | 0.09±0.01 | 0.12±0.03 |
| CX32 | 5.3±0.72 | 3.39±0.93 | 5.21±0.53 | 6.34±0.88 | 4.53±0.40 | 4.53±0.62 | 3.52±1.03 | 3.47±0.07 | 2.18±0.31 | 1.92±0.16 | 2.79±0.47 | 2.32±0.65 |
| GAPDH | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Consistent relative higher or lower expression of each gene in comparison to passage 1 (P1) is indicated in boldface, respectively. Data are normalized to and given as a percentage of GAPDH expression. Colonies of fetal hepatic stem cells (n=3) were analyzed after 10 days in culture on plastic;

Represented the significant statistically difference (p<0.05) of the gene expression for these two methods.
P1, passage 1; CSP, collagenase perfusion, gravity sedimentation, and Percoll density gradient centrifugation method; MACS, magnetic-activated cell sorting; ALB, albumin; AFP, alpha-fetoprotein; DPP4, dipeptylpeptidase 4; C3A4, cytochrome P450 3A4; EpCAM, epithelial cell adhesion molecule; CK19, cytokeratin 19; NCAM, neural cell adhesion molecule; CKIT, c-kit (v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog); PEPCK, phosphoenolpyruvate carboxykinase; CLDN, claudin; TRF, transferrin; CX, connexin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Comparison of surface markers and gene expression profiles of hFLSCs isolated by the CSP and MACS methods
Cells isolated with MACS were also analyzed by flow cytometry from P1 to P20 for cell markers. The results demonstrated that there were no significant differences in the percentage of positive cells from P1 to P10 isolated by the CSP and MACS methods. Significantly lower expression of the main stem cell markers, such as EpCAM, c-kit, CD44, and OV-6, was found in P20 cells initially isolated by MACS than those by the CSP method (Fig. 4A–D, p<0.05). Compared with cells isolated by the CSP method, the expression of AFP and ALB by cells isolated using the MACS method increased significantly from P15 to P20 (Fig. 4F, I, p<0.05). For gene expression profiles, the liver-specific genes (e.g., PEPCK, TRF, CX26, and C3A4) almost showed no expression in the entire culture process for these two methods; levels of expression of AFP and ALB were slightly increased at P15 and P20 for cells isolated by MACS, and there was significant difference compared with the CSP method (p<0.05). In these two methods, the cells in all passages showed a high expression of c-kit, EpCAM, CK19, NCAM, and CLDN3, and there was no significant difference between them (Table 2, p>0.05).
Pluripotent differentiation of hFLSCs
As progenitor cells, the hFLSCs have the potential to differentiate into different lineages. Following the addition of carefully chosen differentiation factors in the culture medium, hFLSCs showed the ability to differentiate to different types of cells, including hepatocytes, cholangiocytes, osteoblasts, adipocytes, and endothelium cells.
When grown with FGF4, cells spread into a monolayer with a progressively increased size, and presented a hepatocyte-like polygonal shape (Fig. 5A) but not in negative control (Fig. S5A). Differentiated cells stored glycogen within the cytoplasm, and 90% of the cells became strongly positive for PAS staining (Fig. 5B–D). Immunocytochemical analysis showed that HGF and FGF4 greatly increased G6Pase activity (Fig. 5E–H). G6Pase activity was not detected at the time of plating (Fig. 5E), but it became apparent approximately 12 days after HGF and FGF4 treatment (Fig. 5F). After 30 days, most hepatic cells exhibited high G6Pase activity (Fig. 5G, H). In contrast, no activity was present in untreated cultures of the equivalent age (Fig. S5). We also investigated the functional state of the mature hepatocytes using ICG and 1,1-dioctadecyl-3,3,3,3-tetramethyl-indocarbocyanine perchlorate (Dil)-Ac-labeled LDL. The results of the ICG and LDL uptake assay demonstrated that the differentiated cells were mature enough to process ICG dye (Fig. 5I–L) and LDL (Fig. 5M–R). In addition, the synthesis and release of ALB and urea were increased during differentiation by hFLSCs. To compare the ALB and urea secretion of the un-differentiated and differentiated cells, medium collected every 2 days from cultures was analyzed by nephelometry or spectrophotometer. Total secretion of ALB and urea into the medium was increased daily and reached the peak at day 14 (Fig. 5S, T), whereas no difference was found in untreated cultures. These data demonstrated that HGF and FGF4 induced hepatocyte differentiation of cultured hFLSCs.
FIG. 5.
Human fetal liver stem cells (hFLSCs) were differentiated to hepatocytes. When cultured with 10 ng/mL fibroblast growth factor 4 (FGF4), hFLSCs flatten and increase in size to become polygonal cells characteristic of hepatocytes (A, magnification 400×), differentiated hepatocytes express glycogen deposits, which stain pink with Periodic Acid Schiff (PAS) (B, magnification 400×, negative control; C, magnification 400×; D, magnification 200×). (E–H) Effect of human growth factor (HGF) and FGF4 on the activity of glucose 6-phosphatase (G6Pase), which is shown as the brown–black precipitates (E, magnification 400×, negative control; F, magnification 400×, 12 days after HGF and FGF4 treatment; G and H, magnification 400×30 days after treatment). The Indocyanine Green (ICG) uptake assay illustrated that the cells were able to process the ICG dye (I and J, magnification 400×, the undifferentiated group; K, magnification 400×, ICG uptake cells; L, magnification 400×, ICG excretion after cells cultured 6 hr). (M–R) Fluorescence images showing that mature hepatocytes process low-density lipoprotein (LDL) (1,1-dioctadecyl-3,3,3,3-tetramethyl-indocarbocyanine perchlorate (Dil)-Ac-labeled LDL [Dil-Ac-LDL], red, magnification 400×). (S and T) A total of 2×106 cells were cultured without fetal bovine serum (FBS) in the presence of 10 ng/mL HGF and FGF4 (●), and cells were cultured in the absence of growth factors (▪). Scale bar, 100 μm except for D (50 μm). DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/rej
In the cholangiocyte induction medium, the cells switched to a short spindle, triangular and round shape, and became similar to biliary epithelial cells cultured in vitro. These cells were stained for CK7 (red) and CK19 (green) positively (Fig. 6). In the bone induction medium, cells became elongated with irregular processes, deposited calcium into the extracellular matrix; the nacarat mineralized nodes were stained with Alizarin Red (Fig. 7A–C). Moreover, Von Kossa staining also showed calcium deposition in the extracellular matrix (Fig. 7D–F). In the adipose induction medium, cultured cells increased in size, and 40% of the cells accumulated coalescing vacuoles that were positive for Oil Red O staining (Fig. 7G–I). In the endothelium induction medium, hFLSCs became spindle-shaped (Fig. 7J) and expressed endothelial markers CD31 (AEC stain, Fig. 7K, L) and Factor VIII-related antigen (FITC stain; Fig. 7M, N), which were negative under un-differentiation.
FIG. 6.
The human fetal liver stem cells (hFLSCs) were differentiated to cholangiocytes. When cultured in biliary differentiation medium, the cells switched to short spindle, triangular, and round shapes and were similar to biliary epithelial cells cultured in vitro (A–C, magnification 400×). These cells were stained by CK7 (tetramethylrhodamine [TRITC]) (D–F, magnification, 400×) and CK19 (fluorescein isothiocyanate [FITC]) (G–I, magnification 400×) in different weeks (0, 2, 4 weeks). Scale bar, 100 μm. Color images available online at www.liebertpub.com/rej
FIG. 7.
The pluripotency potential of isolated human fetal liver stem cells (hFLSCs). The hFLSCs were differentiated to osteoblasts (A–F), adipocytes (G–I), and endothelial cells (J–N) with different induction media. Bone induction: Differentiated osteoblasts present a longitudinal and interwoven conformation, deposit calcium into the extracellular matrix, which show nacarat mineralized nodes with Alizarin Red stain (A, magnification 400×, negative control; B, magnification 200×; and C, magnification 400×) and show black with von Kossa stain (D, magnfication 400×, negative control; E, magnification 400×); counterstained with 1% Neutral Red for cytoplasm which show red (F, magnification 400×). Adipose tissue induction: Differentiated cells show increased size and accumulate vacuoles that are positive for Oil Red O stain (G, magnification 400×, negative control; H, magnification 400× and I, 400×). Endothelium induction: Differentiated endothelial cells become spindle-shaped with formation of linear channels between cells (J, magnification 400×); more than 90% of cells are positive for CD31 (K, magnification 400×, negative control; L, magnification 400×; red 3-amino-9-ethyl-carbazole [AEC] stain) and Factor VIII-related antigen (M, magnification 400×, negative control; N, magnification 400×; fluorescein isothiocyanate [FITC] stain). Scale bar, 100 μm except for B (50 μm). Color images available online at www.liebertpub.com/rej
Discussion
In this study, a more convenient and efficient method to isolate hFLSCs with high proliferation was established, as compared with the MACS method. First, we found that after the first passage, the main hFLSCs and functional hepatocellular markers, such as EpCAM and AFP, remained stable with subsequent passages. For the CSP method, not only could the hFLSCs be obtained in 10 days, but they also possessed high proliferative capacity and could be passaged serially for more than 20 passages without differentiation. This might be time saving in the exponential growth phase and may represent a longer-lasting differentiation ability. Second, the hFLSCs were isolated quickly and efficiently by a combination of two-step in situ collagenase perfusion, gravity sedimentation, and Percoll density gradient centrifugation. With two-step in situ collagenase perfusion, the red blood cells could be excluded, and the cells isolated could keep specific function longer.11
Because the surface of liver stem cells expressed adhesion molecules such as E-cadherin, claudin, and connexin, which induce adhesion between cells,47 they could be separated from other cells effectively by disaggregation and sedimentation. Percoll density gradient centrifugation is of low viscosity, producing a non-osmotic effect or no toxicity toward cells, and has been used for isolation of various types of animal cells.48,49 Liu et al. isolated rat fetal liver stem cells under the condition of continuous gradient centrifugation with 40% Percoll at 20,000×g for 90 min.20 The rat fetal liver stem cells (rFLSCs) that they isolated were close to what was obtained by MACS, which illustrated that high purity and proliferative stem cells could be obtained by combination with other physical methods. Being similar to our method, their method did not require any specific cell markers. The whole physical process might cause less damage to cells,11 and the cell re-activation time may be shortened. They successfully used the specific markers to monitor the process of enriching pure rFLSCs and related the morphological characters of rFLSCs to the specific markers in their study. However, the parameters of their method were suited to isolating FLSCs from rats.
Liu et al. did not describe how long and how many of these cells could be maintained and serially passaged without differentiation. Moreover, the characteristics of these isolated rFLSCs were not confirmed by in vitro differentiation to multiple lineages. In addition, with the exception of the time that cells entered into the exponential growth phase, the three stages for isolation and acquisition of fetal liver stem cells were also time-consuming (taking as much as about 1 week), and the 90-min high-speed centrifugation time was longer than our method, which may affect cell reactivation.
We used a series of different density layers by diluting Percoll (30%, 50%, 70%, and 80%) and found that the optimal density range for isolating hFLSCs was between 50% and 70% (from 1.090 to 1.067 grams/mL) at 12,000×g for 30 min, which proved to be more efficient and more user friendly for obtaining hFLSCs than the MACS method. Freshly isolated cells with the CSP and MACS methods had similar surface markers and gene expression profiles as well as the same capacity for pluripotent differentiation. However, the operation time and process of the CSP method were shorter and simpler. Moreover, the CSP method was mostly a physical process, which caused less damage to cells.11 The cells obtained by the CSP method had more single clonal formations, shorter recovery time, and longer stem cell function. Liver fibroblasts could be excluded by adding 0.19 gram/L EDTA to the digestive buffer as they were digested and spread into single cell layer along with the perfusion buffer after the two-step in situ collagenase perfusion.50,51
In addition, some important biofactors and the peritoneal fluid of fetuses were added to the culture medium to shorten the latent period of hFLSCs and maintain the high proliferative ability. The peritoneal fluid contains peritoneal cells, mainly macrophages and lymphocytes, which could secrete some cytokines, such as SCF, IL-2, and IL-6, that provide necessary growth conditions for hFLSCs.52,53 Because peritoneal cells can not proliferate in vitro and die within a week, these “feeder” cells would not affect the growth and purity of hFLSCs. A number of growth factors, including HGF, EGF, SCF, and LIF, were added into the culture medium. HGF promotes DNA synthesis of liver cells whereas bFGF promotes cell adhesion.17 LIF and SCF leave cells in an un-differentiated state and enable cells to proliferate faster.17 Following the above treatment, small colonies of hFLSCs that appeared as flattened blast-like cells could be observed after 5–7 days, and they entered into the exponential growth phase by day 10. Moreover, after multiple sub-passages or cryopreservation, the isolated hFLSCs still maintained robust proliferation and other vital activities. The cryopreserved cells showed the same growth kinetics as fresh cultures after resuscitation.
Several studies have shown that the aforementioned isolated flattened blast-like cells were fetal liver stem cells.25,29 The results of immunohistochemistry, immunofluorescence, and flow cytometry showed that the isolated hFLSCs were positive for the general mesenchymal stem cell markers CD44 and EpCAM and oval cell marker OV-6.29,54 The stem cell marker c-kit was also positive, which indicated that the blast-like cells in culture may be similar to mesenchymal stem cells being capable of generating hepatocytes in vivo, as reported by Avital et al.55 In addition, CK19, the bile duct epithelial cell–specific marker, and AFP, a specific marker of immature fetal liver cells, were also weakly positive. These results indicated that these cells may have the potential to differentiate into hepatic and biliary lineages, which was consistent with the characteristics of the reported liver stem cells.6,56
Furthermore, expression of mRNA for specific genes that have been reported to be positive for hFLSCs was investigated by RT-qPCR. hFLSCs at all pasaages showed highly similar expression profiles after being isolated by either the MACS or CSP method. The stem cell phenotype comprised expression of c-kit, EpCAM, NCAM, CK19, CLDN3, and weak levels of AFP but no expression of ALB or adult liver-specific proteins, such as PEPCK, dipeptidyl peptidase 4 (DPP4), or P450s.29 More important, the expression of both hepatic (AFP, CK18) and biliary (CK19) markers reflects the bi-potential nature of the hepatic stem cells toward both lineages of the hepatic and biliary, respectively.14,57 The results of our study confirmed the expression profiles of mRNA and suggested that the isolated cells had the phenotype of stem cells.
Finally, the characteristics of these isolated hFLSCs were further confirmed by in vitro differentiation to multiple lineages. These cells from the human fetal liver were capable of differentiating into hepatocytes, cholangiocytes, osteoblasts, adipocytes, and endothelial cells. Therefore, on the basis of current experimental results, we could consider that the isolated flattened blast-like cells were FLSCs.
In summary, we developed a more convenient and efficient approach to successful isolation of highly proliferative hFLSCs than the MACS method, and this study suggests that these pluripotent hepatic progenitors can be stably preserved in their phenotypes throughout in vitro culture. Moreover, hFLSCs could enter into the exponential growth phase in just 10 days, and they could maintain sufficient proliferative activity to permit subculture for at least 20 passages without differentiation.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81372947 and 81202163).
Author Disclosure Statement
No competing financial interests exist.
All authors have contributed to this article. X.G. and S.W. completed the main experiments and drafted the full manuscript. Y.L.D. was responsible for sample collection. Z.L.C. and X.F.G. completed part of the trials for cell isolation. Z.Q.S. and Z.G.Q. were the consultants for data analysis. J.W.L. and M.J. supervised the whole study and edited the manuscript.
References
- 1.Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, Raj TA, Tiwari SK, Kumaresan K, Venkateswarlu J, Pande G, Habibullah CM. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant 2010;19:409–418 [DOI] [PubMed] [Google Scholar]
- 2.Wertheim J, Baptista P, Soto-Gutierrez A. Cellular therapy and bioartificial approaches to liver replacement. Curr Opin Organ Transplant 2012;17:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Defresne F, Tondreau T, Stephenne X, Smets F, Bourgois A, Najimi M, Jamar F, Sokal EM. Biodistribution of adult derived human liver stem cells following intraportal infusion in a 17-year-old patient with glycogenosis type 1A. Nucl Med Biol 2014;41:371–375 [DOI] [PubMed] [Google Scholar]
- 4.Pietrosi G, Vizzini G, Gerlach J, Chinnici C, Luca A, Amico G, M DA, Conaldi PG, Petri SL, Spada M, Tuzzolino F, Alio L, Schmelzer E, Gridelli B. Phase I–II matched case-control study of human fetal liver cell transplantation for treatment of chronic liver disease. Cell Transplant 2014; doi: 10.3727/096368914X682422 [DOI] [PubMed] [Google Scholar]
- 5.Oertel M. Fetal liver cell transplantation as a potential alternative to whole liver transplantation? J Gastroenterol 2011;46:953–965 [DOI] [PubMed] [Google Scholar]
- 6.Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol 2009;6:724–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl 2010;16:229–237 [DOI] [PubMed] [Google Scholar]
- 8.Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006;24:2840–2850 [DOI] [PubMed] [Google Scholar]
- 9.Dianat N, Steichen C, Vallier L, Weber A, Dubart-Kupperschmitt A. Human pluripotent stem cells for modelling human liver diseases and cell therapy. Curr Gene Ther 2013;13:120–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toshima T, Yoshizumi T, Uchiyama H, Ikegami T, Soejima Y, Ikeda T, Kawanaka H, Yamashita Y, Morita M, Oki E, Mimori K, Sugimachi K, Saeki H, Watanabe M, Shirabe K, Maehara Y. Effect of CD133-positive stem cells in repeated recurrence of hepatocellular carcinoma after liver transplantation: A case report. Fukuoka Igaku Zasshi 2013;104:383–388 [PubMed] [Google Scholar]
- 11.Gridelli B, Vizzini G, Pietrosi G, Luca A, Spada M, Gruttadauria S, Cintorino D, Amico G, Chinnici C, Miki T, Schmelzer E, Conaldi PG, Triolo F, Gerlach JC. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl 2012;18:226–237 [DOI] [PubMed] [Google Scholar]
- 12.Bernstein HS, Hyun WC. Strategies for enrichment and selection of stem cell-derived tissue precursors. Stem Cell Res Ther 2012;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piscaglia AC, Campanale M, Gasbarrini A, Gasbarrini G. Stem cell-based therapies for liver diseases: State of the art and new perspectives. Stem Cells Int 2010;2010:259461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells 2006;24:1852–1858 [DOI] [PubMed] [Google Scholar]
- 15.Makin E, Davenport M. Fetal and neonatal liver tumours. Early Hum Dev 2010;86:637–642 [DOI] [PubMed] [Google Scholar]
- 16.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science 2008;322:1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duret C, Gerbal-Chaloin S, Ramos J, Fabre JM, Jacquet E, Navarro F, Blanc P, Sa-Cunha A, Maurel P, Daujat-Chavanieu M. Isolation, characterization, and differentiation to hepatocyte-like cells of nonparenchymal epithelial cells from adult human liver. Stem Cells 2007;25:1779–1790 [DOI] [PubMed] [Google Scholar]
- 18.Zaret KS. Regulatory phases of early liver development: Paradigms of organogenesis. Nat Rev Genet 2002;3:499–512 [DOI] [PubMed] [Google Scholar]
- 19.Gabriel E, Schievenbusch S, Kolossov E, Hengstler JG, Rotshteyn T, Bohlen H, Nierhoff D, Hescheler J, Drobinskaya I. Differentiation and selection of hepatocyte precursors in suspension spheroid culture of transgenic murine embryonic stem cells. PLoS One 2012;7:e44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W-h, Wang X, You N, Tao K-s, Wang T, Tang L-j, Dou K-f. Efficient enrichment of hepatic cancer stem-like cells from a primary rat HCC model via a density gradient centrifugation-centered method. PloS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki A, Zheng YW, Fukao K, Nakauchi H, Taniguchi H. Liver repopulation by c-Met-positive stem/progenitor cells isolated from the developing rat liver. Hepatogastroenterology 2004;51:423–426 [PubMed] [Google Scholar]
- 22.Fujii T, Zen Y, Harada K, Niwa H, Masuda S, Kaizaki Y, Watanabe K, Kawashima A, Nakanuma Y. Participation of liver cancer stem/progenitor cells in tumorigenesis of scirrhous hepatocellular carcinoma—human and cell culture study. Hum Pathol 2008;39:1185–1196 [DOI] [PubMed] [Google Scholar]
- 23.Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, Shetty K, Johnson L, Reddy EP. Liver stem cells and hepatocellular carcinoma. Hepatology 2009;49:318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci 2002;115:2679–2688 [DOI] [PubMed] [Google Scholar]
- 25.Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci USA 2006;103:9912–9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong A, Austin TW, Lagasse E, Uchida N, Tamaki S, Bordier BB, Weissman IL, Glenn JS, Millan MT. Isolation of human fetal liver progenitors and their enhanced proliferation by three-dimensional coculture with endothelial cells. Tissue Eng Part A 2008;14:995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyamath P, Alvi A, Habeeb A, Khosla S, Khan AA, Habibullah CM. Characterization of hepatic progenitors from human fetal liver using CD34 as a hepatic progenitor marker. World J Gastroenterol 2007;13:2319–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masson NM, Currie IS, Terrace JD, Garden OJ, Parks RW, Ross JA. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am J Physiol Gastrointest Liver Physiol 2006;291:G45–G54 [DOI] [PubMed] [Google Scholar]
- 29.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME, Reid LM. Human hepatic stem cells from fetal and postnatal donors. J Exp Med 2007;204:1973–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noto FK, Duncan SA. Generation of hepatocyte-like cells from human pluripotent stem cells. Stem Cells Handbook 2013:139–147 [Google Scholar]
- 31.Gaudio E, Carpino G, Cardinale V, Franchitto A, Onori P, Alvaro D. New insights into liver stem cells. Dig Liver Dis 2009;41:455–462 [DOI] [PubMed] [Google Scholar]
- 32.Dolle L, Best J, Mei J, Al Battah F, Reynaert H, van Grunsven LA, Geerts A. The quest for liver progenitor cells: A practical point of view. J Hepatol 2010;52:117–129 [DOI] [PubMed] [Google Scholar]
- 33.Jang Y-Y, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 2004;6:532–539 [DOI] [PubMed] [Google Scholar]
- 34.Hay D, Zhao D, Fletcher J, Hewitt Z, McLean D, Urruticoechea-Uriguen A, Black J, Elcombe C, Ross J, Wolf R. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells (Dayton, Ohio) 2008;26:894. [DOI] [PubMed] [Google Scholar]
- 35.Mathijs K, Kienhuis AS, Brauers KJ, Jennen DG, Lahoz A, Kleinjans JC, van Delft JH. Assessing the metabolic competence of sandwich-cultured mouse primary hepatocytes. Drug Metab Dispos 2009;37:1305–1311 [DOI] [PubMed] [Google Scholar]
- 36.He Y-F, Liu Y-K, Gao D-M, Chen J, Yang P-Y. An efficient method of sorting liver stem cells by using immuno-magnetic microbeads. World J Gastroenterol 2006;12:3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006;24:2840–2850 [DOI] [PubMed] [Google Scholar]
- 38.Teutsch HF. Improved method for the histochemical demonstration of glucose-6-phosphatase activity. Histochemistry 1978;57:107–117 [DOI] [PubMed] [Google Scholar]
- 39.Toyoda T, Nakamura K, Yamada K, Thanseem I, Anitha A, Suda S, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, Iwata Y, Suzuki K, Matsuzaki H, Kawai M, Sekine Y, Tsuchiya K, Sugihara G, Ouchi Y, Sugiyama T, Takei N, Yoshikawa T, Mori N. SNP analyses of growth factor genes EGF, TGFbeta-1, and HGF reveal haplotypic association of EGF with autism. Biochem Biophys Res Commun 2007;360:715–720 [DOI] [PubMed] [Google Scholar]
- 40.Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells 2002;20:146–154 [DOI] [PubMed] [Google Scholar]
- 41.Dianat N, Dubois-Pot-Schneider H, Steichen C, Desterke C, Leclerc P, Raveux A, Combettes L, Weber A, Corlu A, Dubart-Kupperschmitt A. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology 2014;60:700–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 43.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nat Rev Cancer 2002;2:826–835 [DOI] [PubMed] [Google Scholar]
- 44.Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 1985;56:351–358 [PMC free article] [PubMed] [Google Scholar]
- 45.Mota LA, Roberto Neto J, Monteiro VG, Lobato CS, Oliveira MA, Cunha M, D'Avila H, Seabra SH, Bozza PT, DaMatta RA. Culture of mouse peritoneal macrophages with mouse serum induces lipid bodies that associate with the parasitophorous vacuole and decrease their microbicidal capacity against Toxoplasma gondii. Mem Inst Oswaldo Cruz 2014;109:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki A, Nakauchi H, Taniguchi H. In vitro production of functionally mature hepatocytes from prospectively isolated hepatic stem cells. Cell Transplant 2003;12:469–473 [DOI] [PubMed] [Google Scholar]
- 47.Hirose T, Yasuchika K, Fujikawa T, Fujii H, Oe S, Azuma H, Ikai I, Yamaoka Y. “Blastosphere”: A new culture method for human fetal hepatic progenitor cells. Gastroenterology 2001;120:A542–A543 [Google Scholar]
- 48.Che X, Guo J, Wang B, Bai Y. Rapid isolation of muscle-derived stem cells by discontinuous Percoll density gradient centrifugation. In Vitro Cell Dev Biol Anim 2011;47:454–458 [DOI] [PubMed] [Google Scholar]
- 49.Pösel C, Möller K, Fröhlich W, Schulz I, Boltze J, Wagner D-C. Density gradient centrifugation compromises bone marrow mononuclear cell yield. PloS One 2012;7:e50293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 2007;282:23337–23347 [DOI] [PubMed] [Google Scholar]
- 51.Dudas J, Mansuroglu T, Batusic D, Ramadori G. Thy-1 is expressed in myofibroblasts but not found in hepatic stellate cells following liver injury. Histochem Cell Biol 2009;131:115–127 [DOI] [PubMed] [Google Scholar]
- 52.Masuda H, Tanaka R, Fujimura S, Ishikawa M, Akimaru H, Shizuno T, Sato A, Okada Y, Iida Y, Itoh J, Itoh Y, Kamiguchi H, Kawamoto A, Asahara T. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J Am Heart Assoc 2014;3:e000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sodhi A, Pandey AK. In vitro activation of murine peritoneal macrophages by recombinant YopJ: Production of nitric oxide, proinflammatory cytokines and chemokines. Immunobiology 2011;216:358–366 [DOI] [PubMed] [Google Scholar]
- 54.Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang L, Yan HX, Fu J, Chen Y, Zhang HL, Tang L, Zheng LY, He YQ, Li YQ, Wu FQ, Zou SS, Li Z, Wu MC, Feng GS, Wang HY. OV6(+) tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol 2012;57:613–620 [DOI] [PubMed] [Google Scholar]
- 55.Avital I, Inderbitzin D, Aoki T, Tyan D, Cohen A, Ferraresso C, Rozga J, Arnaout W, Demetriou A. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun 2001;288:156–164 [DOI] [PubMed] [Google Scholar]
- 56.Mancino MG, Carpino G, Onori P, Franchitto A, Alvaro D, Gaudio E. Hepatic “stem” cells: State of the art. Ital J Anat Embryol 2007;112:93–109 [PubMed] [Google Scholar]
- 57.Duret C, Gerbal‐Chaloin S, Ramos J, Fabre JM, Jacquet E, Navarro F, Blanc P, Sa‐Cunha A, Maurel P, Daujat‐Chavanieu M. Isolation, characterization, and differentiation to hepatocyte-like cells of nonparenchymal epithelial cells from adult human liver. Stem Cells 2007;25:1779–1790 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.