Abstract
Objective: We aimed to determine the effect of an open-label 8 week Vitamin D3 supplementation on manic symptoms, anterior cingulate cortex (ACC) glutamate, and γ-aminobutyric acid (GABA) in youth exhibiting symptoms of mania; that is, patients with bipolar spectrum disorders (BSD). We hypothesized that an 8 week Vitamin D3 supplementation would improve symptoms of mania, decrease ACC glutamate, and increase ACC GABA in BSD patients. Single time point metabolite levels were also evaluated in typically developing children (TD).
Methods: The BSD group included patients not only diagnosed with BD but also those exhibiting bipolar symptomology, including BD not otherwise specified (BD-NOS) and subthreshold mood ratings (Young Mania Rating Scale [YMRS] ≥8 and Clinical Global Impressions - Severity [CGI-S] ≥3). Inclusion criteria were: male or female participants, 6–17 years old. Sixteen youth with BSD exhibiting manic symptoms and 19 TD were included. BSD patients were asked to a take daily dose (2000 IU) of Vitamin D3 (for 8 weeks) as a supplement. Neuroimaging data were acquired in both groups at baseline, and also for the BSD group at the end of 8 week Vitamin D3 supplementation.
Results: Baseline ACC GABA/creatine (Cr) was lower in BSD than in TD (F[1,31]=8.91, p=0.007). Following an 8 week Vitamin D3 supplementation, in BSD patients, there was a significant decrease in YMRS scores (t=−3.66, p=0.002, df=15) and Children's Depression Rating Scale (CDRS) scores (t=−2.93, p=0.01, df=15); and a significant increase in ACC GABA (t=3.18, p=0.007, df=14).
Conclusions: Following an 8 week open label trial with Vitamin D3, BSD patients exhibited improvement in their mood symptoms in conjunction with their brain neurochemistry.
Introduction
Bipolar disorder (BD) has a 3.9% lifetime prevalence in the United States adult population (Kessler et al. 2005b), and is characterized by shifts in mood between mania and depression. The prevalence is as low as 1.8% (Van Meter et al. 2011) in the pediatric population; however, when broader diagnostic criteria are used, the rates rise up to 6.7% in the United States (Van Meter et al. 2011). BD is associated with significant morbidity and oftentimes the pharmacotherapy may be inadequate in resolving symptoms (Deckersbach et al. 2014; Pfennig et al. 2014). More than 50% of patients diagnosed with BD have onset of their illness prior to 20 years of age (Lish et al. 1994; Morselli et al. 2003; Kessler et al. 2005a; Blumberg 2008), and subsyndromal symptoms may be experienced years earlier (Chang 2007). Also, in pediatric populations, symptoms may differ from those seen in adults, making the diagnosis and management of BD in pediatric populations even more challenging (DeFilippis et al. 2013; Rocha et al. 2013).
Previous studies have implicated the glutamatergic neurotransmitter system in the pathophysiology of BD, showing abnormalities in glial cells and neurons (postmortem methods) (Benes et al. 2000; Cotter et al. 2002) and brain glutamate levels (in vivo proton magnetic resonance spectroscopy [1H MRS] studies) (Hyder et al. 2006; Bhagwagar et al. 2007; Moore et al. 2007; Singh et al. 2010; Gigante et al. 2012), specifically within the anterior cingulate cortex (ACC) (Eastwood et al. 2010). The ACC, a critical center of the brain's self-regulatory system (Bush et al. 2000) has been associated with BD (Cerullo et al. 2009; Palermo et al. 2014). Abnormalities within the γ-aminobutyric acid (GABA)ergic neurotransmitter system have also been implicated in the pathophysiology of BD through GABA receptor gene polymorphisms (Benes and Berretta 2001; Massat et al. 2002; Otani et al. 2005), postmortem (Benes and Berretta 2001; Knable et al. 2004; Torrey et al. 2005; Woo et al. 2007) and MRS studies (Wang et al. 2006; Bhagwagar et al. 2007; Brady et al. 2013). Specifically, irregularities in ACC GABA have been shown in patients with BD (Bhagwagar et al. 2007; Woo et al. 2007; Brady et al. 2013). The changes in excitatory (i.e., glutamatergic) and inhibitory (i.e., GABAergic) neurotransmission may be synergistic: Glial produced glutamine is the source for both neuronal glutamate and GABA.
Recent evidence indicated that Vitamin D3, a neuroactive steroid, may act on the GABAergic system and play a role in mood regulation. Neuroactive steroids, acting on the nervous system, present neuroprotective properties through different signaling pathways (Melcangi and Panzica 2006, 2009), including modulatory effects on the release of neurotransmitters such as glutamate and GABA (Zheng 2009). For example, alterations in neuroactive steroid levels may modify GABAA receptor subunits, impacting the level of inhibition (Maguire et al. 2009) and hence influencing mood (Carta et al. 2012). Vitamin D3 (cholecalciferol) is known for its classic role in systemic calcium (Ca2+) homeostasis (Holick 2003); however, in recent years, it has been suggested that Vitamin D3 meets the criteria for a neuroactive steroid (Melcangi and Panzica 2009; Harms et al. 2011).
Several observations have indicated that Vitamin D3 is synthesized directly in the nervous system, regulating neuronal activities and exerting neuroprotective effects (Melcangi and Panzica 2009) (i.e., in autism [Cannell 2008], schizophrenia [McGrath 1999], multiple sclerosis [Garcion et al. 1998; Correale et al. 2009] and traumatic brain injury [Cekic et al. 2009]). Furthermore, the relationship between Vitamin D3 and seasonal affective disorder (Stumpf et al. 1989; Gloth et al. 1999) led to investigations of antidepressant effects of Vitamin D3 (Khoraminya et al. 2013; Li et al. 2014; Robinson et al. 2014; Spedding 2014). Khoraminya et al. compared the therapeutic effects of fluoxetine, with and without Vitamin D3, in major depressive disorder, and showed significantly more improvement in depression scales for patients supplemented with Vitamin D3 (Khoraminya et al. 2013).
Lower serum Vitamin D3 levels during pregnancy were identified as a risk factor for development of postpartum depression (Robinson et al. 2014). On the other hand, meta-analysis investigating effect of Vitamin D3 supplementation on depressed patients did not warrant conclusive results (Li et al. 2014), possibly because of study design flaws (Spedding 2014). However, analysis of the comprehensive evidence-based studies concluded that there were positive results in the management of depression with Vitamin D3 supplementation (Spedding 2014). Vitamin D3 is inactive and is converted in the liver to 25-hydroxy Vitamin D3 [25 (OH)D], a stably circulating form; and 25(OH)D is further converted in the kidneys and brain to an active form of Vitamin D3; that is, 1-α-25-dihydroxy Vitamin D3 [1,25 (OH)2D]. Specifically, low levels of serum 25(OH)D have been associated with depressive symptoms (Kjaergaard et al. 2012). 1,25 (OH)2D has been shown to affect neurotrophins (McCann et al. 2008; Humble 2010; Khoraminya et al. 2013), which are secreted proteins that support survival and differentiation of neurons in mammals (Woo and Lu 2006); further supporting the neuroprotective impact of Vitamin D3. In addition, there is evidence that Vitamin D receptors may contribute to BD, specifically by alteration in the expression of the dopamine receptor gene (Ahmadi et al. 2012); and Vitamin D receptors are expressed in key brain regions that may potentially play a crucial role in mood regulation (Prufer et al. 1999; Eyles et al. 2005, 2011).
It has been suggested that glutamatergic and GABAergic abnormalities observed in BD fit into a model wherein stress-induced cortisol increases synaptic glutamate, leading to overactivation of postsynaptic N-Methyl-d-aspartate (NMDA) glutamate receptors and subsequent increased intracellular Ca2+ concentration (Moghaddam et al. 1994) as well as mania. Vitamin D3 has been associated with reductions in brain Ca2+ levels (Kalueff et al. 2004). 1,25 (OH)2D may modulate GABAA in the brain in a similar fashion to other neuroactive steroids (Losel et al. 2003). One could further speculate that increase in neuroactive steroid 1,25 (OH)2D may be related to decrease in Ca2+ concentration (Moghaddam et al. 1994), which can help modulate the GABAergic system, resulting in improvement in manic symptoms.
Given the glutamatergic and GABAergic abnormalities in BD and the possible regulatory impact of Vitamin D3 acting as a neuroactive steroid, we investigated the role of Vitamin D3 as a supplemental treatment for children and adolescents exhibiting manic symptoms (Hafeman et al. 2013). In particular, we investigated the effect of an open-label 8 week Vitamin D3 supplement on manic symptoms, on serum Vitamin D3 and ACC glutamate and GABA levels in children and adolescents exhibiting symptoms of mania (i.e., patients with bipolar spectrum disorders [BSD] [Merikangas et al. 2011]). In addition, single time point serum Vitamin D3 and ACC glutamate and GABA levels were also evaluated in typically developing children and adolescents (TD). We hypothesized that 1) BSD would have higher ACC glutamate and lower ACC GABA than TD at baseline; and 2) an 8 week open-label trial with Vitamin D3 supplementation would improve symptoms of mania, decrease ACC glutamate, and increase ACC GABA in BSD.
Materials and Methods
Participants
Sixteen children and adolescents with BSD exhibiting manic symptoms and 19 typically TD participated in this study. The BSD group included patients not only diagnosed with BD (according to Diagnostic and Statistical Manual of Mental Disorder, 4th ed., Text Revision [DSM-IV-TR]) but also those exhibiting the bipolar symptomatology (American Psychiatric Association 2000; Merikangas et al. 2011), including BD not otherwise specified (BD-NOS) (Birmaher et al. 2006) and subthreshold mood ratings (Merikangas et al. 2011). The participants were recruited through the Child and Adolescents NeuroDevelopment Initiative (CANDI) at the University of Massachusetts Medical School (UMMS), from the current pool of clinic patients, word of mouth, advertisements in local newspapers, and online advertising. A telephone screen was administered to determine participant eligibility. Inclusion criteria were male or female participants ages 6–17 years at the time of enrollment. For the BSD patients, the screen visit Young Mania Rating Scale (YMRS) score was ≥8 and the Clinical Global Impressions-Severity Score (CGI-S) was ≥3. The mood ratings recorded during the screen visit were only used to determine eligibility and were not utilized for data analysis. All BSD participants needed to be able ingest the Vitamin D3 orally. If patients were taking psychotropic medications, they were required to be stably medicated (same dose for 4 weeks prior to enrollment) and willing to maintain that dose for the duration of the study. Similarly, if participants were taking a daily multivitamin or vitamin supplement, they had to continue with that supplement for the duration of the study. Exclusion criteria included: history of an uncontrolled general medical disorder; a history of neurological illness, schizophrenia, or psychosis; history of head trauma with loss of consciousness; substance dependence; suicidal or homicidal ideation; and contraindications to MRI. Moreover, patients who had a disorder that would interfere with the action, absorption, distribution, metabolism or excretion of Vitamin D3, that might pose a safety concern, or interfere with the accurate assessment of safety and efficacy, were excluded. Finally, participants who had an Axis I diagnosis or a family history of a mood disorder in a first degree relative were not included in the TD group.
All participants provided written consent/assent as approved by UMMS Institutional Review Board (Docket # 13821). For participants 6–15 year old, parental written consent and participants' written assent were obtained. For participants 16 and 17 years old, participants and their parents provided written consent for the study.
All participants underwent a baseline clinical and semistructured research interview, a blood draw, and a neuroimaging scanning session. BSD patients also participated in an 8 week 2000 IU Vitamin D3 open-label supplementation trial, which was monitored weekly (by phone or face-to-face) and followed by an end-point clinical research interview, blood draw, and neuroimaging session.
Clinical interview
All participants underwent a clinical interview as well as a semistructured research interview, administered by a child and adolescent psychiatrist (A.A.L.N.) or an advanced practice nurse (D.S.). The subjects' demographics and medical history were obtained, and psychiatric diagnoses were assessed via the Kiddie-SADS-Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997). In addition, medication use and severity of illness using the CGI-S (Guy 1976) were determined. In each clinical interview, mood ratings, YMRS (Young et al. 1978) and Children's Depression Rating Scale (CDRS) (Poznanski et al. 1979) were administered. Suicidal ideation and behavior were checked using the Columbia-Suicide Severity Rating Scale (CSSR-S). Finally, participants underwent Wechsler Intelligence Scale for Children – IV (WISC-IV) to assess intelligence quotient (IQ), and had their height and weight measured.
Vitamin D3 supplementation
BSD patients were asked to take a daily dose (2000 IU) of Vitamin D3 for 8 weeks as an adjunctive supplement. The clinical status of the participants was monitored, using CGI-S, mood scales, and CSSR-S, through weekly interviews (by phone or face-to-face). In addition to the screen, baseline and 8 week visits (as described), each patient was seen at the clinic at least three times.
Neuroimaging data acquisition
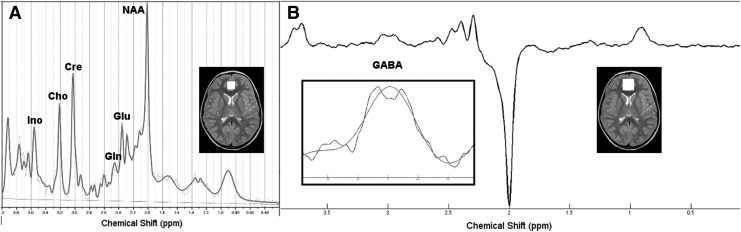
Neuroimaging data were acquired on a 3.0 Tesla Philips Achieva whole-body MR system (Philips Healthcare, Best, the Netherlands) with an eight element phased-array head coil at the Advanced MRI Center, UMMS. For diagnostic and localization purposes, T1-weighted anatomical MRI (magnetization-prepared rapid acquisition with gradient echo [MPRAGE] sequence) was collected; 1H MRS data were acquired using the single voxel point-resolved spectroscopy sequence (PRESS) (TE, 28 msec; TR, 2000 msec; 128 averages; voxel, 20 mm×20 mm×20 mm) to quantify brain glutamate and Mescher-Garwood point-resolved spectroscopy sequence (MEGA-PRESS) (TE, 68 msec; TR, 2000 msec; 16 averages×20 dynamic scans; voxel, 30 mm×30 mm×20 mm) to quantify brain GABA (Mescher et al. 1998; Waddell et al. 2007). Voxels were placed in the ACC by a trained spectroscopist (E.M.S.) (Fig. 1).
FIG. 1.
Representative proton spectra with anterior cingulate cortex (ACC) voxel placement for (A) point-resolved spectroscopy (PRESS) and (B) Mescher-Garwood point-resolved spectroscopy sequence (MEGA-PRESS). White squares superimposed on the horizontal brain slices represent the location of the voxels. For B) the signal around the GABA resonance was fit using a Gaussian function as shown inside the square (zoomed signal and the fit).
A board certified neuroradiologist evaluated the diagnostic scans, and no anatomical brain abnormalities were identified for any participants.
Neuroimaging data analysis
Glutamate concentrations was obtained using LCModel (Version 6.3.0) (Provencher 1993, 2001) and a simulated basis set (including alanine, aspartate, creatine (Cr), phosphocreatine, GABA, glucose, glutamate, glutamine, glycerophosphocholine, phosphocholine, glutathione, inositol, lactate, N-acetyl aspartate, N-acetyl aspartyl glutamate, scyllo-inositol, and taurine, as well as macromolecules and lipids). Cramer–Rao lower bounds (CRLB) (estimated error of the metabolite quantification) were >15% for glutamate and glutamate+glutamine (Glx) classified the peak as not reliably detected (Provencher 1993, 2001); however, for all the participants, CRLB for glutamate and Glx peaks were ≤7%; therefore, all were included in the analysis.
GABA concentrations were determined using in-house MATLAB (The Mathworks, MA) software, which determined the ratios of GABA with respect to Cr levels. Prior to Fourier transformation of the MRS signal, 3 Hz exponential line broadening was applied. The difference-edited GABA signal at 3 ppm and the Cr peak from the unsuppressed MEGA-PRESS signal were fit with Gaussian and Lorentzian models, respectively (r>0.95), and the area under these model fits was calculated to determine the GABA to total Cr ratio (GABA/Cr) (Edden and Barker 2007; Dydak et al. 2011; Zhu et al. 2011; Edden et al. 2014).
Finally, structural MRI scans were analyzed using Statistical Parameter Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/); white or gray matter or cerebrospinal fluid (CSF) in each MRS voxel were estimated using MATLAB code (provided by Drs. Nia Goulden and Paul Mullins at Bangor University [http://biu.bangor.ac.uk/projects.php.en]) (Gasparovic et al. 2006).
Statistical analysis
Statistical analysis was performed using SPSS 21 (IBM Corp., Armonk, NY). An outlier analysis for brain glutamate and GABA within each group of participants was conducted. Values that were two standard deviations from the mean of their corresponding groups were eliminated from further analysis. The remaining brain glutamate and GABA levels underwent statistical analysis. Univariate ANOVA was used compare the effects of mania; that is, pre-Vitamin D3 supplementation BSD patients versus TD. In addition, a paired t test was performed to investigate the effects of 8 weeks of Vitamin D3 supplementation on the clinical assessments, blood serum levels (calcium, phosphorous, parathyroid hormone [PTH], 25-hydroxy Vitamin D3 [25(OH)D] and 1-α-25-dihydroxy Vitamin D3 [1,25 (OH)2D levels]), and brain glutamate and GABA levels in patients with BSD. Because we had an a priori hypothesis (see Introduction), we did not perform a multiple comparison analysis. Finally, as an exploratory analysis, Pearson's correlation analyses were conducted to investigate the relationships between the ACC glutamate and GABA, and mood ratings considering only baseline BSD data. Similarly, we examined the relationships between the ACC glutamate and GABA and baseline blood serum levels [25(OH)D and 1,25 (OH)2D levels], including all the participants.
Results
Participant demographics
A total of 35 children and adolescents were recruited for this study: 16 patients with BSD (mean age 12.3±3.2 years; 12 males) and 19 matched TD (mean age 11.9±3.6 years; 8 males). Patients with BSD exhibited symptoms of mania during their screen visit: YMRS scores ≥8 and CGI-S scores ≥3. Eight of the 16 BSD patients met DSM-IV-TR diagnosis for BD-1 as determined by semistructured clinical interview and K-SADS-PL, and 2 patients were diagnosed with BD-NOS (Birmaher et al. 2006). In addition, six participants had YMRS ≥8 during their screen visit but did not meet criteria for BD-1 or BD-NOS (i.e., at risk of developing BD). In sum, 16 participants met the criteria for BSD (Merikangas et al. 2011). YMRS scores were ≤1 and CGI-S scores were 1 for all participants within the TD group. IQ was ≥75 for all participants (BSD, 103.3±16.6; TD, 118.7±11.4; p=0.01). There were no statistical differences in demographic information among groups (p>0.05) except for WISC-IV I.Q. Table 1 lists group averages regarding demographic and clinical information. Table 2 gives detailed individualized demographic and clinical information on BSD patients, including age, gender, diagnosis (including comorbidities), medication and mood ratings before and after the Vitamin D3 supplementation.
Table 1.
Demographic Information, Mood Ratings, Blood Serum, and Brain Glutamate and GABA Levels
| TD | BSD pre-Vit D3 | BSD post-Vit D3 | |
|---|---|---|---|
| Age (Years) | 11.9±3.6 (n=19) | 12.3±3.2 (n=16) | |
| Gender | 8 M, 11 F | 12 M, 4 F | |
| WISC-IV IQ | 118.7±11.4 (n=12) | 103.3±16.6 (n=15) | |
| YMRS | 0.28±0.67 (n=18) | 19.81±10.26 (n=16) | 11.25±8.90 (n=16) |
| CDRS | 17.76±0.97 (n=17) | 33.25±12.29 (n=16) | 25.31±5.59 (n=16) |
| CGI-S | 1.00±0.00 (n=19) | 4.00±0.89 (n=16) | 2.50±1.10 (n=16) |
| Calcium (ng/mL) | 9.62±0.32 (n=16) | 9.75±0.34 (n=16) | 9.66±0.35 (n=16) |
| Phosphorus (ng/mL) | 4.35±0.60 (n=16) | 4.19±0.66 (n=16) | 4.13±0.50 (n=16) |
| PTH (ng/mL) | 29.69±15.57 (n=16) | 35.13±13.99 (n=16) | 38.69±24.40 (n=16) |
| 25(OH)D (ng/mL) | 25.81±7.54 (n=16) | 21.13±8.53 (n=16) | 32.31±9.85 (n=16) |
| 1,25 (OH)2D (ng/mL) | 57.88±13.19 (n=16) | 62.07±16.45 (n=15) | 67.56±13.25 (n=16) |
| Glutamate (IU) | 10.93±0.69 (n=19) | 10.58±0.89 (n=15) | 10.80±0.66 (n=15) |
| Glx (IU) | 14.41±0.85 (n=18) | 13.89±1.22 (n=15) | 13.85±0.75 (n=15) |
| GABA/Cr | 0.082±0.012 (n=18) | 0.066±0.018 (n=15) | 0.080±0.016 (n=16) |
| Cr | 5.36±0.45 (n=18) | 5.20±0.51 (n=15) | 5.33±0.27 (n=16) |
Group averages (with standard deviations) for typically developing children and adolescents (TD), and bipolar spectrum disorder (BSD) patients (baseline [pre-Vit D3] and following 8 weeks of Vitamin D3 supplementation [post-Vit D3] visits).
GABA, γ-aminobutyric acid; WISC-IV, Wechsler Intelligence Scale for Children – IV; IQ, intelligence quotient; YMRS, Young Mania Rating Scale; CDRS, Children's Depression Rating Scale; CGI-S, Clinical Global Impressions – Severity; PTH, parathyroid hormone; Glx, glutamate+glutamine; Cr, creatine.
Table 2.
Individual Demographic and Clinical Information on BSD Patients
| ID | Age | Gender | Diagnosis | Medication (generic names) | YMRS pre-Vit D3 | YMRS post-Vit D3 | CDRS pre-Vit D3 | CDRS post-Vit D3 | CGI-S pre-Vit D3 | CGI-S post-Vit D3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | M | BD-1, SAD, ADHD, Asperger's | Sertraline, lamotrigine, melatonin | 29 | 19 | 26 | 30 | 4 | 2 |
| 2 | 8 | M | BD-1 | Risperidone, lamotrigine, melatonin | 20 | 17 | 31 | 26 | 4 | 3 |
| 3 | 11 | M | BD-1, PTSD, ADHD, ODD | Clonidine, melatonin, fish oil | 15 | 14 | 26 | 24 | 5 | 4 |
| 4 | 11 | F | BD-1, SAD, social phobia, GAD, ADHD, ODD | Aripiprazole, lamotrigine, melatonin | 39 | 20 | 49 | 31 | 4 | 3 |
| 5 | 13 | M | BD-1, GAD | Lithium ER, valproic acid, levothyroxine | 24 | 5 | 30 | 19 | 3 | 1 |
| 6 | 16 | F | BD-1, panic disorder, ADC, social phobia, GAD, OCD, PTSD, ADHD, ODD | Oxcarbazepine, hydroxyzine, bupropion, clonidine, omeprazole, quetiapine | 15 | 8 | 52 | 24 | 4 | 3 |
| 7 | 17 | M | BD-1, GAD, ADHD | Gabapentin, atomoxetine, risperidone, lamotrigine | 12 | 3 | 28 | 25 | 3 | 3 |
| 8 | 9 | M | BD-NOS, ODD | Not medicated | 22 | 4 | 32 | 25 | 4 | 1 |
| 9 | 12 | F | BD-NOS | Lithium | 6 | 1 | 28 | 17 | 4 | 1 |
| 10 | 17 | M | BD-1, SAD, simple phobia, agoraphobia, PTSD, ADHD, Asperger's | Dexmethylphenidate, sertraline, risperidone | 27 | 17 | 22 | 24 | 5 | 3 |
| 11 | 10 | M | GAD, ADHD, ODD | Aripiprazole, clonidine, melatonin | 10 | 15 | 23 | 35 | 5 | 4 |
| 12 | 8 | M | DD NOS, SAD, ADC, simple phobia, encopresis, ADHD, ODD | Dextroamphetamine/amphetamine, clonidine | 24 | 32 | 53 | 32 | 6 | 4 |
| 13 | 8 | M | DD NOS, SAD, simple phobia, enuresis, ADHD, conduct disorder | Methylphenidate | 40 | 13 | 58 | 33 | 4 | 3 |
| 14 | 14 | M | ADHD | Risperidone, dextroamphetamine/amphetamine, clonidine | 14 | 0 | 20 | 20 | 3 | 2 |
| 15 | 12 | F | ADC, social phobia, GAD, ODD | Not medicated | 8 | 0 | 26 | 17 | 3 | 1 |
| 16 | 15 | M | DD NOS, ADHD, ODD | Not medicated | 12 | 12 | 28 | 23 | 3 | 2 |
Mood ratings were recorded during baseline (pre-Vit D3) and following 8 weeks of Vitamin D3 supplementation (post-Vit D3) visits.
ADC, avoidant disorder of childhood; ADHD, attention-deficit/hyperactivity disorder; BD-1, bipolar disorder 1; BD-NOS, bipolar disorder not otherwise specified; BSD, bipolar spectrum disorder; DD, depressive disorder; GAD, generalized anxiety disorder; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; ODD, oppositional defiant disorder; PTSD, posttraumatic stress disorder; SAD, separation anxiety disorder; CDRS, Children's Depression Rating Scale; CGI-S: Clinical Global Impressions-Severity; YMRS, Young Mania Rating Scale.
Mood assessments
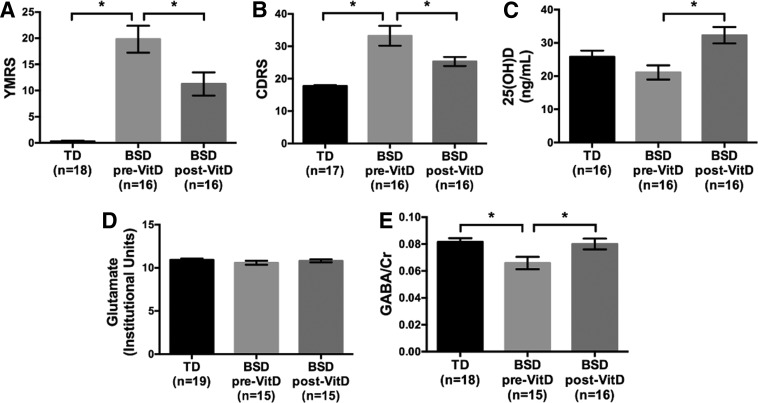
At baseline, pre-Vitamin D3 supplementation, YMRS scores were significantly higher for the BSD patients than for the TD (F[1,32]=65.14, p<0.0001) (TD, 0.28±0.67 vs. pre-Vitamin D3 BSD, 19.81±10.27). Following an 8 week Vitamin D3 supplementation, in the BSD patients, there was a significant decrease in YMRS scores (t=−3.66, p=0.002, df=15) (post-Vitamin D3 BSD, 11.25±8.90) (Fig. 2A). Similarly, at baseline, CDRS scores were higher for BSD patients than for the TD group (F[1,31]=26.85, p<0.0001) (TD, 17.76±0.97 vs. pre-Vitamin D3 BSD, 33.25±12.29), some of the subjects exhibited mixed symptoms, and these scores significantly decreased following 8 weeks of Vitamin D3 supplementation (t=−2.93, p=0.01, df=15) (post-Vitamin D3 BSD, 25.31±5.59) (Fig. 2B) (see Tables 1 and 2).
FIG. 2.
(A) Young Mania Rating Scale (YMRS), (B) Children's Depression Rating Scale (CDRS), (C) blood serum 25(OH)D levels, (D) anterior cingulate cortex (ACC) glutamate concentrations, (E) ACC γ-aminobutyric acid/creatine (GABA/Cr) concentrations (group averages±standard errors) for typically developing children and adolescents (TD) and patients with bipolar spectrum disorder (BSD) before and after 8 week Vitamin D3 supplementation. *p<0.05.
Serum levels
Serum calcium, phosphorous, PTH, 25(OH)D, and 1,25 (OH)2D levels were measured for both groups of participants (see Table 1). Vitamin D3 is converted to 25(OH)D, which is usually utilized as the serum measurement to indicate Vitamin D3 levels. Baseline 25(OH)D was lower for BSD patients than TD at a trend level (F[1,30]=2.71, p=0.11). There were no statistical differences on any of the other serum levels between the BSD patients and TD. Following 8 weeks of Vitamin D3 supplementation, the BSD patients showed a significant increase in serum 25(OH)D levels (t=6.15, p<0.001, df=15) (Fig. 2C), but there were no significant changes for any of the other serum metabolites (see Table 1).
ACC glutamate (and Glx) and GABA levels
There was no statistical difference in baseline ACC glutamate (or Glx) between BSD patients and TD (p>0.05). ACC glutamate (or Glx) did not show any changes following 8 weeks of Vitamin D3 supplementation (p>0.05) (Fig. 2D) (see Table 1).
Baseline ACC GABA (GABA/Cr) were statistically lower for BSD patients than for the TD group (F[1,31]=8.91, p=0.005). Because there was a statistically significant difference in WISC-IV IQ between participants with BSD and TD, we covaried for WISC-IV score IQ, but this did not significantly change our findings. GABA/Cr remained lower for BSD patients when compared with TD (F[1,22]=3.61, p=0.07). Following 8 weeks of Vitamin D3 supplementation, a statistically significant increase in ACC GABA/Cr levels was observed in BSD patients (t=3.18, p=0.007, df=14) (Fig. 2E) (see Table 1). It is of note that total Cr levels were not significantly different between BSD and TD (F[1,31]=0.91, p=0.35), nor did total Cr levels change pre- to post-Vitamin D3 supplementation (t=1.02, p=0.33, df=14).
There was no statistically significant difference between the groups in terms of ACC voxel tissue content (p>0.05). Therefore, gray matter/white matter/CSF quantities were not included in our analysis of the metabolic differences.
Further subanalysis
As criteria for hypomania is defined to be YMRS >12, we performed a subanalysis in which we eliminated three patients whose pre-Vitamin D3 YMRS scores were <12. The findings were in line with those reported previously: Pre-Vitamin D3 supplementation, mood ratings were significantly higher for BSD patients than for TD (YMRS, F[1,29]=101.87, p<0.0001; CDRS, F[1,28]=29,79, p<0.0001); however following 8 week Vitamin D3 supplementation, in BSD patients, there was a significant decrease in YMRS and CDRS scores (YMRS, t=−3.75, p=0.003, df=12; CDRS: t=−3.13, p=0.009, df=12). Baseline 25(OH)D was lower for BSD patients than for TD at a trend level (F[1,27]=2.98, p=0.1), and following 8 weeks of Vitamin D3 supplementation, the BSD patients showed a significant increase in serum 25(OH)D levels (t=5.56, p<0.001, df=12). Similarly, GABA/Cr levels were significantly higher for TD than for BSD patients (pre-Vitamin D3 supplementation) (F[1,28]=6.27, p=0.018); and after Vitamin D3 supplementation, GABA/Cr levels were significantly increased (t=2.79, p=0.017, df=11).
Relationship between baseline mood ratings and ACC glutamate (and Glx) and GABA levels
There was no statistically significant association between baseline mood ratings (YMRS and CDRS) and ACC glutamate (or Glx) levels, or between baseline mood ratings and ACC GABA/Cr levels (p>0.1), for the BSD patients. Baseline CDRS scores inversely correlated with glutamate (r=−0.47, p=0.08, df=15) and Glx (r=−0.48, p=0.07, df=15) levels at the trend level, for the BSD patients.
Relationship between baseline serum levels and ACC glutamate (and Glx) and GABA levels
There was no statistically significant association between baseline blood serum [25(OH)D and 1,25 (OH)2D] levels and ACC glutamate (or Glx) (p>0.1) or baseline blood serum levels and ACC GABA/Cr (p>0.1), for the BSD patients.
Discussion
Motivated by the glutamatergic and GABAergic abnormalities in BD and possible regulatory impact of Vitamin D3, especially on brain GABAA receptors, we investigated the effect of an 8 week open label Vitamin D3 supplement on children and adolescents exhibiting manic or mixed symptoms. As we hypothesized, improved mood symptoms were observed at the end of the 8 week Vitamin D3 supplementation trial. Although there were no differences in baseline ACC glutamate (or Glx) between BSD patients and TD, baseline ACC GABA was significantly lower for BSD patients than for TD, and the ACC GABA significantly increased following the 8 week trial with Vitamin D3.
Studies using MRS to examine brain glutamate levels in patients with BD have shown mixed results. Although one study (Xu et al. 2013) found no significant difference in ACC glutamate or Glx between unmedicated adults with BD and healthy adults, two other studies (Michael et al. 2003; Ongur et al. 2008) reported higher Glx in the prefrontal cortex of medicated adults with BD-mania; however glutamate differences in the latter studies were not significant (Ongur et al. 2008). In addition to these studies conducted with adult populations, there exist only a few studies using MRS to examine ACC glutamate and/or Glx in adolescents. Singh et al. observed reductions in ACC glutamate levels in youth with BD-1 or BD-2 relative to the glutamate concentrations from TD as well as from youth with subsyndromal symptoms of mania (Singh et al. 2010). However, the majority of studies investigating ACC glutamate and/or Glx in adolescents meeting DSM-IV-TR BD criteria for a manic or mixed episode did not observe any significant difference in ACC glutamate or Glx when compared with TD (Davanzo et al. 2001, 2003; Moore et al. 2007; Strawn et al. 2012). The lack of a significant result for glutamate or Glx seen in our study is in line with previous investigations in similarly aged patients. The findings may be a consequence of the younger age group of our participants and/or the subthreshold status of some of our participants.
There are inconsistencies across studies investigating adult brain GABA levels, using MRS, in BD. Previous work with adults reported elevations in the occipital (Wang et al. 2006) and parieto-occipital cortex (Brady et al. 2013), reductions in the occipital lobe (Bhagwagar et al. 2007), or no change in the parieto-occipital cortex for GABA levels of BD patients (Kaufman et al. 2009) when compared with healthy controls. Medial prefrontal/ACC (Wang et al. 2006) and ACC (Brady et al. 2013) GABA were investigated in two of the above mentioned studies, and both reported elevated GABA for patients with BD in comparison with healthy controls. The differences in these findings may be the result of the disease status/process and/or pharmacological interventions received by the study participants. Here, we present the first investigation of brain GABA levels in a pediatric BSD population. Consistent with our hypothesis, we observed lower ACC GABA in patients with manic or mixed episodes when compared with TD. Overall, our findings are in agreement with studies implicating the GABAergic neurotransmitter system (Schousboe 2003; Bak et al. 2006; Wang et al. 2006; Bhagwagar et al. 2007; Woo et al. 2007; Brady et al. 2013) in the pathophysiology of BD.
Many pharmacotherapeutic interventions used in the treatment of BD affect either the glutamatergic and/or GABAergic systems (Nonaka et al. 1998; Hashimoto et al. 2002; Zarate et al. 2002; Cooper et al. 2003; Sanacora et al. 2008). Given that this study was to examine Vitamin D3 as a supplemental intervention, 13 of the 16 BSD participants were receiving psychotropic treatment, in addition to the Vitamin D3. We speculate that patient medications may already have corrected some glutamate imbalances, whereas the GABAergic imbalances remained.
We investigated patients who covered a range of manic symptoms; that is, bipolar spectrum, rather than restricting the patient population to those meeting criteria; that is, YMRS >12 for hypomania or YMRS >20 for mania. However, when we excluded the three BSD patients whose initial YMRS was <12, our findings remained the same.
Overall, we expected 1,25 (OH)2D, the active form of Vitamin D3, to act in a similar fashion as neuroactive steroids (Losel et al. 2003) and possibly decrease elevated Ca2+ concentration (Moghaddam et al. 1994; Kalueff et al. 2004) and modulate the GABAergic system (Losel et al. 2003) in BSD patients. Our findings of improved mood and increased ACC GABA levels at the end of 8 week Vitamin D3 supplementation support the proposed model. In addition, following Vitamin D3 supplementation, we observed increases in 25(OH)D serum levels, which is the circulating form of Vitamin D3 and a precursor to the active form of 1,25 (OH)2D.
Limitations and future considerations
The major limitation of this study was the open label nature; one cannot rule out a placebo effect. However, we noted a 43% improvement in manic symptoms in the individuals with BSD treated with Vitamin D3, which compares favorably with the 20% improvement usually seen with placebo (Oliveira et al. 2009). We also had a relatively small sample size. Further research with larger patient populations may investigate the effect of Vitamin D3 in a double-blind randomized controlled trial design.
Almost all the patients with mania were on medication, as this was a supplemental treatment study. All were stably medicated (same dose for 4 weeks prior to enrolment) and remained stably medicated throughout the 8 weeks on Vitamin D3. However, possible medication effects remain as a confounding factor for this study. Future directions would be to investigate Vitamin D3 supplementation as an add-on to specific therapeutics; or to investigate the response of unmedicated children and adolescents with BSD to Vitamin D3 supplementation. In addition, the majority of the participants within our study (BSD, 13/16; TD, 12/19) were Vitamin D3 deficient at baseline. Future studies may want to investigate the role of 25(OH)D deficiency in mood symptoms.
Finally, although a significant decrease in manic symptoms (∼43% across 16 patients) was observed in the BSD patients following the 8 week Vitamin D3 supplementation, manic symptoms still persisted in the majority. Ten of the 16 patients had YMRS values ≥8 (9 and 2 of the 16 patients had YMRS values ≥12 and ≥20, respectively), at the end of the open label trial. For this study, patients with BSD took a daily dose of 2000 IU of Vitamin D3 for 8 weeks as an adjunctive supplement. Future studies may consider higher doses of Vitamin D3.
Conclusion and Clinical Significance
Following a “research domain criteria (RDoc)” approach, this study investigated patients with a range of symptoms on a bipolar spectrum, with the common indicator being that all were showing manic or mixed symptoms. We illustrated differences in brain GABA levels between children and adolescents with manic symptoms; that is, patients with BSD and TD. In addition, following an 8 week open label trial with Vitamin D3 supplementation, patients with BSD exhibited improvement in their mood in conjunction with their neurochemistry. However, although a significant decrease in manic symptoms was observed in the BSD patients following the 8 week Vitamin D3' supplementation, manic symptoms still persisted in the majority.
Acknowledgments
The authors thank the Department of Radiology and the Advanced MR Imaging Core at the University of Massachusetts Medical School.
Disclosures
Dr. Frazier has received research support from Alcobra, GlaxoSmithKline, Neuren, Pfizer, Inc., Roche, Seaside Therapeutics, and SyneuRX International, and has served on a Data Safety Monitoring Board for Forest Pharmaceuticals. The other authors have nothing to disclose.
References
- Ahmadi S, Mirzaei K, Hossein-Nezhad A, Shariati G:Vitamin D receptor FokI genotype may modify the susceptibility to schizophrenia and bipolar mood disorder by regulation of dopamine D1 receptor gene expression. Minerva Med 103:383–391, 2012 [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS: The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98:641–653, 2006 [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S: GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25:1–27, 2001 [DOI] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS, Logiotatos P, Williams M: Glutamate decarboxylase(65)–immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat 20:259–269, 2000 [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ: Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry 61:806–812, 2007 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M: Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 63:175–183, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP: The next wave in neuroimaging research in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 47:483–485, 2008 [DOI] [PubMed] [Google Scholar]
- Brady RO, Jr., McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, Renshaw PF, Ongur D: Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord 15:434–439, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222, 2000 [DOI] [PubMed] [Google Scholar]
- Cannell JJ: Autism and vitamin D. Med Hypotheses 70:750–759, 2008 [DOI] [PubMed] [Google Scholar]
- Carta MG, Bhat KM, Preti A: GABAergic neuroactive steroids: A new frontier in bipolar disorders? Behav Brain Funct 8:61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Sayeed I, Stein DG: Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Front Neuroendocrinol 30:158–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo MA, Adler CM, Delbello MP, Strakowski SM: The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry 21:314–322, 2009 [DOI] [PubMed] [Google Scholar]
- Chang K: Adult bipolar disorder is continuous with pediatric bipolar disorder. Can J Psychiatry 52:418–425, 2007 [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH: The Biochemical Basis of Neuropharmacology. New York: Oxford University Press; 2003 [Google Scholar]
- Correale J, Ysrraelit MC, Gaitan MI: Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 132:1146–1160, 2009 [DOI] [PubMed] [Google Scholar]
- Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I: The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry 51:377–386, 2002 [DOI] [PubMed] [Google Scholar]
- Davanzo P, Thomas MA, Yue K, Oshiro T, Belin T, Strober M, McCracken J: Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology 24:359–369, 2001 [DOI] [PubMed] [Google Scholar]
- Davanzo P, Yue K, Thomas MA, Belin T, Mintz J, Venkatraman TN, Santoro E, Barnett S, McCracken J: Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry 160:1442–1452, 2003 [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Peters AT, Sylvia L, Urdahl A, Magalhaes PV, Otto MW, Frank E, Miklowitz DJ, Berk M, Kinrys G, Nierenberg A: Do comorbid anxiety disorders moderate the effects of psychotherapy for bipolar disorder? Results from STEP-BD. Am J Psychiatry 171:178–186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis M, Wagner KD: Bipolar disorder in adolescence. Adolesc Med 24:433–445, ix, 2013 [PubMed] [Google Scholar]
- Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, Edden RA, Hu S, Fu X, Long Z, Mo XA, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W: In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect 119:219–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ: Markers of glutamate synaptic transmission and plasticity are increased in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry 67:1010–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Barker PB: Spatial effects in the detection of gamma-aminobutyric acid: Improved sensitivity at high fields using inner volume saturation. Magn Reson Med 58:1276–1282, 2007 [DOI] [PubMed] [Google Scholar]
- Edden RA, Puts NAJ, Harris AD, Barker PB, Evans J: Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 40:1445–1452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D, Burne T, McGrath J: Vitamin D in fetal brain development. Semin Cell Dev Biol 22:629–636, 2011 [DOI] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ: Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29:21–30, 2005 [DOI] [PubMed] [Google Scholar]
- Garcion E, Sindji L, Montero–Menei C, Andre C, Brachet P, Darcy F: Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia 22:282–294, 1998 [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA: Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55:1219–1226, 2006 [DOI] [PubMed] [Google Scholar]
- Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN: Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: A meta-analysis. Bipolar Disord 14:478–487, 2012 [DOI] [PubMed] [Google Scholar]
- Gloth FM, 3rd, Alam W, Hollis B: Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging 3:5–7, 1999 [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; 1976 [Google Scholar]
- Hafeman D, Axelson D, Demeter C, Findling RL, Fristad MA, Kowatch RA, Youngstrom EA, Horwitz SM, Arnold LE, Frazier TW, Ryan N, Gill MK, Hauser–Harrington JC, Depew J, Rowles BM, Birmaher B: Phenomenology of bipolar disorder not otherwise specified in youth: A comparison of clinical characteristics across the spectrum of manic symptoms. Bipolar Disord 15:240–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms LR, Burne TH, Eyles DW, McGrath JJ: Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab 25:657–669, 2011 [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM: Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem 80:589–597, 2002 [DOI] [PubMed] [Google Scholar]
- Holick MF.Vitamin D: A millenium perspective. J Cell Biochem 88:296–307, 2003 [DOI] [PubMed] [Google Scholar]
- Humble MB: Vitamin D, light and mental health. J Photochem Photobiol B 101:142–149, 2010 [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG: Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab 26:865–877, 2006 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Eremin KO, Tuohimaa P: Mechanisms of neuroprotective action of vitamin D(3). Biochemistry 69:738–741, 2004 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, Renshaw PF, Pollack MH: Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 33:427–434, 2009 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE: Lifetime prevalence and age–of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602, 2005a [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE: Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoraminya N, Tehrani–Doost M, Jazayeri S, Hosseini A, Djazayery A:Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry 47:271–275, 2013 [DOI] [PubMed] [Google Scholar]
- Kjaergaard M, Waterloo K, Wang CE, Almas B, Figenschau Y, Hutchinson MS, Svartberg J, Jorde R: Effect of vitamin D supplement on depression scores in people with low levels of serum 25–hydroxyvitamin D: Nested case–control study and randomised clinical trial. Br J Psychiatry 201:360–368, 2012 [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador–Woodruff J, Torrey EF: Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 9:609–620, 544, 2004 [DOI] [PubMed] [Google Scholar]
- Li G, Mbuagbaw L, Samaan Z, Falavigna M, Zhang S, Adachi JD, Cheng J, Papaioannou A, Thabane L: Efficacy of vitamin D supplementation in depression in adults: A systematic review. J Clin Endocrinol Metab 99:757–767, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lish JD, Dime–Meenan S, Whybrow PC, Price RA, Hirschfeld RM: The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord 31:281–294, 1994 [DOI] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol–Haseroth K, Wehling M: Nongenomic steroid action: Controversies, questions, and answers. Physiol Rev 83:965–1016, 2003 [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I: Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology 34 Suppl 1:S84–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massat I, Souery D, Del-Favero J, Oruc L, Noethen MM, Blackwood D, Thomson M, Muir W, Papadimitriou GN, Dikeos DG, Kaneva R, Serretti A, Lilli R, Smeraldi E, Jakovljevic M, Folnegovic V, Rietschel M, Milanova V, Valente F, Van Broeckhoven C, Mendlewicz J: Excess of allele1 for alpha3 subunit GABA receptor gene (GABRA3) in bipolar patients: A multicentric association study. Mol Psychiatry 7:201–207, 2002 [DOI] [PubMed] [Google Scholar]
- McCann JC, Ames BN: Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J 22:982–1001, 2008 [DOI] [PubMed] [Google Scholar]
- McGrath J: Hypothesis: Is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res 40:173–177, 1999 [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Panzica G: Neuroactive steroids: An update of their roles in central and peripheral nervous system. Psychoneuroendocrinology 34 Suppl 1:S1–8, 2009 [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Panzica GC: Neuroactive steroids: Old players in a new game. Neuroscience 138:733–739, 2006 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina–Mora ME, Ono Y, Posada–Villa J, Sagar R, Wells JE, Zarkov Z: Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 68:241–251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R: Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266–272, 1998 [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Gossling M, Arolt V, Heindel W, Pfleiderer B: Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology 168:344–346, 2003 [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein–Behrens B, Sapolsky R: Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res 655:251–254, 1994 [DOI] [PubMed] [Google Scholar]
- Moore CM, Frazier JA, Glod CA, Breeze JL, Dieterich M, Finn CT, Frederick B, Renshaw PF: Glutamine and glutamate levels in children and adolescents with bipolar disorder: A 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry 46:524–534, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli PL, Elgie R: GAMIAN-Europe/BEAM survey I—global analysis of a patient questionnaire circulated to 3450 members of 12 European advocacy groups operating in the field of mood disorders. Bipolar Disord 5:265–278, 2003 [DOI] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM: Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A 95:2642–2647, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira IR, Nunes PM, Coutinho DM, Sena EP: Review of the efficacy of placebo in comparative clinical trials between typical and atypical antipsychotics. Rev Bras Psiquiatr 31:52–56, 2009 [DOI] [PubMed] [Google Scholar]
- Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF: Abnormal glutamatergic neurotransmission and neuronal–glial interactions in acute mania. Biol Psychiatry 64:718–726, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani K, Ujike H, Tanaka Y, Morita Y, Katsu T, Nomura A, Uchida N, Hamamura T, Fujiwara Y, Kuroda S: The GABA type A receptor alpha5 subunit gene is associated with bipolar I disorder. Neurosci Lett 381:108–113, 2005 [DOI] [PubMed] [Google Scholar]
- Palermo S, Cauda F, Costa T, Duca S, Gallino G, Geminiani G, Keller R, Amanzio M: Unawareness of bipolar disorder: The role of the cingulate cortex. Neurocase 16:1–10, 2014 [DOI] [PubMed] [Google Scholar]
- Pfennig A, Correll CU, Marx C, Rottmann–Wolf M, Meyer TD, Bauer M, Leopold K: Psychotherapeutic interventions in individuals at risk of developing bipolar disorder: A systematic review. Early Interv Psychiatry 8:3–11, 2014 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ: A depression rating scale for children. Pediatrics 64:442–450, 1979 [PubMed] [Google Scholar]
- Provencher SW: Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14:260–264, 2001 [DOI] [PubMed] [Google Scholar]
- Provencher SW: Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679, 1993 [DOI] [PubMed] [Google Scholar]
- Prufer K, Veenstra TD, Jirikowski GF, Kumar R: Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat 16:135–145, 1999 [DOI] [PubMed] [Google Scholar]
- Robinson M, Whitehouse AJ, Newnham JP, Gorman S, Jacoby P, Holt BJ, Serralha M, Tearne JE, Holt PG, Hart PH, Kusel MM: Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch Womens Ment Health 17:213–219, 2014 [DOI] [PubMed] [Google Scholar]
- Rocha TB, Zeni CP, Caetano SC, Kieling C: Mood disorders in childhood and adolescence. Rev Bras Psiquiatr 35 Suppl 1:S22–31, 2013 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK: Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7:426–437, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A: Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem Res 28:347–352, 2003 [DOI] [PubMed] [Google Scholar]
- Singh M, Spielman D, Adleman N, Alegria D, Howe M, Reiss A, Chang K: Brain glutamatergic characteristics of pediatric offspring of parents with bipolar disorder. Psychiatry Res 182:165–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding S: Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 6:1501–1518, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Patel NC, Chu WJ, Lee JH, Adler CM, Kim MJ, Bryan HS, Alfieri DC, Welge JA, Blom TJ, Nandagopal JJ, Strakowski SM, DelBello MP: Glutamatergic effects of divalproex in adolescents with mania: a proton magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry 51:642–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf WE, Privette TH: Light, vitamin D and psychiatry. Role of 1,25 dihydroxyvitamin D3 (soltriol) in etiology and therapy of seasonal affective disorder and other mental processes. Psychopharmacology 97:285–294, 1989 [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador–Woodruff JH, Knable MB: Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry 57:252–260, 2005 [DOI] [PubMed] [Google Scholar]
- Van Meter AR, Moreira AL, Youngstrom EA: Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry 72:1250–1256, 2011 [DOI] [PubMed] [Google Scholar]
- Waddell KW, Avison MJ, Joers JM, Gore JC: A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging 25:1032–1038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Sailasuta N, Chandler RA, Ketter TA: Magnetic resonance spectroscopic measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatrica 18:120–126, 2006 [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B: Regulation of cortical interneurons by neurotrophins: From development to cognitive disorders. Neuroscientist 12:43–56, 2006 [DOI] [PubMed] [Google Scholar]
- Woo TU, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM: Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr Res 96:46–61, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dydak U, Harezlak J, Nixon J, Dzemidzic M, Gunn AD, Karne HS, Anand A: Neurochemical abnormalities in unmedicated bipolar depression and mania: A 2D 1H MRS investigation. Psychiatry Res 213:235–241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: Reliability, validity and sensitivity. Br J psychiatry 133:429–435, 1978 [DOI] [PubMed] [Google Scholar]
- Zarate CA, Quiroz J, Payne J, Manji HK: Modulators of the glutamatergic system: Implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull 36:35–83, 2002 [PubMed] [Google Scholar]
- Zheng P: Neuroactive steroid regulation of neurotransmitter release in the CNS: Action, mechanism and possible significance. Prog Neurobiol 89:134–152, 2009 [DOI] [PubMed] [Google Scholar]
- Zhu H, Edden RA, Ouwerkerk R, Barker PB: High resolution spectroscopic imaging of GABA at 3 Tesla. Magn Reson Med 65:603–609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]