Summary
Objective
In order to gain a better understanding of the timing of emergent symptoms of osteoarthritis, we sought to investigate the existence, duration and nature of a prodromal symptomatic phase preceding incident radiographic knee osteoarthritis (ROA).
Design
Data were from the incidence cohort of the Osteoarthritis Initiative (OAI) public use datasets. Imposing a nested case–control design, ten control knees were selected for each case of incident tibiofemoral ROA between 2004 and 2010 from participants aged 45–79 years. Candidate prodromal symptoms were Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) subscale scores and individual items, available up to 4 years prior to the time of incident ROA. Multi-level models were used to estimate the length of the prodromal phases.
Results
The prodromal phase for subscale scores ranged from 29 months (KOOS Other Symptoms) to 37 months (WOMAC Pain). Pain and difficulty on activities associated with higher dynamic knee loading were associated with longer prodromal phases (e.g., pain on twisting/pivoting (39 months, 95% confidence interval: 13, 64) vs pain on standing (25 months: 7, 42)).
Conclusions
Our analysis found that incident ROA is preceded by prodromal symptoms lasting at least 2–3 years. This has potential implications for understanding phasic development and progression of osteoarthritis and for early recognition and management.
Keywords: Knee osteoarthritis, Symptoms, Epidemiology, Case–control, Prodrome
Introduction
Osteoarthritis has a substantial impact on the health of individuals and populations worldwide1,2. Symptoms often precede the appearance on plain radiographs of features traditionally used to define disease incidence (e.g., marginal osteophytes, joint space narrowing)3–5, implying the existence of a potentially detectable ‘prodromal phase’ (period of premonitory symptoms) in the transition from pre-radiographic to radiographic stages of osteoarthritis. In the pre-radiographic stage, magnetic resonance imaging reveals lesions in articular cartilage, subchondral bone, bone marrow, and meniscus that appear to be associated with symptoms6–8, suggesting a range of plausible sources of pain in this hypothesised prodromal phase. The investigation of prodromes has been an important focus for research in several recurrent-relapsing and chronic long-term conditions9–12 where its significance is seen in terms of the prospects of early intervention, targeted search for biomarkers, understanding pathogenesis and the process of developing illness or disease, and enriched sampling for efficient trial design. These are also major concerns in osteoarthritis research and our desire to understand the contribution of simple patient-reported information to these goals was a strong motivation behind the current study. However, until recently it was not possible to undertake a prospective investigation of the timing of symptom changes before the occurrence of gross pathological changes on radiographs due to the lack of longitudinal studies that have obtained sufficiently frequent repeated images of the joint and measures of symptoms in persons at risk of developing radiographic knee osteoarthritis (ROA).
The Osteoarthritis Initiative (OAI) was established in 2001. It is a nationwide, multi-centre, longitudinal, prospective observational study of knee osteoarthritis, funded by private-public partnership, and developed to provide a unique publicly accessible research resource13. In a nested case–control analysis using data from participants enrolled between 2004 and 2006 and with annual plain radiography and measures of symptoms, we hypothesised that the incidence of ROA would be preceded by an increase in symptoms in which the symptoms that were earliest to appear and that had the strongest association with future incidence would be those experienced during more demanding activities involving high mechanical loads on the knee14.
Methods
Study setting and population
The study population comprises participants of the OAI ‘incidence sub-cohort’15. Between 2004 and 2006, 3,284 persons aged 45–79 years and at high risk of developing ROA were enrolled at 4 recruitment centers (Baltimore, MD; Pawtucket, RI; Columbus, OH; Pittsburgh, PA) from a combination of focused mailings, websites, and local advertisements, presentations and meetings. Potential participants were screened by telephone and clinic visit and were enrolled in the incidence sub-cohort if they did not have symptomatic ROA in either knee but were at high risk according to the presence of predetermined age-specific combinations of known risk factors (e.g., frequent knee symptoms, overweight, knee surgery). Individuals with an existing diagnosis of rheumatoid arthritis or other inflammatory arthritis were excluded. Extensive data were collected from participants at enrolment including self-complete questionnaires, personal interview, physical examination and plain radiography. Measures are repeated at annual clinic visits (94%, 89%, 85%, and 80% successfully followed up at 1, 2, 3, and 4 years respectively). All participants signed informed consent, and the study was approved by the institutional review board. Data used in the preparation of this article were obtained from the OAI database, which is available for public access at http://www.oai.ucsf.edu/. Specific datasets used were: “enrollees; version 18”, “outcomes99; version 3”, “allclinical00; version 0.2.2”, “allclinical01; version 1.2.1”, “allclinical03; version 3.2.1”, “allclinical05; version 5.2.1”, “allclinical06; version 6.2.1”, “physexam00; version 0.2.2”, “physexam01; 1.2.1”, “physexam03; 3.2.1”, “physexam05; version 5.2.1”, “physexam06; version 6.2.1”, “subjectchar00; version 0.2.2”, “subjectchar01; version 1.2.1”, “subjectchar03; version 3.2.1”, “subject char05; version 5.2.1”, “subjectchar06; version 6.2.1”, “medhist00; version 0.2.2”, “jointsx00; version 0.2.2”, “kxr_sq_bu00; version 0.6”, “kxr_sq_bu01; version 1.6”, “kxr_sq_bu03; version 3.5”, “kxr_sq_bu05; version 5.5” and “kxr_sq_bu06; version 6.3”.
Selection of cases and controls
We sampled knees that developed incident ROA during follow-up up to 4 years, which were without knee symptoms on most days at enrolment, in order to control for the potential effect of the control group being entered into the OAI for different ‘at risk’ factors than the cases. Incident ROA, ascertained from fixed-flexion knee radiographs repeated at annual visits, was defined as the new onset of combined definite osteophyte and joint space narrowing in the tibiofemoral joint16. This outcome definition is the same as that used in previous studies investigating early disease biomarkers17, and has been found to be associated with increased risk of further future disease progression18. The annual visit at which incident ROA was first identified was denoted in our analysis as time-zero (t0). Initially, the parameter of interest was the incidence rate ratio and so for each case knee, we used concurrent sampling19 to select 10 control knees, matched for annual visit but not side (left/right) of the incident knee. Under this approach, matched odds ratios estimate incidence rate ratios20.
Potential prodromal symptoms
Information on potential prodromal symptoms was available at each annual visit from two well-validated and recommended knee-specific self-complete questionnaires21: the Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC LK 3.122) and the Knee injury and Osteoarthritis Outcome Score (KOOS23). The WOMAC questionnaire comprises items on Pain on activity (5 items, summative subscale score 0–20), Stiffness (2 items, 0–8), and Physical Functioning (17 items, 0–68) with higher scores indicating more severe problems. The KOOS comprises items, with some overlapping content with WOMAC, on Pain (9 items), Other Symptoms (7 items), Function in Daily Living (17 items), Function in Sport and Recreation (5 items), and Knee-related Quality of Life (4 items) which are reverse-scored to provide 0–100 subscale scores with lower scores indicating more severe problems. For both measures all questions were answered separately for each knee with response options typically ‘none/mild/moderate/severe/extreme’, and the timeframe being ‘within the past 7 days’. In our study we used some of these subscale scores (WOMAC Pain, Physical Function and Stiffness, and KOOS Pain and Other Symptoms), categorised as 1 if they had indicated moderate/severe/extreme symptoms on at least 1 of the individual items in the scale and 0 if not, due to the non-normal distribution of the subscale scores, as well as a selection of individual items from these measures (dichotomised into none/mild vs moderate/severe/extreme or never/rarely vs sometimes/often/always) and pain on most days of the month in the last 12 months, to explore the nature of prodromal symptoms. The timing of symptom report was anchored to the timing of incident ROA, i.e., symptoms reported 1, 2, 3, and 4 years before incident ROA were denoted by t0-1, t0-2, t0-3, and t0-4.
Statistical analysis
All analyses were completed in Stata/MP 13.1 (Stata Corporation, TX, USA). The data was set up as survival time data which was then converted to case–control data using the -sttocc- command. Conditional logistic regression, with standard errors adjusted (clustered sandwich estimator) for the clustering between knees within the same person, was used to provide initial estimates of the crude associations between outcome at each time point, i.e., incident ROA, and either subscale scores or dichotomised items. Subscale scores and individual items that differed significantly (Wald test, P < 0.05) between cases and controls 1 year before incident ROA, i.e., t0-1, were considered for further modelling. Multi-level logistic regression models were then fitted using the -runmlwin- command24,25 to estimate the trajectories of the probability of scoring at least 1 item ‘moderate’, ’severe’ or ’extreme’ in each subscale and the probability of scoring ‘moderate’, ‘severe’, or ’extreme’ for each individual item in turn over time for cases and controls, as repeated measures of symptoms were nested within knees and knees were nested within people. The length of prodrome was estimated as the time at which the odds ratio equalled unity, i.e., cases had no increased odds of scoring higher for that subscale/item compared to controls (see Web Appendix). The probabilities of a case and of a control scoring moderate, severe or extreme (or sometimes, often or always) at each time were plotted for each item.
Each model included the same factors: a dummy variable for case–control status, time of visit before t0 measured continuously in years, a product term for interaction between case–control status and time, age measured continuously centred on the mean, sex and a constant term. Time was considered as a non-linear effect by using fractional polynomials. Random slope models were fitted to the subscales and individual items and Restricted Iterative Generalised Least Squares (RIGLS) estimation was used when estimating the models in order to obtain unbiased parameter estimates.
To evaluate how sensitive our findings were to our approach to sampling we repeated the above analyses but selected the first incident knee only per case (if both knees were classed as ‘incident’ at the same annual visit, a knee was randomly selected) from the sample of knees which had recorded incident ROA by the 4 year follow. Ten control individuals were sampled for each case from those who did not develop incident knee ROA in either knee at any point during the 4-year follow-up. The particular knee for each control was selected at random, using random number generation. Multi-level models were then fitted to the data for the subscale scores only, with the repeated observations at level 1 and the individual knees at level 2. The results were compared to those estimated from concurrent sampling.
Results
Patient characteristics
169 knees from 161 persons satisfying the criteria for developing incident ROA during the first 4 years of follow-up of the OAI cohort were selected (68, 31, 47 and 23 knees at years 1, 2, 3 and 4 respectively) and were matched by time to 1690 control knees. Cases were slightly older, more likely to be female, and had higher body mass index (Table I). On entry to the OAI cohort, cases were more likely than controls to have a Kellgren–Lawrence Grade 1 score, to report previous exposure to lifting or moving objects ≥25 pounds on most days, and previous knee surgery (Table I).
Table I.
Characteristics of cases and controls at baseline (t0) and frequency of eligibility factors on enrolment into the study, data from the OAI, United States of America, 2004–2010
| Cases∗ (n = 169) | Controls∗ (n = 1690) | |
|---|---|---|
| Characteristics† | ||
| Sex (Female) | 117 (69.2) | 993 (58.8) |
| Age in years; mean (SD) | 65.2 (9.2) | 64.5 (9.6) |
| BMI in kg/m2; mean (SD) | 29.2 (4.6) | 27.2 (4.6) |
| Eligibility factors‡ | ||
| Kellgren–Lawrence grade 1 | 122 (72.2) | 494 (23.2) |
| Above cut-off for overweight | 58 (34.3) | 421 (24.9) |
| Frequent knee bending activity | 129 (77.3) | 1204 (72.0) |
| Climb ≥10 flights, on most days | 94 (56.6) | 954 (57.0) |
| Kneel ≥30 min, on most days | 17 (10.1) | 203 (12.1) |
| Squat or deep knee bend ≥30 min, on most days | 22 (13.0) | 226 (13.5) |
| Lift or move objects ≥25lbs, on most days | 73 (43.5) | 575 (34.4) |
| Immediate family had knee replacement | 25 (15.0) | 277 (16.6) |
| Hard bumps on joints closest to fingertips | 59 (35.1) | 604 (35.9) |
| Medication use for knee pain | 23 (13.6) | 203 (12.0) |
| Knee injury | 72 (42.6) | 658 (39.2) |
| Knee surgery | 35 (20.7) | 229 (13.6) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Values are Number (%) unless otherwise stated.
Measured at t0.
Measured on entry into the OAI cohort.
Numbers and percentages with at least 1 item in subscale recorded as moderate/severe/extreme (for subscales) or reporting moderate/severe/extreme symptoms (for individual items) are given in Web Table S1.
Data on candidate prodromal symptoms were well completed; only 2 out of 40 outcome variables had >10% missing data at a given time point. These were ‘difficulty getting in/out of the bathtub’ (15–30% missing), and ‘pain when twisting/pivoting on the knee’ (4–12% missing). Generally, the missing rate was similar for cases and controls, but the total subscale non-response tended to be slightly higher in controls than cases, particularly at t0.
Prodromal symptoms
On unadjusted univariable analysis, all WOMAC and KOOS subscales, 31 of 34 dichotomised individual items reported at t0-1 (i.e., one year before incident ROA was first recorded among cases) and pain on most days were associated with outcome (P < 0.05) (Web Table S1). Frequent awareness of a problem with the knee, frequent knee pain, and pain on most days of at least one month in the past 12 months were the commonest symptoms associated with outcome (frequency among cases 54.9%, 35.8%, and 27.6% respectively). Items with strong crude associations at t0-1 and relatively high frequency included pain walking on a flat surface (odds ratio 5.13: 95% CI 2.97, 8.86; frequency among cases 11.6%) and self-reported swelling of the knee (4.12: 2.23, 7.61; 9.4%).
Modelling the prodromal period
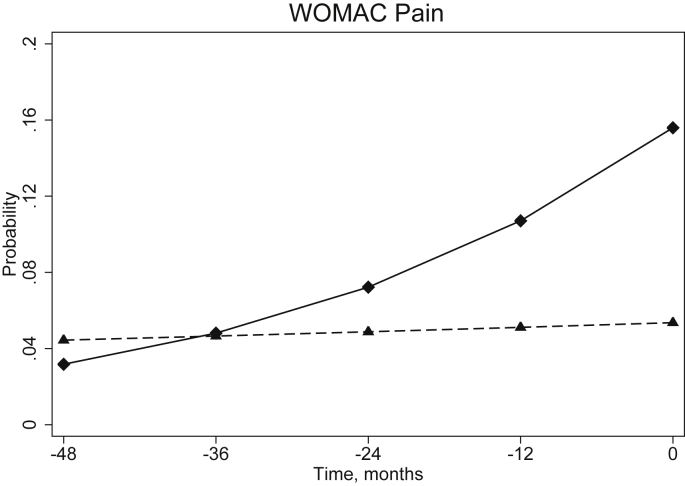
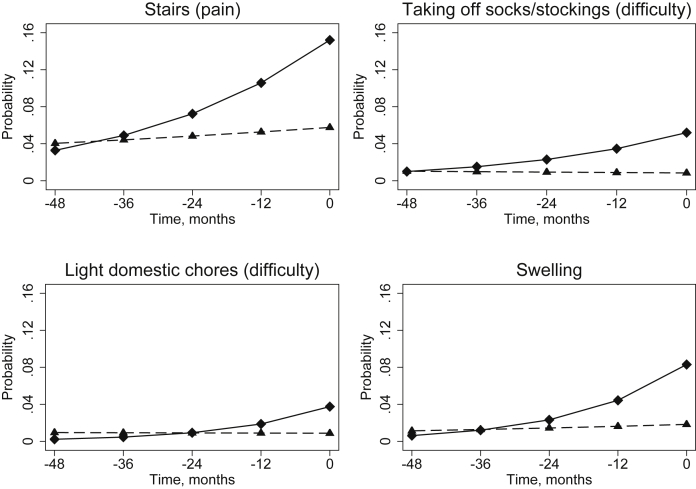
From multi-level models, the estimated length of prodrome – taken as the point at which the probability of scoring at least 1 item in the subscale as moderate/severe/extreme were equal for cases and controls – ranged from almost 29 months for the KOOS Other Symptoms (Table II) to 37 months for the WOMAC Pain (Fig. 1). For dichotomised individual items, the estimated length of prodrome – defined as the point when the estimated probability of that symptom being moderate/severe/extreme was equal for cases and controls – ranged from 24 months (difficulty with light domestic chores) to 47 months (difficulty taking off socks/stockings). Fig. 2 illustrates 4 examples of items with contrasting changes in the probabilities of the cases having the symptom. The 95% confidence intervals for the estimated length of prodrome crossed zero for an additional five individual items not displayed in Table II (difficulty descending stairs, difficulty standing, difficulty walking, difficulty with heavy domestic chores and pain on straightening the knee fully). At each level, only random intercept models were fitted, as when random slope models were fitted, the variance around the slope was consistently not significantly different from zero. There was little difference to the overall shape of the growth model when using fractional polynomials to consider time as a non-linear effect and so the simpler model whereby time was linear was fitted.
Table II.
Coefficients and estimated lengths of prodromes from multi-level models fitted to subscale scores and individual items, data from the OAI, United States of America, 2004–2010
| Multi-level model |
Estimated length of prodrome (months) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Case–control status |
Time |
Interaction |
||||||
| β# | 95% CI | β∗∗ | 95% CI | ↆ | 95% CI | Length | 95% CI | |
| Subscale scores‡‡ | ||||||||
| WOMAC Pain | 1.18 | 0.77, 1.60 | −0.05 | −0.14, 0.04 | −0.38 | −0.64, −0.13 | 37.1 | 17.0, 57.1 |
| WOMAC Stiffness | 1.34 | 0.90, 1.79 | 0.06 | −0.04, 0.16 | −0.55 | −0.82, −0.27 | 29.6 | 17.5, 41.6 |
| WOMAC Physical Function | 1.08 | 0.67, 1.48 | 0.01 | −0.07, 0.10 | −0.38 | −0.61, −0.14 | 34.4 | 17.0, 51.8 |
| KOOS Pain | 1.32 | 0.96, 1.68 | −0.01 | −0.07, 0.06 | −0.53 | −0.74, −0.32 | 29.7 | 20.4, 39.0 |
| KOOS Other Symptoms | 1.08 | 0.70, 1.46 | −0.03 | −0.10, 0.04 | −0.45 | −0.67, −0.24 | 28.7 | 17.6, 39.8 |
| Individual items§§ (in ascending order of estimated length of prodrome) | ||||||||
| Difficulty taking off socks/stockings‡ | 1.86 | 1.00, 2.73 | 0.05 | −0.19, 0.29 | −0.47 | −1.00, 0.05 | 47.1 | 2.3, 91.8 |
| Pain on stairs∗ | 1.08 | 0.62, 1.53 | −0.09 | −0.19, 0.01 | −0.32 | −0.60, −0.04 | 40.0 | 11.2, 68.8 |
| Pain on twisting/pivoting§ | 1.41 | 0.89, 1.92 | −0.09 | −0.22, 0.03 | −0.44 | −0.78, −0.10 | 38.5 | 13.3, 63.8 |
| Pain on bending knee fully§ | 1.29 | 0.75, 1.84 | −0.13 | −0.27, 0.00 | −0.40 | −0.77, −0.04 | 38.4 | 9.1, 67.7 |
| Difficulty putting on socks/stockings‡ | 1.77 | 0.97, 2.58 | 0.18 | −0.01, 0.37 | −0.58 | −1.05, −0.10 | 36.9 | 11.2, 62.7 |
| Pain walking on flat surface∗ | 1.59 | 0.99, 2.20 | −0.02 | −0.17, 0.14 | −0.54 | −0.95, −0.13 | 35.4 | 12.6, 58.3 |
| Pain when sitting or lying down∗ | 1.15 | 0.40, 1.91 | 0.06 | −0.11, 0.23 | −0.39 | −0.87, 0.08 | 35.4 | 0.4, 70.5 |
| Swelling‖ | 1.58 | 0.95, 2.21 | −0.12 | −0.29, 0.05 | −0.55 | −0.98, −0.11 | 34.6 | 10.7, 58.6 |
| Stiffness later in day† | 1.09 | 0.59, 1.60 | 0.02 | −0.09, 0.13 | −0.40 | −0.70, −0.09 | 33.2 | 12.0, 54.4 |
| Difficulty rising from sitting‡ | 1.13 | 0.58, 1.68 | −0.02 | −0.14, 0.10 | −0.41 | −0.76, −0.07 | 32.7 | 9.6, 55.8 |
| Difficulty bending to floor‡ | 0.88 | 0.38, 1.38 | −0.02 | −0.12, 0.08 | −0.33 | −0.63, −0.03 | 32.4 | 8.1, 56.6 |
| Difficulty getting in/out of car‡ | 1.05 | 0.48, 1.61 | 0.06 | −0.06, 0.17 | −0.40 | −0.74, −0.06 | 31.3 | 9.0, 53.7 |
| Difficulty lying in bed‡ | 1.69 | 0.96, 2.42 | 0.08 | −0.10, 0.27 | −0.68 | −1.18, −0.18 | 29.8 | 10.9, 48.7 |
| Frequent awareness of a problem with knee¶ | 0.75 | 0.44, 1.07 | 0.08 | 0.03, 0.13 | −0.30 | −0.47, −0.14 | 29.7 | 16.9, 42.6 |
| Pain at night while in bed∗ | 1.47 | 0.82, 2.13 | 0.06 | −0.10, 0.22 | −0.60 | −1.05, −0.15 | 29.7 | 11.0, 48.5 |
| Knee catch or hang up when moving‖ | 1.09 | 0.48, 1.69 | 0.02 | −0.11, 0.14 | −0.44 | −0.81, −0.07 | 29.6 | 8.5, 50.7 |
| Stiffness in morning† | 1.11 | 0.62, 1.60 | 0.07 | −0.02, 0.17 | −0.45 | −0.74, −0.16 | 29.5 | 13.6, 45.4 |
| Frequent knee pain§ | 1.10 | 0.76, 1.43 | −0.00 | −0.06, 0.06 | −0.45 | −0.65, −0.26 | 28.9 | 18.7, 39.1 |
| Difficulty shopping‡ | 1.35 | 0.57, 2.14 | −0.02 | −0.20, 0.17 | −0.57 | −1.10, −0.04 | 28.5 | 5.0, 52.1 |
| Pain on most days | 1.41 | 1.04, 1.77 | −0.04 | −0.12, 0.03 | −0.60 | −0.84, −0.36 | 28.2 | 18.8, 37.6 |
| Difficulty getting out of bed‡ | 1.32 | 0.63, 2.02 | 0.04 | −0.10, 0.19 | −0.56 | −0.99, −0.14 | 28.1 | 9.2, 47.1 |
| Difficulty ascending stairs‡ | 0.96 | 0.42, 1.50 | −0.08 | −0.20, 0.04 | −0.42 | −0.78, −0.05 | 27.6 | 7.4, 47.8 |
| Difficulty getting in/out of bathtub‡ | 1.66 | 0.94, 2.38 | 0.12 | −0.05, 0.28 | −0.75 | −1.25, −0.25 | 26.6 | 11.3, 41.9 |
| Difficulty sitting‡ | 1.55 | 0.67, 2.42 | −0.01 | −0.24, 0.21 | −0.73 | −1.40, −0.05 | 25.5 | 4.7, 46.3 |
| Pain on standing∗ | 1.39 | 0.64, 2.14 | −0.01 | −0.18, 0.17 | −0.68 | −1.21, −0.14 | 24.7 | 7.2, 42.2 |
| Difficulty with light domestic chores‡ | 1.48 | 0.63, 2.33 | 0.02 | −0.19, 0.23 | −0.73 | −1.37, −0.10 | 24.3 | 5.5, 43.0 |
Abbreviations: CI, confidence interval; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Parent scale for individual items.
WOMAC Pain.
WOMAC Stiffness.
WOMAC Function.
KOOS Pain.
KOOS Other symptoms.
KOOS Quality of Life.
Coefficient from multi-level models for case–control status.
Coefficient from multi-level models for time (measured continuously in years).
Coefficient from multi-level models for the interaction between case–control status and time.
Modelled using multi-level linear regression models.
Modelled using multi-level logistic regression models.
Fig. 1.
Probability of cases (solid line, diamond marker) and controls (dashed line, triangle marker) responding moderate, severe or extreme to at least 1 item in the WOMAC pain subscale at each time prior to t0 (incidence for the cases)estimated from multi-level model. The length of the prodrome is estimated as the time point where these lines cross. Data from the OAI, United States of America, 2004–2010.
Fig. 2.
Probability of cases (solid line, diamond marker) and controls (dashed line, triangle marker) responding moderate, severe or extreme to individual items at each time prior to t0 (incidence for the cases). The length of the prodrome is estimated as the time point where these lines cross. Data from the OAI, United States of America, 2004–2010.
Sensitivity analysis
When we used only the first knee to develop ROA to define cases and selected controls from those free of ROA at the end of follow-up (i.e., at 4 year follow-up in OAI), the estimated strengths of association between prodromal symptoms at t0-1 and outcome were similar to those estimated when using concurrent sampling, but the confidence intervals were slightly narrower. The estimated lengths of prodrome were slightly shorter than those estimated in the primary analysis using concurrent sampling (Web Table S2).
Discussion
In this exploratory study, the incidence of ROA was associated with a prior trajectory of increasing knee pain, stiffness, and difficulties with functional tasks. Consistent with the generally favourable long-term trajectory of knee pain with minimal osteoarthritis26,27, control knees which did not develop ROA exhibited, on average, no change in symptoms over the 4-year study period. We estimated that the symptom trajectory of cases began to diverge from control knees generally between 25 and 30 months before the outcome of incident ROA.
Perceived difficulty due to the knee in taking off sock/stockings, knee pain on twisting and pivoting, and knee joint stiffness were among the symptoms that appeared earliest. The finding that self-reported swelling was also more common in this prodromal phase is intriguing. The validity of this marker as an indicator of the presence and degree of underlying joint inflammation is unknown. Yet in spite of likely random misclassification28 this observed association may be consistent with effusion synovitis (but not cysts29) predicting future cartilage loss reported in recent magnetic resonance imaging studies30–32.
A prodrome is defined as “an early symptom indicating the onset of a disease or illness”33. The outcome in the present study – the new onset of combined definite osteophyte and joint space narrowing in the tibiofemoral joint – is neither the beginning of the pathological process of osteoarthritis nor even the first indication from plain radiographs of gross morphological changes. The appearance of ‘doubtful osteophytes’ (Kellgren–Lawrence Grade I) strongly predicts the outcome used in our study34–36. There were only 117 incident Grade I cases in the first four years of follow-up in the OAI cohort but we would also defend our choice of a more advanced stage of disease on the grounds that it permits direct comparison of findings with other studies using the same outcome to investigate early disease biomarkers (e.g.,17) and that it may be more strongly associated with symptoms37. While Kellgren & Lawrence Grade I changes on plain radiographs were strongly associated with the outcome of future definite osteophyte and joint space narrowing (data not shown), the persistence of an association between prodromal symptoms and outcome after adjusting for Grade I implies that the pathologic process represented by transition from doubtful to definite osteophyte cannot fully explain the existence of prodromal symptoms. These arguments illustrate the difficulties in applying the concept of a prodrome to a pathological disease process that does not have an unambiguously defined discrete point of onset, which comprises repair and damage processes, and in which new imaging modalities and biomarkers are redefining ‘early disease’38. A critical assumption in our study is that the new onset of combined definite osteophyte and joint space narrowing represents a meaningful and important stage in the osteoarthritic process39, but in some cases radiological changes might not be the first sign of the disease, as symptoms can be present without radiological changes, and this may constitute ‘real disease’. The methods used in our study could be applied to explore prodromal symptoms at other putative stages in that process.
The OAI is the largest suitable dataset for this study with the advantage of participants selected to be at high risk of future incident ROA. Nevertheless, there were only a small number of incident cases over the 4-year follow-up. We confirmed on qualitative inspection that there were no major differences in the strength or direction of the associations when the observations were split into four mutually exclusive groups dependent on the study visit that was assigned as the baseline time (i.e., t0 at 1 year, 2 year, 3 year or 4 year follow up visit). This was investigated to rule out any effect of regression to the mean after unusually high measurements at the first OAI visit related to selection into the cohort in our analysis. As a result of the small number of incident cases, estimates for the length of the prodrome are quite imprecise and it was not possible to quantitatively explore different patterns of prodromal symptoms among cases. It was also necessary to dichotomise the ordinal responses to individual items which will have resulted in a loss of information. For all items we chose a standard cut-point, a priori, corresponding to what we feel may be a meaningful distinction between ‘mild’ and ‘moderate’ severity and which we have used previously40. The annual intervals between repeated measures of symptoms also limit the ability to fit the actual timing of prodromal symptom change, including non-linear trajectories. It is also worth stressing that in conducting this nested case–control study within a cohort of ‘high risk’ patients, the length of prodrome is likely to be under-estimated relative to a study conducted in an unselected population sample.
The association between prodromal symptoms and the outcome of radiographic evidence of osteophytes and joint space narrowing is hypothesized to result from a shared common cause: pathologic tissue damage that is part of the underlying osteoarthritic process and that acts as a peripheral nociceptive driver. Adjusting for covariates that are believed to cause this disease process – for example, high body mass index, prior significant injury or surgery to the knee, and a family history of osteoarthritis – is not indicated. Our findings of a prodrome for incident radiographic knee OA are adjusted for potential confounding by age and gender. Future larger studies may be able to investigate hypothesized causal mechanisms that give rise to the observed prodromal symptoms.
The mean scores on WOMAC subscales in this population are very low, the differences between cases and controls small, and each prodromal symptom was reported by 5–20% of cases in the prodromal phase. While these findings are comparable with those from studies of early imaging biomarkers in the same cohort, they emphasize the need for multivariable models for individual risk prediction as well as the continued need for measurements that are more sensitive to change in symptoms in the early stages of disease. It is worth noting that a fairly large proportion of the cases had no symptoms at the time of incidence (44% had a WOMAC Pain subscale score of zero at t0).
In conclusion, we have studied the nature and timing of symptoms preceding the incidence of ROA and found a trajectory of increasing pain, stiffness, and functional limitation that appears to begin, on average, at least two to three years before this disease stage is reached. This does not mark the beginning of the osteoarthritic process but may be related to phasic progression of the condition. The value of prodromal symptoms in helping target early intervention may warrant further investigation.
Author contributions
RC contributed to the design of the study, prepared the data, carried out the statistical analysis and contributed to the interpretation of the findings and drafting of the manuscript. ET contributed to the study design, data preparation, statistical analysis, and interpretation of findings. EC contributed to the interpretation of the findings. GP initially proposed the study, contributed to study design and interpretation of findings, and the drafting of the manuscript. All authors reviewed the draft manuscript and approved the final version. RC (r.l.case@keele.ac.uk) takes responsibility for the integrity of the work as a whole.
Role of the funding source
RC is funded by a National Institute for Health Research (NIHR) Research Methods Fellowship.
This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests
None declared.
Acknowledgements
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. The funders did not contribute to data collection, analysis or interpretation of the data, manuscript preparation or submission.
The authors wish to thank Elaine Nicholls, Martin Thomas and Kelvin Jordan for comments on the draft manuscript.
Contributor Information
R. Case, Email: r.l.case@keele.ac.uk.
E. Thomas, Email: elainethomas37@hotmail.co.uk.
E. Clarke, Email: e.clarke@keele.ac.uk.
G. Peat, Email: g.m.peat@keele.ac.uk.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Peat G., McCarney R., Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart D.J., Doyle D.V., Spector T.D. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42:17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. 2-E. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C., Snow S., McAlindon T.E., Kellingray S., Stuart B., Coggon D. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Kerkhof H.J., Bierma-Zeinstra S.M., Arden N.K., Metrustry S., Castano-Betancourt M., Hart D.J. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203620. [DOI] [PubMed] [Google Scholar]
- 6.Javaid M.K., Lynch J.A., Tolstykh I., Guermazi A., Roemer F., Aliabadi P. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthr Cartil. 2010;18:323–328. doi: 10.1016/j.joca.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guermazi A., Niu J., Hayashi D., Roemer F.W., Englund M., Neogi T. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma L., Chmiel J.S., Almagor O., Dunlop D., Guermazi A., Bathon J. Significance of pre-radiographic MRI lesions in persons at higher risk for knee osteoarthritis. Arthritis Rheumatol. 2014 doi: 10.1002/art.38611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yung A.R., McGorry P.D. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 10.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Peres K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 11.Schapira A.H., Tolosa E. Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nat Rev Neurol. 2010;6:309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- 12.Tabak A.G., Jokela M., Akbaraly T.N., Brunner E.J., Kivimaki M., Witte D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OAIOnline . 2014. Osteoarthritis Initiative. A Knee Health Study. [Google Scholar]
- 14.Hawker G.A., Stewart L., French M.R., Cibere J., Jordan J.M., March L. Understanding the pain experience in hip and knee osteoarthritis–an OARSI/OMERACT initiative. Osteoarthr Cartil. 2008;16:415–422. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Nevitt M.C., Felson D.T., Lester G. 2006. The Osteoarthritis Initiative: Protocol for the Cohort Study. v1.1. [Google Scholar]
- 16.Felson D.T., Niu J., Guermazi A., Sack B., Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70:1884–1886. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neogi T., Bowes M.A., Niu J., De Souza K.M., Vincent G.R., Goggins J. Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Rheum. 2013;65:2048–2058. doi: 10.1002/art.37987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felson D., Niu J., Sack B., Aliabadi P., McCullough C., Nevitt M.C. Progression of osteoarthritis as a state of inertia. Ann Rheum Dis. 2013;72:924–929. doi: 10.1136/annrheumdis-2012-201575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miettinen O. Estimability and estimation in case-referent studies. Am J Epidemiol. 1976;103:226–235. doi: 10.1093/oxfordjournals.aje.a112220. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S., Thomas D.C. On the need for the rare disease assumption in case–control studies. Am J Epidemiol. 1982;116:547–553. doi: 10.1093/oxfordjournals.aje.a113439. [DOI] [PubMed] [Google Scholar]
- 21.Collins N.J., Misra D., Felson D.T., Crossley K.M., Roos E.M. Measures of knee function: International knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellamy N. 1996. WOMAC Osteoarthritis Index. A Users Guide. [Google Scholar]
- 23.Roos E.M., Roos H.P., Lohmander L.S., Ekdahl C., Beynnon B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 24.Leckie G., Charlton C. Volume 52. 2013. pp. 1–40. (runmlwin: A Program to Run the MLwiN Multilevel Modeling Software from within Stata). [Google Scholar]
- 25.Rasbash J., Charlton C., Browne W.J., Healy M., Cameron B. 2009. MLwiN. [Google Scholar]
- 26.Holla J.F., van der Leeden M., Heymans M.W., Roorda L.D., Bierma-Zeinstra S.M., Boers M. Three trajectories of activity limitations in early symptomatic knee osteoarthritis: a 5-year follow-up study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202984. [DOI] [PubMed] [Google Scholar]
- 27.Collins J.E., Katz J.N., Dervan E.E., Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr Cartil. 2014;22:622–630. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peat G., Wood L., Wilkie R., Thomas E. KNE-SCI Study Group. How reliable is structured clinical history-taking in older adults with knee problems? Inter- and intraobserver variability of the KNE-SCI. J Clin Epidemiol. 2003;56:1030–1037. doi: 10.1016/s0895-4356(03)00204-x. S089543560300204X. [DOI] [PubMed] [Google Scholar]
- 29.Guermazi A., Hayashi D., Roemer F.W., Niu J., Yang M., Lynch J.A. Cyst-like lesions of the knee joint and their relation to incident knee pain and development of radiographic osteoarthritis: the MOST study. Osteoarthr Cartil. 2010;18:1386–1392. doi: 10.1016/j.joca.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill C.L., Hunter D.J., Niu J., Clancy M., Guermazi A., Genant H. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roemer F.W., Zhang Y., Niu J., Lynch J.A., Crema M.D., Marra M.D. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–780. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemer F.W., Guermazi A., Felson D.T., Niu J., Nevitt M.C., Crema M.D. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anonymous Oxford Dictionary of English. Oxford University Press; Oxford: 2005. [Google Scholar]
- 34.Lachance L., Sowers M.F., Jamadar D., Hochberg M. The natural history of emergent osteoarthritis of the knee in women. Osteoarthr Cartil. 2002;10:849–854. doi: 10.1053/joca.2002.0840. S106345840290840X. [DOI] [PubMed] [Google Scholar]
- 35.Hart D.J., Spector T.D. Kellgren & Lawrence grade 1 osteophytes in the knee–doubtful or definite? Osteoarthr Cartil. 2003;11:149–150. doi: 10.1053/joca.2002.0853. S1063458402908538. [DOI] [PubMed] [Google Scholar]
- 36.Leyland K.M., Hart D.J., Javaid M.K., Judge A., Kiran A., Soni A. The natural history of radiographic knee osteoarthritis: a fourteen-year population-based cohort study. Arthritis Rheum. 2012;64:2243–2251. doi: 10.1002/art.34415. [DOI] [PubMed] [Google Scholar]
- 37.Neogi T., Felson D., Niu J., Nevitt M., Lewis C.E., Aliabadi P. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter D.J., Arden N., Conaghan P.G., Eckstein F., Gold G., Grainger A. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthr Cartil. 2011;19:963–969. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutton C.W. Can the distribution of symptomatic and radiographic osteoarthritis within the human body be explained? Br J Rheumatol. 1989;28:143. doi: 10.1093/rheumatology/28.2.143. [DOI] [PubMed] [Google Scholar]
- 40.Duncan R., Peat G., Thomas E., Hay E., McCall I., Croft P. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007;66:86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.