Abstract
Background
Type-specific high-risk HPV (hrHPV) infection is related to cervical carcinogenesis. The prevalence of hrHPV infection varies geographically, which might reflect the epidemiological characteristics of cervical cancer among different populations. To establish a foundation for HPV-based screening and vaccination programs in China, we investigated the most recent HPV prevalence and genotypic distributions in different female age groups and geographical regions in China.
Methods
In 2012, a total of 120,772 liquid-based cytological samples from women enrolled for population- or employee-based cervical screening in 37 Chinese cities were obtained by the Laboratory of Molecular Infectious Diseases of Guangzhou KingMed. A total of 111,131 samples were tested by Hybrid Capture II and the other 9,641 were genotyped using the Tellgenplex™ HPV DNA Assay.
Results
The total positive rate for hrHPV was 21.07 %, which ranged from 18.42 % (Nanchang) to 31.94 % (Haikou) and varied by region. The regions of Nanchang, Changsha, Hangzhou, Chengdu, Fuzhou, Guangdong, and Guiyang could be considered the low prevalence regions. Age-specific prevalence showed a “two-peak” pattern, with the youngest age group (15–19 years) presenting the highest hrHPV infection rate (30.55 %), followed by a second peak for the 50–60-year-old group. Overall, the most prevalent genotypes were HPV16 (4.82 %) and HPV52 (4.52 %), followed by HPV58 (2.74 %). Two genotypes HPV6 (4.01 %) and HPV11 (2.29 %) were predominant in the low-risk HPV (lrHPV) type, while the mixed genotypes HPV16 + 52 and HPV52 + 58 were most common in women with multiple infections.
Conclusions
This study shows that HPV infection in China has increased to the level of an “HPV-heavy-burden” zone in certain regions, with prevalence varying significantly among different ages and regions. Data from this study represent the most current survey of the nationwide prevalence of HPV infection in China, and can serve as valuable reference to guide nationwide cervical cancer screening and HPV vaccination programs.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-015-0998-5) contains supplementary material, which is available to authorized users.
Keywords: Human papillomavirus, hrHPV prevalence, HPV genotyping, Cervical cancer, China
Background
HPV infection can cause a variety of genital diseases, and type-specific persistent infection of high-risk HPV (hrHPV) cases is strongly associated with cervical carcinogenesis [1]. Cervical cancer is the third most common type of cancer in women worldwide [2]. Effective implementation of cervical screening programs in developed countries has resulted in a steady reduction in the incidence of cervical cancer [3]. However, in China, the most populous country, cervical cancer remains the second leading cause of cancer deaths among 15- to 44-year-old females [4]. It is estimated that 75,434 women are diagnosed with cervical cancer annually (11.3/100,000) and 33,914 (45.0 %) of those women die because of cervical cancer [5, 6].
To date, more than 200 HPV genotypes have been identified, and ~40 HPV genotypes have been detected in the female genital tract. HPV16 and HPV18 are well known as oncogenic genotypes; additionally, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV68, HPV69, and HPV 82 are also closely associated with cervical cancer. Therefore, all of these genotypes are classified as “high-risk” HPV. Meanwhile, “low-risk” genotypes, including HPV6, HPV11, HPV42, HPV43, and HPV44 are the causative agents for benign or low-grade changes in cervical cells, such as genital warts [7, 8].
The current hrHPV detection is supposed to serve as an additional approach for the early diagnosis of cervical cancer, and was implemented to complement less sensitive and non-objective cytology-based methods [3]. The high negative predictive value of hrHPV testing is applicable for the indication of a low-risk population, in which the cervical cancer screening interval can be safely extended [3]. Based on the results of clinical trials, several European countries will implement hrHPV testing as the primary screening modality [9]. In addition to HPV screening, HPV vaccination has been shown to be an effective strategy against HPV infection and has been recently implemented in most western countries. Although Cervarix (HPV16/18) and Gardasil (HPV6/11/16/18) protect against infection by HPV16 and HPV18, these vaccines provide no effect on some of the hrHPV types found in at least 25 % of cervical cancers [10]. Furthermore, the role of non-vaccine HPV types in the development of lesions remains unknown, and it remains possible that non-vaccine HPV types could replace these vaccine types as the causative agents for cervical precancerous lesions and cancer in vaccinated cohorts without sufficiently broad cross-protection [10].
The prevalence of HPV infection and type-specific distribution vary greatly both between nations [11], and between different regions within countries [12]. Additionally, several other risk factors can influence the prevalence of HPV, including genetic variation, sexual behavior (age at first sexual intercourse and individuals with multiple sex partners), biological predisposition of the immature cervix, and immunodeficiency [13]. Hence, surveillance of the general population is needed to assess the clinical benefits of screening and vaccination strategies.
Obtaining large-scale information about the epidemiological features of HPV in China is critical for global HPV prevention strategies, because of the need to understand the geographic diversity and age distribution of HPV infections. Although a pilot project aimed to measure the hrHPV infection rate and cervical cancer screening was conducted in a few Chinese areas in 1999 [14], and several similar larger scale investigations were carried out that covered more regions that were conducted in 2003, 2008, 2009, and 2012 [15–18], the available data remain insufficient [9] and outdated. Obtaining a more current dataset will also provide a reference for effective screening and vaccination. Herein, we describe a nationwide cross-sectional and large-scale study that had the following aims: 1) to reveal the prevalence of HPV in regions not yet investigated; 2) to follow-up potential changes in HPV infection in regions that have been previously studied; 3) to clarify the genotypic distribution of HPV in different regions and age-grouped populations. These data should expand the data available regarding HPV-related cervical cancer and aid in future research, screening, and vaccination efforts.
Methods
Ethics Statement
This study was approved by the Ethics Committee of Tianjin Medical University in accordance with the Ethical Principles for Biomedical Research Involving Human Subjects (Ministry of Health of the People’s Republic of China) and Declaration of Helsinki for Human Research of 1974 (last modified in 2000). Samples were originally obtained from clinical settings for laboratory diagnoses. After diagnostic testing, excess samples were anonymized and kept for this study. Informed consent was obtained from each female participant. For those individuals younger than 18 years old, the consent form was signed by the parents of each participant.
Study population
KingMed Diagnostics is the largest reference laboratory in China and provides diagnostic testing services for over 13,000 hospitals in 18 provinces and 4 municipalities across the nation. From January to December of 2012, a total of 120,772 samples were obtained for population- or employee-based screening from 37 cities, belonging to 18 regions (Guangdong province was regarded as one region as 20 cities in this investigation were located in that province). Fig. 1 shows a map of all of the geographical sites in China and the 18 regions were further grouped into 4 macro-geographical regions (East, West, South, and North).
Fig. 1.
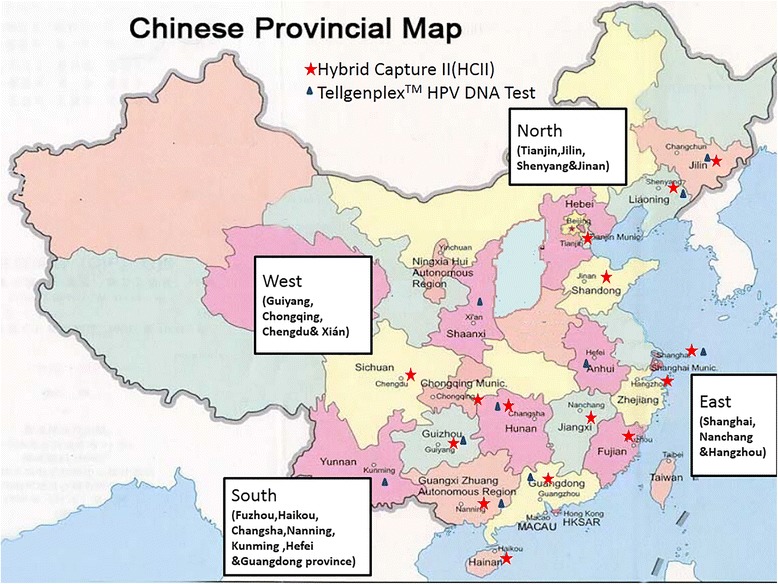
National map of China showing all the geographical sites included in this study
The women enrolled from population- or employee-based cervical cancer screening programs ranged in age from 15 to 60 years old, were sexually active, and had no history of cervical treatment before the screening. Exclusion criteria included current pregnancy, <3 months post-partum, HIV-seropositivity, and a history of either hysterectomy or treatment for cervical cancer.
Among all of the samples, 111,131 were collected from 15 regions (Haikou, Chongqing, Jinan, Jilin, Shenyang, Tianjin, Shanghai, Nanning, Guangdong, Guiyang, Fuzhou, Hangzhou, Chengdu, Changsha, and Nanchang; Fig. 1) and were analyzed using Hybrid Capture II (HCII); 105,069 (94.5 %) of these samples could be grouped by age. The other 9641 samples from 10 regions (Shanghai, Guiyang, Xi’an, Guangdong, Nanning, Changsha, Hefei, Kunming, Shenyang, and Jilin; Fig. 1) were genotyped using the Tellgenplex™ HPV DNA Test and age data were available for 9,194 (95.4 %) samples.
Specimen collection
Following currently accepted protocols of practice, cervico-vaginal cells at the transformation zone of the uterine cervix were collected by a gynecologist or a trained gynecologist assistant with a standard cytobrush (with a spatula), then were resuspended in a standard transport medium (STM) and stored at 4 °C [19]. All specimens were coded without knowledge of the subjects. Subsequently, all samples were shipped to the lab of KingMed Diagnostics for HPV testing within 24 h.
Hybrid Capture II (HCII)
Liquid-based samples were processed by following the instructions included in the Digene sample conversion kit. The hrHPV DNA panel of 13 pooled types (HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV68) was examined using the HCII HPV DNA test (Digene Corporation, Gaithersburg, MD, USA). Data were calculated as a ratio of mean relative light unit (RLU) for the sample to the mean RLU values for the assay of a positive calibrator (PC). A RLU-to-PC ratio >1 (~1.08 pg DNA/ml) was defined as a positive result [20, 21].
Tellgenplex™ HPV DNA Test
HPV genotyping was performed using the Tellgenplex™ HPV DNA Test (Tellgen Life Science Co., Shanghai, China). The assay can be used to detect and genotype 26 HPV genotypes including 19 hrHPV genotypes (HPV16, HPV18, HPV26, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV53, HPV55, HPV56, HPV58, HPV59, HPV66, HPV68, HPV82, and HPV83) and 7 lrHPV genotypes (HPV6, HPV11, HPV40, HPV42, HPV44, HPV61, and HPV73). The Tellgenplex HPV™ kit was used to simultaneously examine the presence or absence of the most common 26 HPV genotypes in a single test by multiplex PCR combined with Luminex technology [22, 23]. The three steps of DNA extraction, PCR amplification, and hybridization were included in the procedure and a template of 10–20 pg/ml HPV DNA was needed for each assay.
Statistical analyses
Region-specific prevalence of HPV
The HPV infection rate in each region was calculated by dividing the number of HPV-positive samples by the total number of samples that were successfully tested for HPV. A binomial 95 % confidence interval (95 % CI) was estimated for each calculation of the prevalence of HPV. Chi-squared (χ2) tests were used to compare differences among all regions and every two regions.
Age-specific prevalence
The HPV infection rate was estimated within 5 age groups (15–19, 20–29, 30–39, 40–49, and 50–60). A binomial 95 % confidence interval (95 % CI) was estimated, and P-values for age trends of HPV infection were analyzed using the linear-by-linear association test. Differences between each pairing of two age groups were compared by the chi-squared (χ2) test. Multiple comparisons were performed using the Bonferoni step-down procedure to minimize an inflated risk of type 1 error [24]; P < 0.05 was considered statistically significant.
HPV-type-specific prevalence
The frequency of each hrHPV and lrHPV genotype was presented in hrHPV-positive samples and lrHPV-positive samples, respectively.
All statistical analyses were conducted using SPSS20.0 software (SPSS lnc., Chicago, IL, USA).
Results
The total prevalence of hrHPV infection
The total hrHPV infection rate was 21.07 % (95 % Cl 20.83–21.31 %). The prevalence of hrHPV infection differed significant among the various regions (P < 0.001). The regions with the highest hrHPV prevalence were Haikou (31.94 %) and Chongqing (27.29 %). By contrast, Nanchang, Changsha, Hangzhou, Chengdu, Fuzhou, Guangdong, and Guiyang could be grouped into the low prevalence regions (Table 1).
Table 1.
Region-specific prevalence of hrHPV infection by HCII
| Regions | Positive samples | Total samples | Infection | 95 % CI of infection |
|---|---|---|---|---|
| rate (%) | rate (%) | |||
| Haikou | 221 | 692 | 31.94 | 28.47–35.41 |
| Chongqing | 191 | 700 | 27.29 | 23.99–30.59 |
| Jinan | 2,651 | 10,306 | 25.72 | 24.88–26.57 |
| Shenyang | 98 | 387 | 25.32 | 20.99–29.65 |
| Jilin | 360 | 1,423 | 25.3 | 23.04–27.56 |
| Tianjin | 807 | 3,220 | 25.06 | 23.57–26.56 |
| Shanghai | 118 | 522 | 22.61 | 19.02–26.20 |
| Nanning | 1,976 | 8,869 | 22.28 | 21.41–23.15 |
| Guiyang | 597 | 2,919 | 20.45 | 18.99–21.91 |
| Guangdong | 14,567 | 72,763 | 20.02 | 19.73–20.31 |
| Fuzhou | 441 | 2,213 | 19.93 | 18.26–21.59 |
| Chengdu | 375 | 1,886 | 19.88 | 18.08–21.68 |
| Hangzhou | 649 | 3,269 | 19.85 | 18.49–21.22 |
| Nanchang | 290 | 1,574 | 18.42 | 16.51–20.34 |
| Total | 23,413 | 111,131 | 21.07 | 20.83–21.31 |
HrHPV infection rate was different among all the regions using chi-squared test (P < 0.001). Subsequently, multiple comparisons were performed using the Bonferoni step-down procedure: marginal differences between the two most heavy-burdened cities of Haikou and Chongqing (P = 0.057), and no difference was found among the second group cities, including Jinan, Shenyang, Jilin, and Tianjin (P = 0.892), as well as the low infection rate group that included Nanchang, Changsha, Hangzhou, Chengdu, Fuzhou, Guangdong, and Guiyang (P = 0.758)
As shown in Fig. 2, hrHPV infection was associated with age. The group of 15–19-year-olds showed the highest prevalence (30.55 %), followed by the 50–60-year-olds (23.30 %). The total infection rate of hrHPV was associated with age (P < 0.001, Additional file 1: Table S1). After the highest peak, the prevalence declined, but then the infection rate significantly increased again in the 50–60-year-old group compared with the 40-49-year-old group (P < 0.001, Additional file 1: Table S1). Among the four macro-regions, only the south showed a similar trend with the overall age-specific hrHPV prevalence, whereas the other three macro-geographic regions did not show significant differences among all five age groups (Fig. 2, Additional file 1: Table S1).
Fig. 2.
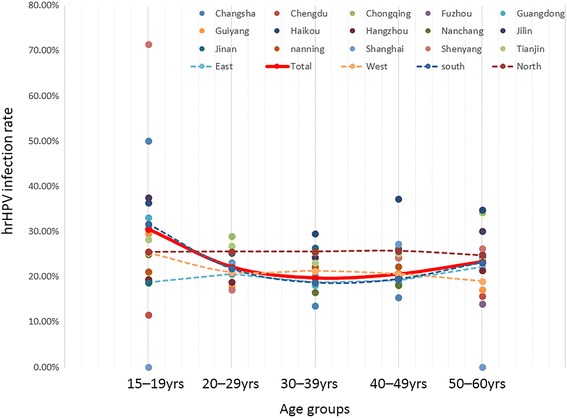
Age-specific hrHPV infection by HCII
The distribution of variant HPV genotypes
Among the well-recognized 26 HPV genotypes, all genotypes except HPV73 were examined using the Tellgenplex technique. The total infection rate of each genotype among all of the samples and the distributions of each genotype in the HPV-positive individuals were respectively evaluated. In descending order, the infection rate and distribution are shown in Table 2: the most common hrHPV types were HPV16 (18.02 %) and HPV52 (16.9 %) (P = 0.288), moreover, HPV6 (45.80 %), HPV11 (26.15 %), and HPV61 (14.20 %) were common in lrHPV.
Table 2.
The prevalence of each HPV genotype by the Tellgenplex™ HPV DNA Test
| HrHPV Genotypes | Positive Samples | Infection Rate (in 9,641 total samples) % | Proportion (in 2,580 hrHPV-positive samples) % |
|---|---|---|---|
| HPV16 | 465 | 4.82 | 18.02 |
| HPV52 | 436 | 4.52 | 16.90 |
| HPV58 | 264 | 2.74 | 10.23 |
| HPV59 | 158 | 1.64 | 6.12 |
| HPV56 | 157 | 1.63 | 6.09 |
| HPV39 | 154 | 1.60 | 5.97 |
| HPV18 | 143 | 1.48 | 5.54 |
| HPV68 | 135 | 1.40 | 5.23 |
| HPV51 | 111 | 1.15 | 4.30 |
| HPV33 | 105 | 1.09 | 4.07 |
| HPV31 | 81 | 0.84 | 3.14 |
| HPV66 | 74 | 0.77 | 2.87 |
| HPV82 | 69 | 0.72 | 2.67 |
| HPV55 | 60 | 0.62 | 2.33 |
| HPV53 | 59 | 0.61 | 2.29 |
| HPV45 | 44 | 0.46 | 1.71 |
| HPV35 | 38 | 0.39 | 1.47 |
| HPV83 | 19 | 0.20 | 0.74 |
| HPV26 | 8 | 0.08 | 0.31 |
| LrHPV Genotypes | Positive Samples | Infection Rate (in 9,641 total samples)% | Proportion (in 844 lrHPV-positive samples) |
| HPV6 | 387 | 4.01 | 45.80 |
| HPV11 | 221 | 2.29 | 26.15 |
| HPV61 | 120 | 1.24 | 14.20 |
| HPV40 | 46 | 0.48 | 5.56 |
| HPV44 | 46 | 0.48 | 5.44 |
| HPV42 | 24 | 0.25 | 2.84 |
Significant differences among the proportion of all genotypes using the chi-squared (χ 2) test (P < 0.0001). Multiple comparisons were further performed using the Bonferoni step-down procedure, and the proportion of the most common genotypes HPV16 and HPV52 (P = 0.288), as well as the proportion of HPV59, HPV56, HPV39, HPV18, and HPV68 (P = 0.583) were not significantly different
Regarding the region-specific distribution of hrHPV, the top three genotypes were analyzed in each region. HPV16, HPV58, and HPV52 were dominant in six regions—Guiyang, Xi’an, Guangdong, Nanning, Changsha, and Shenyang—although the orders of the three genotypes showed variation in different regions (Additional file 2: Table S2). However, three different top patterns were observed: HPV16, HPV18, and HPV83 in Shanghai; HPV16, HPV33, and HPV82 in Hefei; HPV16, HPV56, and HPV59 in Kunming; as well as HPV16, HPV52, and HPV58 in Jilin (Additional file 2: Table S2).
For lrHPV, HPV11 was the most common genotype in Shanghai and Kunming, while HPV6 was the most frequent genotype in all of the other regions (Additional file 2: Table S2). The distribution of the top three HPV genotypes was also determined on the basis of age. For hrHPV, HPV16, HPV52, and HPV58 were dominant among all of the age groups, except the group of 15–19-year-olds, in which HPV52, HPV16, and HPV59 were the major genotypes (Additional file 1: Table S3). For lrHPV, HPV6 was the leading genotype in all of the age groups, and the second most commonly detected genotype was HPV11 in the younger age groups (i.e., the groups of 15–19, 20–29, and 30–39), while in the older groups (40–49 and 50–60) HPV61 was the most prevalent lrHPV type (Additional file 1: Table S3).
Infection with multiple HPVs was detected in a total of 486 specimens (5.04 %), among which 434 (16.82 %) and 52 (6.16 %) were infected with hrHPVs and lrHPVs, respectively. In the multiple hrHPV infection individuals, the frequencies of 6, 5, 4, 3, and 2 genotypes were 0.23 %, 1.84 %, 4.61 %, 17.74 %, and 75.58 %, respectively, and three genotypes showed higher positive rates, i.e., HPV16 (35.02 %), HPV52 (32.26 %), and HPV58 (21.20 %) (Fig. 3). The top most common combinations of strains were HPV16 + HPV52 (26 cases) and HPV52 + HPV58 (14 cases) (Additional file 1: Table S3.). For the individuals with multiple lrHPV infections, the proportion of those with 4, 3, and 2 genotypes were 1.92 %, 3.85 %, and 94.23 %, respectively.
Fig. 3.
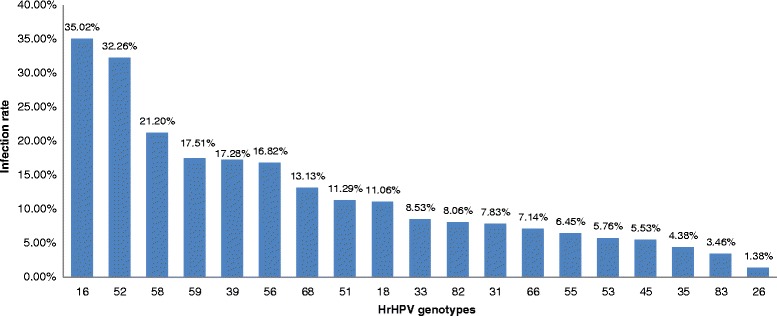
The prevalence of each genotype hrHPV in multiple infections by Tellgenplex™ HPV DNA Test
Among different regions, the highest incidence of multiple hrHPV and lrHPV infections were in Shanghai (19.51 %) and Nanning (3.13 %), respectively. The distribution of dual, triple, quad and up multiple hrHPV infections in each city showed in Fig. 4. Similar to the overall age trend, of 472 cases with accessible age information, after the first peak in the 15–19-year-olds, another peak was observed in 50–60-year-old individuals both for hrHPV and lrHPV (P < 0.001; Additional file 1: Fig. S1.)
Fig. 4.
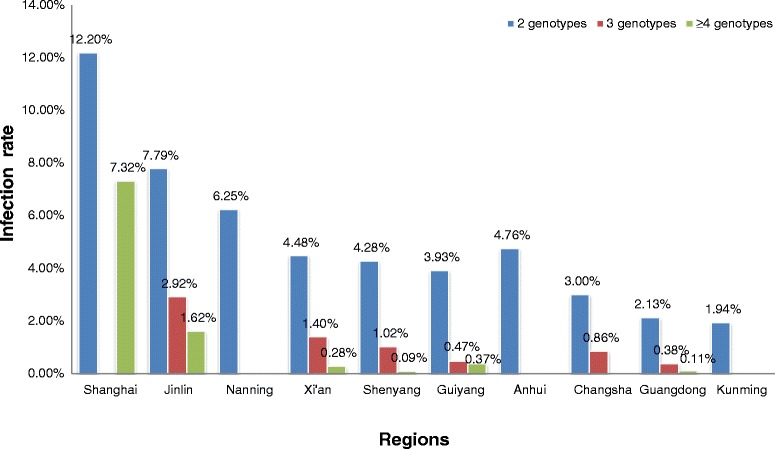
Region-specific multiple infections of hrHPV by Tellgenplex™ HPV DNA Test
Discussion
This is one of the few nationwide investigations of high- and low-risk HPV in a large-scale screen of the Chinese population. Because of regional differences, the population composition, and the sampling periods, the reported prevalence varies from study to study, but the most the heavily burdened HPV regions are Sub-Saharan Africa (24.0 %), Eastern Europe (21.4 %), Latin America (16.1 %), and Southeastern Asia (14 %) [25]. In this present survey, the overall hrHPV-positive rate was 21.07 % (95 % Cl 20.83–21.31 %), which increased in levels within HPV-heavy-burden countries and was higher than the average global level.
It is possible that the variable HPV prevalence in China occurs because of the large Chinese population and its territories. Meanwhile, economic conditions, cultural habits, and population migrations have affected Chinese lifestyles and health [11, 15, 26]. Based on population-based screening results that have been previously reported, the overall prevalence of hrHPV varies from 9.9–27.5 % in China [15]. The highest infection rate is in Shanxi, a region with a heavy burden of cervical cancer in China. By contrast, the lowest infection rate was detected in Beijing, the capital of China with prosperous economic and more robust healthcare system. The infection rates in other regions were ~15–20 %, and a pool analysis that included 17 populations from 9 regions showed that the positive rate of hrHPV infection was 17.7 % [27]. Together, the overall hrHPV-positive rate in this present study was found to have increased slightly.
Compared with region-based data, the rates obtained in this present study were higher than those previously reported for Shanghai [28, 29], Shenyang [28], Guangdong [30, 31], and Hangzhou [32] (Additional file 1: Table S5). Additionally, some newly studied regions in this present survey showed high hrHPV incidences, for example HaiKou (31.94 %) and Chongqing (27.29 %), and several cities in the North, including Jinan, Jilin, and Tianjin. These data showed that hrHPV infection is becoming more serious, as the infection rate is increasing in many regions, and some of those regions may soon reach the level of a heavy burden of infection. Notably, great advances in screening strategy and laboratory methods could also have partially contributed to the increased prevalence.
Regarding the hrHPV prevalence in different age groups, a meta-analysis conducted by Bruni et al. [25] showed a bimodal age distribution, with the peak of HPV infection occurring within a younger age group (just after beginning sexual relations). Globally, the lower prevalence of infection in the middle age group was accompanied by a gradual reduction in the incidence in developing countries or a second peak in developed countries [25]. The trend wherein hrHPV infection shows high rates in younger groups and low rates in middle age groups reflects the natural history of HPV infection. Young females are sensitive to HPV soon after beginning of sexual activity because of immature immune protection. Nevertheless, most cases of HPV infection are usually temporary, so in 70 % and 91 % of women infected with HPV, the virus could be cleared within one or two years, respectively [33]. The slight increase in the HPV infection rate in older females might reflect the viral persistence or reactivation of latent HPV, likely because of the physiological and immunological disorders that can result from hormone fluctuations during the menopausal transition [34]. In this study, the general age distribution showed a first peak of hrHPV in the age group of 15-year-old patients (30.55 %), then gradually decreasing in middle age, which is consistent with data from Bruni et al. [25]. However, the hrHPV infection rate was significantly increased in 50–60-year-old individuals compared with women in their 40s, which is a similar trend that has been observed in most developed countries. The prosperous economy in most major cities could influence the culture and sexual behavior of Chinese individuals [15]. Nonetheless, the exact mechanisms for the increase in HPV prevalence still remain unclear.
Data concerning the distribution of HPV genotypes is important for both vaccine development and HPV-based screening design, particularly for selecting the testing spectrum of HPV genotypes [35] and detecting multiple HPV infections. It is a prerequisite for the genotyping assays in cervical cancer screening programs. However, few HPV devices have been cleared by the FDA, including the Roche Cobas 4800, which also provides limited genotyping data [36]. Therefore, the Tellgenplex™ HPV DNA Test, an established genotyping method [23], was performed in this survey. By following CAP (College of American Pathologists) principles, the validation of Tellgenplex™ HPV DNA Test were conducted compare to HCII in the molecular biology lab of KingMed, and the correlations that we detected were found to be (coincident rate = 90.5 %, Kappa = 0.88).
Consistent with the data generated by some Chinese population-specific investigations, HPV16, HPV52, and HPV58 were found to be the dominant hrHPV types [37, 38]. Compared with the data of Wu et al. [28], a population-based investigation from five regions (Beijing, Shanghai, Xinjiang, Henan, and Shangxi) in China, the infection rates of HPV16 (4.82 %), HPV52 (4.52 %), and HPV58 (2.74 %) were all higher than in the HPV16 (2.9 %), HPV52 (1.7 %), and HPV58 (1.5 %) strains obtained in this present study. Regarding the proportion of these three genotypes, except for HPV52 (16.9 % and 11.9 %, P = 0.006), which is higher than that reported in the present study, there were no significant differences detected in the proportion of HPV16 (20.2 % and 18.02 %, P = 0.245) or HPV58 (10.8 % and 10.23 %, P = 0.686). The proportion of HPV16 in this survey was even lower than that among the normal cytology samples reported by Guan et al. and Bruni et al. (20.4 % and 22.5 %, respectively) [25, 39]. Notably, HPV52 and HPV58 accounted for 27.13 % of infections, which is markedly higher than the global rate of 14.37 % [39]. Although both HPV52 and HPV58 were all common among Asian populations, the significance of the two genotypes remains unknown. Zhao et al. [40] reported that HPV52 infections are more common among healthy individuals, whereas HPV58 has been linked to cervical cancer. Some studies conducted in the South and West regions of China, which only included CIN or cancer samples, indicated that HPV58 is more prevalent than HPV52 [18, 30, 41, 42]. HPV18, apart from HPV16, is also important for cervical carcinogenesis [43]. However, in our present study, it was the seventh most common infection, and the infection rate (1.48 %) was in line with the study of Wu et al. (P = 0.871) [28]. These findings indicated that in addition to HPV16 and HPV18, the HPV vaccine in China should also include the HPV52 and HPV58 genotypes.
In addition to carcinogenesis, many benign cutaneous warts, mucosal lesions, and low-grade cervical intraepithelial lesions generate a considerable health burden that is associated with lrHPV infection [44]. Specifically, HPV6 and HPV11 cause 90 % of genital warts, over 95 % of recurrent respiratory papillomatosis cases, and ~10 % of early cervical lesions [45]. The infection can become more serious in immune-compromised individuals [44]. In our present survey, the incidence of HPV6 (4.01 %) infection was inconsistent with the results of Bruni et al. [25], who showed that HPV6 was most frequent among Americans (2.9 %) and was less frequent in Asian individuals (0.2 %), followed by HPV11 and HPV61.
Characterization of the prevalence of multiple HPV infections might be important for understanding its effects on cervical carcinogenesis. Herrero et al. [10] reported that women infected with HPV16 alone were at a similar or higher risk for cervical cancer than those infected with both HPV16 and another HPV type. Lee et al. [46] reported an association between infection with multiple HPV types and an increased risk of cervical cancer. In a recent study, Schmitt et al. [47] confirmed that co-infection would increase the duration of infection. Furthermore, patients with multiple high viral loads showed a 4- to 6-fold increased risk of cervical precancerous cytological lesions compared with patients with single high viral loads. In this present study, 434 hrHPV-positive samples (4.5 %) were multiple-infections. The incidence was in the same range (3.5–5.3 %) as was previously reported in China [37], but was higher than the global average (3.2 %) [25]. Furthermore, the rate of infection (14.19 %) was lower than reported for both domestic (25.8 %) [28] and international (20 %) [25] populations. The most common combinations of two types were HPV16 + HPV52 (26 cases) and HPV52 + HPV58 (14 cases), and the genotypes HPV52 and HPV58 were more likely to be involved in co-infections. Regarding region-specific surveillance, some geographical features were observed. For example, in Shanghai, a city with a large internal population, the situation was close to the world average regarding HPV prevalence, genotypic distribution, and multiple infections.
This present study confirmed the high overall incidence of HPV in China and strongly argues for the necessity of developing national population-based screening programs. However, the appropriate management of this HPV screening program for a large number of women with HPV-positive specimens and the absence of cytological evidence of cervical pre-cancer or cancer remains a major concern [48]. HPV genotyping could be an option to stratify the HPV-positive women. Furthermore, a stainable HPV detection and close follow-up program for hrHPV carrier women should be implemented. Thus, more cost-effective techniques, such as the Cervista™ HR HPV test, COBAS HPV test, and some other genotyping platforms, might be good alternatives for molecular detection of HPV [49].
Conclusions
In conclusion, China has large population and a variety of territories; meanwhile, economic conditions, cultural habits, and population migrations have dramatically affected Chinese lifestyle and health, for instance, cancers related to sexual transmitted diseases. Therefore, attention should be paid to the prevalence of HPV infection in a timely and regional basis because it has been commonly recognized that HPV infection plays a critical role in the occurrence of cervical cancer and its increase incidence. These surveillance findings indicated that a national plan for a cervical screening program is urgently needed, not only because of the increase in hrHPV infection rate in some previously reported regions, but also because of the high infection rate in most newly investigated regions. In brief, the most significant findings of this study are as follows: (1) the prevalence of hrHPV infection has reached a level that cannot be ignored, and the rate of increase is growing with time; (2) the prevalence of hrHPV infection showed population variations in age and region, and also reflected economic, cultural, and lifestyle relevance; (3) the HPV16, HPV52, and HPV58 strains consistently constituted the three dominant genotypes in different Chinese populations, a characteristic pattern that was significantly different from the epidemiological features in most of industrial countries. These findings defined principles for future proposals for cervical screening and vaccination in China.
Acknowledgements
Rong Wang is appointed to a collaborative project between University of Groningen in the Netherlands and Tianjin medical University of China. This study was supported by the grand from natural science foundation of Tianjin (12JCYBJC33700).
Abbreviations
- HPV
Human papillomavirus
- hrHPV
High-risk HPV
- lrHPV
Low- risk HPV
- HCII
Hybrid capture II
- CAP
College of American Pathologists
Additional files
Age-specific HPV infection in each region by HCII. Table S3. Age-specific prevalence of each subtype HPV by Tellgenplex™ HPV DNA Test. Table S4. The common combinations of double infections by Tellgenplex™ HPV DNA Test. Table S5. HPV Prevalence in Women from population-based Screening Studies. Figure S1. Age-specific multiple infection by Tellgenplex™ HPV DNA Test.
Region-specific hrHPV and lr HPV subtype prevalence by Tellgenplex™ HPV DNA Test.
Footnotes
Rong Wang and Xiao-lei Guo contributed equally to this work.
Competing interests
Shangwei Wu is Medical Director of Kingmed Diagnostics. Ed Schuuring is a member of the scientific advisory board of Roche, Hologic and QCMD, received travel reimbursements from Roche, Abbott, Hologic Inc. and QCMD.
Authors’ contributions
RW, XLG WFW and ZZY carried out the sample collection,laboratory detection and drafted the manuscript. GBAW, ES and HZ participated in the design of the study and performed the statistical analysis. SWW and RW conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Rong Wang, Email: wangrong825@126.com.
Xiao-lei Guo, Email: labguoxiaolei@kingmed.com.cn.
G. Bea. A. Wisman, Email: g.b.a.wisman@umcg.nl
Ed Schuuring, Email: e.schuuring@umcg.nl.
Wen-feng Wang, Email: wangwenfeng840825@163.com.
Zheng-yu Zeng, Email: labzzy@kingmed.com.cn.
Hong Zhu, Email: zhuhongepi@126.com.
Shang-wei Wu, Email: lab-wushangwei@kingmed.com.cn.
References
- 1.Zur Hausen H. Papillomaviruses in human cancers. Molecular carcinogenesis. 1988;1(3):147–150. doi: 10.1002/mc.2940010302. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Priebe AM. 2012 cervical cancer screening guidelines and the future role of HPV testing. Clinical obstetrics and gynecology. 2013;56(1):44–50. doi: 10.1097/GRF.0b013e3182836b6a. [DOI] [PubMed] [Google Scholar]
- 4.Papillomavirus H, Cancers R. WHO/ICO HPV information centre summary report (september 15. 2010. [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.Internation Agency for Research on Cancer(IRAC). IARC handbooks of cancer prevention: cervix cancer screening, chapter 2. Lyon: IARC Press.2005;10:1-299
- 7.de Villiers EM, Fauquet C, Broker TR. Bernard HU, zur Hausen H: Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England journal of medicine. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 9.Castle PE, de Sanjose S, Qiao YL, Belinson JL, Lazcano-Ponce E, Kinney W. Introduction of human papillomavirus DNA screening in the world: 15 years of experience. Vaccine. 2012;30(Suppl 5):F117–122. doi: 10.1016/j.vaccine.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. The Journal of infectious diseases. 2009;199(7):919–922. doi: 10.1086/597308. [DOI] [PubMed] [Google Scholar]
- 11.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet infectious diseases. 2007;7(7):453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 12.Krul EJ, Van De Vijver MJ, Schuuring E, Van Kanten RW, Peters AA, Fleuren GJ. Human papillomavirus in malignant cervical lesions in Surinam, a high-risk country, compared to the Netherlands, a low-risk country. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 1999;9(3):206–211. doi: 10.1046/j.1525-1438.1999.99020.x. [DOI] [PubMed] [Google Scholar]
- 13.Kliucinskas M, Nadisauskiene RJ, Minkauskiene M. Prevalence and risk factors of HPV infection among high-risk rural and urban Lithuanian women. Gynecologic and obstetric investigation. 2006;62(3):173–180. doi: 10.1159/000093572. [DOI] [PubMed] [Google Scholar]
- 14.Belinson J, Qiao Y, Pretorius R, Zhang W, Keaton K, Elson P, et al. Prevalence of cervical cancer and feasibility of screening in rural China: a pilot study for the Shanxi Province Cervical Cancer Screening Study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 1999;9(5):411–417. doi: 10.1046/j.1525-1438.1999.99055.x. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Huang R, Schmidt JE, Qiao YL. Epidemiological features of Human Papillomavirus (HPV) infection among women living in Mainland China. Asian Pacific journal of cancer prevention : APJCP. 2013;14(7):4015–4023. doi: 10.7314/APJCP.2013.14.7.4015. [DOI] [PubMed] [Google Scholar]
- 16.Zhao R, Zhang WY, Zhang SW, Wu MH, Wang JD, Xie Z, et al. Study on subtype of human papillomavirus infection among aged 25–54 reproductive women in Beijing from 2006 to 2008. Zhonghua fu chan ke za zhi. 2011;46(3):184–187. [PubMed] [Google Scholar]
- 17.Wu D, Cai L, Huang M, Zheng Y, Yu J. Prevalence of genital human papillomavirus infection and genotypes among women from Fujian province, PR China. European journal of obstetrics, gynecology, and reproductive biology. 2010;151(1):86–90. doi: 10.1016/j.ejogrb.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Mei J, Wang X, Hu L, Lin Y, Yang P. Human papillomavirus type-specific prevalence in women with cervical intraepithelial neoplasm in Western China. Journal of clinical microbiology. 2012;50(3):1079–1081. doi: 10.1128/JCM.06214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han J, Swan DC, Smith SJ, Lum SH, Sefers SE, Unger ER, et al. Simultaneous amplification and identification of 25 human papillomavirus types with Templex technology. Journal of clinical microbiology. 2006;44(11):4157–4162. doi: 10.1128/JCM.01762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boers A, Slagter-Menkema L, van Hemel BM, Belinson JL, Ruitenbeek T, Buikema HJ, et al. Comparing the Cervista HPV HR test and Hybrid Capture 2 assay in a Dutch screening population: improved specificity of the Cervista HPV HR test by changing the cut-off. PloS one. 2014;9(7) doi: 10.1371/journal.pone.0101930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boers A, Wang R, Slagter-Menkema L, van Hemel BM, Ghyssaert H, van der Zee AG, et al. Clinical validation of the Cervista HPV HR test according to the international guidelines for human papillomavirus test requirements for cervical cancer screening. Journal of clinical microbiology. 2014;52(12):4391–4393. doi: 10.1128/JCM.02716-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei W, Shi Q, Guo F, Zhang BY, Chen C, Zhang NS, et al. The distribution of human papillomavirus in tissues from patients with head and neck squamous cell carcinoma. Oncology reports. 2012;28(5):1750–1756. doi: 10.3892/or.2012.1990. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Qian HZ, Sun J, Gao L, Yin L, Li X, et al. Anal human papillomavirus infection among HIV-infected and uninfected men who have sex with men in Beijing. China. J Acquir Immune Defic Syndr. 2013;64(1):103–114. doi: 10.1097/QAI.0b013e31829b6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludbrook J. Multiple comparison procedures updated. Clinical and experimental pharmacology & physiology. 1998;25(12):1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 25.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. The Journal of infectious diseases. 2010;202(12):1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 26.Bayo S, Bosch FX, de Sanjose S, Munoz N, Combita AL, Coursaget P, et al. Risk factors of invasive cervical cancer in Mali. International journal of epidemiology. 2002;31(1):202–209. doi: 10.1093/ije/31.1.202. [DOI] [PubMed] [Google Scholar]
- 27.Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li LY, Zhang QM, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. International journal of cancer Journal international du cancer. 2012;131(12):2929–2938. doi: 10.1002/ijc.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu EQ, Liu B, Cui JF, Chen W, Wang JB, Lu L, et al. Prevalence of type-specific human papillomavirus and pap results in Chinese women: a multi-center, population-based cross-sectional study. Cancer causes & control : CCC. 2013;24(4):795–803. doi: 10.1007/s10552-013-0162-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Shi TY, Ren Y, Lu H, Wei ZH, Hou WJ, et al. Risk factors for human papillomavirus infection in Shanghai suburbs: a population-based study with 10,000 women. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013;58(1):144–148. doi: 10.1016/j.jcv.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Xie LX, Qing ZR, Li LJ, Luo ZY, Lin M, et al. Epidemiologic characterization of human papillomavirus infection in rural Chaozhou, eastern Guangdong Province of China. PloS one. 2012;7(2) doi: 10.1371/journal.pone.0032149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu RF, Dai M, Qiao YL, Clifford GM, Liu ZH, Arslan A, et al. Human papillomavirus infection in women in Shenzhen City, People's Republic of China, a population typical of recent Chinese urbanisation. International journal of cancer Journal international du cancer. 2007;121(6):1306–1311. doi: 10.1002/ijc.22726. [DOI] [PubMed] [Google Scholar]
- 32.Ye J, Cheng X, Chen X, Ye F, Lu W, Xie X. Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virology journal. 2010;7:66. doi: 10.1186/1743-422X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2005;32(Suppl 1):S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Hildesheim A, Wang SS. Host and viral genetics and risk of cervical cancer: a review. Virus research. 2002;89(2):229–240. doi: 10.1016/S0168-1702(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 35.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International journal of cancer Journal international du cancer. 2007;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 36.Cui M, Chan N, Liu M, Thai K, Malaczynska J, Singh I, et al. Clinical performance of Roche Cobas 4800 HPV Test. Journal of clinical microbiology. 2014;52(6):2210–2211. doi: 10.1128/JCM.00883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CY, Wang CH: Human papillomavirus type distribution in southern china and taiwan. Human papillomavirus and related diseases from bench to bedside-research aspects 2012;19-36
- 38.Jing L, Zhong X, Huang W, Liu Y, Wang M, Miao Z, et al. HPV genotypes and associated cervical cytological abnormalities in women from the Pearl River Delta region of Guangdong province. China: a cross-sectional study. BMC infectious diseases. 2014;14:388. doi: 10.1186/1471-2334-14-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. International journal of cancer Journal international du cancer. 2012;131(10):2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 40.Zhao FH, Tiggelaar SM, Hu SY, Xu LN, Hong Y, Niyazi M, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: suggestions for optimal age of human papillomavirus vaccination in China. Cancer epidemiology. 2012;36(4):384–390. doi: 10.1016/j.canep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Wei H, Wang N, Zhang S, Zhang Y, Ruan Q, et al. The prevalence and role of human papillomavirus genotypes in primary cervical screening in the northeast of China. BMC cancer. 2012;12:160. doi: 10.1186/1471-2407-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu SS, Chan KY, Leung RC, Chan KK, Tam KF, Luk MH, et al. Prevalence and risk factors of Human Papillomavirus (HPV) infection in southern Chinese women - a population-based study. PloS one. 2011;6(5) doi: 10.1371/journal.pone.0019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;38(2):260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 44.Leto M, Santos Junior GF, Porro AM, Tomimori J. Human papillomavirus infection: etiopathogenesis, molecular biology and clinical manifestations. Anais brasileiros de dermatologia. 2011;86(2):306–317. doi: 10.1590/S0365-05962011000200014. [DOI] [PubMed] [Google Scholar]
- 45.Preaud E, Largeron N: Economic burden of non-cervical cancers attributable to human papillomavirus: a European scoping review. Journal of medical economics 2013;16(6):763-76 [DOI] [PubMed]
- 46.Lee SA, Kang D, Seo SS, Jeong JK, Yoo KY, Jeon YT, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer letters. 2003;198(2):187–192. doi: 10.1016/S0304-3835(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 47.Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, Pawlita M: Multiple HPV infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. Journal of clinical microbiology 2013;51(5):1458-64. [DOI] [PMC free article] [PubMed]
- 48.Gage JC, Ajenifuja KO, Wentzensen NA, Adepiti AC, Eklund C, Reilly M, et al. The age-specific prevalence of human papillomavirus and risk of cytologic abnormalities in rural Nigeria: implications for screen-and-treat strategies. International journal of cancer Journal international du cancer. 2012;130(9):2111–2117. doi: 10.1002/ijc.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munson E, Du Chateau BK, Bellerose B, Czarnecka J, Griep J. Clinical laboratory evaluation of Invader(R) chemistry and hybrid capture for detection of high-risk human papillomavirus in liquid-based cytology specimens. Diagnostic microbiology and infectious disease. 2011;71(3):230–235. doi: 10.1016/j.diagmicrobio.2011.07.004. [DOI] [PubMed] [Google Scholar]


