Abstract
The potential of medications to cause kidney injury is well known. Although nephrotoxicity is most commonly associated with injury in the tubulointerstitial compartment as either acute tubular necrosis or acute interstitial nephritis, a growing body of literature has also highlighted the potential for drug-induced glomerular lesions. This review surveys the three primary patterns of drug-induced glomerular diseases stratified by the cell type at which the glomerular lesion is focused: visceral epithelial cell (or podoctye) injury, endothelial cell injury, and mesangial cell injury. A number of commonly prescribed medications, including IFNs, bisphosphonates, nonsteroidal anti-inflammatory drugs, antiplatelet agents, and antiangiogenesis drugs, that are both prescribed and available over the counter, have been implicated in these iatrogenic forms of glomerular disease. Recognition of these drug-induced etiologies of glomerular disease and rapid discontinuation of the offending agent are critical to maximizing the likelihood of renal function recovery.
Keywords: drug nephrotoxicity, glomerular disease, GN
Introduction
Medications are a common cause of kidney injury, because they have the capacity to damage cells within all compartments of the kidney. Nephrotoxicity is most commonly described as producing tubular or interstitial injury, resulting in acute tubular necrosis (ATN) or acute interstitial nephritis (AIN), respectively. Much less attention has been paid to drug-induced glomerular injury.
The three main cell types within the glomerulus are the visceral epithelial cell (VEC; also referred to as the podocyte), the endothelial cell, and the mesangial cell. This review will focus on patterns of nephrotoxicity that primarily target each of the three cell types (Table 1). This is a simplified approach, because a medication may directly affect a target cell, its metabolites may induce the effect, or the agent may modify the immune system or another cell type, resulting in a specific pattern of glomerular injury. Although the distinction may be subtle, drug-induced glomerular injury may primarily affect the immune system and lead to the production of autoantibodies, resulting in various drug-induced glomerulopathies that are covered in an accompanying review article.
Table 1.
Drug–induced glomerular lesions
| Epithelial cells (podocytes) |
| Minimal change disease |
| IFN, pamidronate, lithium, nonsteroidal anti–inflammatory drugs |
| FSGS |
| IFN, pamidronate, lithium, sirolimus, anabolic steroids |
| Endothelial cells |
| Thrombotic microangipathy |
| Antiangiogenesis drugs, mitomycin-C, gemcitabine |
| IFN, thienopyridines (antiplatelet agents), quinine |
| Mesangial cells |
| Idiopathic nodular glomerulosclerosis (mesangial sclerosis) |
| Heavy tobacco smoking |
Epithelial Cell Injury
The drug-induced podocytopathies consist of minimal-change disease (MCD) and FSGS, including both FSGS not otherwise specified (FSGS-NOS) and collapsing FSGS (C-FSGS). It is notable that, although the relationship between MCD and FSGS has been an area of longstanding debate and the majority of patients with MCD do not progress to FSGS or vice versa, multiple therapeutic agents are associated with both conditions (Table 2).
Table 2.
Medications associated with minimal change disease and FSGS
| Minimal change disease |
| IFN-α and -β |
| Pamidronate |
| Lithium |
| Nonsteroidal anti-inflammatory drugs |
| Cyclooxygenase-2 inhibitors |
| FSGS |
| IFN-α and -γ |
| Pamidronate |
| Lithium |
| Sirolimus |
| Anabolic steroids |
| Collapsing FSGS |
| IFN-α, -β, and -γ |
| Pamidronate (rarely zoledronate or alendronate) |
| Anabolic steroids |
IFN
The IFNs are cytokines that were initially named for the ability to interfere with viral replication (1). There are three main types of IFNs: α, β, and γ. IFN-α is mainly synthesized by leukocytes (other than lymphocytes), IFN-β is mainly fibroblast derived, and IFN-γ originates from T cells and natural killer cells. IFN-α and -β are mainly produced by virally infected cells, act through common receptors, and induce changes in neighboring cells that block viral replication. In contrast, IFN-γ acts to stimulate macrophage activation and MHC expression. Collectively, the IFNs are a critical component of the innate immune system that protects against viral infection.
IFNs are indicated for the treatment of multiple medical conditions. IFN-α is used in the treatment of hepatitis B and C and various malignancies. IFN-β is extensively used to treat multiple sclerosis. IFN-γ is indicated for chronic granulomatous disease and malignant osteopetrosis. All three forms are administered through the subcutaneous or intramuscular route and are associated with constitutional effects and laboratory abnormalities that limit their use.
Treatment with IFN has been associated with nephrotic syndrome (NS) with histologic findings of MCD, FSGS-NOS, or C-FSGS (2). Review of the literature on IFN-α reveals six patients with MCD, nine patients with FSGS-NOS, and nine patients with C-FSGS; 14 of the 24 patients were treated for the hepatitis C virus. IFN-β has been associated with two reports of MCD and three reports of C-FSGS, whereas there are two reports of C-FSGS and one report of FSGS-NOS in patients treated with IFN-γ (2). In virtually all instances, IFN was discontinued, and a significant percentage of patients was treated with steroids. Outcomes were largely determined by the histology pattern of injury, with complete or partial remission seen in all patients with MCD. Nearly all patients with FSGS-NOS or C-FSGS improved after discontinuation of IFN, although less than one half reached partial or complete remission (2).
The reports of C-FSGS after treatment with IFN are distinctive for a strong black racial predominance and the ultrastructural finding of endothelial tubuloreticular inclusions. Tubuloreticular inclusions are cytoplasmic microtubular structures found in endothelial cells that can be induced in animal models after administration of IFN (“IFN footprints”) (3). Recent studies have provided insight into the mechanism by which IFN may lead to the development of FSGS (4,5). APOL1 gene variants confer a significantly greater risk for the development of FSGS and more rapid progression to ESRD in black patients (4). DNA was analyzed from six black patients and one Hispanic patient who developed C-FSGS while undergoing treatment with IFN, and all seven patients were found to be homozygous for high-risk APOL1 alleles (5). IFN increases APOL1 expression in vitro and quite possibly, in vivo. These studies raise the possibility that innate IFN production in response to viral infection may play a role in inducing renal disease in patients with the APOL1 high-risk phenotype and that treatment with exogenous IFN may act as a second hit in patients with high-risk APOL1 alleles (5).
Bisphosphonates
Bisphosphonates are a class of drugs that are used to prevent bone resorption. Oral bisphosphonates are used to treat osteoporosis and are not thought to be nephrotoxic. The nitrogen-containing intravenous bisphosphonates are most commonly used to treat metastatic bone disease, hypercalcemia of malignancy, and multiple myeloma. The dosage for this indication is substantially higher and associated with nephrotoxicity. Two bisphosphonates are approved for this indication in the United States: pamidronate and zoledronic acid. NS has been reported after the use of pamidronate (6,7). In contrast, the main pattern of nephrotoxicity associated with zoledronic acid is ATN (7,8).
The most common pattern of VEC injury associated with pamidronate is C-FSGS (Figure 1) (6,7). The initial report of C-FSGS after pamidronate therapy included seven patients with NS and renal insufficiency (6). Our experience has subsequently expanded to 19 patients (7), and at least 10 additional patients have been reported. Patients with pamidronate-associated C-FSGS present with florid NS and renal insufficiency, often have received doses of pamidronate that exceed recommended levels, and in most cases, have poor outcomes, with approximately one half showing improvement in renal function after drug discontinuation but none returning to baseline. In one patient, a partial remission was seen after discontinuation of pamidronate, the drug was later reintroduced, and proteinuria immediately worsened (9). There are rare reports of MCD and FSGS-NOS with pamidronate; not surprisingly, the best outcomes are seen in patients with MCD (10). There are also rare reports of C-FSGS after treatment with intravenous zoledronate (11,12), and there was a single case after the use of oral alendronate in a recipient of a liver transplant (13).
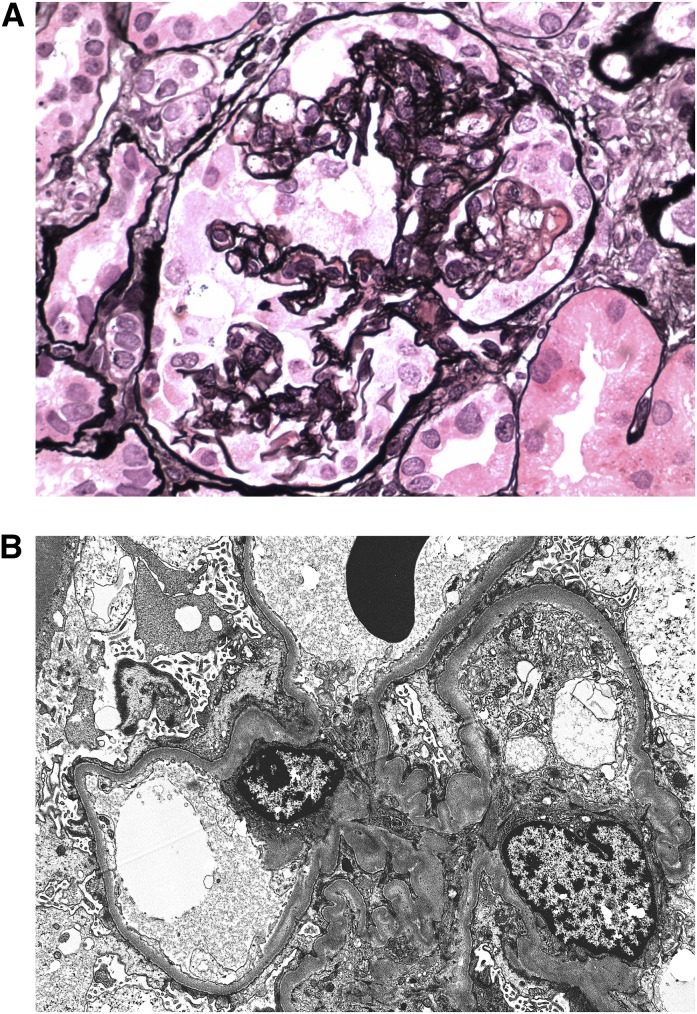
Figure 1.
Drug-induced epithelial cell injury. (A) A glomerulus from a patient treated with pamidronate exhibits collapsing FSGS with global wrinkling and retraction of the glomerular basement membrane associated with swelling and proliferation of overlying epithelial cells. Jones methenamine silver, ×600. (B) In this patient treated for multiple sclerosis with IFN-β, glomeruli appear unremarkable by light microscopy. On ultrastructural evaluation, complete foot process effacement, diagnostic of minimal change disease, is seen (×6,000).
The mechanism by which pamidronate produces C-FSGS is unknown, although the pattern of injury favors direct or indirect podocyte effects. The nitrogen-containing intravenous bisphosphonates mainly exert their therapeutic effects on osteoclasts, a cell type with a complex, branching cytoarchitecture that resembles the podocyte. Within the osteoclast, pamidronate inhibits the mevalonate pathway, leading to altered cell signaling and apoptosis as well as impaired cellular energetics and disruption of the cytoskeleton assembly (7). Similar effects may be operant in podocytes.
Lithium
Lithium carbonate is widely used in the treatment of bipolar disorder. Lithium therapy is associated with multiple adverse renal effects, most frequently nephrogenic diabetes insipidus. Acute lithium intoxication is associated with AKI, with histologic findings of ATN (14). Chronic lithium toxicity, most commonly encountered after ≥10 years of therapy, is a distinctive chronic tubulointerstitial nephropathy (CTIN) characterized by distal tubular cysts (15,16). Treatment with lithium is also associated with NS and histologic findings of MCD (17–19).
Lithium-induced MCD was first reported in 1980 (17). A woman developed NS 10 months after commencing therapy. Lithium was withdrawn, NS resolved; lithium was reinstituted 2 years later, and NS recurred (17). During both NS episodes, renal biopsy revealed MCD. A patient with lithium-associated MCD was reported in 1989, and eight additional reports were reviewed (18). Patients ranged in age from 14 to 60 years and included six women and three men. MCD was associated with abrupt onset of NS usually during the initial 12 months of treatment, and after discontinuation, NS resolved over 2–10 weeks. Three of nine patients had a recurrence of NS when rechallenged with lithium (18). A later report described two patients with MCD accompanied by AKI; complete remission followed lithium discontinuation and treatment with steroids (19). The mechanism by which lithium leads to MCD is not known, although hypotheses have highlighted the interactions between the positively charged lithium and negatively charged glomerular basement membrane and the effects of lithium on lymphocyte function, possibly leading to release of lymphocyte-derived factors that alter the glomerular filtration barrier (19).
The association between lithium and FSGS is more nebulous (16,20,21). In 1988, three patients who developed FSGS while treated with lithium were described (20). Although all had nephrotic-range proteinuria, none had full NS. Two patients had significant CKD and marked tubulointerstitial scarring on renal biopsy. In the largest report on pathologic findings in chronic lithium nephrotoxicity, the CTIN characteristic of this condition was accompanied by FSGS in 12 of 24 patients (16). Among 12 patients with FSGS lesions, six patients had nephrotic-range proteinuria, including three patients with NS. These 12 patients also had moderate to severe tubulointerstitial scarring, and most had glomerulomegaly, favoring a secondary form of FSGS resulting from hyperfiltration in remnant nephrons. In most patients with lithium-associated CTIN, the finding of FSGS is a secondary occurrence, but in rare instances, the presentation and extensive foot process effacement favor a primary podocyte injury akin to lithium-associated MCD (16). Of note, a recent report described an 11-year-old boy with 3 g/d proteinuria in the absence of edema or hypoalbuminemia in the setting of lithium therapy. Renal biopsy revealed FSGS, lithium was discontinued, and the proteinuria resolved within 3 weeks (21).
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a broad class of medications with analgesic, antipyretic, and anti-inflammatory properties. These widely used agents inhibit cyclooxygenase-1 (COX-1) and COX-2, thereby blocking prostaglandin, prostacyclin, and thromboxane production. NSAIDs are associated with multiple patterns of nephrotoxicity, including hemodynamically-mediated ATN, AIN, MCD, papillary necrosis, and, rarely, membranous nephropathy.
NSAID-associated MCD was first reported in three patients with AKI and NS who had biopsy findings of both AIN and MCD that remitted after NSAID discontinuation (22). A subsequent literature review noted that NSAID-associated MCD may occur in the absence of AIN (23). This report included 24 patients with MCD, among whom 15 patients received fenoprofen. Patients presented with full-blown NS and had excellent outcomes after NSAID discontinuation, often in the absence of corticosteroids (23). NSAIDs subsequently have been recognized as a drug-induced etiology of MCD. Unlike IFN, lithium, and bisphosphonates, which are associated with MCD and FSGS, available data do not support a relationship between NSAIDs and FSGS. The observation that MCD occurs with multiple classes of NSAIDs with distinct chemical structures suggests that the pathogenesis of MCD and other NSAID-associated nephropathies may involve shunting of AA metabolites into alternative pathways that modify immune function (24). Of note, the COX-2 inhibitors, a newer class of NSAIDs that selectively inhibits COX-2, have also been associated rarely with MCD (25). Although drug discontinuation is often associated with remission of MCD, a course of steroids may be beneficial when NS persists despite drug withdrawal.
Additional Therapeutics Agents Associated with FSGS
Treatment of recipients of renal transplants with sirolimus, a mammalian target of rapamycin inhibitor, is commonly accompanied by an increase in proteinuria, which is usually subnephrotic (26). In 2007, eight cases of biopsy-proven FSGS were reported in recipients of renal transplants treated with sirolimus, including three patients treated de novo and five patients who previously received a calcineurin inhibitor (CNI) (27). All three patients treated de novo had urine protein >6 g/d, whereas the mean urine protein increased from 0.28 to 2.54 g/d in five patients who previously received a CNI. When the patients treated de novo were switched to a CNI, proteinuria markedly diminished (27). Pathologic evaluation revealed FSGS-NOS with immunohistochemical evidence of podocyte dysregulation. In cell culture, podocytes exposed to sirolimus exhibit reduced synaptopodin, nephrin, and podocin expression, increased apoptosis, and actin cytoskeletal reorganization, forming a potential basis for this toxicity (28). Ironically, sirolimus has been used with limited success as a treatment for FSGS in the native kidney (29).
C-FSGS and FSGS-NOS have been reported in bodybuilders after long-term anabolic steroid use. In 2010, Herlitz et al. (30) described 10 patients with mean urine protein of 10.1 g/d and serum creatinine of 3.0 mg/dl, including three patients with classic NS. In seven patients with available follow-up who discontinued anabolic steroid use, stabilization or improvement in proteinuria and kidney function was noted. Rechallenge in one patient led to rapid worsening of proteinuria and renal dysfunction. The pathogenesis may involve hyperfiltration in response to increased (lean) body mass as well as direct nephrotoxic effects of anabolic steroids (30).
Endothelial Cell Injury
Thrombotic microangiopathy (TMA) is a term that has been applied to multiple seemingly unrelated conditions that share clinical and pathologic features. Clinically, TMA is characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and end organ injury (31,32). Pathologic findings in TMA target the endothelial cell and include endothelial swelling and necrosis, glomerular and vascular thrombosis, mesangiolysis, glomerular basement membrane duplication with cellular interposition, mucoid intimal edema, and fibrin deposition (31,32). Conditions associated with TMA include hereditary and acquired disorders and may present acutely with catastrophic effects or gradually with chronic end organ injury (31,32). Medications are an important acquired cause of TMA (Table 3). This section will briefly review drug-induced forms of TMA, with the exception of CNIs, that are of interest to nephrologists.
Table 3.
Medications associated with thrombotic microangiopathy
| Antineoplastic agents |
| Antiangiogenesis drugs |
| Mitomycin-C |
| Gemcitabine |
| Cisplatin/carboplatin |
| Estramustine/lomustine |
| Cytarabine |
| Tamoxifen |
| Bleomycin |
| Daunorubicin |
| Hydroxyurea |
| Antiplatelet agents |
| Ticlopidine, clopidogrel, prasugrel |
| Dipyradamole |
| Defribrotide |
| IFNs |
| IFN-α and -β |
| Immunosuppressive agents |
| Calcineurin inhibitors |
| Anti-CD33 (OKT3) |
| Antimicrobial agents |
| Valacyclovir |
| Penicillins |
| Rifampin |
| Metronidazole |
| Tetracycline |
| Sulfasoxazole |
| Albendazole |
| Nonsteroidal anti–inflammatory drugs |
| Diclofenac |
| Piroxicam |
| Ketorolac |
| Hormones |
| Conjugated estrogens alone or combined with progestins |
| Contraceptives, combination |
| Others |
| Quinine |
| Intravenous oxymorphone (Opana) ER |
| Simvastatin |
| Iodine |
| Cocaine |
ER, extended release.
Antiangiogenesis Drugs
Antiangiogenesis drugs (AADs) are used to treat a number of cancers as well as various neovascular eye disorders (33). AADs include monoclonal antivascular endothelial growth factor (anti-VEGF) antibodies (bevacizumab), circulating VEGF decoy-receptor molecule (aflibercept), and VEGF receptor tyrosine-kinase inhibitors (sunitinib, sorafenib, axitinib, and pazopanib) (33). Their anticancer efficacy is on the basis of observations that many malignancies proliferate and disseminate by promoting unregulated tumor angiogenesis through dysregulation of the VEGF signaling pathway (33). Antiangiogenesis is also effective in nonmalignant neovascular processes, where nephrotoxicity is a rare occurrence (33).
Adverse renal effects of AADs result from disturbance of the normal housekeeping role and tissue repair of VEGF-associated angiogenesis in the kidneys. Constitutive expression and secretion of VEGF by podocytes preserve glomerular integrity through paracrine and, possibly autocrine binding of endothelial and epithelial receptors, respectively (34–36). This cross-talk between the VEC and endothelial cell plays a major role in maintaining the structure and function of the filtering unit (34–36). Experimental evidence supports the importance of an intact VEGF pathway. Progressive inhibition of VEGF expression is associated with glomerular endotheliosis, glomerular aneurysmal change, mesangiolysis, and full-blown TMA (34–36).
The most common renal manifestations of AAD-associated TMA are proteinuria and hypertension (HTN); in more severe cases, AKI is seen (37–40). This pattern of renal injury, which is more common with anti-VEGF antibodies or decoy-receptor therapy than with tyrosine-kinase inhibitors, has been described in over 100 patients (41,42). These drugs produce a clinical-pathologic picture quite similar to preeclampsia, which is driven, in part, by a similar molecular mechanism. MAHA and thrombocytopenia are only observed in approximately 50% of patients, despite the findings of acute or chronic TMA in the kidney (36,41). HTN occurs in >80% of patients, whereas proteinuria is nearly universal and ranges from low grade to nephrotic range (36,41). Urine sediment can be active with cylinduria and cellular/granular casts or bland. Kidney function ranges from mild injury to severe AKI requiring dialysis. BP control and drug withdrawal can be associated with return of kidney function, which is relatively uncommon for TMA associated with others agents (43). Interestingly, adverse renal effects often correlate with effective antitumor activity (44). Thus, nephrologists and oncologists have to balance drug withdrawal for nephrotoxicity against effective cancer therapy. We suggest that HTN and proteinuria should prompt antihypertensive therapy, whereas AKI is an indication to interrupt drug therapy (42,45).
Chemotherapeutic Agents
Mitomycin-C.
Mitomycin-C is an alkylating agent used to treat various malignancies (46). After the recognition of the association between mitomycin-C therapy and TMA, a registry identified 85 patients, and subsequent clinical trials noted a TMA incidence ranging from 4% to 15% (46,47). Kidney injury is dose dependent, with a cumulative dose >60 mg significantly increasing TMA risk. The mechanism is unknown, but direct toxic endothelial injury is favored over an immune-mediated process involving ADAMTS-13 or the alternative complement pathway (46,47).
Although TMA can develop during drug exposure, it more often develops weeks to months after the last dose (mean of 75 days) (46,47). AKI is a defining feature of mitomycin-C–associated TMA (46,47) and is often severe; one third of 85 registry patients required dialysis (46). Renal recovery can occur, but most patients are left with CKD. Systemic manifestations of MAHA and thrombocytopenia are common, whereas neurologic symptoms are rare (16%) (46,47). Treatment rests primarily on drug withdrawal, because steroids, plasma exchange/pheresis (PE/PH), and anticoagulation are generally ineffective. TMA-related mortality is approximately 44% (46,47).
Gemcitabine.
Gemcitabine is a cell cycle–specific pyrimidone antagonist that is used to treat a variety of malignancies (43). TMA (Figure 2) was described as a complication within a few years following approval, with an incidence ranging from 0.015% to 2.4% (43,46,48). Higher cumulative dose and prior exposure to other chemotherapeutic agents increase the risk for TMA (43,46,48). Drug-induced endothelial injury is the probable mechanism. Disruption of endothelial integrity may cause release of vWf multimers and plasminogen activator inhibitor, reduce synthesis of prostacyclin and tissue plasminogen activator, and expose a denuded endothelial surface that favors platelet adhesion, leading to fibrin and platelet deposition in the renal microvasculature (43,46,48).
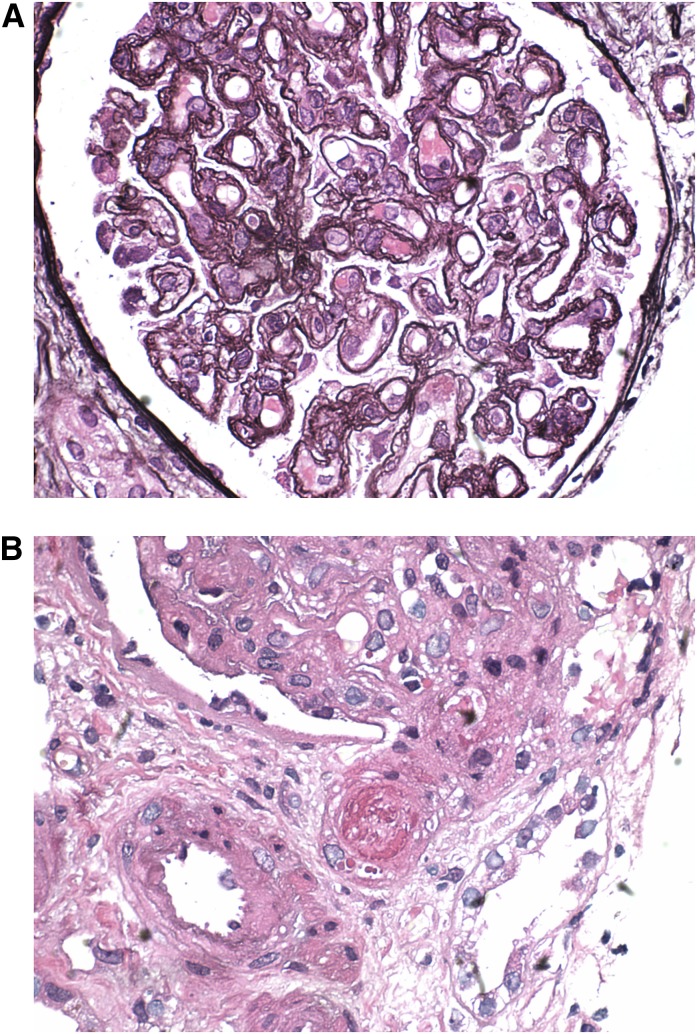
Figure 2.
Drug induced TMA. (A) A glomerulus from a patient treated with gemcitabine exhibits subacute findings of thrombotic microangiopathy, including global double contours of the glomerular basement membrane (tram tracking), cellular interposition, and dissolution of the mesangial matrix consistent with mesangiolysis. Mild swelling of endothelial and visceral epithelial cells is also noted. Jones methenamine silver, ×600. (B) A glomerulus from the same patient exhibits a fresh fibrin thrombus in a preglomerular arteriole. Hematoxylin and eosin, ×600. TMA, thrombotic microangiopathy.
TMA most often develops weeks to months after initiating gemcitabine therapy (43,46,48). AKI is present in virtually all cases. In a case series of 29 patients with gemcitabine-associated TMA, all patients had MAHA, thrombocytopenia, and AKI, whereas new or worsening HTN, proteinuria, and hematuria were noted in 26 of 29 patients (49). Drug discontinuation or future avoidance resulted in full (n=8) or partial (n=11) recovery with CKD in three patients and ESRD in seven patients (25%). PE/PH had minimal or no effect. Reported mortality is wide-ranging (0%–90%), with 15% a reasonable estimate for TMA-specific death (43,46,48,49).
IFN
In addition to their association with MCD and FSGS, the IFNs are associated with the development of TMA (50,51). Over 30 patients with IFN-α–associated TMA have been reported, most often in the setting of chronic myelogenous leukemia (50,52). High-dose and prolonged regimens are most often associated with TMA (50,52). Patients present with HTN, severe AKI often requiring dialysis, proteinuria, and active urine sediment, whereas MAHA and thrombocytopenia are variably present (50,52). In the patients reviewed, treatment included drug discontinuation and BP control, whereas PE/PH was performed in approximately two thirds of patients; antiplatelet agents and steroids were occasionally used (50,52). CKD and ESRD requiring dialysis are fairly common—nearly one half of patients reported developed long-standing kidney disease. Mortality occurs from TMA, but most deaths are caused by underlying malignancy (50).
IFN-β causes TMA less commonly than IFN-α, with experience limited to a small number of cases and regulatory reports of patients with relapsing multiple sclerosis (51). Clinical features, treatment, and outcomes are similar to those described with IFN-α (51,53,54). The mechanism of IFN-associated TMA remains unclear, although three reports describe ADAMTS-13 autoantibody/deficiency (50–54).
Thienopyridines
Ticlopidine, clopidogrel, and prasugrel are antiplatelet agents that target the P2Y12ADP receptor, and they are used to prevent and/or treat cerebrovascular accidents and coronary artery events, including stent thrombosis (55,56). Paradoxically, this class of drugs is one of the most commonly implicated in drug-induced TMA (55,56).
Ticlopidine was Food and Drug Administration (FDA)-approved in 1991, and a case series subsequently described 60 patients with TMA (55,56). The incidence of TMA is approximately 0.02%, with the syndrome developing 2–12 weeks after drug exposure. Neurologic complications (72%) of TMA dominate, with AKI noted to be less severe and uncommon (29%) (55,56). MAHA and thrombocytopenia are often present and can be severe. In contrast to direct endothelial injury, ticlopidine appears to cause TMA through reducing ADAMTS-13 activity (55,56). Production of an inhibiting autoantibody is described, although how ticlopidine induces this effect is unknown. Molecular mimicry and hapten formation have been suggested. Interestingly, concomitant complement factor H mutations and ADAMTS-13 deficiency were described in four patients with ticlopidine-induced TMA, suggesting that two hits may be required in some patients (57). Although discontinuation of ticlopidine is required in patients with TMA, PE/PH provided added benefit, presumably by removing autoantibodies that promote thrombosis. Patients treated with PE/PH more commonly recover and have lower mortality rates (0%–40%) compared with drug discontinuation alone (50%–67%) (55,56). CKD is an uncommon complication.
Clopidogrel entered clinical practice as an antiplatelet agent in 1998 driven by clinical trials showing better safety (no TMA in >20,000 patients) than ticlopidine (55,56). Currently, in part because of its sheer volume of use, clopidogrel has become the leading cause of drug-induced TMA (55,56). As of 2011, the FDA received 197 reports of clopidogrel-associated TMA, with an incidence that approximates 0.012%. In contrast to ticlopidine, TMA develops more rapidly, often within 2 weeks of exposure. AKI is more common (55%) and typically more severe, whereas thrombocytopenia is mild (55,56). Interestingly, most cases are not explained by ADAMTS-13 deficiency/inhibitor, favoring direct drug-induced endothelial injury, enhanced release of ultralarge vWF, or an unknown mechanism (55,56). In addition to drug discontinuation, PE/PH has been used but seems to require prolonged treatment periods (3 weeks) for response in contrast to the 3–5 days required for ticlopidine-associated TMA. Recovery is common, but CKD is an established complication. Mortality from clopidogrel-associated TMA with and without PE/PH therapy is reported to be 28% and 33%, respectively (55,56).
Prasugrel was FDA approved in 2009 for coronary stent thrombosis prevention, and as of 2011, 14 patients with TMA have been reported to the FDA (55,56). Notably, no TMA cases were described in clinical trials containing 1769 patients treated with prasugrel (55). As with clopidogrel, TMA generally develops within 2 weeks of exposure. Drug discontinuation and PE/PH are currently recommended.
Quinine
Quinine has been used to treat muscle cramps and malaria, and despite an FDA ban on over-the-counter marketing, it remains widely available by prescription, in beverages and nutrition/health store products, and on internet sites (58,59). Although well recognized as a cause of immune thrombocytopenia, quinine is also an important cause of TMA (58,59). Quinine-induced TMA is often characterized by oliguric or anuric AKI, frequently requiring dialysis (up to 85%), and laboratory manifestations of MAHA with severe thrombocytopenia (58,59).
The rapid development of TMA after a single quinine tablet bespeaks an immune-mediated process (37,38). Quinine-dependent IgG antibodies drive this process by binding to a broad range of cells, including platelets, white blood cells, and endothelial cells (59,60). Both antibody-induced endothelial injury and platelet activation may underlie the development of TMA (59,60). Quinine is not associated with ADAMTS-13 autoantibody or deficiency (58–60). Drug discontinuation and plasmapheresis are indicated to remove the culprit antibody. Existing literature suggests that ESRD and CKD develop commonly (approximately 50%) after TMA, with a mortality of 21% in one large series (58–60). Relapse may occur with re-exposure.
Mesangial Cell Injury
Nodular glomerulosclerosis (NGS) has emerged as a specific glomerular lesion associated with cigarette smoking. The finding of nodular mesangial sclerosis with glomerular and tubular basement thickening, in the absence of immune deposits, is the classic description of nodular diabetic glomerulosclerosis. However, this same pattern of NGS (Figure 3) has been reported in patients without clinical evidence of diabetes. The lesion was first reported as “diabetic glomerulosclerosis without diabetes” (61,62) and subsequently rebranded “idiopathic nodular mesangial sclerosis” or “idiopathic nodular glomerulosclerosis” (63,64). However, the publication of the first substantial case series on this entity in 2002, which included 23 patients compared with a total of 20 patients previously reported over 31 years and 13 publications (65), presented strong evidence that heavy cigarette smoking was linked to this lesion (66); 21 of 23 patients (91%) reported a history of tobacco use, with a mean cumulative smoking history of 52.9 pack-years, and more than one half of patients were still smoking at the time of biopsy. Longstanding HTN (mean duration of 15.1 years) was a similar common feature present in 22 of 23 patients. A more recent series detailed 15 patients with idiopathic NGS over a 10-year period (67). Ten patients reported a history of smoking (mean cumulative smoking history of 54.2 pack-years), and all were actively smoking at the time of biopsy. It has been proposed that this distinct clinicopathologic entity should more appropriately be called “smoking-associated nodular glomerulosclerosis” (68).
Figure 3.

Nodular glomerulosclerosis of smoking. Pathologic findings in smoking-associated nodular glomerulus resemble changes seen in nodular diabetic glomerulosclerosis and include nodular mesangial sclerosis with thickening of glomerular and tubular basement membranes. Periodic acid–Schiff, ×500.
Tobacco-induced formation of advanced glycation end products (AGEs) may be a mechanism of injury in this form of NGS. In diabetic glomerulosclerosis, AGE through interaction with receptor to AGE activates signaling cascades that promote mesangial cell synthesis of profibrogenic cytokines, including platelet–derived growth factor and TGF-β (69). Reactive glycation products, present in extracts of tobacco and tobacco smoke (70), can react rapidly with proteins to form AGEs, and increased levels of AGE have been found in the lenses and blood vessels of smokers (71).
Most patients with smoking-associated NGS present with evidence of renal dysfunction (mean creatinine >2.0 mg/dl) and nephrotic-range proteinuria. Predictably, given this clinical presentation, prognosis is generally poor, with a high likelihood of progression to ESRD (66–68). Smoking cessation may be an important intervention in modifying this natural history. In one series with >2 years follow-up postbiopsy, none of the patients who had discontinued smoking reached ESRD, whereas all of the patients who continued to smoke reached ESRD over a median time of 11 months (66).
Conclusion
A variety of drugs, both prescription and over the counter, have the capacity to induce direct cellular injury at the level of the glomerulus. The specific glomerular cell that is targeted by these nephrotoxins, in turn, influences the clinical presentation and histopathology. These drug-induced glomerular diseases should be part of the differential diagnosis in patients presenting with proteinuria, hematuria, and/or renal insufficiency. Recognition of a drug-induced etiology and withdrawal of the offending agent provide the best hope for renal recovery. For most drugs, it impossible to predict the time course for recovery of kidney function. As a result, the clinician needs to observe closely and individualize patient management.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Isaacs A, Lindenmann J: Viral interference. I. The interferon. Proc R Soc Lond B Biol Sci 147: 258–267, 1957 [PubMed] [Google Scholar]
- 2.Markowitz GS, Nasr SH, Stokes MB, D’Agati VD: Treatment with IFN-alpha, -beta, or -gamma is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 607–615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman D, Hoar RM, Niemann WH, Valentine T, Cukierski M, Hendrickx AG: Tubuloreticular inclusions in placental chorionic villi of rhesus monkeys after maternal treatment with interferon. Am J Obstet Gynecol 155: 413–424, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D’Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Perazella MA, Markowitz GS: Bisphosphonate nephrotoxicity. Kidney Int 74: 1385–1393, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, Park J, Nasr SH, Hoh S, Siegel DS, D’Agati VD: Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 64: 281–289, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Markowitz GS, Fine PL, D’agati VD: Nephrotic syndrome after treatment with pamidronate. Am J Kidney Dis 39: 1118–1122, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Barri YM, Munshi NC, Sukumalchantra S, Abulezz SR, Bonsib SM, Wallach J, Walker PD: Podocyte injury associated glomerulopathies induced by pamidronate. Kidney Int 65: 634–641, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bodmer M, Amico P, Mihatsch MJ, Haschke M, Kummer O, Krähenbühl S, Mayr M: Focal segmental glomerulosclerosis associated with long-term treatment with zoledronate in a myeloma patient. Nephrol Dial Transplant 22: 2366–2370, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Neyra JA, Vaidya OU, Hendricks A, Sambandam KK: Collapsing focal segmental glomerulosclerosis resulting from a single dose of zoledronate. Nephron Extra 4: 168–174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual J, Torrealba J, Myers J, Tome S, Samaniego M, Musat A, Djamali A: Collapsing focal segmental glomerulosclerosis in a liver transplant recipient on alendronate. Osteoporos Int 18: 1435–1438, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Lavender S, Brown JN, Berrill WT: Acute renal failure and lithium intoxication. Postgrad Med J 49: 277–279, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hestbech J, Hansen HE, Amdisen A, Olsen S: Chronic renal lesions following long-term treatment with lithium. Kidney Int 12: 205–213, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D’Agati VD: Lithium nephrotoxicity: A progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439–1448, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Richman AV, Masco HL, Rifkin SI, Acharya MK: Minimal-change disease and the nephrotic syndrome associated with lithium therapy. Ann Intern Med 92: 70–72, 1980 [DOI] [PubMed] [Google Scholar]
- 18.Wood IK, Parmelee DX, Foreman JW: Lithium-induced nephrotic syndrome. Am J Psychiatry 146: 84–87, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Tam VKK, Green J, Schwieger J, Cohen AH: Nephrotic syndrome and renal insufficiency associated with lithium therapy. Am J Kidney Dis 27: 715–720, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Santella RN, Rimmer JM, MacPherson BR: Focal segmental glomerulosclerosis in patients receiving lithium carbonate. Am J Med 84: 951–954, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Sakarcan A, Thomas DB, O’Reilly KP, Richards RW: Lithium-induced nephrotic syndrome in a young pediatric patient. Pediatr Nephrol 17: 290–292, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Brezin JH, Katz SM, Schwartz AB, Chinitz JL: Reversible renal failure and nephrotic syndrome associated with nonsteroidal anti-inflammatory drugs. N Engl J Med 301: 1271–1273, 1979 [DOI] [PubMed] [Google Scholar]
- 23.Feinfeld DA, Olesnicky L, Pirani CL, Appel GB: Nephrotic syndrome associated with use of the nonsteroidal anti-inflammatory drugs. Case report and review of the literature. Nephron 37: 174–179, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Pirani CL, Valeri A, D’Agati V, Appel GB: Renal toxicity of nonsteroidal anti-inflammatory drugs. Contrib Nephrol 55: 159–175, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Alper AB, Jr., Meleg-Smith S, Krane NK: Nephrotic syndrome and interstitial nephritis associated with celecoxib. Am J Kidney Dis 40: 1086–1090, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Murakami N, Riella LV, Funakoshi T: Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: A systematic review and meta-analysis. Am J Transplant 14: 2317–2327, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi MN, Helal I, Noël LH, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Müller-Krebs S, Weber L, Tsobaneli J, Kihm LP, Reiser J, Zeier M, Schwenger V: Cellular effects of everolimus and sirolimus on podocytes. PLoS ONE 8: e80340, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsagalis G, Psimenou E, Iliadis A, Nakopoulou L, Laggouranis A: Rapamycin for focal segmental glomerulosclerosis: A report of 3 cases. Am J Kidney Dis 54: 340–344, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D’Agati VD: Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George JN, Nester CM: Syndromes of thrombotic microangiopathy. N Engl J Med 371: 654–666, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Shenkman B, Einav Y: Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: Diagnosis and classification. Autoimmun Rev 13: 584–586, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Gurevich F, Perazella MA: Renal effects of anti-angiogenesis therapy: Update for the internist. Am J Med 122: 322–328, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE: Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 17: 724–735, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Eremina V, Baelde HJ, Quaggin SE: Role of the VEGF—a signaling pathway in the glomerulus: Evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol 106: 32–37, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, Khayat D, Spano JP: Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol 20: 807–815, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC: VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer 46: 439–448, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Wu S, Dahut WL, Parikh CR: Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 49: 186–193, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, Stergiopoulos K, Wu S: Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta-analysis. Acta Oncol 48: 9–17, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Izzedine H, Escudier B, Lhomme L, Pautier P, Rouvier P, Gueutin V, Baumelou A, Derosa L, Bahleda R, Hollebecque A, Soria JC: Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): An 8-year observational study at a single center. Medicine (Baltimore) 93: 333–339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izzedine H, Mangier M, Ory V, Zhang SY, Sendeyo K, Bouachi K, Audard V, Péchoux C, Soria JC, Massard C, Bahleda R, Bourry E, Khayat D, Baumelou A, Lang P, Ollero M, Pawlak A, Sahali D: Expression patterns of RelA and c-mip are associated with different glomerular diseases following anti-VEGF therapy. Kidney Int 85: 457–470, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Izzedine H, Isnard-Bagnis C, Launay-Vacher V, Mercadal L, Tostivint I, Rixe O, Brocheriou I, Bourry E, Karie S, Saeb S, Casimir N, Billemont B, Deray G: Gemcitabine-induced thrombotic microangiopathy: A systematic review. Nephrol Dial Transplant 21: 3038–3045, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ: Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 103: 763–773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bollée G, Patey N, Cazajous G, Robert C, Goujon JM, Fakhouri F, Bruneval P, Noël LH, Knebelmann B: Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant 24: 682–685, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Zakarija A, Bennett C: Drug-induced thrombotic microangiopathy. Semin Thromb Hemost 31: 681–690, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Lesesne JB, Rothschild N, Erickson B, Korec S, Sisk R, Keller J, Arbus M, Woolley PV, Chiazze L, Schein PS, Neefe JR: Cancer-associated hemolytic-uremic syndrome: Analysis of 85 cases from a national registry. J Clin Oncol 7: 781–789, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Humphreys BD, Sharman JP, Henderson JM, Clark JW, Marks PW, Rennke HG, Zhu AX, Magee CC: Gemcitabine-associated thrombotic microangiopathy. Cancer 100: 2664–2670, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Glezerman I, Kris MG, Miller V, Seshan S, Flombaum CD: Gemcitabine nephrotoxicity and hemolytic uremic syndrome: Report of 29 cases from a single institution. Clin Nephrol 71: 130–139, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Zuber J, Martinez F, Droz D, Oksenhendler E, Legendre C; Groupe D’stude Des Nephrologues D’ile-de-France (GENIF): Alpha-interferon-associated thrombotic microangiopathy: A clinicopathologic study of 8 patients and review of the literature. Medicine (Baltimore) 81: 321–331, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Hunt D, Kavanagh D, Drummond I, Weller B, Bellamy C, Overell J, Evans S, Jackson A, Chandran S: Thrombotic microangiopathy associated with interferon beta. N Engl J Med 370: 1270–1271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deutsch M, Manesis EK, Hadziyannis E, Vassilopoulos D, Archimandritis AJ: Thrombotic thrombocytopenic purpura with fatal outcome in a patient with chronic hepatitis C treated with pegylated interferon-a/2b. Scand J Gastroenterol 42: 408–409, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Sallée M, Crétel E, Jean R, Chiche L, Bourlière M, Poullin P, Lefèvre P, Durand JM: Thrombotic thrombocytopenic purpura complicating interferon therapy in chronic C hepatitis. Gastroenterol Clin Biol 32: 145–146, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Orvain C, Augusto JF, Besson V, Marc G, Coppo P, Subra JF, Sayegh J: Thrombotic microangiopathy due to acquired ADAMTS13 deficiency in a patient receiving interferon-beta treatment for multiple sclerosis. Int Urol Nephrol 46: 239–242, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Jacob S, Dunn BL, Qureshi ZP, Bandarenko N, Kwaan HC, Pandey DK, McKoy JM, Barnato SE, Winters JL, Cursio JF, Weiss I, Raife TJ, Carey PM, Sarode R, Kiss JE, Danielson C, Ortel TL, Clark WF, Rock G, Matsumoto M, Fujimura Y, Zheng XL, Chen H, Chen F, Armstrong JM, Raisch DW, Bennett CL: Ticlopidine-, clopidogrel-, and prasugrel-associated thrombotic thrombocytopenic purpura: A 20-year review from the Southern Network on Adverse Reactions (SONAR). Semin Thromb Hemost 38: 845–853, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zakarija A, Kwaan HC, Moake JL, Bandarenko N, Pandey DK, McKoy JM, Yarnold PR, Raisch DW, Winters JL, Raife TJ, Cursio JF, Luu TH, Richey EA, Fisher MJ, Ortel TL, Tallman MS, Zheng XL, Matsumoto M, Fujimura Y, Bennett CL: Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): Review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989-2008). Kidney Int Suppl 112: S20–S24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapin J, Eyler S, Smith R, Tsai HM, Laurence J: Complement factor H mutations are present in ADAMTS13-deficient, ticlopidine-associated thrombotic microangiopathies. Blood 121: 4012–4013, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Kojouri K, Vesely SK, George JN: Quinine-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: Frequency, clinical features, and long-term outcomes. Ann Intern Med 135: 1047–1051, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Park YA, Hay SN, King KE, Matevosyan K, Poisson J, Powers A, Sarode R, Shaz B, Brecher ME: Is it quinine TTP/HUS or quinine TMA? ADAMTS13 levels and implications for therapy. J Clin Apher 24: 115–119, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Glynne P, Salama A, Chaudhry A, Swirsky D, Lightstone L: Quinine-induced immune thrombocytopenic purpura followed by hemolytic uremic syndrome. Am J Kidney Dis 33: 133–137, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Nash DA, Jr., Rogers PW, Langlinais PC, Bunn SM, Jr.: Diabetic glomerulosclerosis without glucose intolerance. Am J Med 59: 191–199, 1975 [DOI] [PubMed] [Google Scholar]
- 62.Mactier RA, Luger A, Khanna R: Diabetic glomerulosclerosis without concurrent diabetes mellitus. South Med J 81: 1573–1577, 1988 [DOI] [PubMed] [Google Scholar]
- 63.Alpers CE, Biava CG: Idiopathic lobular glomerulonephritis (nodular mesangial sclerosis): A distinct diagnostic entity. Clin Nephrol 32: 68–74, 1989 [PubMed] [Google Scholar]
- 64.Herzenberg AM, Holden JK, Singh S, Magil AB: Idiopathic nodular glomerulosclerosis. Am J Kidney Dis 34: 560–564, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Kuppachi S, Idris N, Chander PN, Yoo J: Idiopathic nodular glomerulosclerosis in a non-diabetic hypertensive smoker—case report and review of literature. Nephrol Dial Transplant 21: 3571–3575, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Markowitz GS, Lin J, Valeri AM, Avila C, Nasr SH, D’Agati VD: Idiopathic nodular glomerulosclerosis is a distinct clinicopathologic entity linked to hypertension and smoking. Hum Pathol 33: 826–835, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Li W, Verani RR: Idiopathic nodular glomerulosclerosis: A clinicopathologic study of 15 cases. Hum Pathol 39: 1771–1776, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Nasr SH, D’Agati VD: Nodular glomerulosclerosis in the nondiabetic smoker. J Am Soc Nephrol 18: 2032–2036, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Throckmorton DC, Brogden AP, Min B, Rasmussen H, Kashgarian M: PDGF and TGF-beta mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int 48: 111–117, 1995 [DOI] [PubMed] [Google Scholar]
- 70.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A: Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 94: 13915–13920, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicholl ID, Stitt AW, Moore JE, Ritchie AJ, Archer DB, Bucala R: Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol Med 4: 594–601, 1998 [PMC free article] [PubMed] [Google Scholar]