Abstract
Drug-induced autoimmune disease was initially described decades ago, with reports of vasculitis and a lupus-like syndrome in patients taking hydralazine, procainamide, and sulfadiazine. Over the years, multiple other agents have been linked to immune-mediated glomerular disease, often with associated autoantibody formation. Certain clinical and laboratory features may distinguish these entities from their idiopathic counterparts, and making this distinction is important in the diagnosis and management of these patients. Here, drug-induced, ANCA-associated vasculitis, drug-induced lupus, and drug-associated membranous nephropathy are reviewed.
Keywords: GN, ANCA, SLE, membranous nephropathy, drug nephrotoxicity
Introduction
Exposure to certain drugs can elicit an immune response that results in the generation of autoantibodies and clinical autoimmune disease, including immune complex or pauci-immune GN. Awareness of these associations by clinicians is important in discerning drug-associated syndromes from their primary counterparts, a distinction that may affect prognosis and treatment. These entities may also lend insight into the underlying pathogenesis of primary autoimmune glomerular disease. Here, we review drug-induced, ANCA-associated vasculitis (AAV), drug-induced lupus (DIL), and drug-associated membranous nephropathy (MN).
Drug-Induced AAV
History and Clinical Presentation
Reports emerged linking drugs to vasculitis as early as the 1940s. This hypothesis was strengthened after the discovery of ANCAs and their target antigens proteinase 3 (PR3) and myeloperoxidase (MPO) in the 1980s, with case series of patients who were ANCA positive and exposed to medications, such as hydralazine and propylthiouracil (PTU); some developed vasculitis (1–4).
Choi et al. (5) conducted the largest retrospective analysis of drug-associated AAV in 250 patients with MPO-positive AAV. The 30 patients with the highest anti-MPO antibody titers were reviewed for the use of 11 candidate medications; 60% (18 of 30) of patients had been exposed to one of these medications (hydralazine, n=10; PTU, n=3; penicillamine, n=2; allopurinol, n=2; sulfasalazine, n=1), and nine of 10 patients with hydralazine AAV had evidence of renal involvement, with five exhibiting pauci-immune necrotizing GN on kidney biopsy. Four of the remaining eight patients also had renal involvement (one patient each for PTU, penicillamine, allopurinol, and sulfasalazine). Lung involvement (manifesting as pulmonary hemorrhage/hemoptysis, hemothorax, radiographic infiltrates or nodules, or lung biopsy–proven AAV) was more common in 12 patients without detectable drug exposure (83% versus 38% with hydralazine/PTU exposure; P<0.05), but the frequencies of other clinical manifestations, death, and dialysis dependence were not different between groups.
The presence of additional autoantibodies in patients with drug-associated AAV was also noted; nine of 10 patients on hydralazine, all three patients on PTU, and four of five patients on penicillamine/allopurinol/sulfasalazine also had detectable antibodies to elastase or lactoferrin (other antigens for perinuclear ANCA [p-ANCA]). No patient was positive for anti-PR3 ANCA. Both patients with culprit drug exposure (16 of 18) and nonexposure (eight of 12) were often antinuclear antibody (ANA) positive, but positivity for antidouble–stranded DNA antibodies (dsDNAs) was uncommon (four of 30). On the basis of this report and others, drug-associated AAV should be considered in patients with a history of drug exposure, high-titer anti-MPO antibodies, and the presence of other autoantibodies. Table 1 lists drugs most commonly associated with AAV.
Table 1.
Drugs commonly implicated in ANCA-associated vasculitis
| Drug | Evidence | Renal Involvement | Comments |
| Cocaine and levamisole | Multiple case series and case reports | 44%a | Skin manifestations in 61%a |
| Neutropenia in 28%a | |||
| Hydralazine | Case series and case reports | 80%–90%b | Combined pulmonary-renal syndrome is rare (15 patients to date) |
| Lupus-like syndrome is common | |||
| Antithyroid medications | PTU: multiple case series and case reports | May be common | Animal models also support association with PTU |
| Carbimazole and methimazole: case reports | |||
| Minocycline | Small case series and case reports | No reported cases of renal involvement with small-vessel vasculitis | Conflicting data on ANCA seroconversion with minocycline use (45,46) |
| PAN with p-ANCA positivity and renal involvement reported | |||
| Allopurinol | Case reports | Reported | Pulmonary-renal syndrome rarely reported |
| Penicillamine | Case reports | Reported | No seroconversion noted in analysis of scleroderma trial (46) |
| Sulfasalazine | Case reports | Reported | Pulmonary-renal syndrome also reported |
| No seroconversion noted in analysis of the CSSRD Trial (46) |
PTU, propylthiouracil; PAN, polyarteritis nodosa; p-ANCA, perinuclear ANCA; CSSRD, Cooperative Systematic Studies of the Rheumatic Diseases.
Data from the largest case series of cocaine- and levamisole-associated, ANCA-associated vasculitis (21).
Renal Histology
Kidney biopsies from patients with drug AAV exhibit necrotizing and crescentic GN (Figure 1) that is pauci-immune by immunofluorescence. Although two studies found less severe histologic findings in Chinese patients with PTU AAV versus primary AAV (6,7), these differences have not otherwise been reported. The histologic classification scheme used in idiopathic AAV to predict clinical prognosis (8) has not been validated in drug-associated AAV.
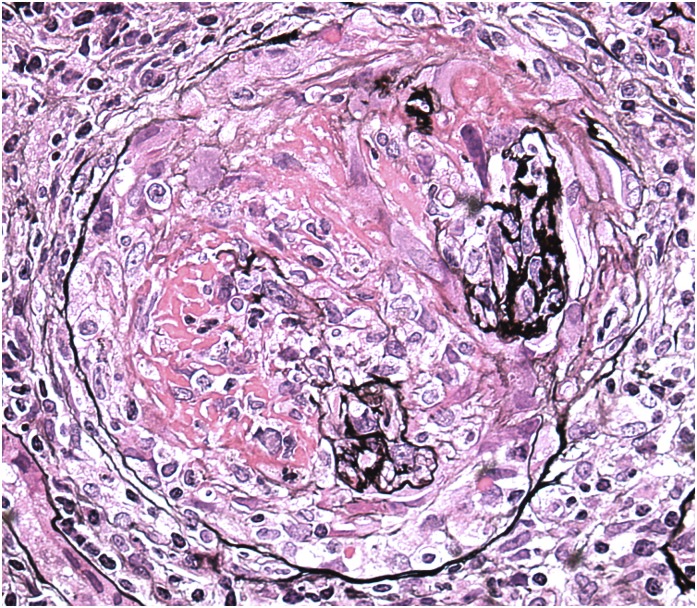
Figure 1.
A glomerulus from a patient who used cocaine that contained levamisole and presented with rapidly progressive GN and high-titer anti-myeloperoxidase ANCA. The glomerulus exhibits fibrinoid necrosis, multifocal rupture of the glomerular basement membrane, and an overlying, circumferential cellular crescent. Jones methenamine silver, ×400.
Treatment
No trials have been conducted in the treatment of drug-associated AAV. In mild cases, stopping the offending drug may lead to resolution of AAV. However, in severe cases, particularly with pulmonary or renal involvement, aggressive treatment with immunosuppression regimens used in idiopathic AAV (9,10) (corticosteroids with cyclophosphamide or rituximab and plasma exchange for pulmonary hemorrhage and rapidly progressive GN) should be considered. Importantly, patients should be educated about future drug avoidance, with the culprit drug added as an allergen in the patient’s medical record. Other controversial aspects in managing drug-associated AAV include using a shorter duration of induction therapy and foregoing maintenance immunosuppression.
Drugs Associated with AAV
Cocaine- and Levamisole-Associated Vasculitis.
Multiple reports describe the development of vasculitis in cocaine users, including cutaneous vasculitis, cerebral vasculitis (11), and cocaine-induced midline destructive lesions (12). Recently, the adulterant levamisole has been implicated as a culprit in cocaine-associated AAV.
Levamisole has been used in humans for pediatric nephrotic syndrome (NS), colon cancer, inflammatory bowel disease, and rheumatoid arthritis (RA). Reports exist describing cutaneous vasculitis (13,14) and the presence of autoantibodies, including ANCAs (15,16), in patients exposed to levamisole. Levamisole was withdrawn from the market in the United States in 2000 because of cases of treatment-associated agranulocytosis.
Levamisole has been detected with increased frequency in the illicit cocaine supply entering the United States and Europe over the last decade (17,18), with a 2011 Drug Enforcement Agency report noting contamination in 82% of the United States supply. Levamisole shares similar physical properties (appearance, smell, and taste) with cocaine, and it may mimic or potentiate the euphoric effects of cocaine through sympathetic nervous system stimulation. One hypothesis is that the availability of levamisole in South America is because of its veterinary use as an antihelminth agent.
There is a temporal association between the rise in the number of patients with cocaine AAV and the detection of levamisole in the United States cocaine supply. In 2009, cases emerged of cocaine users who presented with agranulocytosis (19) followed by AAV with characteristic skin findings of small- (palpable purpura) and medium-vessel (retiform purpura) vasculitis (Figure 2). Neutropenia occurred in some patients with cocaine AAV, and levamisole was detected in some patients (20).
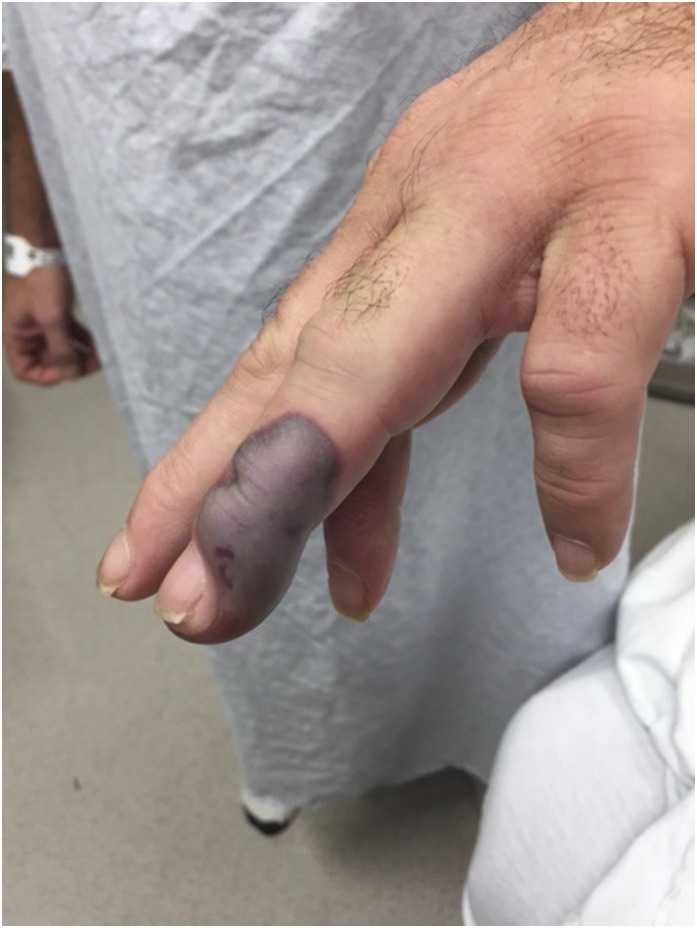
Figure 2.
Digital cutaneous vasculitis of a patient with levamisole-associated, ANCA-associated vasculitis. Reprinted from Joan Von Feldt and Robert Michelleti, with permission.
McGrath et al. (21) describe the largest case series of levamisole-associated AAV. Of 327 patients with newly positive ANCA titers between 2009 and 2010, 30 had evidence of active cocaine use by report or toxicology. On presentation, 83% had arthralgias, 61% had skin manifestations, 44% had ear/nose/throat involvement, 44% had evidence of renal involvement (defined by abnormal urine dipstick or urine microscopy), 28% were neutropenic, and 17% had pulmonary hemorrhage. No patient had pulmonary-renal syndrome. Two patients had severe AKI, one of whom underwent a kidney biopsy showing pauci-immune crescentic GN. Both of these patients were left with significant renal impairment, despite immunosuppression.
Serologically, all patients were anti-MPO positive, and one half were anti-PR3 ANCA positive. Consistent with earlier observations in drug-associated AAV (5), patients with cocaine-associated AAV had higher anti-MPO levels (15 times) than patients with idiopathic AAV over the same period (range =1075–7988 versus median =112; P<0.01), and many patients had additional abnormal serologies (ANA positive, 14 of 17; low C3 and/or C4, seven of 11; anti-dsDNA antibody positive, three of nine; positive lupus anticoagulant, six of nine). During the same time period, 15 of 21 new patients with AAV that were dual-ANCA positive (anti-PR3 and anti-MPO) were attributed to cocaine. The mechanism of levamisole-associated AAV is unclear. HLA B27 positivity has been shown to increase the risk of levamisole-associated agranulocytosis (22,23), but its association with AAV has not been explored.
Treatment has focused on cessation of cocaine use and the use of immunosuppression in severe cases. However, cocaine’s widespread use and addictive nature present a unique challenge. Health care providers should have a high level of suspicion for cocaine/levamisole AAV in patients with high-titer anti-MPO ANCA, coexistent MPO and PR3 ANCA, other autoimmune serologies, leukopenia, severe cutaneous lesions, and recurrent AAV episodes.
Antithyroid Drugs.
Reports emerged in the 1990s of AAV, some with crescentic/pauci-immune GN, in patients treated with PTU, carbimazole, and/or methimazole for hyperthyroidism (4,24–28). Gunton et al. (29) then explored the link between antithyroid drugs and ANCAs, finding that only one of 10 newly diagnosed patients developed ANCAs (atypical cytoplasmic ANCA and high anti-MPO titer) 8 months after starting carbimazole in contrast to eight of 30 (27%) long-term patients. Patients who were ANCA positive were mostly p-ANCA/anti-MPO antibody positive, on PTU (seven of eight), and had a longer mean drug exposure (8.9 versus 2.8 years). Four of these patients had possible vasculitis symptoms that resolved after stopping the medications, although renal disease was not specifically mentioned. Five of eight patients became ANCA negative within 6 months of stopping antithyroid therapy. Gunton et al. (29) concluded that ANCA positivity associated with long-term use of antithyroid medications.
A cross-sectional analysis of 207 patients with hyperthyroidism in The Netherlands found that exposure to antithyroid medication (PTU, methimazole, and carbimazole) was associated with an 11.8 times higher odds (95% confidence interval, 1.5 to 93.3) of developing a positive ANCA serology (p-ANCA, cytoplasmic ANCA, or atypical p-ANCA on immunofluorescence or ELISA positive for anti-MPO, PR3, or human lactoferrin antibody) versus nonexposure (30); four of 13 patients with positive ANCA serologies had clinical signs or symptoms of vasculitis, three of whom had kidney biopsies showing necrotizing and crescentic GN. However, the association between antithyroid medications and development of a positive ANCA serology was no longer observed when patients with only anti–human lactoferrin antibody (a nonpathogenic ANCA) were excluded, and ANCA positivity was not related to any individual antithyroid drug or treatment duration. A second study by Afeltra et al. (31) detected a positive ANCA in 29% (six of 21) of patients with Graves Disease not being treated with PTU versus 9% (one of 11) of patients with Hashimotos thryoiditis and zero of 20 controls. These two studies support the hypothesis that the risk for a positive ANCA serology may be linked to underlying autoimmunity rather than drug exposure.
The pathogenesis of drug AAV with these medications is poorly understood. One study showed a higher reactivity of sera in patients with PTU AAV against specific MPO fragments versus both patients with idiopathic AAV and patients with PTU-associated anti-MPO antibodies without clinical vasculitis (32). Other hypotheses include (1) PTU and/or its metabolites accumulate in neutrophils and bind to MPO, altering its configuration and promoting autoantibody formation (29,33–35), (2) PTU oxidization in the presence of activated neutrophils creates reactive drug metabolites that stimulate ANCA production (30,36), and (3) PTU decreases the degradation of neutrophil extracellular traps, resulting in autoimmunity (37).
Hydralazine.
Evidence for hydralazine-associated vasculitis dates to the pre-ANCA era, including rapidly progressive GN. A 2009 review of the literature found 68 hydralazine vasculitis reports (mean duration of drug exposure =4.7 years; mean dose =142 mg/d) (38). Similar to the findings by Choi et al. (5), kidney disease was common on presentation (81%), and patients had additional serologic evidence of an autoimmune process (96% ANA positive, 26% anti-dsDNA antibody positive, and 44% hypocomplementemia). Combined pulmonary-renal syndrome with hydralazine-associated AAV is rare, with only 15 suspected cases in the literature (39–42). Given the overlap in the clinical presentation of hydralazine-associated SLE and AAV, both diagnoses should be considered.
Hypotheses for the mechanism of hydralazine-associated AAV include (1) neutrophil apoptosis in response to hydralazine MPO binding, resulting in the production of multiple autoantibodies (38), (2) increased expression of neutrophil autoantigens through hydralazine-induced reversal of epigenic silencing of MPO and PR3, and (3) a break in tolerance in slow versus fast acetylators of hydralazine (43).
Other Drugs.
Initial reports linked minocycline to AAV (44), but subsequent data on this association are conflicting. A cross-sectional study in patients of dermatology found ANCA positivity in 12 of 174 (7%) of patients with past/current minocycline use compared with zero of 71 patients without exposure (45). However, Choi et al. (46) found that no patient developed a positive ANCA after 48 weeks of minocycline in a smaller RA cohort. Polyarteritis nodosa with p-ANCA positivity, sometimes with renal involvement, has also been reported in patients exposed to minocycline (47,48). Fifteen patients with nonpolyarteritis nodosa minocycline AAV have been reported to date, but none had renal involvement (47).
Exposures to penicillamine (n=2), sulfasalazine (n=1), and allopurinol (n=2) were noted in patients with high anti-MPO titers in the series by Choi et al. (5). However, in a second study, Choi et al. (46) found no ANCA seroconversions in patients given high- or low-dose penicillamine for scleroderma or sulfasalazine for rheumatologic diseases. Allopurinol-associated vasculitis has been noted in case reports (49), but reports of serology-positive AAV are rare (50).
The association between the use of TNF-α inhibitors and AAV is not clear. A French survey–based registry of TNF-α inhibitor AAV found it in 39 of 1200 patients, five of whom were ANCA positive (51). Another prospective series of patients with ankylosing spondylitis did not show a significant increase in ANCA seroconversion in treated patients (52). Six patients with TNF-α inhibitor AAV have been reported to date (53,54).
Case reports exist (some with renal involvement) for IFN AAV during treatment for hepatitis C (55,56). This is a challenging diagnosis, because autoantibodies (including ANCAs) may be seen with hepatitis C infection (commonly anti-PR3) (57); also, the presentation of AAV may overlap clinically with cryoglobulinemic vasculitis. Cutaneous vasculitis has been described after treatment with granulocyte factors and GM-CSFs (58,59), but ANCA titers were not checked in most patients, and renal disease has not been described. Both isoniazid and rifampin have been implicated as causes of vasculitis in case reports (60,61), but the association between these drugs and AAV is confounded by studies that have shown a high prevalence of positive ANCA serologies in patients with tuberculosis (62). Evidence for the association of other medications and AAV is limited to isolated case reports (63–65).
DIL
Epidemiology, Clinical Presentation, and Pathogenesis
Since the first description of lupus in 1945 with sulfadiazine therapy (66), many drugs have been associated with the production of autoantibodies, but true DIL is uncommon. DIL may be limited to the skin or resemble SLE. Although no prospective studies exist in DIL, it is estimated that there are 15,000–30,000 patients with DIL annually (67). Previously, the drugs most commonly associated with DIL were hydralazine and procainamide, with incidences as high as 5%–8% and 20%, respectively, during the first year of therapy (68). There has been a reduction in DIL in parallel with decreased use of these agents (69). However, the newer biologics (e.g., anti–TNF-α therapy) have been found to carry a risk for DIL, which was reported to be 0.1% in a registry study (70). Table 2 lists the most common drugs reported to be associated with lupus-like disease. Although herbal compounds, such as l-canavanine in alfalfa supplements and Echinacea, have been implicated in causing lupus, the data in humans are sparse (71,72).
Table 2.
Drugs and risk for developing drug-induced lupus
| Drug Category | Risk for Drug-Induced Lupus | |||
| High | Moderate | Low | Very Low | |
| Antiarrhythmics | Procainamide | Quinidine | Disopyramide, propafenone, amiodarone | |
| Antihypertensives | Hydralazine | Methyldopa, captopril, acebutolol | Enalapril, lisinopril, clonidine, atenolol, labetalol, pindolol, minoxidil, prazosin | |
| Antipsychotics | Chlorpromazine | Phenelzine, chlorprothixene, lithium | ||
| Antibiotics | Isoniazid, minocycline | Nalidixic acid, sulfamethoxazole, quinine | ||
| Anticonvulsants | Carbamazepine | Clobazam, phenytoin, trimethadione, primidone, ethosuximide, valproic acid | ||
| Antithyroid | Propylthiouracil | |||
| Diuretics | Chlorthalidone, hydrochlorothiazide | |||
| Biologics | TNF-α inhibitors | IFN-α | ||
| Miscellaneous | Statins, levodopa, aminoglutethimide, timolol drops, ticlodipine | |||
Modified from ref. 73.
DIL has only a minor increase in preponderance in women and tends to occur in older individuals compared with idiopathic SLE. Systemic symptoms, including fever, anorexia, weight loss, and arthralgia, are common. Skin manifestations (including macular, maculopapular, urticarial, or vasculitic rashes) are less common than in classic SLE. Although arthritis, serositis, and hepatosplenomegaly may occur, major organ involvement is rare (67,73). GN is uncommon but has been reported with hydralazine (74), sulfasalazine (75), PTU (76), penicillamine (77), and anti–TNF-α therapy (78).
Serologic abnormalities in procainamide and hydralazine DIL are similar but share notable differences from idiopathic SLE. Antihistone antibodies are common in DIL, with (H2A-H2B)-DNA subnucleosome being the predominant antigen in procainamide DIL (79) and H1 and the H3-H4 complex being the predominant antigens in hydralazine DIL (80). Anti-dsDNA antibodies have been observed in patients with TNF-α inhibitor DIL (81) but are rare in procainamide and hydralazine DIL (68). The serologic profile of minocycline DIL is also different from other DIL with the occurrence of positive ANA, anti-dsDNA antibodies, and p-ANCA (82). Complement levels are variably reduced in DIL, and hypocomplementemia is seen more commonly with quinidine and TNF-α inhibitors (67,69). The clinical and serologic manifestations of DIL differ somewhat between drugs as discussed below and noted in Table 3.
Table 3.
Comparison of clinical and laboratory features of three drugs associated with drug-induced lupus
| Drug | Clinical Features | Laboratory Features | Positive Antibody Tests |
| Hydralazine | Rash, fever, myalgias, pleuritis, polyarthritis nephritis <10% | Anemia, leukopenia | ANA, anti-dsDNA, ANCA, antihistone |
| Procainamide | Polyarthritis, polyarthralgias serositis nephritis <10% | Anemia | Anti-dsDNA, antihistone, anticardiolipin |
| TNF-α inhibitors | Systemic symptoms predominant (nephritis in 7%); skin manifestations dominate | Thrombocytopenia, hypocomplementemia | ANA, anti-dsDNA, antinucleosome, anticardiolipin |
ANA, antinuclear antibody; dsDNA, double-stranded DNA antibody.
One clue to the presence of drug-induced autoimmunity is the presence of multiple additional abnormal serologies (e.g., the presence of p-ANCA antibodies with multiple specificities along with antiphospholipid antibodies and antihistone antibodies) (83).
DIL is a type B (hypersensitivity) reaction. Research on DIL pathogenesis has focused on drug-specific generation of autoimmunity and predisposing factors in affected patients, mechanisms that are poorly understood and likely drug specific. Jiang et al. (36) showed that activated neutrophils convert multiple DIL agents (including procainamide and hydralazine) into cytotoxic products in vitro through an MPO-dependent pathway. Several mechanisms have been proposed to explain the production of autoantibodies in DIL, including disruption of central T cell tolerance by culprit drug metabolites, leading to the production of reactive T cells that lead to a peripheral autoantibody response (73). A T cell response may also be provoked by drugs or their oxidative metabolites (84). Unlike in SLE, the clearance of circulating immune complexes by the reticuloendothelial system seems to be intact in DIL. The antibodies in DIL are true autoantibodies and not antibodies to drug products (73). Patients with slow acetylation phenotypes may be predisposed to certain drug-induced etiologies of DIL. The evidence for drug-specific DIL is presented below.
Diagnosis
Although no firm criteria exist, some works (67,85) have proposed diagnostic guidelines for DIL: (1) sufficient (at least 1 month) and continuing exposure to a specific drug, (2) at least one symptom compatible with SLE (such as arthralgia, myalgia, malaise, fever, serositis, and/or rash), (3) no history suggestive of SLE before starting the drug, and (4) resolution of symptoms within weeks (sometimes months) after discontinuation of the putative offending agent (67). The latter observation is critical to separate DIL from drug exacerbation or unmasking of SLE, where the clinical symptoms persist, despite stopping the drug. It is important to note that there is no characteristic syndrome associated with a particular drug. Thus, placing heavy reliance on the presence/absence of organs involved and specific antibody patterns is not recommended.
Treatment and Prognosis
There are few data and no randomized trials or guidelines for the treatment of DIL. The first step is to withdraw the offending agent. Symptoms typically resolve in days to weeks, although serologic abnormalities may take months to resolve. If disease persists, both antimalarials and corticosteroids have been used for symptoms, such as serositis, and nonsteroidal anti-inflammatory drugs (NSAIDs) have been used for arthritis. The involvement of major organs (especially kidneys, brain, lungs, and heart) requires escalation of immunosuppressive therapy, such as in SLE, with high-dose corticosteroids, antimetabolites, or alkylating agents (86). The prognosis for recovery is good, except when severe major organ involvement occurs. Significant organ damage and death have been reported under these circumstances, especially with TNF-α inhibitors (87).
Drugs Associated with DIL
Procainamide.
DIL is most closely associated with procainamide; 80%–90% of patients develop a positive ANA over 2 years of therapy, and approximately 20%–30% develop DIL. Middle-aged men are most commonly affected, likely because of increased prescription in this population. Presentation with pulmonary symptoms and serositis is common, whereas arthritis and major organ involvement (including the kidney) are rare (69,88).
Although the exact mechanism of procainamide DIL is unclear, in a small study of 20 patients receiving chronic procainamide therapy, patients who were slow acetylators developed antinuclear antibodies sooner (2.9 months) than fast acetylators (7.3 months) (89). Procainamide was also found to be a potent T cell activator through a hypomethylation pathway in mice, leading to autoreactivity and autoimmunity (90).
Hydralazine.
Renal involvement is uncommon in hydralazine DIL. In one case series, hydralazine DIL occurred in 14 of 281 (5%) patients. Six patients had evidence of renal involvement. These six patients were all women, were taking 50–300 mg/d hydralazine for 0.5–7 years, and commonly had positive ANA (100%), anti-dsDNA antibody (66%), and hypocomplementemia (50%). All patients were slow acetylators, and four had HLA-DR4 genotype. The patients improved after stopping hydralazine and treatment with immunosuppressive therapy (74).
Like with procainamide, slow acetylators have an increased risk of hydralazine DIL versus fast acetylators (91), and studies have found an increased frequency of HLA-DR4 antigens in patients with hydralazine DIL (92). Another study showed a higher prevalence of complement C4 null alleles in patients with hydralazine DIL compared with the general population, implying higher lupus risk through activation of the classic complement pathway (93).
Biologic Agents, Including TNF-α Inhibitors.
Piga et al. (87) found 26 patients with biologic-associated (including TNF-α inhibitors) autoimmune renal disease through a cohort study analysis and literature review. On the basis of clinical manifestations and renal histology, patients were classified into GN associated with systemic vasculitis (GNSV), GN in lupus-like syndrome, and isolated autoimmune renal disorders (IARDs). TNF-α inhibitors included etanercept (15 patients; 51.7%), adalimumab (nine patients; 31.0%), and infliximab (three patients; 10.3%). Other drugs associated with DIL included tocilizumab and abatacept (one patient each; 3.4% each); 13 of 29 (44.8%) patients were classified with IARD, 12 (41.3%) patients were classified with GNSV, and four (13.9%) patients were classified with GN in lupus-like syndrome. A worse outcome was associated with GNSV and continued use of biologics; end stage renal failure was reported in three patients with GNSV and one patient with IARD, and one death was reported in GNSV (87).
In a review of 25 published case reports of TNF-α inhibitor DIL (86), skin involvement occurred in 67% of patients, renal manifestations were noted in 7% of patients, anti-dsDNA antibodies were common (72%), and low complements occurred in 17%. Similarly, in a French Registry, cutaneous manifestations alone were seen in 10 patients, and systemic symptoms (without renal involvement) were found in 12 of 866 patients (70). Symptoms began approximately 6 months after drug initiation and took 1–4 months to resolve after discontinuation (87).
The proposed pathogenesis of anti–TNF-α DIL differs from that of other agents. Low TNF-α levels lead to severe lupus-like autoimmunity (94), and recombinant TNF-α delays the development of lupus (95) in the NZB mouse model. Several theories have been proposed to explain TNF-α inhibitor DIL. In the cytokine shift paradigm, TNF-α blockade suppresses production of Th1 cytokines, driving the immune response toward Th2 cytokine production, IL-10, and IFN-α. The resulting cytokine imbalance results in autoantibody production and lupus manifestations (96,97). Furthermore, anti–TNF-α drugs may induce apoptosis in inflammatory cells, releasing autoantigens that stimulate autoantibody formation (98,99). It is important to note that anti–TNF-α agents are given to patients for the treatment of autoimmune diseases, such as RA and inflammatory bowel diseases, which themselves are associated with other autoimmune diseases, such as lupus. Improvement in lupus symptoms after stopping anti–TNF-α drugs is the only way to determine whether the latter agent was implicated in causing DIL.
Drugs That Exacerbate SLE
Many drugs have been associated with SLE exacerbations, including antibiotics (penicillin), anticonvulsants (mesantoin), hormones, NSAIDs, sulfonamides, para-aminosalacylic acid, hydrochlorothiazide, and cimetidine. It is of interest that patients with SLE are significantly more prone to develop drug allergies, especially to antibiotics, such as sulfonamides, penicillin/cephalosporin, and erythromycin (100).
Drug-Induced MN
Epidemiology, Pathogenesis, Histology, and Treatment
MN is the most common etiology of primary NS in white adults (101). The majority of patients with MN have primary disease, and most patients with primary MN have autoantibodies directed against the phospholipase A2 receptor (PLA2R) (102). In contrast, 25%–30% of patients have secondary forms of disease (103,104). In a review of nine published series on MN, 6.6% of patients represented drug-induced disease (103). A more recent cohort identified a drug-induced etiology in 14% of patients (104). Testing for anti-PLA2R antibodies should be performed on renal biopsies or serum in patients with MN. On the basis of our understanding, the finding of anti-PLA2R antibodies in glomeruli or serum strongly supports the diagnosis of primary MN and excludes a secondary drug–induced form of disease.
The pathogenesis of drug-induced MN (DIMN) likely involves an immune response to a therapeutic agent or its byproduct. The most plausible mechanism is that cationic drug–derived antigens traverse the glomerular basement membrane (GBM), are planted at the subepithelial aspect of the GBM, and become bound in situ to circulating antibodies directed against these antigens. This mechanism underlies the early-childhood MN that occurs in response to cationic BSA present in cow’s milk (105).
Pathologically, MN is defined by subepithelial immune complex deposits and associated GBM alterations that include intervening spike formation, overly neomembrane formation, and remodeling (Figure 3). Pathologic findings do not distinguish between primary MN and DIMN, highlighting the importance of obtaining a detailed clinical history regarding drug use.
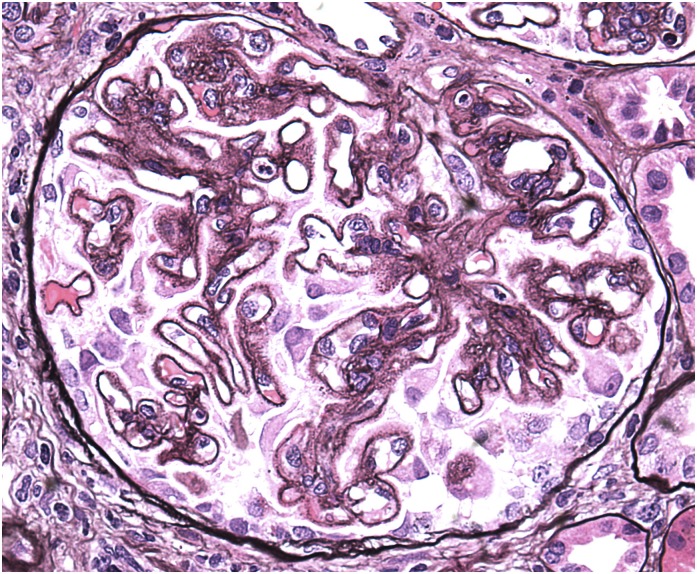
Figure 3.
A glomerulus from a patient who developed nephrotic syndrome while receiving a nonsteroidal anti-inflammatory drug for arthritis. The glomerulus exhibits global thickening of the glomerular basement with spike formation, which is characteristic of stage 2 membranous changes. Jones methenamine silver, ×400.
Treatment of DIMN begins with withdrawal of the culprit drug. In the case of some therapeutic agents, such as gold salts, penicillamine, and bucillamine, proteinuria and MN are so frequent that urine monitoring is required. With severe symptoms of NS and/or lack of improvement after withdrawal, immunosuppressive therapy may be warranted.
Drugs Associated with MN
Gold Therapy.
Gold salts, including oral and parenteral preparations, have been used in the treatment of RA for more than three quarters of a century, but recently, they have been largely replaced by safer, more efficacious agents. The most common side effect of gold is proteinuria, which develops in 3%–7% of patients (106,107). Renal biopsy most commonly reveals stage 1 or 2 MN, indicating early detection because of screening. After withdrawal, proteinuria resolves in most patients (106,108). Experimental studies have shown that gold inclusions mainly localize to proximal tubules, and the same is true in a rodent model of gold-induced MN, suggesting that gold targets tubular epithelia and leads to release of tubular antigens that cross-react with podocyte antigens, akin to the mechanism of Heymann nephritis (109,110).
Penicillamine and Bucillamine.
Similar to gold, penicillamine and bucillamine are used to treat RA, proteinuria is the most frequent indication for discontinuing therapy, and the most common biopsy finding is MN stage 1 (111–113). Penicillamine has been used to treat RA for nearly 50 years, and the incidence of proteinuria may exceed 10% (111). Outcomes after penicillamine withdrawal are excellent, with complete resolution of proteinuria in the absence of immunosuppression in 32 of 33 patients in one series (112).
Bucillamine is a more recently developed antirheumatic drug that is mainly used in Asia and differs from penicillamine by an additional sulfhydryl group. Similar to penicillamine, proteinuria resolves in nearly all patients after withdrawal (113). The mechanism by which penicillamine and bucillamine produce MN is unknown but may involve modification of the immune response and/or hapten formation.
Mercury.
Mercury exposure is associated with severe toxicity involving multiple organ systems, most notably the central nervous system. Sources of mercury include contaminated foods (in particular, fish), dental amalgams, cosmetics, occupational exposures, and skin-lightening creams. Chronic mercury exposure is infrequently associated with NS and in most cases, MN (114). After discontinuation, remission of proteinuria occurs in most patients (114). A recent report described two patients who developed DIMN after the use of skin creams that contained mercury (115). The absence of anti-PLA2R antibodies in these two patients as well as the previously established rat model of mercury-induced MN (116) provide support for the association between mercury and MN.
Captopril.
Captopril is an angiotensin-converting enzyme inhibitor (ACE-I) that is commonly used to treat hypertension and reduce proteinuria. Ironically, captopril seems to be the only ACE-I associated with the development of NS and in most cases, biopsy findings of MN (117). This complication of therapy, which is seen in up to 1% of patients (118), has been attributed to a sulfhydryl group, which is unique to captopril among the ACE-Is but a feature that it shares in common with penicillamine and bucillamine (119). After discontinuation, significant reduction in proteinuria commonly occurs.
NSAIDs.
NSAIDs are widely used for their analgesic, antipyretic, and anti-inflammatory properties. NSAIDs inhibit cyclooxygenase-1 (COX-1) and COX-2, thereby blocking prostaglandin, prostacyclin, and thromboxane production. MN has been reported after the use of multiple classes of NSAIDs with varying chemical structures, including proprionic acid derivatives (ketoprofen, fenoprofen, and ibuprofen), acetic acid derivatives (diclofenac, sulindac, and tolmetin), enolic acid derivatives (piroxicam), and selective COX-2 inhibitors (etodolac and celecoxib) (120,121). The development of MN with these diverse agents suggests a mechanism of DIMN that likely involves the common pharmacologic effects of NSAIDs on mediators of inflammation.
Given the widespread use of NSAIDs, the incidence of NSAID-associated MN is difficult to determine. A stringent criterion for this diagnosis is the requirement for rapid remission after withdrawal. Radford et al. (120) examined NSAID-associated MN over a 20-year period at the Mayo Clinic. Considering only patients with stage 1 or early stage 2 MN, 29 of 125 patients were taking NSAIDs, and 13 had a rapid remission after drug withdrawal, suggesting that NSAID-associated MN represented 10% of patients with early MN.
Additional Drug-Induced Etiologies of MN.
There are many published case reports describing an association between MN and individual therapeutic agents as well as small series describing DIMN after treatment with tiopronin for cystinuria (122) or trimethadione, an anticonvulsant (123).
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Nässberger L, Sjöholm AG, Jonsson H, Sturfelt G, Akesson A: Autoantibodies against neutrophil cytoplasm components in systemic lupus erythematosus and in hydralazine-induced lupus. Clin Exp Immunol 81: 380–383, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nässberger L, Johansson AC, Björck S, Sjöholm AG: Antibodies to neutrophil granulocyte myeloperoxidase and elastase: Autoimmune responses in glomerulonephritis due to hydralazine treatment. J Intern Med 229: 261–265, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Almroth G, Eneström S, Hed J, Samuelsson I, Sjöström P: Autoantibodies to leucocyte antigens in hydralazine-associated nephritis. J Intern Med 231: 37–42, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Dolman KM, Gans RO, Vervaat TJ, Zevenbergen G, Maingay D, Nikkels RE, Donker AJ, von dem Borne AE, Goldschmeding R: Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet 342: 651–652, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Merkel PA, Walker AM, Niles JL: Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: Prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum 43: 405–413, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chen YX, Zhang W, Chen XN, Yu HJ, Ni LY, Xu J, Pan XX, Ren H, Chen N: Propylthiouracil-induced antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis versus primary ANCA-associated renal vasculitis: A comparative study. J Rheumatol 39: 558–563, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Lin W: Clinical study of renal impairment in patients with propylthiouracil-induced small-vessel vasculitis and patients with primary ANCA-associated small-vessel vasculitis. Exp Ther Med 5: 1619–1622, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh D, Walsh M, Westman K, Jayne DR; European Vasculitis Study Group: Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211–220, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Fessler BJ, Ding L, Viviano L, Tchao NK, Phippard DJ, Asare AL, Lim N, Ikle D, Jepson B, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Mueller M, Sejismundo LP, Mieras K, Stone JH; RAVE-ITN Research Group: Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 369: 417–427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkel PA, Koroshetz WJ, Irizarry MC, Cudkowicz ME: Cocaine-associated cerebral vasculitis. Semin Arthritis Rheum 25: 172–183, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Trimarchi M, Gregorini G, Facchetti F, Morassi ML, Manfredini C, Maroldi R, Nicolai P, Russell KA, McDonald TJ, Specks U: Cocaine-induced midline destructive lesions: Clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine (Baltimore) 80: 391–404, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane DG, Bacon PA: Levamisole-induced vasculitis due to circulating immune complexes. BMJ 1: 407–408, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheinberg MA, Bezerra JB, Almeida FA, Silveira LA: Cutaneous necrotising vasculitis induced by levamisole. BMJ 1: 408, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laux-End R, Inaebnit D, Gerber HA, Bianchetti MG: Vasculitis associated with levamisole and circulating autoantibodies. Arch Dis Child 75: 355–356, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rongioletti F, Ghio L, Ginevri F, Bleidl D, Rinaldi S, Edefonti A, Gambini C, Rizzoni G, Rebora A: Purpura of the ears: A distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol 140: 948–951, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Wolford A, McDonald TS, Eng H, Hansel S, Chen Y, Bauman J, Sharma R, Kalgutkar AS: Immune-mediated agranulocytosis caused by the cocaine adulterant levamisole: A case for reactive metabolite(s) involvement. Drug Metab Dispos 40: 1067–1075, 2012 [DOI] [PubMed] [Google Scholar]
- 18.UNODC: World Drug Report, 2011, pp 85–126 [Google Scholar]
- 19.Zhu NY, Legatt DF, Turner AR: Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 150: 287–289, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Lazareth H, Peytavin G, Polivka L, Dupin N: The hairy-print for levamisole-induced vasculitis. BMJ Case Rep 2012: bcr2012006602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath MM, Isakova T, Rennke HG, Mottola AM, Laliberte KA, Niles JL: Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol 6: 2799–2805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodinka L, Géher P, Merétey K, Gyódi EK, Petrányi GG, Bozsóky S: Levamisole-induced neutropenia and agranulocytosis: Association with HLA B27 leukocyte agglutinating and lymphocytotoxic antibodies. Int Arch Allergy Appl Immunol 65: 460–464, 1981 [DOI] [PubMed] [Google Scholar]
- 23.Veys EM, Mielants H, Verbruggen G: Levamisole-induced adverse reactions in HLA B27-positive rheumatoid arthritis. Lancet 1: 148, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Stankus SJ, Johnson NT: Propylthiouracil-induced hypersensitivity vasculitis presenting as respiratory failure. Chest 102: 1595–1596, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Vogt BA, Kim Y, Jennette JC, Falk RJ, Burke BA, Sinaiko A: Antineutrophil cytoplasmic autoantibody-positive crescentic glomerulonephritis as a complication of treatment with propylthiouracil in children. J Pediatr 124: 986–988, 1994 [DOI] [PubMed] [Google Scholar]
- 26.D’Cruz D, Chesser AM, Lightowler C, Comer M, Hurst MJ, Baker LR, Raine AE: Antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis associated with anti-thyroid drug treatment. Br J Rheumatol 34: 1090–1091, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Kudoh Y, Kuroda S, Shimamoto K, Iimura O: Propylthiouracil-induced rapidly progressive glomerulonephritis associated with antineutrophil cytoplasmic autoantibodies. Clin Nephrol 48: 41–43, 1997 [PubMed] [Google Scholar]
- 28.Kawachi Y, Nukaga H, Hoshino M, Iwata M, Otsuka F: ANCA-associated vasculitis and lupus-like syndrome caused by methimazole. Clin Exp Dermatol 20: 345–347, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Gunton JE, Stiel J, Clifton-Bligh P, Wilmshurst E, McElduff A: Prevalence of positive anti-neutrophil cytoplasmic antibody (ANCA) in patients receiving anti-thyroid medication. Eur J Endocrinol 142: 587, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Slot MC, Links TP, Stegeman CA, Tervaert JW: Occurrence of antineutrophil cytoplasmic antibodies and associated vasculitis in patients with hyperthyroidism treated with antithyroid drugs: A long-term followup study. Arthritis Rheum 53: 108–113, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Afeltra A, Paggi A, De Rosa FG, Manfredini P, Addessi MA, Amoroso A: Antineutrophil cytoplasmic antibodies in autoimmune thyroid disorders. Endocr Res 24: 185–194, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Gou SJ, Xu PC, Zhao MH, Chen M: Epitope analysis of anti-myeloperoxidase antibodies in propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res Ther 15: R196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee E, Hirouchi M, Hosokawa M, Sayo H, Kohno M, Kariya K: Inactivation of peroxidases of rat bone marrow by repeated administration of propylthiouracil is accompanied by a change in the heme structure. Biochem Pharmacol 37: 2151–2153, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Waldhauser L, Uetrecht J: Antibodies to myeloperoxidase in propylthiouracil-induced autoimmune disease in the cat. Toxicology 114: 155–162, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Waldhauser L, Uetrecht J: Oxidation of propylthiouracil to reactive metabolites by activated neutrophils. Implications for agranulocytosis. Drug Metab Dispos 19: 354–359, 1991 [PubMed] [Google Scholar]
- 36.Jiang X, Khursigara G, Rubin RL: Transformation of lupus-inducing drugs to cytotoxic products by activated neutrophils. Science 266: 810–813, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, Nishio S, Kasahara M, Ishizu A: Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: Implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 64: 3779–3787, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Yokogawa N, Vivino FB: Hydralazine-induced autoimmune disease: Comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol 19: 338–347, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Dobre M, Wish J, Negrea L: Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis: A case report and literature review. Ren Fail 31: 745–748, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Kalra A, Yokogawa N, Raja H, Palaniswamy C, Desai P, Zanotti-Cavazzoni SL, Rajaram SS: Hydralazine-induced pulmonary-renal syndrome: A case report. Am J Ther 19: e136–e138, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Marina VP, Malhotra D, Kaw D: Hydralazine-induced ANCA vasculitis with pulmonary renal syndrome: A rare clinical presentation. Int Urol Nephrol 44: 1907–1909, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Agarwal G, Sultan G, Werner SL, Hura C: Hydralazine induces myeloperoxidase and proteinase 3 anti-neutrophil cytoplasmic antibody vasculitis and leads to pulmonary renal syndrome. Case Rep Nephrol 2014: 868590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pendergraft WF, 3rd, Niles JL: Trojan horses: Drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol 26: 42–49, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Elkayam O, Yaron M, Caspi D: Minocycline induced arthritis associated with fever, livedo reticularis, and pANCA. Ann Rheum Dis 55: 769–771, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzo-Ortega H, Baxter K, Strauss RM, Drysdale S, Griffiths B, Misbah SA, Gough A, Cunliffe WJ, Emery P: Is minocycline therapy in acne associated with antineutrophil cytoplasmic antibody positivity? A cross-sectional study. Br J Dermatol 156: 1005–1009, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Choi HK, Slot MC, Pan G, Weissbach CA, Niles JL, Merkel PA: Evaluation of antineutrophil cytoplasmic antibody seroconversion induced by minocycline, sulfasalazine, or penicillamine. Arthritis Rheum 43: 2488–2492, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Lenert P, Icardi M, Dahmoush L: ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: Case-based review. Clin Rheumatol 32: 1099–1106, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Kermani TA, Ham EK, Camilleri MJ, Warrington KJ: Polyarteritis nodosa-like vasculitis in association with minocycline use: A single-center case series. Semin Arthritis Rheum 42: 213–221, 2012 [DOI] [PubMed] [Google Scholar]
- 49.ten Holder SM, Joy MS, Falk RJ: Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother 36: 130–147, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Choi HK, Merkel PA, Niles JL: ANCA-positive vasculitis associated with allopurinol therapy. Clin Exp Rheumatol 16: 743–744, 1998 [PubMed] [Google Scholar]
- 51.Saint Marcoux B, De Bandt M; CRI (Club Rhumatismes et Inflammation): Vasculitides induced by TNFalpha antagonists: A study in 39 patients in France. Joint Bone Spine 73: 710–713, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Arends S, Lebbink HR, Spoorenberg A, Bungener LB, Roozendaal C, van der Veer E, Houtman PM, Griep EN, Limburg PC, Kallenberg CG, Wolbink GJ, Brouwer E: The formation of autoantibodies and antibodies to TNF-α blocking agents in relation to clinical response in patients with ankylosing spondylitis. Clin Exp Rheumatol 28: 661–668, 2010 [PubMed] [Google Scholar]
- 53.Hirohama D, Hoshino J, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N, Takemoto F, Ubara Y, Hara S, Ohashi K, Takaichi K: Development of myeloperoxidase-antineutrophil cytoplasmic antibody-associated renal vasculitis in a patient receiving treatment with anti-tumor necrosis factor-α. Mod Rheumatol 20: 602–605, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Tosovský M, Bradna P, Laco J, Podhola M, Soukup T, Brozík J: Case 1-2012: ANCA associated glomerulonephritis in combination with IgG4-positive mediastinal mass in a patient with ankylosing spondylitis treated with TNF alpha inhibitors. Acta Med (Hradec Kralove) 55: 42–46, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Oono Y, Takeshita E, Kobayashi Y, Tanaka Y, Joko K, Ooshiro Y: Case of ANCA associated vasculitis induced by interferon therapy for HCV infection. Nippon Shokakibyo Gakkai Zasshi 105: 1787–1793, 2008 [PubMed] [Google Scholar]
- 56.Marx J, Schwenger V, Blank N, Stremmel W, Encke J: Hemoptysis and acute renal failure in a 29-year-old patient with chronic hepatitis C infection. Internist (Berl) 49: 1120–1125, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Palazzi C, Buskila D, D’Angelo S, D’Amico E, Olivieri I: Autoantibodies in patients with chronic hepatitis C virus infection: Pitfalls for the diagnosis of rheumatic diseases. Autoimmun Rev 11: 659–663, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Jain KK: Cutaneous vasculitis associated with granulocyte colony-stimulating factor. J Am Acad Dermatol 31: 213–215, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Johnson ML, Grimwood RE: Leukocyte colony-stimulating factors. A review of associated neutrophilic dermatoses and vasculitides. Arch Dermatol 130: 77–81, 1994 [DOI] [PubMed] [Google Scholar]
- 60.Tan CD, Smith A, Rodriguez ER: Systemic necrotizing vasculitis induced by isoniazid. Cardiovasc Pathol 23: 181–182, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Lee NK, Song SH, Rhee H, Seong EY, Kim IY, Lee SB, Kwak IS: A case of rifampin-induced crescentic glomerulonephritis. Korean J Med 82: 236–240, 2012 [Google Scholar]
- 62.Flores-Suárez LF, Cabiedes J, Villa AR, van der Woude FJ, Alcocer-Varela J: Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology (Oxford) 42: 223–229, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Parry RG, Gordon P, Mason JC, Marley NJ: Phenytoin-associated vasculitis and ANCA positivity: A case report. Nephrol Dial Transplant 11: 357–359, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Feriozzi S, Muda AO, Gomes V, Montanaro M, Faraggiana T, Ancarani E: Cephotaxime-associated allergic interstitial nephritis and MPO-ANCA positive vasculitis. Ren Fail 22: 245–251, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Sakai N, Wada T, Shimizu M, Segawa C, Furuichi K, Kobayashi K, Yokoyama H: Tubulointerstitial nephritis with anti-neutrophil cytoplasmic antibody following indomethacin treatment. Nephrol Dial Transplant 14: 2774, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Hoffman BJ: Sensitivity to sulfadiazine resembling acute disseminated lupus erythematosus. AMA Arch Derm Syphilol 51: 190–192, 1945 [Google Scholar]
- 67.Borchers AT, Keen CL, Gershwin ME: Drug-induced lupus. Ann N Y Acad Sci 1108: 166–182, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Rubin RL: Drug-induced lupus. Toxicology 209: 135–147, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Xiao X, Chang C: Diagnosis and classification of drug-induced autoimmunity (DIA). J Autoimmun 48-49: 66–72, 2014 [DOI] [PubMed] [Google Scholar]
- 70.De Bandt M, Sibilia J, Le Loët X, Prouzeau S, Fautrel B, Marcelli C, Boucquillard E, Siame JL, Mariette X; Club Rhumatismes et Inflammation: Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: A French national survey. Arthritis Res Ther 7: R545–R551, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akaogi J, Barker T, Kuroda Y, Nacionales DC, Yamasaki Y, Stevens BR, Reeves WH, Satoh M: Role of non-protein amino acid L-canavanine in autoimmunity. Autoimmun Rev 5: 429–435, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Petri M: Systemic lupus erythematosus: 2006 update. J Clin Rheumatol 12: 37–40, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Rubin RL: Drug-induced lupus. Expert Opin Drug Saf 14: 361–378, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Ihle BU, Whitworth JA, Dowling JP, Kincaid-Smith P: Hydralazine and lupus nephritis. Clin Nephrol 22: 230–238, 1984 [PubMed] [Google Scholar]
- 75.Gunnarsson I, Kanerud L, Pettersson E, Lundberg I, Lindblad S, Ringertz B: Predisposing factors in sulphasalazine-induced systemic lupus erythematosus. Br J Rheumatol 36: 1089–1094, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Prasad GV, Bastacky S, Johnson JP: Propylthiouracil-induced diffuse proliferative lupus nephritis: Review of immunological complications. J Am Soc Nephrol 8: 1205–1210, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Donnelly S, Levison DA, Doyle DV: Systemic lupus erythematosus-like syndrome with focal proliferative glomerulonephritis during D-penicillamine therapy. Br J Rheumatol 32: 251–253, 1993 [DOI] [PubMed] [Google Scholar]
- 78.Stokes MB, Foster K, Markowitz GS, Ebrahimi F, Hines W, Kaufman D, Moore B, Wolde D, D’Agati VD: Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant 20: 1400–1406, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Burlingame RW, Rubin RL: Autoantibody to the nucleosome subunit (H2A-H2B)-DNA is an early and ubiquitous feature of lupus-like conditions. Mol Biol Rep 23: 159–166, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Portanova JP, Arndt RE, Tan EM, Kotzin BL: Anti-histone antibodies in idiopathic and drug-induced lupus recognize distinct intrahistone regions. J Immunol 138: 446–451, 1987 [PubMed] [Google Scholar]
- 81.Eriksson C, Engstrand S, Sundqvist KG, Rantapää-Dahlqvist S: Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis 64: 403–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlienger RG, Bircher AJ, Meier CR: Minocycline-induced lupus. A systematic review. Dermatology 200: 223–231, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Wiik A: Clinical and laboratory characteristics of drug-induced vasculitic syndromes. Arthritis Res Ther 7: 191–192, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Engler OB, Strasser I, Naisbitt DJ, Cerny A, Pichler WJ: A chemically inert drug can stimulate T cells in vitro by their T cell receptor in non-sensitised individuals. Toxicology 197: 47–56, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Sarzi-Puttini P, Atzeni F, Capsoni F, Lubrano E, Doria A: Drug-induced lupus erythematosus. Autoimmunity 38: 507–518, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA: Autoimmune diseases induced by TNF-targeted therapies: Analysis of 233 cases. Medicine (Baltimore) 86: 242–251, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Piga M, Chessa E, Ibba V, Mura V, Floris A, Cauli A, Mathieu A: Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: Systematic literature review and analysis of a monocentric cohort. Autoimmun Rev 13: 873–879, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Araújo-Fernández S, Ahijón-Lana M, Isenberg DA: Drug-induced lupus: Including anti-tumour necrosis factor and interferon induced. Lupus 23: 545–553, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Woosley RL, Drayer DE, Reidenberg MM, Nies AS, Carr K, Oates JA: Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med 298: 1157–1159, 1978 [DOI] [PubMed] [Google Scholar]
- 90.Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC: Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol 154: 3025–3035, 1995 [PubMed] [Google Scholar]
- 91.Perry HM, Jr., Tan EM, Carmody S, Sakamoto A: Relationship of acetyl transferase activity to antinuclear antibodies and toxic symptoms in hypertensive patients treated with hydralazine. J Lab Clin Med 76: 114–125, 1970 [PubMed] [Google Scholar]
- 92.Batchelor JR, Welsh KI, Tinoco RM, Dollery CT, Hughes GR, Bernstein R, Ryan P, Naish PF, Aber GM, Bing RF, Russell GI: Hydralazine-induced systemic lupus erythematosus: Influence of HLA-DR and sex on susceptibility. Lancet 1: 1107–1109, 1980 [DOI] [PubMed] [Google Scholar]
- 93.Speirs C, Fielder AH, Chapel H, Davey NJ, Batchelor JR: Complement system protein C4 and susceptibility to hydralazine-induced systemic lupus erythematosus. Lancet 1: 922–924, 1989 [DOI] [PubMed] [Google Scholar]
- 94.Kontoyiannis D, Kollias G: Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol 30: 2038–2047, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Gordon C, Ranges GE, Greenspan JS, Wofsy D: Chronic therapy with recombinant tumor necrosis factor-alpha in autoimmune NZB/NZW F1 mice. Clin Immunol Immunopathol 52: 421–434, 1989 [DOI] [PubMed] [Google Scholar]
- 96.Gonnet-Gracia C, Barnetche T, Richez C, Blanco P, Dehais J, Schaeverbeke T: Anti-nuclear antibodies, anti-DNA and C4 complement evolution in rheumatoid arthritis and ankylosing spondylitis treated with TNF-alpha blockers. Clin Exp Rheumatol 26: 401–407, 2008 [PubMed] [Google Scholar]
- 97.Singh VK, Mehrotra S, Agarwal SS: The paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity and allergy. Immunol Res 20: 147–161, 1999 [DOI] [PubMed] [Google Scholar]
- 98.Lorenz HM, Herrmann M, Winkler T, Gaipl U, Kalden JR: Role of apoptosis in autoimmunity. Apoptosis 5: 443–449, 2000 [DOI] [PubMed] [Google Scholar]
- 99.D’Auria F, Rovere-Querini P, Giazzon M, Ajello P, Baldissera E, Manfredi AA, Sabbadini MG: Accumulation of plasma nucleosomes upon treatment with anti-tumour necrosis factor-alpha antibodies. J Intern Med 255: 409–418, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Petri M, Allbritton J: Antibiotic allergy in systemic lupus erythematosus: A case-control study. J Rheumatol 19: 265–269, 1992 [PubMed] [Google Scholar]
- 101.Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 102.Beck LH, Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Glassock RJ: Secondary membranous glomerulonephritis. Nephrol Dial Transplant 7[Suppl 1]: 64–71, 1992 [PubMed] [Google Scholar]
- 104.Rihova Z, Honsova E, Merta M, Jancova E, Rysava R, Reiterova J, Zabka J, Tesar V: Secondary membranous nephropathy—one center experience. Ren Fail 27: 397–402, 2005 [PubMed] [Google Scholar]
- 105.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P: Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 364: 2101–2110, 2011 [DOI] [PubMed] [Google Scholar]
- 106.Katz WA, Blodgett RC, Jr., Pietrusko RG: Proteinuria in gold-treated rheumatoid arthritis. Ann Intern Med 101: 176–179, 1984 [DOI] [PubMed] [Google Scholar]
- 107.Silverberg DS, Kidd EG, Shnitka TK, Ulan RA: Gold nephropathy. A clinical and pathologic study. Arthritis Rheum 13: 812–825, 1970 [DOI] [PubMed] [Google Scholar]
- 108.Hall CL, Fothergill NJ, Blackwell MM, Harrison PR, MacKenzie JC, MacIver AG: The natural course of gold nephropathy: Long term study of 21 patients. Br Med J (Clin Res Ed) 295: 745–748, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brun C, Olsen S, Raaschou F, Sorensen AW: The localization of gold in the human kidney following chrysotherapy: A biopsy study. Nephron 1: 265–276, 1964 [DOI] [PubMed] [Google Scholar]
- 110.Nagi AH, Alexander F, Barabas AZ: Gold nephropathy in rats—light and electron microscopic studies. Exp Mol Pathol 15: 354–362, 1971 [DOI] [PubMed] [Google Scholar]
- 111.Day AT, Golding JR, Lee PN, Butterworth AD: Penicillamine in rheumatoid disease: A long-term study. BMJ 1: 180–183, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hall CL, Jawad S, Harrison PR, MacKenzie JC, Bacon PA, Klouda PT, MacIver AG: Natural course of penicillamine nephropathy: A long term study of 33 patients. Br Med J (Clin Res Ed) 296: 1083–1086, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Obayashi M, Uzu T, Harada T, Yamato M, Takahara K, Yamauchi A: Clinical course of bucillamine-induced nephropathy in patients with rheumatoid arthritis. Clin Exp Nephrol 7: 275–278, 2003 [DOI] [PubMed] [Google Scholar]
- 114.Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, Liu ZH: Mercury-induced membranous nephropathy: Clinical and pathological features. Clin J Am Soc Nephrol 5: 439–444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chakera A, Lasserson D, Beck LH, Jr., Roberts IS, Winearls CG: Membranous nephropathy after use of UK-manufactured skin creams containing mercury. QJM 104: 893–896, 2011 [DOI] [PubMed] [Google Scholar]
- 116.Icard P, Pelletier L, Vial MC, Mandet C, Pasquier R, Michel A, Druet P: Evidence for a role of antilaminin-producing B cell clones that escape tolerance in the pathogenesis of HgCl2-induced membranous glomerulopathy. Nephrol Dial Transplant 8: 122–127, 1993 [PubMed] [Google Scholar]
- 117.Hoorntje SJ, Kallenberg CG, Weening JJ, Donker AJ, The TH, Hoedemaeker PJ: Immune-complex glomerulopathy in patients treated with captopril. Lancet 1: 1212–1215, 1980 [DOI] [PubMed] [Google Scholar]
- 118.Rosendorff C: Captopril—an overview. S Afr Med J 62: 593–599, 1982 [PubMed] [Google Scholar]
- 119.Jaffe IA: Adverse effects profile of sulfhydryl compounds in man. Am J Med 80: 471–476, 1986 [DOI] [PubMed] [Google Scholar]
- 120.Radford MG, Jr., Holley KE, Grande JP, Larson TS, Wagoner RD, Donadio JV, McCarthy JT: Reversible membranous nephropathy associated with the use of nonsteroidal anti-inflammatory drugs. JAMA 276: 466–469, 1996 [PubMed] [Google Scholar]
- 121.Markowitz GS, Falkowitz DC, Isom R, Zaki M, Imaizumi S, Appel GB, D’Agati VD: Membranous glomerulopathy and acute interstitial nephritis following treatment with celecoxib. Clin Nephrol 59: 137–142, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Ferraccioli GF, Peri F, Nervetti A, Mercadanti M, Cavalieri F, Dall’Aglio PP, Savi M, Ferrari C: Tiopronin-nephropathy: Clinical, pathological, immunological and immunogenetic characteristics. Clin Exp Rheumatol 4: 9–15, 1986 [PubMed] [Google Scholar]
- 123.Heymann W: Nephrotic syndrome after use of trimethadione and paramethadione in petit mal. JAMA 202: 893–894, 1967 [PubMed] [Google Scholar]