Abstract
Background and objectives
Sedentary behavior is associated with increased mortality in the general population. Whether replacing sedentary behavior with low- or light-intensity activities confers a survival benefit in the general or CKD populations is unknown.
Design, setting, participants, & measurements
This observational analysis of the 2003–2004 National Health and Nutrition Examination Survey examined the associations of low- and light-intensity activities with mortality. On the basis of the number of counts/min recorded by an accelerometer, durations of sedentary (<100/min), low (100–499/min), light (500–2019/min), and moderate/vigorous (≥2020/min) activity were defined and normalized to 60 minutes. The mortality associations of 2 min/hr less sedentary duration in conjunction with 2 min/hr more (tradeoff) spent in one of the low, light, or moderate/vigorous activity durations while controlling for the other two activity durations were examined in multivariable Cox regression models in the entire cohort and in the CKD subgroup.
Results
Of the 3626 participants included, 383 had CKD. The mean sedentary duration was 34.4±7.9 min/hr in the entire cohort and 40.8±6.8 in the CKD subgroup. Tradeoff of sedentary duration with low activity duration was not associated with mortality in the entire cohort or the CKD subgroup. Tradeoff of sedentary duration with light activity duration was associated with a lower hazard of death in the entire cohort (hazard ratio, 0.67; 95% confidence interval, 0.48 to 0.93) and CKD subgroup (hazard ratio, 0.59; 95% confidence interval, 0.35 to 0.98). Tradeoff of sedentary duration with moderate/vigorous activity duration had a nonsignificant lower hazard in the entire cohort and CKD subgroup.
Conclusions
Patients with CKD are sedentary nearly two thirds of the time. Interventions that replace sedentary duration with an increase in light activity duration might confer a survival benefit.
Keywords: chronic kidney disease, quality of life, obesity
Introduction
Physical function (1–3) and physical activity (PA) (4,5) are diminished in the CKD and ESRD populations. Compared with the non-CKD subgroup, those with CKD are more sedentary (4,6,7). Sedentary activities with low energy expenditure (approximately 1.0–1.3 metabolic equivalents [METs]) have emerged as an important risk factor for obesity (8), insulin resistance (9), diabetes (10), and increased mortality (11–13) in the general population. The term exercise is typically applied to moderate (3–6 METs of energy expenditure) or vigorous (>6 METs) physical activities (14). The Physical Activity Guidelines for Americans recommends at least 150 minutes of moderate-intensity activity (3–6 METs) per week or 75 minutes of vigorous-intensity activity (>6 METs) per week (14). In LOOK-AHEAD, a large randomized controlled trial, an intensive lifestyle intervention of increasing moderate activity to 175 min/wk did not reduce the rate of cardiovascular events in overweight or obese patients with type 2 diabetes mellitus (15). This raises the question of whether interventions that focus on sedentary behavior—the other extreme of the PA spectrum—might confer a survival benefit.
There is a general consensus that sedentary behavior must be decreased, but as noted in the United Kingdom guidelines on this topic (16), the critical unresolved questions are how much of the sedentary activities must be replaced and by which kind of activity. It is unlikely that moderate/vigorous physical activities (MVPAs) could be an effective replacement for sedentary activities because most Americans do not even reach the current goals for MVPA (17,18). Furthermore, assuming 16 awake hours per day, achieving the currently recommended duration of MVPA would account for only 2% of the total awake time (2.5 hours out of 112 awake hours per week). Therefore, decreasing sedentary activities must involve an increase in activities that are less intensive than MVPAs. These are low- or light-intensity activities (Table 1). Because standing, a low-intensity activity, is by definition nonsedentary (i.e., not sitting or lying down), one might consider replacing sitting duration with standing duration in order to decrease sedentary behavior. However, to our knowledge, the relative importance of low- and light-intensity activities for reducing sedentary behavior has not been established in the general or CKD populations.
Table 1.
Definition and examples of physical activity intensity levels
| Physical Activity Level | Accelerometer Counts | Examples |
|---|---|---|
| Sedentary | <100 | Sitting quietly, watching television, lying down |
| Low intensity | 100–499 | Sitting in class, studying, note taking, standing, making bed |
| Light intensity | 500–2019 | Casual walking, light gardening, cleaning (sink/toilet) |
| Moderate or vigorous intensity | ≥2200 | Brisk walking or running, lifting heavy weights |
Because sedentary PA, low- and light-intensity PA, and MVPAs are mutually exclusive, decreasing sedentary behavior involves a tradeoff of sedentary duration with the duration of one of the other three activities. Therefore, we examined the mortality associations of 2 min/hr less sedentary duration in conjunction with 2 min/hr more time (tradeoff) spent in one of the low- or light-intensity PAs or MVPAs while controlling for the durations of the other two activities. We used objective PA data obtained with an accelerometer in the 2003–2004 National Health and Nutrition Examination Survey (NHANES). We also used theoretical calculations to estimate the energy expenditure for tradeoff of 1–5 min/hr of sedentary duration with equal durations of low- or light-intensity activities.
Materials and Methods
Study Population
The National Center for Health Statistics is conducting NHANES to obtain a sample of a representative population of the noninstitutionalized United States population. Of the 5041 individuals aged ≥20 years in the 2003–2004 NHANES dataset, 4070 had accelerometry performed and 3786 were deemed to have reliable accelerometry data. The final cohort included 3626 individuals (3243 without CKD and 383 with CKD) who wore an accelerometer for at least 10 hours a day and for at least 4 days for whom follow-up mortality data were available.
Baseline Data
NHANES data collection details have been published elsewhere (17). In brief, physician-diagnosed history of comorbid conditions and mobility limitations were per self-report. Mobility limitation was defined as special equipment needed to walk or inability to walk up 10 steps of stairs. PA was objectively assessed with an ActiGraph Model 7164 accelerometer (ActiGraph, Ft. Walton Beach, FL) (19). Participants were asked to wear the device while they were awake and to take it off for swimming or bathing (20). The uniaxial ActiGraph measures and records vertical acceleration as counts, indicating the intensity of PA associated with locomotion. Data were recorded in 1-minute epochs. PA intensity during awake time that the device was worn was categorized as sedentary, low intensity, light intensity, and moderate/vigorous according to the accelerometer counts (Table 1). Further technical details of PA intensity definitions are provided in the Supplemental Material.
Definition of CKD
The most recent CKD-Epidemiology Collaboration equation was used to estimate GFR (21). CKD was defined as eGFR<60 ml/min per 1.73 m2.
Mortality Data
A Linked Mortality File through December 31, 2006, was created by the National Center for Health Statistics using a probabilistic match between NHANES and death certificate records from the National Death Index (22).
Statistical Analyses
NHANES is based on a complex probability sample design. We used the svy suite of commands in Stata software, version 12 (Stata Corp., College Station, TX) and followed the NHANES analytical guidelines (23). Hence, all reported results (including linear and Cox regression models) take survey weights into account.
Univariate descriptive statistics, including means and SDs, and designated quantiles, including medians and 25th, 75th percentiles, were obtained for such numeric variables as PA intensity durations; proportions and their associated 95% confidence intervals (95% CIs) were obtained to describe distributions of categorical variables. MVPA duration was positively skewed; therefore, it was square root transformed for subsequent statistical analyses. Numeric and categorical factors were compared between the tertiles of light-activity duration using an ANOVA test with 3 degrees of freedom for numeric factors and chi-squared tests for categorical factors.
Multiple linear regression was used to estimate the mean differences in durations of low-intensity and light-intensity PA and MVPA in conjunction with sedentary activity duration of 2 min/hr more; we adjusted for age, sex, race, education, smoking, alcohol use, lung disease, and an indicator variable for the presence of mobility limitations. Similar multiple linear regression analyses were used to estimate mean differences in the remaining activity durations in conjunction with 2 min/hr more in durations of low-intensity or light-intensity PA and MVPA.
Separate Cox regression analyses were performed to relate mortality individually to each PA intensity duration, with covariate adjustment for factors that are unlikely in the causal pathway between sedentary behavior and mortality (age, sex, race, education, smoking, alcohol use, lung disease, and an indicator variable for the presence of mobility limitations). A subsequent multivariable Cox regression model was used to jointly relate to durations of the low and light PAs and MVPAs with the same covariates, with sedentary duration treated as the reference category. Because the durations of the four activity intensity levels (sedentary, low, light, and moderate/vigorous) were normalized to add to 60 min/hr, exponentiation of appropriately defined linear contrasts of the Cox regression coefficients provided estimates of the hazard ratios (HRs) associated with 2 minutes more and 2 minutes less of the durations for each pair of the four activity levels; the analysis controlled for the remaining two activity levels and the remaining covariates.
In additional analyses, the above models were adjusted for variables that might be in the causal pathway of the associations of PA intensity with mortality. These included comorbid conditions (history of congestive heart failure, coronary heart disease, stroke, diabetes, hypertension, and cancer), waist circumference, serum high-sensitivity C-reactive protein, and urine albumin-to-creatinine ratio.
We also examined the mortality associations of tradeoff of 1–5 min/hr of low- and light-intensity PA durations with sedentary duration. We also estimated the energy expenditure associated with tradeoff of 1–5 min/hr of low- and light-intensity PA durations with sedentary duration, as described in the Supplemental Material.
All analyses were repeated in the CKD subgroup.
Results
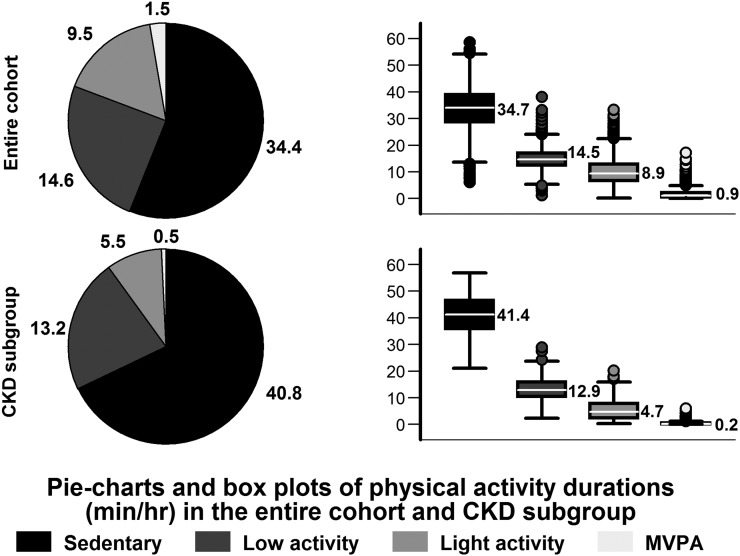
The mean accelerometer wear time was 14.0±1.4 hr/d. Figure 1 describes the distribution of the duration of the PA intensity levels in the entire cohort and CKD subgroup. Those with CKD were more sedentary and spent nearly two thirds of their time in sedentary activities.
Figure 1.
Distribution of physical activity intensity durations per 60 minutes. Normalized means are presented in the pie charts. Normalized medians and 25th and 75th percentiles are presented in box plots, and whiskers represent the 10th and 90th percentiles. MVPA, moderate/vigorous-intensity physical activity.
Mean baseline eGFR was 93.5±17.1 and 48.5± 12.9 ml/min per 1.73 m2 in the entire cohort and CKD subgroup, respectively. Baseline clinical characteristics by tertiles of duration of light-intensity activity in the entire cohort and CKD subgroup are summarized in Table 2. In general, longer duration of light-intensity activity was associated with younger age; male sex; and lower prevalence of diabetes, hypertension, and cardiovascular conditions.
Table 2.
Baseline clinical characteristics by tertiles of duration of light-intensity activity in the entire cohort and CKD subgroup
| Characteristic | Light-Intensity Activity Duration | |||||
|---|---|---|---|---|---|---|
| Entire Cohort (n=3626) | CKD Subgroup (n=383) | |||||
| Lowest Tertile (<7.1 min/hr) (n=1209 [28.%]) | Middle Tertile (7.1–11.1 min/hr) (n=1209 [35.6%]) | Highest Tertile (>11.1 min/hr) (n=1208 [36.4%]) | Lowest Tertile (<3.3 min/hr) (n=128 [28.0]) | Middle Tertile (3.4–7.0 min/hr (n=128 [32.7%]) | Highest Tertile (>7.0 min/hr) (n=127 [39.3%]) | |
| Demographic | ||||||
| Age (yr)a,b | 55±16 | 46±12 | 41±10 | 76±14 | 73±11 | 66±14 |
| Male (%)a | 44 | 42 | 57 | 37 | 40 | 41 |
| Black race (%) | 9 | 11 | 9 | 5 | 7 | 7 |
| High school education or more (%)c | 80 | 88 | 81 | 68 | 69 | 76 |
| Clinical | ||||||
| Diabetes (%)a | 17 | 7 | 5 | 32 | 31 | 19 |
| Hypertension (%)a | 51 | 38 | 27 | 80 | 79 | 75 |
| Coronary heart disease (%)a | 7 | 3 | 1 | 15 | 13 | 10 |
| Congestive heart failure (%)a,d | 6 | 2 | 1 | 20 | 14 | 8 |
| Stroke (%)a | 6 | 2 | 1 | 18 | 11 | 9 |
| Cancer (%)a | 15 | 8 | 5 | 30 | 25 | 20 |
| Chronic bronchitis or emphysema (%)a | 12 | 7 | 5 | 12 | 18 | 11 |
| Smoking (%)a | 24 | 25 | 31 | 8 | 15 | 13 |
| Alcohol use (%)a | 61 | 68 | 72 | 46 | 44 | 51 |
| Waist circumference (cm)d,e | 99.6±13.6 | 96.8±11.9 | 96.5±10.8 | 103.5±17.4 | 100.6±17.9 | 98.4±14.8 |
| High-sensitivity C-reactive protein (mg/dl)e,a | 2.4 (1.1, 5.3) | 2.0 (0.8, 4.7) | 1.6 (0.7, 3.7) | 3.0 (1.5, 6.2) | 2.3 (1.2, 4.4) | 2.0 (1.0, 5.0) |
| Urine albumin-to-creatinine ratio (μg/mg)c,d,e | 6.8 (4.3, 14.6) | 5.8 (4.0, 9.7) | 5.4 (3.8, 9.1) | 11.5 (5.6, 53.9) | 11.2(5.9, 28.5) | 7.2 (5.4, 22.9) |
| eGFR (ml/min per 1.73 m2)a,d | 84.3±20.7 | 94.0±15.6 | 99.9±13.7 | 45.3±15.2 | 48.7±13.3 | 50.7±10.0 |
| Special equipment needed to walk or unable to walk up 10 steps of stairs (%)a,b | 27 | 10 | 7 | 64 | 33 | 21 |
| Physical activity duration (min/hr) | ||||||
| Sedentary durationa,b | 42±4 | 35±3 | 27±4 | 47±5 | 41±4 | 34±5 |
| Low-intensity activity durationa,b | 13±3 | 15±2 | 16±3 | 11±4 | 14±4 | 15±4 |
| Light-intensity activity durationa,b | 5±1 | 9±1 | 15±3 | 2±1 | 5±1 | 10±3 |
| Moderate- to vigorous-intensity activity durationa,b | 0.4 (0.1,1.1) | 1.1 (0.6,1.8) | 2.0 (1.1,3.2) | 0.1 (0.0,0.2) | 0.2 (0.1,0.4) | 0.5 (0.3,0.8) |
Survey weight-adjusted means ± SDs, medians (interquartile range), or proportions are presented.
P<0.001 for the entire cohort.
P<0.001 for CKD subgroup.
P<0.05 for the entire cohort.
P<0.05 for CKD subgroup.
Waist circumference missing in 108 patients in the entire cohort and 16 in the CKD subgroup, urine albumin-to-creatinine ratio missing in 32 and 10, and C-reactive protein missing in 2 and 0, respectively.
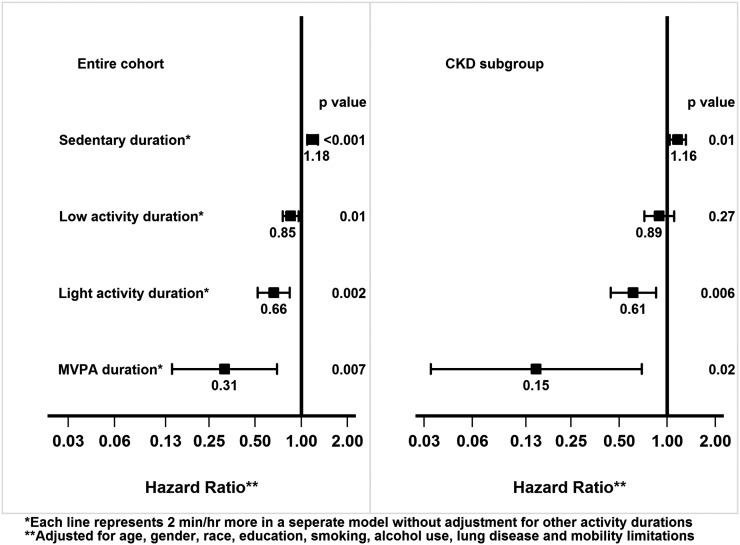
The mean follow-up duration was 2.86±0.64 years. There were 137 deaths over 10,390 person-years of follow-up (mortality rate of 1.32 deaths/100 person-years) in the entire cohort and 50 deaths over 1048 person-years of follow-up (mortality rate of 4.77 deaths/100 person-years) in CKD subgroup. As shown in Figure 2, longer sedentary duration was associated with higher mortality risk in the entire cohort (HR, 1.18; 95% CI, 1.09 to 1.28) and the CKD subgroup (HR, 1.16; 95% CI, 1.04 to 1.31) subgroup. On the other hand, there appeared to be a graded lower mortality risk with greater intensity of physical activity in the entire cohort and CKD subgroup (Figure 2).
Figure 2.
Mortality associations with individual activity durations, without adjustment for other activities. Hazard ratios and 95% confidence intervals are presented. MVPA, moderate/vigorous-intensity physical activity.
Table 3 summarizes the differences in the other three activity levels associated with 2 min/hr more in durations of sedentary activity, low- or light-intensity activity, or MVPA. For instance, each 2 min/hr more in sedentary duration was associated with shorter durations for other activities (1.02 min/hr for low-intensity activity, 0.92 min/hr for light-intensity activity, and 0.06 min/hr for MVPA) in the CKD subgroup. Thus, the association of higher mortality risk with longer sedentary duration described in Figure 2 could reflect less of any of the low-intensity or light-intensity activity or MVPA durations.
Table 3.
Relationships among durations of each intensity of physical activity
| Variable | ∆ Sedentary Duration | ∆ Low-Intensity Activity Duration | ∆ Light-Intensity Activity Dureation | ∆ Moderate- or Vigorous-Intensity Activity Duration |
|---|---|---|---|---|
| Entire cohort | ||||
| ↑ 2 min/hr of sedentary duration | — | −0.72 (−0.77 to −0.68) | −1.11 (−1.15 to −1.07) | −0.17 (−0.19 to −0.15) |
| ↑ 2 min/hr of low-intensity activity duration | −2.77 (−2.89 to −2.64) | — | 0.86 (0.76 to 0.96) | −0.09 (−0.13 to −0.06) |
| ↑ 2 min/hr of light-intensity activity duration | −2.83 (−2.94 to −2.72) | 0.57 (0.48 to 0.67) | — | 0.25 (0.22 to 0.29) |
| ↑ 2 min/hr of moderate or vigorous-intensity activity duration | −6.88 (−7.41 to −6.35) | −0.64 (−0.96 to −0.33) | 4.26 (3.80 to 4.72) | — |
| CKD subgroup | ||||
| ↑ 2 min/hr of sedentary duration | — | −1.02 (−1.15 to −0.90) | −0.92 (−1.02 to −0.82) | −0.06 (−0.09 to −0.03) |
| ↑ 2 min/hr of low-intensity activity duration | −2.77 (−2.92 to −2.61) | — | 0.78 (0.63 to 0.93) | −0.02 (−0.04 to 0.01) |
| ↑ 2 min/hr of light-intensity activity duration | −3.07 (−3.36 to −2.79) | 0.96 (0.69 to 1.24) | — | 0.11 (0.04 to 0.18) |
| ↑ 2 min/hr of moderate or vigorous-intensity activity duration | −6.02 (−8.43 to −3.62) | 0.13 (−0.72 to 0.98) | 3.67 (1.80 to 5.55) | — |
Values are expressed as coefficients (95% confidence intervals). The entries in each row of the table provide survey weight–adjusted estimated mean differences in the durations of each of the remaining three activity levels associated with 2 min/hr more of the activity indicated in the left column. The 2 min/hr more in moderate- or vigorous-intensity physical activity (MPVA) in the bottom row is based on a change from 0.07 min/hr to 2.07 min/hr, which corresponds roughly to an increase from the 25% and 95% percentiles of moderate- or vigorous-intensity in CKD subgroup and 5% and 75% percentiles of moderate- or vigorous-intensity in the entire cohort. Results are based on multiple linear regressions adjusted for age, sex, race, education, smoke, alcohol use, lung disease, and mobility limitations.
Table 4 summarizes mortality risk associated with each 2 min/hr shorter duration of a lower-intensity activity with a corresponding 2 min/hr greater duration of a higher-intensity activity while controlling for the other two activity durations. A tradeoff of sedentary duration with low-intensity PA duration was not associated with survival in the entire cohort or CKD subgroup. On the other hand, 2 min/hr shorter sedentary duration with 2 min/hr greater duration of light-intensity activity duration was significantly associated with lower mortality risk in the entire cohort (HR, 0.67; 95% CI, 0.48 to 0.93) and CKD subgroup (HR, 0.59; 95% CI, 0.35 to 0.98).
Table 4.
Hazard ratios of death per 2 min/hr tradeoff of lower-level activity duration with higher-level activity duration
| Variable | ↑ 2 min/hr of Low-Intensity Activity Duration: HR (95% CI, P Value) | ↑ 2 min/hr of Light-Intensity Activity Duration: HR (95% CI, P Value) | ↑ 2 min/hr of Moderate- or Vigorous-Intensity Activity Duration: HR (95% CI, P Value) |
|---|---|---|---|
| Entire cohorta | |||
| ↓2 min/hr of sedentary duration | 1.01 (0.86 to 1.19; P=0.87) | 0.67 (0.48 to 0.93; P=0.02) | 0.80 (0.42 to 1.51; P=0.46) |
| ↓2 min/hr of low-intensity activity duration | 0.66 (0.42 to 1.05; P=0.08) | 0.79 (0.44 to 1.40; P=0.39) | |
| ↓2 min/hr of light-intensity activity duration | 1.19 (0.52 to 2.73; P=0.66) | ||
| CKD subgroupa | |||
| ↓2 min/hr of sedentary duration | 1.09 (0.82 to 1.44; P=0.54) | 0.59 (0.35 to 0.98; P=0.04) | 0.46 (0.09 to 2.45; P=0.34) |
| ↓2 min/hr of low-intensity activity duration | 0.54 (0.26 to 1.13; P=0.10) | 0.42 (0.08 to 2.15; P=0.28) | |
| ↓2 min/hr of light-intensity activity duration | 0.79 (0.12 to 5.50; P=0.80) |
Hazard ratios from Cox regression models that took survey design into account. For tradeoff: mortality risk associated with each 2 min/hr shorter lower-intensity activity duration with a corresponding 2 min/hr greater higher-intensity activity duration while controlling for the other two activity durations. Adjusted for age, sex, race, education, smoke, alcohol use, lung disease, mobility limitations. HR, hazard ratio; 95% CI, 95% confidence interval.
No missing variables for these main models.
With additional adjustment for factors (listed in the Materials and Methods section) that may be in the causal pathway between physical activity intensity and mortality, tradeoff of each 2 min/hr of sedentary duration with light-intensity activity duration was associated with lower hazard of death in the entire cohort (HR, 0.71; 95% CI, 0.52 to 0.97) and the CKD subgroup (HR, 0.57; 95% CI, 0.35 to 0.95) (Supplemental Table 1).
HRs consistent with reduced risk were observed in the CKD subgroup for 2 min/hr less in sedentary duration with 2 min/hr more in MVPA duration (HR, 0.46; 95% CI, 0.09 to 2.45) and for 2 min/hr less in low-intensity activity duration in conjunction with 2 min/hr more in either light-intensity activity duration (HR, 0.54; 95% CI, 0.26 to 1.11) or MVPA duration (HR, 0.42; 95% CI, 0.08 to 2.15). However, none of the latter three comparisons reached statistical significance (Table 4).
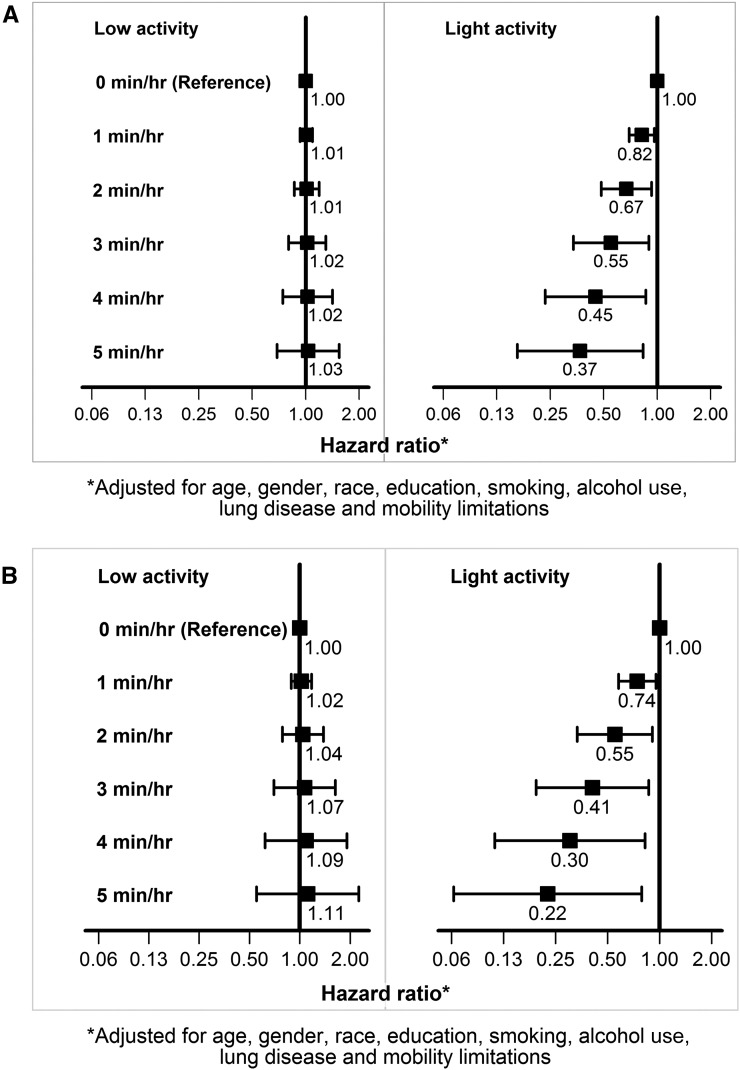
Tradeoff of 1–5 min/hr of sedentary duration with low-intensity activity duration is expected to result in additional weekly expenditure of 30–150 kcal only (Supplemental Figure 1) without any mortality associations (Figure 3). On the other hand, similar tradeoff of sedentary duration with light-intensity activity duration is expected to result in approximately 200–1000 kcal/wk of additional energy expenditure (Supplemental Figure 1) and significantly lower risk of death (Figure 3).
Figure 3.
Mortality associations of tradeoff of 1–5 min/hr of sedentary duration with durations of low- or light-intensity activities. (A) Entire cohort. (B) CKD subgroup.
Discussion
In this study of noninstitutionalized, community-dwelling adults, the participants spent more than half their time in sedentary activities (Figure 1). Those with CKD were even more sedentary and spent more than two thirds of their time in sedentary activities. Furthermore, increase in sedentary duration was associated with increased hazard of death in the entire cohort and CKD subgroup (Figure 2), which is consistent with previous studies of sedentary behavior and mortality (11–13). However, because there are only a fixed number of minutes in an hour, an increase in the duration of any given activity must result in a decrease in at least one of the other three activities. Furthermore, people who are active are likely to have higher levels of not only MVPA but also light-intensity activities. These have important implications for interpretation of the data as discussed below.
We estimated the mortality HRs associated with 2 min/hr tradeoff of sedentary duration with each of the low- and light-intensity activities and MVPAs while controlling for the durations of the remaining two activities.
These data suggest that tradeoff of sedentary duration with low-intensity activity was not associated with a substantial difference in mortality risk in the entire cohort or the CKD subgroup (Table 4). On the other hand, 2 min/hr tradeoff of sedentary duration with an equal duration of light-intensity activity was associated with a lower mortality risk in the entire cohort and the CKD subgroup (Table 4).
According to the concept of nonexercise activity thermogenesis (24,25), the energy expenditure from nonexercise activities provides a biologic framework for the rationale for increasing low- or light-intensity activities. Trading off 1–5 min/hr of sedentary duration for a low-intensity activity (such as standing) will result in an additional energy expenditure of only approximately 30–150 kcal/wk. On the other hand, a 1–5 min/hr tradeoff of sedentary duration with 2.5 METs of light-intensity activities (such as casual walking) is expected to increase energy expenditure by about 200–1000 kcal/wk (Supplemental Figure 1). In comparison, achieving the weekly recommended MVPA duration of 2.5 hr/wk with brisk walking (4 METs) will result in approximately 600 kcal of additional energy expenditure per week. Therefore, increasing light activities has the potential to double the weekly energy expenditure resulting from achieving the MVPA goal alone.
A few pilot studies have examined interventions to decrease sedentary duration (26–28). However, because low-intensity activity does not appear to confer a survival benefit (Table 4) or increase energy expenditure (Supplemental Figure 1), merely replacing sedentary duration by low-intensity activities duration is unlikely to be beneficial. Instead, our analyses suggest that interventions targeting sedentary duration should seek to increase light-intensity activity duration. Randomized, controlled trials are needed to determine whether such interventions will reduce morbidity and mortality.
Each 2 min/hr increment in MVPA duration was associated with the lowest mortality risk (Figure 2). Indeed, the net increase in the physical activity dose was the highest for each 2 min/hr increase in MVPA duration; a net decrease of approximately 7.5 min/hr in duration of sedentary and low-intensity activity with a concomitant increase in duration of light-intensity activities of 4.3 min/hr (Table 3). In comparison, the net increase in the physical activities dose was lower for each 2 min/hr increase in duration of low- or light-intensity activity (Table 3). Thus, these results suggest that the tradeoff of sedentary duration with longer duration of higher-intensity physical activities provides a dose-dependent survival benefit.
Nonetheless, tradeoff of sedentary activity duration with MVPA duration while holding duration of low- and light-intensity activities constant was not associated with statistically significant lower mortality risk (Table 4). This might be because duration of MVPA was too short in this study population.
It may be more realistic, however, to seek to replace sedentary duration with light-intensity activity duration rather than MVPA duration. We believe an optimal strategy should include decreasing sedentary duration by increasing the duration of light- (but not low-) intensity activities in combination with achieving weekly MVPA duration goals. Interventional trials are needed to test this strategy.
The strengths of this study include objective measurement of PA. The technological advances that made objectively measuring PA intensity and duration possible enabled a more comprehensive examination of the associations of duration of PA intensity with mortality in the current study.
The major limitations of this study include those of all observational studies that use existing data. The observational nature of the study prohibits inference beyond associations as unmeasured residual confounding must be considered while interpreting the results and hence, this study should be considered exploratory. Furthermore, activities such as swimming and water aerobics were not recorded because the accelerometers were not waterproof. In addition, the device used in NHANES recorded uniaxial movement; hence, information on use of stationary bikes, elliptical trainers, or equipment that primarily involved upper body movement, such as rowing, may not have been recorded accurately. These might have resulted in underestimation of MVPA (29). However, these issues are unlikely to have led to underestimation of light-intensity activity. In addition, the CKD subgroup in this study is mainly composed of stage III CKD. This study could not examine the associations of PA intensity levels with progression of CKD or incidence of ESRD. Finally, despite the association of light-intensity activity with lower mortality in multivariable models, caution is warranted in interpreting these results because of the relatively short duration of follow-up and low number of mortality events. Hence, longer-term studies are warranted to conclusively establish the relationship of light-intensity activity with mortality in non-CKD and CKD populations.
In summary, the importance of light activity has been underrecognized. Replacement of sedentary activity with light-intensity activity might confer a survival benefit. Survival benefits of interventions that increase light activities in combination with MVPAs need to be tested in both non-CKD and CKD populations.
Disclosures
None.
Supplementary Material
Acknowledgments
This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK091437 and RO1-DK078112) and the University of Utah Study Design and Biostatistics Center (funded in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08410814/-/DCSupplemental.
References
- 1.Padilla J, Krasnoff J, Da Silva M, Hsu CY, Frassetto L, Johansen KL, Painter P: Physical functioning in patients with chronic kidney disease. J Nephrol 21: 550–559, 2008 [PubMed] [Google Scholar]
- 2.Anand S, Chertow GM, Johansen KL, Grimes B, Kurella Tamura M, Dalrymple LS, Kaysen GA: Association of self-reported physical activity with laboratory markers of nutrition and inflammation: The Comprehensive Dialysis Study. J Ren Nutr 21: 429–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S: Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia 38: 565–572, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bharakhada N, Yates T, Davies MJ, Wilmot EG, Edwardson C, Henson J, Webb D, Khunti K: Association of sitting time and physical activity with CKD: A cross-sectional study in family practices. Am J Kidney Dis 60: 583–590, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL: Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14: 479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Akber A, Portale AA, Johansen KL: Pedometer-assessed physical activity in children and young adults with CKD. Clin J Am Soc Nephrol 7: 720–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins MS, Sevick MA, Richardson CR, Fried LF, Arena VC, Kriska AM: Association between physical activity and kidney function: National Health and Nutrition Examination Survey. Med Sci Sports Exerc 43: 1457–1464, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE: Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 289: 1785–1791, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Helmerhorst HJ, Wijndaele K, Brage S, Wareham NJ, Ekelund U: Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes 58: 1776–1779, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB: Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 161: 1542–1548, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Dunstan DW, Barr EL, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N: Television viewing time and mortality: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 121: 384–391, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Koster A, Caserotti P, Patel KV, Matthews CE, Berrigan D, Van Domelen DR, Brychta RJ, Chen KY, Harris TB: Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS ONE 7: e37696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzmarzyk PT, Church TS, Craig CL, Bouchard C: Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998–1005, 2009 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services: Get Active: Newly Released Physical Actiivity Guidelines for Americans. 2012. Available at: http://www.healthfinder.gov/HealthTopics/Category/nutrition-and-physical-activity/physical-activity/get-active. Accessed February 1, 2015
- 15.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ, Look AHEAD Research Group : Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369: 145–154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Department of Health; The Sedentary Behaviour and Obesity Expert Working Group: Sedentary behaviour and obesity: review of the current scientific evidence. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213745/dh_128225.pdf. Accessed February 1, 2015
- 17.Centers for Disease Control and Prevenion, National Center for Health Statistics: NHANES 2003-2004. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes03_04.aspx. Accessed February 1, 2015
- 18.Tucker JM, Welk GJ, Beyler NK: Physical activity in U.S.: Adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med 40: 454–461, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M: Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008 [DOI] [PubMed] [Google Scholar]
- 20.National Health and Nutrition Examination Survey. 2003-2004 Data Documentation, Codebook, and Frequencies: Physical Activity Monitor. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/PAXRAW_C.htm. Accessed February 1, 2015
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. NHANES (1999-2004) linked mortality files. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage.htm. Accessed February 1, 2015
- 23.National Center for Health Statistics: The National Health and Nutrition Examination Survey (NHANES) Analytic and Reporting Guidelines. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Accessed November 25, 2011
- 24.Levine JA, Eberhardt NL, Jensen MD: Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283: 212–214, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM: Interindividual variation in posture allocation: Possible role in human obesity. Science 307: 584–586, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS: Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc 43: 1561–1567, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Otten JJ, Jones KE, Littenberg B, Harvey-Berino J: Effects of television viewing reduction on energy intake and expenditure in overweight and obese adults: A randomized controlled trial. Arch Intern Med 169: 2109–2115, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Gardiner PA, Eakin EG, Healy GN, Owen N: Feasibility of reducing older adults’ sedentary time. Am J Prev Med 41: 174–177, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Painter P, Marcus RL: Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol 8: 861–872, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.