Abstract
This review serves as an introduction to an Immunology Series for the Nephrologist published in CJASN. It provides a brief overview of the immune system, how it works, and why it matters to kidneys. This review describes in broad terms the main divisions of the immune system (innate and adaptive), their cellular and tissue components, and the ways by which they function and are regulated. The story is told through the prism of evolution in order to relay to the reader why the immune system does what it does and why imperfections in the system can lead to renal disease. Detailed descriptions of cell types, molecules, and other immunologic curiosities are avoided as much as possible in an effort to not detract from the importance of the broader concepts that define the immune system and its relationship to the kidney.
Keywords: GN, tolerance, renal transplantation, immunology, ARF
The Beginning of the Journey
Imagine that you are a primitive animal, perhaps a distant predecessor of all mammals. You have lived a long life, thus far relying solely on a basic defense system. If you are invaded by a microbe or parasite, you quickly expel or kill it by releasing chemicals, producing a barrage of defensive protein molecules or unleashing phagocytic cells (1). If all fails, you wall the invader off or regenerate that part of your body reduced to rot by infection. Even if infection proves fatal, your extreme fecundity, which started at a very early age, has already ensured the continuity of your species. This seemingly imaginary scenario is in fact how the more ancient of our ancestors attain near immortality (2).
Now imagine that evolution has something grander in store for you. You are destined to become the progenitor of more sophisticated beings. Your descendants will grow complex organs—robust kidneys that empower them to roam the earth and mighty brains that enable them to rule it. To do so, they will carry their embryos and nurture their young for an extended period of time. Reproduction becomes a later and infrequent event in life, and life itself becomes a much shorter journey. The capacity to regenerate tissues, limbs, and organs dwindles as tissue architecture and function grow increasingly differentiated and complex. Although less abundant, life for your descendants becomes more valuable as failure to survive until a reproductive age spells doom for the species. Faced with these burdens, you quickly realize that you have to devise a more intelligent defense system: one that protects against virtually all pathogens that your successors may encounter during their forays into known and unknown realms, one that provides long-lasting security against infection, and one that is carefully regulated so that it does not attack its own tissues or endanger beneficial cohabitants. You will call this defense system immunity (Figure 1). Defense, after all, is a primitive term that is equally associated with defeat and victory, whereas immunity exudes strength and confidence. So how would you (with the guiding hand of evolution, of course) go about devising such a system, what would it look like, and why will it eventually matter to our kidneys?
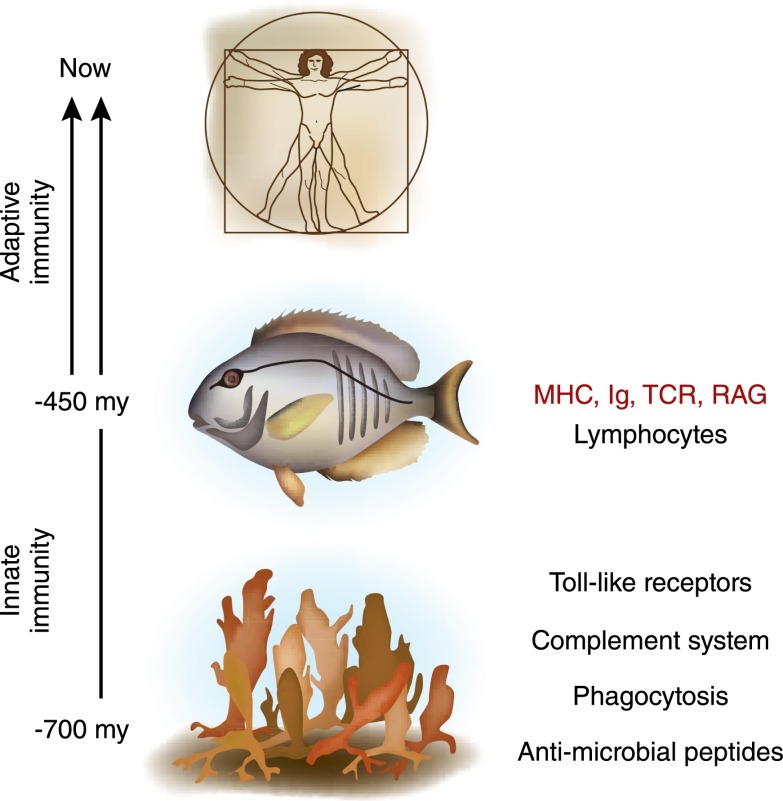
Figure 1.
Evolution of the immune system. Adaptive immunity as we know it in humans did not evolve until the emergence of the first jawed vertebrates (fish) around 450 million years (my) ago. Evolution of adaptive immunity was heralded by the appearance of lymphocytes, the major histocompatibility complex (MHC), immunoglobulin (Ig) molecules, T-cell receptor for antigen (TCR), and recombinase activating genes (RAG) responsible for the diversity in these recognition molecules. Our more ancient ancestors, such as the sponges (−700 my), relied on basic defense systems without the benefit of lymphocytes, antigen receptors with fine molecular specificity, or any noteworthy immunologic memory. An approximate timeline for evolution of innate immune components (antimicrobial peptides, phagocytosis, complement, and Toll-like receptors) is also shown.
Innate and Adaptive Immunity
Devising a sophisticated biologic system, as evolution teaches us, does not require the destruction of preexisting, primitive tools, but instead depends on preserving and building on the best of them (3). Heeding this advice, you take your time, spending hundreds of millions of years, choosing the best and discarding the least useful of your primitive defense mechanisms. You call what is left innate immunity: innate because the defense mechanisms you have chosen are encoded in your germline, having been selected over evolutionary time and passed down from generation to generation with only minor refinements (4). In other words, they have stood the test of time. They include household names such as the complement system, Toll-like receptors (TLRs), and phagocytic cells. Modern-day genome sequencing has established that much of these defense systems are conserved across animal phyla, a true reflection of not only their remarkable effectiveness but also their versatility (3). A complement molecule, a TLR, or a phagocyte is not only essential for detecting and eliminating harmful nonself but is also key to maintaining normal tissue homeostasis, be it sensing and repairing damaged tissues or quietly eliminating senescent or apoptotic cells. Obviously, you have chosen prudently.
However, that is clearly not sufficient. An innate immune system provides immediate albeit incomplete protection against intruders and, at best, has only short-term memory (4,5). Instead of mounting a faster and more effective response upon encountering a known trespasser, it starts sluggishly from scratch each time. Innate immunity has additional shortcomings. Receptors utilized by innate cells, such as the TLR, are adept at discerning self from nonself but lack the molecular specificity required for distinguishing between nonselves, causing them to trigger defenses against both friend and foe. To make matters worse, the imprecise defenses discharged by innate cells can wreak havoc on the surrounding tissue and often the entire organism itself. Imagine, by way of example, a renal abscess full of neutrophils (a typical innate cell) growing unchecked in a hapless patient.
Realizing these dangers, you set out to build a more sophisticated defense system (Figure 1). In a relatively short period (short on the evolutionary time scale, of course), you acquire the tools to create new types of immune cells, known as B and T lymphocytes (6). Lymphocytes possess surface receptors, Igs (or antibodies) on B lymphocytes, and the T-cell receptors (TCRs) for antigen on T lymphocytes that, unlike receptors on innate immune cells, recognize nonself molecules (referred to as antigens in this case) with exquisite specificity. The genes that encode these receptors are not embedded in the germline but are the product of gene recombination during lymphocyte development, a nifty molecular trick that generates a very large number of unique antigen receptors by splicing, rearranging, and linking a finite set of adjacent genes (7). Antigen receptors on either B or T lymphocytes pinpoint the slightest distinction between self and virtually any nonself or between one nonself and another and set off an immune response that only targets the antigen that happens to carry that distinction. Because only one type of antigen receptor, or perhaps a few types at most, is expressed on any given lymphocyte, this exquisite specificity ensures that only pertinent lymphocytes are activated, thus minimizing bystander damage.
However, there is more to the plan. Upon encountering antigens, lymphocytes proliferate extensively to maximize their fighting power and differentiate into specialized subsets to further hone it. B lymphocytes transform into antibody factories known as plasma cells, whereas T lymphocytes differentiate into helper and effector (e.g., cytotoxic) subsets, each with its distinct set of secreted molecules (cytokines). Helper T lymphocyte subsets orchestrate the mounting immune response by dictating what defense strategy is used against a particular intruder, whereas cytotoxic T lymphocytes directly effect the death of cells harboring the intruder. Importantly, immune responses do not march on indefinitely or haphazardly but are tightly regulated by specialized B and T lymphocytes known as regulatory cells (8,9). Moreover, the exponential proliferation and differentiation of lymphocytes responding to an antigen is ultimately restrained by the death of the majority of antigen-specific lymphocytes involved in the response (Figure 2). The precious few that survive become long-lived memory cells. Memory lymphocytes ensure that a second encounter with the same invader is dealt with swiftly and effectively because of the many advantages they have over their inexperienced (naïve) predecessors (10). These include their greater number (for any given antigen), extended lifespan, more rapid response rate, superior proliferation capacity, and wider access to tissues. With the job completed, you marvel at the adaptive features of the lymphocytes you have created (clonal expansion, differentiation, regulation, and memory) and you name this new system adaptive immunity.
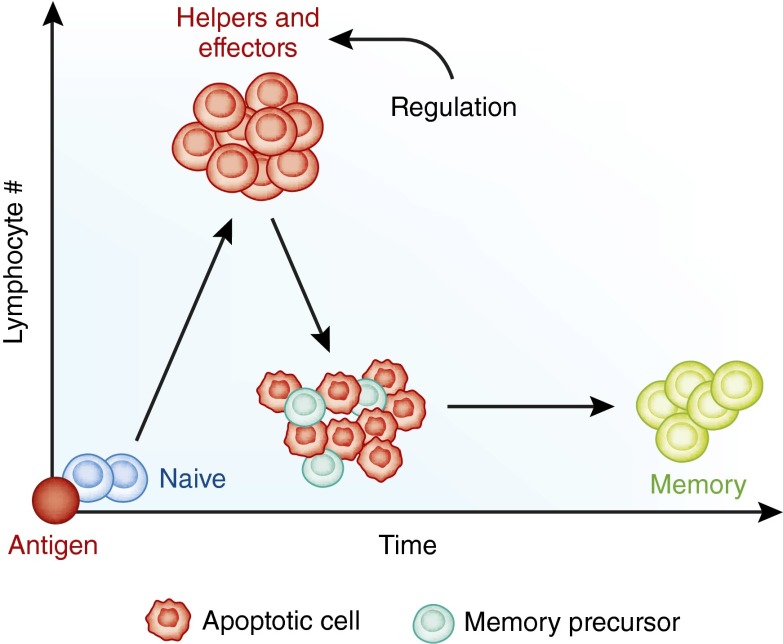
Figure 2.
A two-dimensional view of the adaptive (lymphocyte) immune response. Foreign antigen triggers the exponential proliferation of lymphocytes, which then differentiate into helper and effector cells. Regulatory mechanisms kick in at the peak of the response, the most conspicuous of which is the death of the majority of the lymphocytes by apoptosis. The few that survive become memory precursors and later memory cells. Lymphocyte death is necessary to prevent unwanted immunopathology.
Linking Innate to Adaptive Immunity
What good, however, are two immune systems in one body if they do not communicate with each other? Because the newly devised lymphocytes of the adaptive immune system and the receptors they express are destined to recognize fine molecular specificities on antigens, you co-opt the phagocytic cells of the innate immune system to capture antigens, cut them into small molecular fragments (peptides), and present them to the lymphocytes waiting in anticipation. Immunologists refer to the subset of innate immune cells proficient at processing antigens in this manner as antigen-presenting cells (APCs) and the most skilled among them as dendritic cells (DCs), because of the conspicuous dendrites they extend into every nook and cranny of our tissues (11). To activate the adaptive immune system, DCs package antigenic peptides into major histocompatibility complex (MHC) proteins (human leukocyte antigens in humans), which ensures that virtually any nonself peptide is presented to the T lymphocyte with the optimal TCR specificity and affinity (12). At the same time, DCs provide additional signals, known as costimulatory signals, which guarantee full proliferation and differentiation of the T lymphocyte (13). Parsimoniously, you choose the same molecules utilized by the innate immune system to sense nonself and trigger inflammation to be the ones that stimulate the antigen-presenting and costimulatory capabilities of DCs—that is, induce the maturation of DCs into potent APCs (14). A nonself microbial motif such as lipopolysaccharide (LPS), which unleashes innate immune defenses by binding to its receptor TLR4, also primes DCs through the same receptor to present the myriad foreign antigens that the microbe carries and to activate the appropriate T lymphocytes (15). The innate immune system you have thus far molded has therefore been transformed from a primitive, first-line defense system into an ingenious doorbell that awakens the adaptive immune response (Figure 3). Adaptive immune cells, in turn, cooperate with innate immune cells—driving, fine-tuning, and sometimes regulating them—to maximize the chance that intruders are eliminated at minimal cost to the host. You congratulate yourself on successfully linking the innate and adaptive immune systems and ponder what to do next.
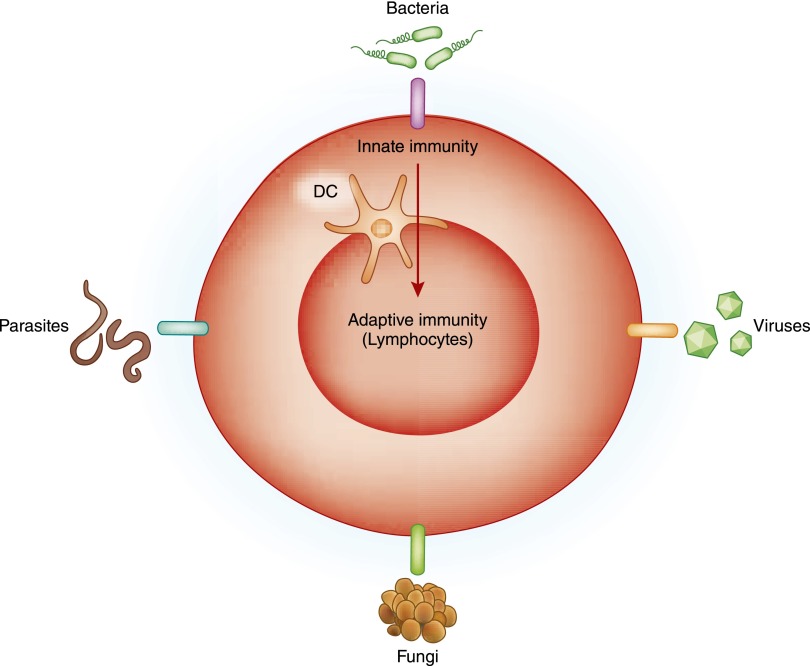
Figure 3.
The role of the innate immune system in activating adaptive immunity. The innate immune system can be envisioned as a doorbell that awakens the adaptive immune system (lymphocytes) upon sensing microbes (bacteria, viruses, fungi, and parasites). The dendritic cell (DC) acts as the link between the innate and adaptive systems by phagocytosing, processing, and presenting microbial antigens to lymphocytes and providing them with the necessary costimulatory signals. Endogenous molecules released by stressed or dying cells participate in acute kidney injury, transplant rejection, or autoimmunity by triggering the same innate immune receptors that sense microbes leading to stimulation of the adaptive immune response.
Lymphoid Organs: A Brief Lesson in Geography
Optimal and timely activation of the adaptive immune response, however, cannot possibly rely on chance encounters between T lymphocytes and the mature DCs that carry the antigenic peptides they recognize. Nor can random wanderings guarantee that B lymphocytes will find their target antigens or the help they need from T lymphocytes to differentiate into antibody-producing plasma cells. After all, your descendants are destined to have large bodies with extensive mucosal surfaces and complex three-dimensional organs, making the surveillance of every tissue fold by lymphocytes an impossible task. So how could one guarantee that rare immune cells find antigen and each other quickly and efficiently? The solution that evolution makes available to you turns out to be a simple one. Your descendants will harbor anatomic structures, known as secondary lymphoid organs or tissues, and will synthesize molecular messages, known as chemokines and adhesion molecules, that bring immune cells together at the right place and time (16). Prime examples of secondary lymphoid organs are the lymph nodes, spleen, and Peyer’s patches in the small intestine. All are organized structures divided into T- and B-cell zones through which naïve T and B lymphocytes circulate constantly or reside for extended periods of time. DCs and other APCs, on the other hand, remain free to live in either secondary lymphoid tissues or virtually any nonlymphoid organ of the body. The kidney, in fact, has an extensive network of such cells. Upon sensing a nonself intruder and capturing its antigens (e.g., an Escherichia coli infecting the urinary tract), DCs migrate along lymphatic channels to the nearest lymph node and, by following chemokine and adhesion molecule cues, strategically position themselves within the lymph node to activate antigen-specific T lymphocytes and subsequently, antigen-specific B lymphocytes. This organized rendezvous between innate and adaptive immune cells generates ample effector and memory lymphocytes that then exit the lymph node and migrate through the bloodstream to the site of antigen entry (e.g., the infected kidney). Effector and memory cell migration to the target tissue is once again guided by chemokines and adhesion molecules and, importantly, by antigen-presenting DCs within the tissue. Some memory T lymphocytes remain in the nonlymphoid tissues as resident memory cells that guard against reinfection with the same pathogen. With the circle completed, you are confident that the noose will tighten around the intruder’s neck.
In addition to secondary lymphoid tissues, evolution has set aside primary lymphoid organs dedicated to the production and education of nascent immune cells. These are the bone marrow and the thymus. The bone marrow is where both innate and adaptive immune cells are born and is the site where B lymphocytes are educated. Newborn T lymphocytes, on the other hand, receive their education in the thymus. So what is lymphocyte education all about and why is it essential? The primary goal of education is to weed out those lymphocytes that recognize self antigens (and therefore could cause harm to the organism itself) by either killing them off or inducing in them a permanent state of unresponsiveness called anergy. This education process, referred to as negative selection (17), is necessary because the specificity of antigen receptors on B and T lymphocytes arose in the first place through random, somatic gene arrangement and not through a predetermined, germline embedded route selected over evolutionary time (such as the case is with innate receptors). Therefore, unless they are carefully selected, emerging B and T lymphocyte populations would harbor an unacceptable level of self-reactivity, unleashing the “horror autotoxicus” described by Paul Ehrlich more than 100 years ago (18). Before negative selection, T lymphocytes also undergo a positive selection step in the thymus, which ensures that only those that recognize self-MHC molecules survive (19). This step is essential because TCR do not engage free-swimming peptides but ones bound to MHC molecules, implying that only those newborn T lymphocytes that express TCR with intrinsic affinity to MHC are useful. In any given individual, T lymphocytes that recognize self-MHC with a reasonable affinity are positively selected and those that engage self-MHC with either too low or too high of an affinity die—the former by neglect and the latter in the negative selection step that ensues. What emerges at the end is a mature lymphocyte repertoire that detects millions of nonself antigens but has a limited ability to mount an immune response against self antigens. Your heirs will be beneficiaries of this well orchestrated educational system but, as we shall see later, will also pay the price for its inherent imperfections.
Blurring the Lines
You have thus far conducted your work carefully, dividing the immune system into innate and adaptive and separating antigens along simple, clean lines into harmless self antigens on one side and harmful nonself microbes on the other. But is all self harmless, and is all nonself harmful? Are all harmful nonselves microbes? And do all immune cells fit neatly into separate innate and adaptive bins?
The immune system that you have assembled is in fact a model of versatility rather than rigid divisions. Not only do innate cells detect traditional harmful intruders (bacteria, viruses, fungi, and other pathogens), but they also respond to a subset of self-molecules (some protein and nucleic acid, others simple chemicals such as uric acid) that alarm the immune system to the presence of tissue damage (20,21). Damage-associated molecules or alarmins are released by dying or stressed cells in infected, ischemic, or injured tissues and serve two purposes: they amplify the immune response to nonself, if nonself is present as is the case with infection, and they enlist the immune system in the tissue repair process. We now know that both lymphoid (e.g., regulatory T cells) and innate myeloid cells (e.g., macrophages) actively participate in and are essential for tissue repair in the kidney and elsewhere (22). In other words, components of both the innate and adaptive systems are ready to react to self when it signals harm or becomes harmful itself.
Your immune system also quickly grasps the reality that not all microbial nonself is harmful. The billions of commensal bacteria and other microbes that will accompany your descendants throughout their life journeys will in fact be essential for their well-being. The immune system therefore promptly takes advantage of the regulatory mechanisms it has to ensure that DCs and lymphocytes at barrier surfaces such as the gut, skin, and lungs are carefully controlled to avoid needless attacks on helpful commensals (23). It also recognizes that nonself that is neither microbial nor pathogenic can also be harmful and must be rejected at times. Take for example a stem cell or fetus in the wrong place or potentially transmissible tumor cells (24,25). This form of nonself, known as allogeneic nonself, triggers powerful adaptive immune responses that are most apparent in the setting of transplant rejection. How lymphocytes recognize allogeneic nonself will be discussed later. How commensals interact with and shape the innate and adaptive immune systems and how the innate immune system distinguishes between self and allogeneic nonself are not entirely clear and will surely intrigue the inquisitive minds of your descendants (26).
Finally, you come to realize that building an immune system based on inflexible distinctions between innate and adaptive immune cells is not possible (27). Evolution is not a predetermined design process; rather, it is one that advances in fits of trial and error as well as chance and necessity. Your successors will carry in them not only the final product of these efforts, a one-and-only perfect immune system, but also the marks and remnants of many immune systems. This is best exemplified, we believe, in the recent discovery of several families of innate lymphoid cells that defy traditional classification (28). On one hand, they lack antigen receptors and therefore do not display antigen specificity. On the other, they secrete classic lymphocyte cytokines and in some cases exhibit classic memory. Among innate lymphoid cells, natural killer cells pose the biggest classification challenge because they are capable of interacting with MHC molecules and generating antigen-specific immunologic memory that mirrors that of adaptive T lymphocytes (29). The precise role that innate lymphoid cells have in immunity in general and in kidney disease in particular remains to be determined.
The Immune System and the Kidney
Pondering the relationship between the kidney and the immune system brings three medical inflictions immediately to mind: autoimmune renal disease, kidney transplant rejection, and AKI (Figure 4). The first two can be thought of as mishaps or unintended consequences of the immunologic design you have put in place, whereas the third is a result of the well intentioned but sometimes overzealous response of the immune system to tissue damage. A fourth connection between the kidney and the immune system is the influence of chronic renal insufficiency on immunity. Uremia weakens crucial defenses required for protection against infection and, paradoxically, also causes generalized inflammation that is linked to excessive cardiovascular disease (30).
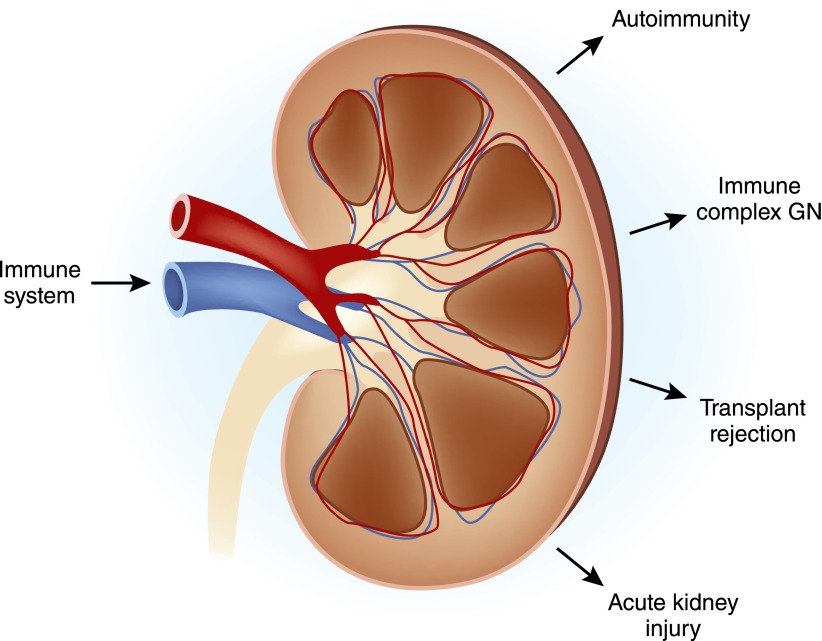
Figure 4.
The relationship between the immune system and kidney disease. The principal renal afflictions in which the immune system plays a major or important role are shown. Conversely, renal insufficiency affects the immune system by weakening immune defenses and by causing systemic inflammation that contributes to cardiovascular disease.
Autoimmune Renal Disease
The kidney can be either the direct target of autoimmunity, whereby a T lymphocyte or antibody that binds a renal antigen elicits renal pathology, or the kidney can be a victim of collateral damage caused by a systemic immune response to self or nonself antigens. In the latter setting, the culprits are usually antibody-antigen complexes (immune complexes) trapped in the glomerular filtration barrier that then instigate local inflammation (31). Autoimmunity is the consequence of the activation of those few self-reactive lymphocytes that the immune system failed to purge in the bone marrow or thymus during ontogeny. Immunologists refer to the purge as central tolerance because it takes place in central or primary lymphoid organs. Fortunately, the activation of self-reactive lymphocytes is a relatively rare event because of additional regulatory mechanisms present outside primary lymphoid organs. Immunologists refer to these as peripheral tolerance because they exert their regulatory functions in secondary lymphoid and nonlymphoid organs—that is, in the periphery. A key component of peripheral tolerance is regulatory T lymphocytes, which ensure that self-reactive lymphocytes are prevented from reacting to self or are quickly silenced if they do. Several events or circumstances, however, can lead to the breakdown of peripheral tolerance and the emergence of autoimmune disease (32). These include genetic mutations that disrupt regulatory T lymphocyte development, maintenance, or function; inflammatory events such as infection that interfere with the function of regulatory T lymphocytes; cross-reactivity between self and nonself antigens whereby T lymphocytes or antibodies specific to microbial antigens, which are readily incited during infection, also happen to bind self antigens; and finally, local tissue accidents that uncover hidden self antigens that had thus far been ignored by the immune system, neither deleted in the process of central tolerance nor regulated in the periphery. Finally, because of its key function in blood filtration, the kidney is often the resting place for antigen-antibody complexes that form elsewhere or sometimes locally after antigen is trapped in the glomerulus (31). Immune complexes can either be the result of a systemic autoimmune process (e.g., SLE) or the product of an immune response to microbes (as may be the case in glomerulonephritides that arise after infection). In both cases, complement activation by antibody molecules appears to play a major role in triggering renal pathology, but the full armamentarium of the immune system, including innate and adaptive cells as well as the cytokines they produce, participates. Through no fault of its own, the kidney obviously can be the target of the wrath of immunity.
Transplant Rejection
Another price that your descendants will pay for the highly sophisticated but imperfect immune system you have bestowed upon them is the rejection of life-saving organ transplants. In the absence of any immunosuppressive drugs, a kidney transplanted from one human to a genetically disparate human (i.e., someone who is not an identical twin with the donor) will be rejected violently. The rejection process is dependent on T lymphocytes, although all other immune defenses participate in one way or another in the rejection process, and the T lymphocyte response to the transplanted organ (the allograft) is characterized by sheer immensity that far exceeds any antimicrobial response (33). So why are T lymphocytes strongly alloreactive if natural selection has indeed been busy perfecting the repulsion of harmful pathogens, not harmless organ transplants? The answer lies first in the fact that any given individual harbors a large number of T lymphocyte clones that recognize and react to MHC antigens, which are the principal histocompatibility antigens responsible for transplant rejection; second, many of these T lymphocyte clones have already acquired memory properties (34). The large number of T lymphocytes that react to MHC antigens is a byproduct of an immune system that selects its T lymphocytes based on their ability to recognize peptides bound to MHC molecules (35). The memory nature of many of the alloreactive T lymphocytes is because TCRs specific for a microbial peptide (presented in the context of self-MHC) are also capable of recognizing allogeneic, nonself MHC—that is, they are cross-reactive (36). For example, memory T lymphocytes generated after exposure to a ubiquitous virus such as the Epstein–Barr virus cross-react with allogeneic MHC molecules and cause vigorous transplant rejection. Therefore, in its obsession to create an immune system that is able to respond to practically any pathogen, evolution put in place a highly diverse (polymorphic) MHC system that can bind virtually any microbial peptide and present it to T lymphocytes, whose TCRs to begin with are biased to bind to and sample all sorts of MHC molecules. Neither you nor evolution, it appears, predicted that some of your descendants will become talented transplant surgeons and nephrologists and that the polymorphic MHC proteins that are essential for antimicrobial immunity will also act as a powerful histocompatibility barrier to organ transplantation.
AKI
A less anticipated and, until recently, overlooked function of the immune system is its role in tissue injury unrelated to infection—so-called sterile tissue injury. AKI, which is the end result of a variety of noninfectious insults such as ischemia, drugs, and toxins, is often accompanied by subtle infiltration of the kidney with leukocytes from the blood and not-so-subtle activation of intrarenal immune cells. The infiltrate is not restricted to innate, myeloid cells (neutrophils and monocytes, for example) but also includes lymphoid cells, both adaptive and innate (37). Similarly, activation of renal cells involves resident macrophages and DCs as well as renal epithelial cells. The latter are increasingly recognized as accomplices of the immune system because they express innate receptors such as the TLR, respond to TLR ligands, and produce a host of inflammatory and immune cytokines (38). The net sum of immune activation after AKI, however, is still puzzling. On one hand, it can lead to more harm by causing excessive inflammation; on the other, it can be beneficial by repairing damaged tissues and cleaning up the mess (31,39). If nephrologists could uncover the secret to striking the right balance, immune therapy of AKI will one day become a reality (40).
Epilogue
It is not often that one biologic system touches so many aspects of human biology in both sickness and health. Although it is seemingly esoteric and beyond comprehension at first blush, the immune system, once viewed through the prism of evolution, is the epitome of versatility and simplicity of purpose. By peeling its layers one at a time, immunologists have succeeded not only in elucidating the inner workings of immunity but have also enabled the translation of their discoveries into real life benefits, such as vaccines that eradicate scourges, immunosuppressive drugs that conquer allograft rejection, cytokine-based therapies that subdue autoimmune disease, and antibodies that unbridle T lymphocytes to attack cancer cells. However, there is still much left for us nephrologists to do and discover. Which immunologic pathways should we target to interrupt or reverse GN? Of the many T lymphocyte, B lymphocyte, cytokine, and complement-based treatments that are now available in the clinic, which ones should we test in our patients? How can we improve long-term renal allograft outcomes without further compromising the immune system and therefore the health of the transplant recipient? What have we missed at a fundamental scientific level that still prevents us from achieving immunologic tolerance to autoantigens or organ transplants in a safe and effective manner, sparing patients the unwanted consequences of global immunosuppression? What immunologic trick can we pull to combat AKI? The list goes on and on as far as the imagination can see. The real journey has only begun.
Glossary
Adaptive Immunity
Adaptive immunity comprises defense mechanisms mediated by immune cells known as lymphocytes (T, B, and natural killer cells) and the specialized molecules required for their function. The term adaptive is applied because lymphocytes rapidly adapt to the situation at hand (e.g., a specific type of microbial infection) generating specialized cells, cytokines, and antibodies as well as long-lasting immunologic memory.
Antigen
Antigen is a nonself molecule, usually a protein, that incites an adaptive immune response.
Cytokines
Cytokines are protein molecules produced by cells of the immune system that mediate diverse defensive functions. These include inflammation, lymphocyte activation and differentiation, and killing of cells harboring foreign antigens. Cytokines play an important role in the pathogenesis of autoimmunity and immune-mediated renal disease.
Dendritic Cells (DCs)
Dendritic cells (DCs) are a specialized myeloid cell that is induced by infection to take up antigens, process them into small peptides, package them inside major histocompatibility complex (MHC) molecules, and present them to T lymphocytes after migrating to secondary lymphoid organs. DCs are prototypical antigen-presenting cells (APCs). They link innate to adaptive immunity.
Innate Immunity
Innate immunity comprises defense mechanisms mediated by the evolutionary more primitive components of our immune system. These include myeloid cells such as macrophages, DCs, and neutrophils and protein molecules such as the complement and coagulation systems. The term innate is used because these defenses are hardwired in the genome, responding in a rather unvarying manner to injury or infection. The innate immune system activates the adaptive immune system, principally via antigen-presenting DCs.
Lymphocytes
Lymphocytes are hematopoietic cells that mediate adaptive immunity. B lymphocytes produce antibodies (humoral immunity), whereas T lymphocytes differentiate into the specialized subpopulations best suited to tackle the offending agent (cellular immunity).
Major Histocompatibility Complex (MHC)
The MHC is a gene complex that codes for a diverse group of related protein molecules expressed on all nucleated cells (in the case of class I MHC molecules) and on immune cells (in the case of class II MHC molecules). In humans, they are known as the human leukocyte antigens. MHC molecules bind antigenic peptides, making possible their detection by T lymphocytes. Therefore, they are necessary for initiating adaptive immune responses. Because of their great diversity in humans, MHC molecules also act as histocompatibility antigens that trigger the rejection of transplanted organs.
Primary Lymphoid Organs
Primary lymphoid organs are organs or tissues where lymphocytes are born and/or trained to recognize and react to nonself antigens but not self-molecules. They include the bone marrow and the thymus.
Secondary Lymphoid Organs
Secondary lymphoid organs are organs or tissues where mature (trained) lymphocytes reside or circulate through. They are the site where lymphocytes encounter antigens and are activated by them to produce antibodies or effector (fighter) cells. Secondary lymphoid organs include the spleen, lymph nodes, and mucosal lymphoid tissues such as the Peyer’s patches in the small intestine.
Toll-Like Receptors (TLRs)
Toll-like receptors (TLRs) are receptors expressed principally on innate immune cells but also present on adaptive immune cells and nonimmune cells. They detect conserved molecular patterns on microbes. The TLR4 receptor, which binds lipopolysaccharide (LPS) of Gram-negative bacteria, is a prototypical example. TLRs also sense tissue damage by binding endogenous molecules released by dying or stressed cells. TLR engagement triggers inflammation as well as DC maturation, leading to enhancement of the adaptive immune response.
Disclosures
None.
Acknowledgments
F.G.L. is supported by grants from the National Institutes of Health (AI049466, AI096553, and AI099465).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gordon S: Elie Metchnikoff: Father of natural immunity. Eur J Immunol 38: 3257–3264, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Augustin R, Bosch TCG: Invertebrate Immunity, edited by Soderhall K, New York, Springer Landes Biosciences, 2010, pp 1–16 [Google Scholar]
- 3.Litman GW, Cooper MD: Why study the evolution of immunity? Nat Immunol 8: 547–548, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R: Innate immune recognition. Annu Rev Immunol 20: 197–216, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Quintin J, van der Meer JW: Trained immunity: A memory for innate host defense. Cell Host Microbe 9: 355–361, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Laird DJ, De Tomaso AW, Cooper MD, Weissman IL: 50 million years of chordate evolution: Seeking the origins of adaptive immunity. Proc Natl Acad Sci U S A 97: 6924–6926, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gearhart PJ: The birth of molecular immunology. J Immunol 173: 4259, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Josefowicz SZ, Lu LF, Rudensky AY: Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri C, Bosma A: Immune regulatory function of B cells. Annu Rev Immunol 30: 221–241, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Mueller SN, Gebhardt T, Carbone FR, Heath WR: Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31: 137–161, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Nussenzweig MC: Ralph Steinman and the discovery of dendritic cells. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2011/steinman_lecture.pdf. Accessed February 9, 2015
- 12.Parham P: Putting a face to MHC restriction. J Immunol 174: 3–5, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Chen L: Turning the tide of lymphocyte costimulation. J Immunol 182: 2557–2558, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Janeway CA, Jr: Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54: 1–13, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Preston-Hurlburt P, Janeway CA, Jr: A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Goodnow CC: Chance encounters and organized rendezvous. Immunol Rev 156: 5–10, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Sprent J: Proving negative selection in the thymus. J Immunol 174: 3841–3842, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich P: On immunity with special reference to cell life: Croonian Lecture. Proc R Soc Lond 66: 424–448, 1900 [Google Scholar]
- 19.Hedrick SM: Positive selection in the thymus: An enigma wrapped in a mystery. J Immunol 188: 2043–2045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzinger P: An innate sense of danger. Semin Immunol 10: 399–415, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kono H, Rock KL: How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burzyn D, Benoist C, Mathis D: Regulatory T cells in nonlymphoid tissues. Nat Immunol 14: 1007–1013, 2013. 24048122 [Google Scholar]
- 23.Hooper LV, Littman DR, Macpherson AJ: Interactions between the microbiota and the immune system. Science 336: 1268–1273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnet FM: “Self-recognition” in colonial marine forms and flowering plants in relation to the evolution of immunity. Nature 232: 230–235, 1971 [DOI] [PubMed] [Google Scholar]
- 25.Pearse AM, Swift K: Allograft theory: Transmission of devil facial-tumour disease. Nature 439: 549, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Oberbarnscheidt MH, Lakkis FG: Innate allorecognition. Immunol Rev 258: 145–149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziauddin J, Schneider DS: Where does innate immunity stop and adaptive immunity begin? Cell Host Microbe 12: 394–395, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Hwang YY, McKenzie AN: Innate lymphoid cells in immunity and disease. Adv Exp Med Biol 785: 9–26, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL: Natural killer cells: Walking three paths down memory lane. Trends Immunol 34: 251–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betjes MG: Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 9: 255–265, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Bluestone JA: Mechanisms of tolerance. Immunol Rev 241: 5–19, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Lakkis FG: Immunotherapy in Transplantation: Principles and Practice, edited by Kaplan B, Burckart GJ, Lakkis FG, West Sussex, UK, Wiley-Blackwell, 2012, pp 3–9 [Google Scholar]
- 34.Lakkis FG, Lechler RI: Origin and biology of the allogeneic response. Cold Spring Harb Perspect Med 3: a014993, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin L, Scott-Browne J, Kappler JW, Gapin L, Marrack P: T cells and their eons-old obsession with MHC. Immunol Rev 250: 49–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macedo C, Orkis EA, Popescu I, Elinoff BD, Zeevi A, Shapiro R, Lakkis FG, Metes D: Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant 9: 2057–2066, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Kinsey GR, Okusa MD: Role of leukocytes in the pathogenesis of acute kidney injury. Crit Care 16: 214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hato T, El-Achkar TM, Dagher PC: Sisters in arms: Myeloid and tubular epithelial cells shape renal innate immunity. Am J Physiol Renal Physiol 304: F1243–F1251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J: Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat Rev Nephrol 10: 625–643, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabb H: The promise of immune cell therapy for acute kidney injury. J Clin Invest 122: 3852–3854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]