Abstract
Background and objectives
Cystinuria is a rare inherited renal stone disease. Mutations in the amino acid exchanger System b0,+, the two subunits of which are encoded by SLC3A1 and SLC7A9, predominantly underlie this disease. The work analyzed the epidemiology of cystinuria and the influence of mutations in these two genes on disease severity in a United Kingdom cohort.
Design, setting, participants, & measurements
Prevalent patients were studied from 2012 to 2014 in the northeast and southwest of the United Kingdom. Clinical phenotypes were defined, and genetic analysis of SLC3A1 and SLC7A9 combining Sanger sequencing and multiplex ligation probe–dependent amplification was performed.
Results
In total, 76 patients (42 men and 34 women) were studied. All subjects had proven cystine stones. Median age of presentation (first stone episode) was 24 years old, but 21% of patients presented after 40 years old. Patients had varied clinical courses, with 37% of patients having ≥10 stone episodes; 70% had evidence of CKD, and 9% had reached ESRD as a result of cystinuria and its complications. Patients with cystinuria received a variety of different therapies, with no obvious treatment consensus. Notably, 20% of patients had staghorn calculi, with associated impaired renal function in 80% of these patients. Genetic analysis revealed that biallelic mutations were present in either SLC3A1 (n=27) or SLC7A9 (n=20); 22 patients had only one mutated allele detected (SLC3A1 in five patients and SLC7A9 in 17 patients). In total, 37 different mutant variant alleles were identified, including 12 novel mutations; 22% of mutations were caused by large gene rearrangements. No genotype-phenotype association was detected in this cohort.
Conclusions
Patients with cystinuria in the United Kingdom often present atypically with staghorn calculi at ≥40 years old and commonly develop significant renal impairment. There is no association of clinical course with genotype. Treatments directed toward reducing stone burden need to be rationalized and developed to optimize patient care.
Keywords: kidney stones, kidney tubule, CKD
Introduction
Cystinuria (OMIM 220100) is an inherited disorder characterized by the urinary loss of cystine, lysine, ornithine, and arginine. This loss leads to the formation of cystine stones in the urinary tract. Cystine is normally reabsorbed from the primary filtrate through a transporter, which consists of the two protein subunits rBAT and b0,+AT (1). rBAT is encoded by SLC3A1 (2), which is located at chromosome 2 (OMIM 104614), whereas b0,+AT is encoded by SLC7A9 (3) at chromosome 19 (OMIM 604144). Mutations in SLC3A1, SLC7A9, or both can lead to cystinuria.
The clinical presentation of cystinuria is usually before the age of 30 years. Clinical management combines lifestyle advice and medical therapy with surgical interventions, when required, to remove problematic stones from the urinary tract. The mainstays of current treatment are increased fluid intake, alkalinization of the urine, and if these measures fail, use of cystine-binding drugs, including penicillamine and tiopronin (alias Thiola), which form soluble heterodimers with cystine (4).
Patients with mutations in SLC3A1 are known as type A, and those with SLC7A9 are known as type B. Genotype types AA and BB denote two mutated alleles in SLC3A1 or SLC7A9, respectively, whereas A and B denote the identification of only one mutated allele (5). In a third rare group, type AB, individuals have one mutation in SLC3A1 and one mutation in SLC7A9 (6). Occasionally, there are more than two mutated alleles present, such as AAB and BBA (5).
Cystinuria caused by mutations in SLC3A1 is usually an autosomal recessive condition. Previous work suggests that heterozygotes for mutations in SLC3A1 have normal urinary cystine and dibasic amino acid levels (6), except that some (but not all) heterozygotes with the duplication of exons 5–9 may have elevated levels of urinary cystine (5) and can develop recurrent cystine stones (7). The inheritance of SLC7A9 mutations is usually autosomal dominant with variable penetrance, with 86% of SLC7A9 heterozygotes having abnormal urinary dibasic amino acid levels (6), and a variable proportion of these develops cystine stones.
Since linkage analysis identified cystinuria involvement of SLC3A1 in 1994 (2,8) and SLC7A9 in 1999 (9), 152 mutations have been reported in SLC3A1, and 104 mutations have been reported in SLC7A9 (10). Cohorts of patients from Europe, Asia, and North America have been genotyped (11–21) along with a recently published group of United Kingdom patients (22).
To build up a large cohort of patients with cystinuria, a United Kingdom National Registry of Rare Kidney Diseases (RaDaR) has been established (23). A national genetic testing service has also been established, and two cohorts of patients from different geographical regions of England underwent detailed genotyping and clinical data collection. We describe the genetic mutations identified and clinical course/phenotype of these patients with a view to personalizing cystinuria management.
Materials and Methods
Patients
Patients recruited to this study all had a clinical diagnosis of cystinuria on the basis of confirmed cystine stone(s) on chemical analysis. Prevalent patients were identified and recruited to the study from October of 2012 to July of 2014 from the southwest and northeast of England (shown in Supplemental Figure 1). We believe that the majority of patients with cystinuria within these regions was identified. No patients with cystinuria declined to be included in the study.
Detailed clinical data were collected retrospectively to inform the genotype/phenotype analysis. The study was approved by the National Research Ethics Service (NRES) Committee South Central (12/SC/0456) and the NRES Committee North East (11/NE/0259), and informed consent was obtained from all participants or their parents/guardians where applicable.
Clinicians completed data collection for each patient with cystinuria under their care, including demographics (age, sex, and ethnicity), age at diagnosis, age at first stone event, number of stone events (subdivided into stones passed spontaneously, lithotripsy sessions, and invasive stone removal procedures, including open, percutaneous, and endourologic procedures), medical treatments (current and previous), and details of relevant blood and urine biochemistry, including eGFR measurements using an abbreviated Modification of Diet in Renal Disease equation (http://egfrcalc.renal.org/). The number of stone events per year was estimated by scoring all spontaneous stones passed with all stone-removing procedures performed since the age of first stone event.
For calculation of prevalence, the number of known patients with cystinuria was used together with estimates of the total population for the geographical regions of study taken from the 2011 United Kingdom National Census.
Genetic Analyses
Patients were genotyped using Sanger sequencing (ABI3730) of all coding exons and flanking intronic regions, including splice sites and branch points of SLC3A1 and SLC7A9. Variant pathogenicity assignment was undertaken using Alamut (version 2.3 rev 1). In addition, all DNA samples were analyzed with multiplex ligation-dependent probe amplification (MLPA) of coding exons using an in-house high-throughput automated MLPA assay (Beckman NX/Beckman CEQ8000). Probes for SLC3A1 and SLC7A9 previously described by Bisceglia et al. (11) were used in conjunction with the Control P200 Probe Kit from MRC-Holland.
Statistical analyses of data were performed using Graphpad Prism, version 5. Where data were not normally distributed, the median and range are stated, and P values were calculated by performing Mann–Whitney U tests (two-tailed) to compare two independent groups of non-Gaussian data. For categorical data, two-tailed Fisher’s exact test was performed. A P value <0.05 was considered statistically significant.
Results
Clinical Features
In total, 76 patients diagnosed with cystinuria were identified comprising 34 (45%) women and 42 (55%) men, including one pediatric patient. All patients were white British, except one patient each from Pakistan and China. Using an estimated combined population size of 7,886,100, the overall prevalence of cystinuria was estimated to be approximately one in 100,000 in this cohort. A positive family history of cystinuria was documented in 26 (34%) patients; 17 patients had only one generation affected (siblings), seven patients had two generations affected, and one patient each had cystinuria in three and four generations.
The median age at first stone event was 24 years old (range =2–62 years old). The majority of patients had their first stone event in their late teens and early 20s. However, 16 (21%) patients were over 40 years old at presentation (Figure 1A). Stone episodes per year varied widely (median frequency of 0.45 stones per year, range of 0.06–78.2), with no significant difference in stone events per year between sexes (P=0.73).
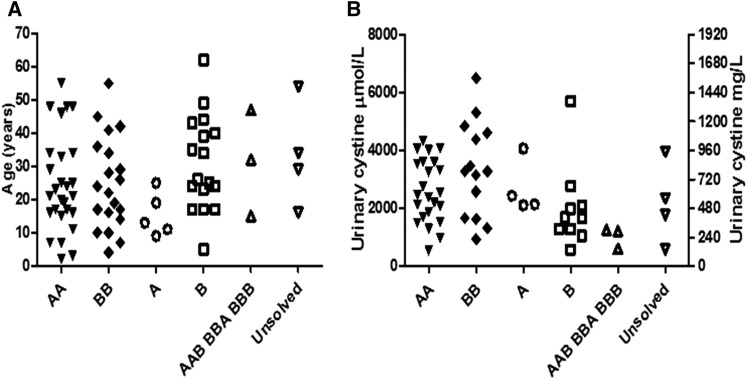
Figure 1.
Genotype-phenotype. A shows the age in years at first stone event for each genotyped group, and B shows 24-hour urinary cystine at first presentation, which shows the considerable overlap between the genotype groups. A, heterozygous SLC3A1; AA, homozygous SLC3A1; AAB, BBA, BBB, triallellic for SLC7A9 mutations or SLC7A9 and SLC3A1 mutations; B, heterozygous SLC7A9; BB, homozygous SLC7A9; unsolved, no mutation detected in SLC3A1 or SLC7A9.
Fifty-three patients (70%) had evidence of CKD (eGFR<90 ml/min per 1.73 m2), including seven patients (9%) with an eGFR<30 ml/min per 1.73 m2 (Table 1). Three of these patients have undergone renal transplantation for ESRD that was attributed to a combination of multiple bilateral cystine stone episodes (including staghorn stone formation), open surgical procedures, and kidney infections.
Table 1.
Comparison of renal function by genotype
| eGFR (ml/min per 1.73 m2) | No. | Percent | AA (B) | A | BB (A or B) | B | AB | Genotype Unknown |
|---|---|---|---|---|---|---|---|---|
| ≥90 | 23 | 30 | 8 | 6 | 8 | 1 | ||
| 60–89 | 37 | 49 | 16 | 4 | 7 | 5 | 3 | 2 |
| 30–59 | 9 | 12 | 2 | 3 | 3 | 1 | ||
| <30 | 4 | 5 | 1 | 1 | 2 | |||
| ESRD with renal transplant | 3 | 4 | 2 | 1 |
All 76 patients had been advised to increase daily intake of fluids, and 13 (17%) patients followed diets low in animal protein. Additional alkalizing and/or cystine-binding treatments were prescribed to 52 of 76 (68%) patients (Table 2). Of 76 patients, 26 (34%) patients received monotherapy, and 24 (32%) patients were given a combination of alkalinization and cystine-binding therapies. Of 26 patients currently or historically prescribed penicillamine, 14 (54%) patients had experienced side effects (Supplemental Table 1). Of 19 patients previously or presently taking tiopronin, only one patient developed a side effect. Three patients were noted to have discontinued alkalinization medication, because they found potassium citrate unpalatable. Overall, there was evidence that 54 of 76 (71%) patients received medical therapy that followed international guidelines (24,25). Despite this, 27 (50%) of 54 patients continued to form kidney stones.
Table 2.
Medications used in the treatment of cystinuria in the English cohort
| Number of Medications Used | Potassium Citrate | Sodium Bicarbonate | Captopril | Penicillamine | Tiopronin | Frequency |
|---|---|---|---|---|---|---|
| None | — | — | — | — | — | 24 |
| Single (n=26) | X | — | — | — | — | 10 |
| — | X | — | — | — | 1 | |
| — | — | X | — | — | 1 | |
| — | — | — | X | — | 9 | |
| — | — | — | — | X | 5 | |
| Dual (n=24) | X | X | — | — | — | 1 |
| X | — | X | — | — | 2 | |
| X | — | — | X | — | 2 | |
| X | — | — | — | X | 10 | |
| — | X | X | — | — | 2 | |
| — | X | — | X | — | 4 | |
| — | X | — | — | X | 3 | |
| Triple | X | — | X | — | X | 2 |
Surgical therapy included lithotripsy in 38 (50%) patients and open, endoscopic, or percutaneous stone removal in 62 (86%) patients.
Fifteen (20%) patients had documented staghorn stones (Table 3). Age at first stone, stone frequency, and sex were not associated with staghorn calculi formation, but the majority of patients had a reduction in eGFR, with 12 of 15 (80%) patients <90 ml/min per 1.73 m2. One patient had reached ESRD and received a renal transplant.
Table 3.
Genetic and clinical characteristics of staghorn calculi formers
| ID | Type | Mutant Allele Type | DNA Variant and Predicted Protein Description | Sex | Age (yr) at First Stone | Stones per Year Since First Stone | eGFR (ml/min per 1.73 m2) |
|---|---|---|---|---|---|---|---|
| 14 | AA | Com het | c.[1400T>C]; [exons 5–9 dup] | M | 2 | 0.32 | 88.5 |
| p.[Met467Thr]; [exons 5–9 dup] | |||||||
| 16 | AA | Com het | c.[1400T>C]; [exons 5–9 dup] | W | 48 | 0.67 | >90 |
| p.[Met467Thr]; [exons 5–9 dup] | |||||||
| 19 | AA | Hom | c.[1400T>C]; [1400T>C] | W | 16 | 0.13 | 62.2 |
| p.[Met467Thr]; [Met467Thr] | |||||||
| 55 F4 | A | Het | c.[exons 5–9dup]; [=] | W | 13 | 0.11 | 65.1 |
| 30 | BB | Com het | c.[368C>T] ; [671C>T] | M | 42 | 0.88 | >90 |
| p.[Thr123Met]; [Ala224Val] | |||||||
| 32 | BB | Com het | c.[414_415delGC]; [exon 12 del] | M | 17 | 2.62 | 80.9 |
| p.[Pro139Leufs*69]; [exon 12 del] | |||||||
| 37 | BB | Hom | c.[614dupA]; [614dupA] | W | 16 | 0.44 | 49.8 |
| p.[Asn206Glufs*3]; [Asn206Glufs*3] | |||||||
| 38 | BB | Hom | c.[614dupA]; [614dupA] | M | 41 | 0.09 | 18 |
| p.[Asn206Glufs*3]; [Asn206Glufs*3] | |||||||
| 41 F3 | BB | Com het | c.[614dupA]; [exon 12 del] | W | 10 | 1.6 | 27 |
| p.[Asn206Glufs*3]; [exon 12 del] | |||||||
| 43 | BB | Com het | c.[671C>T]; [997C>T] | W | 4 | 4.47 | 50 |
| p.[Ala224Val]; [Arg333Trp] | |||||||
| 46 | BB | Com het | c.[1399+4_1399+7del]; [exon 12del] | M | 17 | 2 | >90 |
| 50 | BBB | Com het + Hom | c.[544G>A;1060G>A]; [544G>A] | W | 47 | 0.73 | 83.4 |
| p.[Ala182Thr;Ala354Thr]; [Ala182Thr] | |||||||
| 66 | B | Het | c.[671C>T]; [=] | M | 40 | 0.09 | 39.2 |
| p.[Ala224Val]; [=] | |||||||
| 69 | B | Het | c.[671C>T]; [=] | M | 5 | 0.06 | 52.6 |
| p.[Ala224Val]; [=] | |||||||
| 72 | B | Het | c.[exon 12 del]; [=] | M | 24 | ? | ESRD (transplant) |
ID, identification; F4, family 4; F3, family 3; Com het, compound heterozygote (two different mutations detected—one in each allele); Hom, homozygous (both alleles in the same mutation); Het, heterozygote (single mutated allele detected); M, man; W, woman.
Genetic Analyses
Genetic analysis was performed in all 76 patients (Table 4). At least two distinct genetic mutations were detected in 50 (66%) patients. Of these, three patients had evidence of triallelism. A single mutation was found in 22 (29%) patients, and in four (5%) patients, no pathogenic mutations were detected.
Table 4.
Frequency of genotype in cohorts
| Genotype | No. | Percent of total |
|---|---|---|
| AA | 27 | 36 |
| BB | 20 | 26 |
| AAB | 1 | 1 |
| BBA | 1 | 1 |
| BBB | 1 | 1 |
| A | 5 | 7 |
| B | 17 | 22 |
| Unsolved | 4 | 5 |
| Total | 76 |
In total, 125 mutated alleles were identified, with 37 different distinct variants (Tables 5 and 6). Of the 125 mutated alleles, 27 (22%) were large gene rearrangements, the majority in SLC3A1. We identified 12 previously unreported (novel) mutations: eight in SLC3A1 and four in SLC7A9 (Tables 5 and 6). Only 15 (20%) patients were homozygous for a mutant allele, including seven patients with type AA, six patients with type BB, one patient with type BBA, and one patient with type BBB.
Table 5.
Summary of SLC3A1 alleles detected
| Variant Type | SLC3A1 | Location of Mutation | Predicted Protein Sequence | No. | |
|---|---|---|---|---|---|
| Missense | c.647C>T | Exon 3 | p.Thr216Met | 2 | Known |
| Missense | c.761A>C | Exon 3 | p.Asn254Thr | 2 | Novel |
| Missense | c.1093C>T | Exon 6 | p.Arg365Trp | 2a | Known |
| Missense | c.1354C>T | Exon 8 | p.Arg452Trp | 3 | Known |
| Missense | c.1372G>A | Exon 8 | p.Gly458Arg | 1b | Novel |
| Missense | c.1400T>C | Exon 8 | p.Met467Thr | 14 | Known |
| Missense | c.1412C>G | Exon 8 | p.Thr471Arg | 2 | Knownc |
| Missense | c.1796T>C | Exon 10 | p.Phe599Ser | 1 | Known |
| Missense | c.1799G>A | Exon 10 | p.Gly600Glu | 1 | Known |
| Nonsense | c.1578G>A | Exon 9 | p.Trp526* | 1 | Novel |
| Nonsense | c.1975C>T | Exon 10 | p.Gln659* | 1 | Novel |
| Frameshift | c.161delC | Exon 1 | p.Gln55fs*51 | 1 | Known |
| Frameshift | c.356dupA | Exon 1 | p.Glu120Glyfs16 | 1 | Novel |
| Frameshift | c.2020dupT | Exon 10 | p.Tyr674Leufs*20 | 6 | Novel |
| Splice site | c.1136+2T>C | Intron 6 | 2 | Known | |
| Splice site | c.1332+2T>A | Intron 7 | 1 | Novel | |
| Deletion | Del exon 2 | 1 | Novel | ||
| Deletion | Del exon 2–3 | 2 | Known | ||
| Deletion | Del exon 2–4 | 1 | Known | ||
| Deletion | Del exons 5–10 | 1 | Known | ||
| Deletion | Del exon 10 | 1 | Known | ||
| Duplication | Dup exons 5–9 | 15 | Known | ||
| Total | 62 |
Del, deletion; dup, duplication.
Both alleles in a patient of Pakistani descent.
Novel variants detected in a Chinese patient. cRecently reported in United Kingdom patients.
Table 6.
Summary of SLC7A9 alleles detected
| Variant Type | SLC7A9 | Location of Mutation | Predicted Protein Sequence | No. | |
|---|---|---|---|---|---|
| Missense | c.313G>A | Exon 4 | p.Gly105Arg | 8 | Known |
| Missense | c.368C>T | Exon 4 | p.Thr123Met | 1 | Known |
| Missense | c.544G>A | Exon 5 | p.Ala182Thr | 8 | Known |
| Missense | c.671C>T | Exon 6 | p.Ala224Val | 10 | Known |
| Missense | c.962G>A | Exon 9 | p.Cys321Tyr | 1 | Novel |
| Missense | c.997C>T | Exon 10 | p.Arg333Trp | 1 | Known |
| Missense | c.1060G>A | Exon 10 | p.Ala354Thr | 1 | Known |
| Missense | c.1369T>C | Exon 12 | p.Tyr457His | 1 | Novel |
| Nonsense | c.1353C>A | Exon 12 | p.Tyr451* | 1 | Novel |
| Frameshift | c.411_412delTG | Exon 4 | p.Pro139Leufs*69 | 3 | Novel |
| Frameshift | c.414_415delGC | Exon 4 | p.Pro139Leufs*69 | 1 | Knowna |
| Frameshift | c.614dupA | Exon 6 | p.Asn206Glufs*3 | 15 | Known |
| Splice site | c.1399+4_1399+7del | Intron 12 | 4 | Knowna | |
| Splice site | c.1400–2A>G | Intron 12 | 2 | Knowna | |
| Deletion | Del exon 12 | Exon 12 | 6 | Known | |
| Total | 63 |
Del, deletion; dup, duplication.
Recently reported in United Kingdom patients.
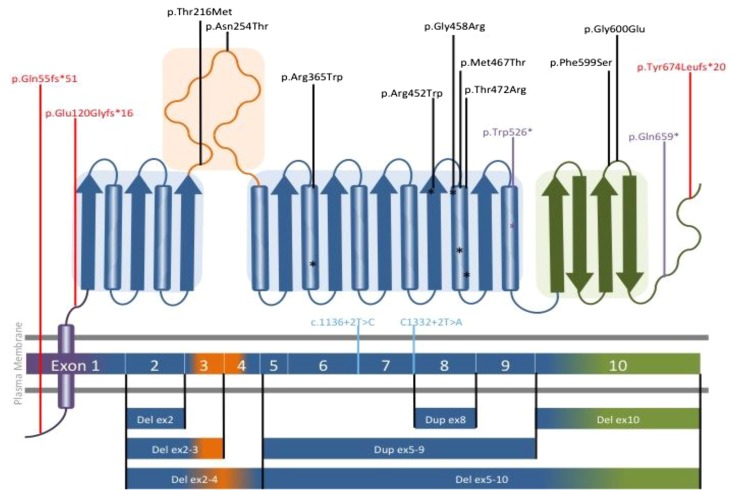
The most frequent mutant allele in SLC3A1 was a duplication of exons 5–9 in 24% (15 of 62) of alleles followed by the missense mutation c.1400T>C, p.(Met467Thr) in 22% (14 of 62). A novel frameshift, c.2020dupT, p.(Tyr674Leufs*20), in exon 10 was detected in 10% (6 of 62) of alleles (Table 5).
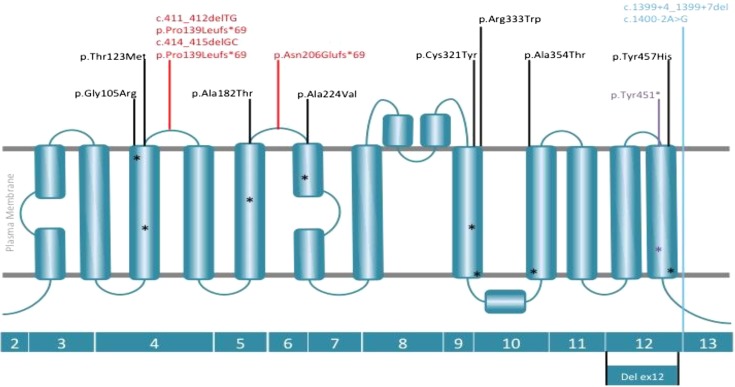
In SLC7A9, the most frequent allele was the frameshift c.614dupA that was observed in 24% (15 of 63). The missense mutations c.671C>T, p.(Ala224Val), c.544G>A, p.(Ala182Thr), and c.313G>A, p.(Gly105Arg) represent 16%, 13%, and 13%, respectively, of SLC7A9 alleles. The deletion of exon 12 also accounted for 10% of SLC7A9 alleles (Table 6).
The distributions of mutations within the exons and protein domains are represented in Figures 2 and 3. In this study, mutations in SLC3A1 occurred throughout all exons, whereas in SLC7A9, mutations were limited to exons 4–6, 9, 10, 12, and 13.
Figure 2.
Distribution of mutations detected in SLC3A1 (rBAT) throughout the exons and protein domains. Schematic diagram of rBAT and a homology model of the extracellular domain of rBAT on the basis of the crystal structure of oligo-1,6-glucosidase from Bacillus cereus (Protein Data Bank ID code 1UOK). The domains of rBAT are shown in purple (TMD), blue (domain A), orange (domain B [subdomain]), and green (domain C). Mutations are labeled as follows: missense in black, nonsense in purple, frameshift in red, and splice site in pale blue. Mutations predicted to fall within α-helices are denoted by asterisks of the appropriate color. TMD, trans-membrane domain.
Figure 3.
Representation of the location of mutations detected in SLC7A9 (b0,+AT) throughout the exons and protein domains. Schematic diagram of b0,+AT and homology model of b0,+AT on the basis of the crystal structure of AdiC protein, an arginine:agmatine antiporter from Escherichia coli (Protein Data Bank ID code 3L1L). SLC7A9 mutations are distributed in exons 4–6, 9, 10, 12, and 13. Mutations are labeled as follows: missense in black, nonsense in purple, frameshift in red, and splice site in pale blue. Mutations predicted to fall within α-helices are denoted by asterisks of the appropriate color.
One novel mutation in SLC3A1 (c.2020dupT) was identified in four patients (two related and two unrelated patients) from the southwest of England, three of whom live in a very geographically remote location, suggestive of a founder effect for this mutation.
Genotype-Phenotype Association
We were unable to show a difference between 27 patients with type AA and 20 patients with type BB in a variety of clinical parameters, including age of first stone event, in which patients with AA presented at a median age of 21 years old (range =2–55 years old) and patients with BB presented at a median of 23 years old (range =4–55 years old; P=0.96); number of stone episodes per year, in which patients with type AA had a median of 0.44 stone episodes per year (range =0.1–7.13) and patients with type BB had a median of 0.48 stone episodes per year (range =0.09–13.3; P=0.32); and renal function, with 70% of patients with type AA and 82% of patients with type BB having an eGFR<90 ml/min per 1.73 m2.
There was overlap between all of the genotypic groups regarding age of first stone event (Figure 1A). Patients with a single mutated allele also had variable disease severity and could not be differentiated from patients with two mutated alleles. Figure 1B shows the variability both between and within the genotypic groups for the urinary cystine levels at first presentation. The level of renal impairment (Table 1) was also similar across all of the genotypes.
We found 13 subgroups who had exactly the same genotype (Supplemental Table 2): four groups for type AA genotype, three groups for type BB genotype, one group for type A genotype, and five groups for type B genotype. Each subgroup showed a wide divergence in stone frequency and age of onset of the first stone, despite having the same underlying mutations. There were pairs of siblings in groups AA, BB, and A who also all had dramatically contrasting clinical courses.
All of the patients in this study had proven cystine stones. Four type A patients (heterozygous for duplication of exons 5–9) had recurrent cystine stone events. Notably, one patient heterozygous for c.761A>C, p.(Asn254Thr) in SLC3A1 had six cystine stone events (patient 51 in Supplemental Table 2).
Discussion
In this study, we have comprehensively studied the genetics and clinical progress of patients with cystinuria from two English regions. We have found that there is no genotype-phenotype correlation in these patients, that stones commonly present in adulthood, and that patients are on a variety of different therapies. Furthermore, our data show that, in both SLC3A1- and SLC7A9-related diseases, having a single detectable mutation is common (and sufficient for a dramatic clinical phenotype) and that a few patients do not have an easily identifiable mutation in the known cystinuria genes.
Cystine stones can occur at any age. In previous studies, >80% of patients developed their first stones within the first two decades (12), and <1% of patients with cystinuria had their first stone when >40 years old (6). However, in this cohort, only 30 of 76 (39%) patients had their first stone by the age of 20 years old (Figure 1A); 16 of 76 (21%) patients did not pass their first stone until they were >40 years old, highlighting the importance of considering this inherited condition as an underlying cause of nephrolithiasis in older age groups. In this cohort, late presentation occurred in five patients with type AA, four patients with type BB, one patient with type BBB, five patients with type B, and one patient with an unsolved genotype.
It is also of note that 20% of patients in this study had staghorn calculi. Staghorn calculi may not always be investigated for underlying cystinuria, because it may be falsely assumed that they are infective stones, struvite in origin, and composed of magnesium ammonium phosphate. Case series reported by Soucy et al. (26) and Viprakasit et al. (27) have shown that between 21% and 44% of staghorn calculi are struvite, and for the remainder, other etiogic causes should be sought. Cystinuria is a known and important cause of staghorn calculi. Remarkably, Cupisti et al. (28) reported a 72-year-old patient with a cystine staghorn calculus, leading to a much delayed diagnosis of cystinuria.
Our cohort showed no associations between genotype and phenotype. There was no difference in age of onset, number of spontaneous stone emissions, or total stone events between those with type AA, BB, A, or B. These findings replicate those from other international studies (6) as well as the recent study from London (22). This clinical heterogeneity together with the fact that four patients in our study had no detectable exonic mutation in SLC3A1 or SLC7A9 suggest that disease-modifying single-nucleotide polymorphisms, intronic variants, or other genes may all contribute to the pathogenesis of cystinuria and should be a focus of future research.
Although there is international guidance and consensus on the optimal treatment strategy for patients with cystinuria (24,25), this was not obviously adhered to by all patients in this cohort, which was probably because of a combination of patient and clinician preferences. Furthermore, current treatments, such as penicillamine (29), often needed to be discontinued because of their serious side effect profile, which includes proteinuria and warrants close surveillance. Side effects from penicillamine in our cohort were similar to previous reports, occurring in around 50% of patients (30). We found that Tiopronin was generally much better tolerated than penicillamine, which has also been described by other groups (31,32). Even with medical therapy, continued stone formation was common. This emphasizes the importance of understanding the molecular basis of cystinuria and developing more effective and better tolerated medications. It would also be interesting to prospectively survey well defined groups of patients with cystinuria and their associated physicians in regard to the attitudes of clinicians and patients with respect to medical therapy prescribing and adherence, but this was not the focus of this study.
From a surgical perspective, we found that a high proportion of patients had received extracorporeal shock wave lithotripsy. There is conflicting evidence on the benefit of extracorporeal shock wave lithotripsy and cystine stone clearance (33,34). However, the benefit of lithotripsy in cystinuria would be better addressed in adequately powered large prospective studies and detailed international registries, such as the International Cystinuria Registry (35) (part of the Rare Kidney Stone Consortium; http://cystinuria.org/the-rare-kidney-stone-consortium-announces-the-cystinuria-registry/) and the proposed RaDaR cystinuria initiative (23).
The duplication of exons 5–9 and the missense mutation c.1400T>C, p.(Met467Thr) were the most frequent SLC3A1 mutations in our cohort, and they were also observed in another United Kingdom cohort (22). The missense mutation c.1400T>C, p.(Met467Thr) is the most common mutation in studies of Italian (11), German (12), Swedish (13), Spanish (14), North American (Texas) (15), and Czech and Slovak (16) cohorts. However, the duplication of exons 5–9 in SLC3A1 is reported much less frequently around the world, except in Germany, where it accounts for 18.8% of SLC3A1 mutations (12). This may be because of some studies failing to incorporate MLPA in their genomic analysis of cystinuria genes. The novel frameshift c.2020dupT, p.(Tyr674Leufs*20) in exon 10 of SLC3A1 was detected in 9% (6 of 64) of alleles in our cohort.
In SLC7A9, the most frequent allele was the frameshift c.614dupA, p.(Asn206Glufs*3) occurring in 23% (15 of 64) of alleles. This allele was also frequently detected in another United Kingdom cohort (22) and a Spanish cohort (14). The SLC7A9 mutation c.671C>T, p.(Ala224Val) described in a German family in 2002 (36) is rare in reported literature (12) but had a high frequency (15%) in our cohort. The deletion of exon 12 accounts for 11% of SLC7A9 alleles in this study but was not described in the recent United Kingdom study (22). Previous cystinuria studies have focused on different clinical aspects of this disease, but many have analyzed genotype-phenotype associations (5,6,11,15,18,22,36–38). These are summarized in Table 7.
Table 7.
Summary of previous large genotype-phenotype studies of cystinuria
| Author and Year | Cohort Origin | Cohort Size | Mutated Alleles Identified | Do All Subjects Have Proven Cystine Stones? | Age (yr) at First Stone/ Diagnosis (Range) | Renal Dysfunction | Is Severity of Clinical Phenotype Linked to Genotype? | No. with Staghorn Calculi | Most Common SLC3A1 Mutation (n/Total) | Most Common SLC7A9 Mutation (n/Total) | Large Gene Rearrangements | DNA Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This study (2015) | England | 76 | 125 | Yes | Median 24 (2–62) | 70% CKD, 9% with ESRD | No | 15 (20%) | Dup exons 5–9 24% (15 of 62) | c.614dupA 24% (15 of 63) | 22% | Sequencing, MLPA |
| Wong et al. 2014 (22) | England | 74 | 143 | No | Mean 23.9 (0–61) | Not studied | One or more SLC3A1 missense mutations had lower urinary ornithine, lysine, and arginine; no other phenotype association seen | Not reported | Dup exons 5–9 27% (24 of 88) | c.614dupA 20% (11 of 55) | 23% (33 of 143) | Sequencing, MLPA |
| Popovska-Jankovic et al. 2013 (18) | Southeastern Europea | 60 | 93 | No | Data incomplete | Not studied | SLC3A1 homozygous for p.Thr216Met had higher urinary dibasic amino acidsb | Not reported | p.Thr216Met 25% (24 of 98) | p.Gly105Arg 21% (21 of 98) | Not reported | Sequencing, SSCP, RFLP |
| Barbosa et al. 2012 (37) | Portugal | 12 | 21 | No | Median 3 (0.6–14) | Not studied | No | Not reported | Dup exons 5–9 (4 of 13) | c.972G>Ac (3 of 8) | 29% (6 of 21) | Sequencing, MLPA |
| Bisceglia et al. 2010 (11) | Italy | 172 | 308 | Yes | Not studied | Not studied | Not studied | Not reported | p.Met467Thr 23% (34 of 145) | p.Gly105Arg 27% (44 of 163) | 11% (35 of 308) | Sequencing, MLPA |
| Font-Llitjós et al. 2005 (5) | Italy, Spain, Israel, Belgium, Portugal, Switzerland, England, Germany | 164 Probands | 282 | Yes | Not studied | Not studied | Not studied | Not reported | p.Met467Thr 26% (33 of 125) | p.Gly105Arg 29% (43 of 147) | SSCP, DHPLC, semiquantitative multiplex PCR, sequencing | |
| Dello Strologo et al. 2002 (6) | Italy, Spain, Israel | 188 had genetic analysis | 302 | No | Median 14 | 17% (30 of 176) mild renal insufficiency,d 1 of 176 ESRD | No | Not reported | Not reported | Not reported | Not studied | SSCP, sequencing |
| Botzenhart et al. 2002 (36) | Germany, Turkey | 31 | 28 | No | Median 7 (0.75–24)e | Not studied | No | Not reported | p.Met467Thr | p.Gly105Arg | Not studied | SSCP |
| Font et al. 2001 (38) | Italy, Spain, North America, Libya (Jewish) | 175 | No | Not studied | Not studied | Not studied | Not reported | Not reported | p.Gly105Arg 25% (29 of 114) | Not reported | Sequencing, RNA SSCP, SSCP | |
| Gitomer et al. 1998 (15) | United States (Texas) | 33 | 34 | No | 3–75 | Not studied | Not studied | Not reported | p.Met467Thr 18% (12 of 66) | Not studied | Not reported | RNA SSCP, RNA MisMatch |
dup, duplication; MLPA, multiplex ligation–dependent probe amplification; SSCP, single–strand conformation polymorphism analysis; RFLP, restriction fragment length polymorphism; DHPLC, denaturing high performance liquid chromatography.
Macedonia, Serbia, Turkey, Kosovo, Montenegro, Croatia, Bulgaria, and Slovenia.
Mutation specific to Gypsy families (Popovska-Jankovic et al. [18] concluded that environmental factors and compliance may influence disease severity).
Mutation that might contribute to expression of disease.
Renal insufficiency defined as plasma creatinine >120 μmol/L.
Majority of subjects were children.
We identified five patients with type A genotype who have developed cystine stones. One patient who was heterozygous for the novel missense mutation c.761A>C in exon 3, p.(Asn254Thr) had multiple stone events since 11 years of age, and four patients with a single duplication of exons 5–9 were cysteine stone formers. This challenges the usual paradigm that those with single heterozygous SLC3A1 mutations are unaffected carriers (except, as previously noted, some heterozygotes of duplication exons 5–9 [5,7]). This is important in assessing stone risk in children, genetic counseling, and when considering initiating preventative therapies.
The clinical data were collected retrospectively, which can limit accuracy, but our findings were comparable with those from the prospective study of patients from London (22). Moreover, a singular clinical and genetic database for United Kingdom patients with cystinuria is currently being established using the RaDaR initiative. Establishing national and international patient cohorts for the study of rare diseases, like cystinuria, is beneficial to develop collaborative research ideas, support patients and their families, and enable the effectiveness of medications to be assessed. Indeed, by genotyping these patients and correlating this information with clinical information held in the database, we hope that it may be possible to individualize therapies and establish treatment algorithms on the basis of specific mutations.
This study broadens the clinical and genetic spectrum of cystinuria, which is not a simple autosomal recessive disease. Rather, it is likely to result from a more complex genetic model involving at least two genes and additional unknown genetic modifiers together accounting for the variable severity of the phenotype.
We also conclude that focused medical and surgical management of patients with cystinuria is needed. Our aim is to encourage national and international comprehensive clinical research networks to study this condition more completely.
Disclosures
J.A.S. has received honoraria from Otsuka, Amgen, and Novartis. R.J.M.C. has an academic grant from Novo Nordisk.
Supplementary Material
Acknowledgments
Thanks to Dr. Jo Taylor, Dr. Coralie Bingham, Dr. Rob Parry and Dr. Jane Tizard for their help in recruiting patients. Dr. L. Bisceglia provided positive control samples for validation of multiplex ligation-dependent probe amplification.
H.L.R. received Kidney Research Training Fellowship UK-TF19/2012 with a supplementary grant from the Royal College of Surgeons of England. S.J.R. was supported by an Medical Research Council Studentship. A.T. acknowledges support from a Wellcome Trust Summer Studentship. D.T.T. and J.A.S. are supported by the Northern Counties Kidney Research Fund. R.J.M.C. is supported by MRC Senior Clinical Fellowship MR/K010492/1.
Footnotes
J.A.S. and R.J.M.C. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10981114/-/DCSupplemental.
References
- 1.Chillarón J, Estévez R, Mora C, Wagner CA, Suessbrich H, Lang F, Gelpí JL, Testar X, Busch AE, Zorzano A, Palacín M: Obligatory amino acid exchange via systems bo,+-like and y+L-like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J Biol Chem 271: 17761–17770, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Calonge MJ, Gasparini P, Chillarón J, Chillón M, Gallucci M, Rousaud F, Zelante L, Testar X, Dallapiccola B, Di Silverio F, Barceló P, Estivill X, Zorzano A, Nunes V, Palacín M: Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genet 6: 420–425, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bisceglia L, Calonge MJ, Totaro A, Feliubadaló L, Melchionda S, García J, Testar X, Gallucci M, Ponzone A, Zelante L, Zorzano A, Estivill X, Gasparini P, Nunes V, Palacín M: Localization, by linkage analysis, of the cystinuria type III gene to chromosome 19q13.1. Am J Hum Genet 60: 611–616, 1997 [PMC free article] [PubMed] [Google Scholar]
- 4.Moe OW, Pearle MS, Sakhaee K: Pharmacotherapy of urolithiasis: Evidence from clinical trials. Kidney Int 79: 385–392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Font-Llitjós M, Jiménez-Vidal M, Bisceglia L, Di Perna M, de Sanctis L, Rousaud F, Zelante L, Palacín M, Nunes V: New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J Med Genet 42: 58–68, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dello Strologo L, Pras E, Pontesilli C, Beccia E, Ricci-Barbini V, de Sanctis L, Ponzone A, Gallucci M, Bisceglia L, Zelante L, Jimenez-Vidal M, Font M, Zorzano A, Rousaud F, Nunes V, Gasparini P, Palacín M, Rizzoni G: Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: A need for a new classification. J Am Soc Nephrol 13: 2547–2553, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C, Vester U, Wagner CA, Lahme S, Hesse A, Hoyer P, Lang F, Zerres K, Eggermann T, Arbeitsgemeinschaft für Pädiatrische Nephrologie : Significant contribution of genomic rearrangements in SLC3A1 and SLC7A9 to the etiology of cystinuria. Kidney Int 64: 1564–1572, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Pras E, Arber N, Aksentijevich I, Katz G, Schapiro JM, Prosen L, Gruberg L, Harel D, Liberman U, Weissenbach J, Pras M, Kastner DL: Localization of a gene causing cystinuria to chromosome 2p. Nat Genet 6: 415–419, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Feliubadaló L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, Golomb E, Centola M, Aksentijevich I, Kreiss Y, Goldman B, Pras M, Kastner DL, Pras E, Gasparini P, Bisceglia L, Beccia E, Gallucci M, de Sanctis L, Ponzone A, Rizzoni GF, Zelante L, Bassi MT, George AL, Jr., Manzoni M, De Grandi A, Riboni M, Endsley JK, Ballabio A, Borsani G, Reig N, Fernández E, Estévez R, Pineda M, Torrents D, Camps M, Lloberas J, Zorzano A, Palacín M, International Cystinuria Consortium : Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat Genet 23: 52–57, 1999 [DOI] [PubMed] [Google Scholar]
- 10.HGMD at the Institute of Medical Genetics in Cardiff: The Human Gene Mutation Database 2013. Available at: http://www.hgmd.org. Accessed October 10, 2014
- 11.Bisceglia L, Fischetti L, Bonis PD, Palumbo O, Augello B, Stanziale P, Carella M, Zelante L: Large rearrangements detected by MLPA, point mutations, and survey of the frequency of mutations within the SLC3A1 and SLC7A9 genes in a cohort of 172 cystinuric Italian patients. Mol Genet Metab 99: 42–52, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Eggermann T, Venghaus A, Zerres K: Cystinuria: An inborn cause of urolithiasis. Orphanet J Rare Dis 7: 19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harnevik L, Fjellstedt E, Molbaek A, Denneberg T, Söderkvist P: Mutation analysis of SLC7A9 in cystinuria patients in Sweden. Genet Test 7: 13–20, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Chillarón J, Font-Llitjós M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacín M: Pathophysiology and treatment of cystinuria. Nat Rev Nephrol 6: 424–434, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Gitomer WL, Reed BY, Ruml LA, Sakhaee K, Pak CY: Mutations in the genomic deoxyribonucleic acid for SLC3A1 in patients with cystinuria. J Clin Endocrinol Metab 83: 3688–3694, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Skopková Z, Hrabincová E, Stástná S, Kozák L, Adam T: Molecular genetic analysis of SLC3A1 and SLC7A9 genes in Czech and Slovak cystinuric patients. Ann Hum Genet 69: 501–507, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Chatzikyriakidou A, Louizou E, Dedousis GV, Bisceglia L, Michelakakis H, Georgiou I: An overview of SLC3A1 and SLC7A9 mutations in Greek cystinuria patients. Mol Genet Metab 95: 192–193, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Popovska-Jankovic K, Tasic V, Bogdanovic R, Miljkovic P, Golubovic E, Soylu A, Saraga M, Pavicevic S, Baskin E, Akil I, Gregoric A, Lilova M, Topaloglu R, Sukarova Stefanovska E, Plaseska-Karanfilska D: Molecular characterization of cystinuria in south-eastern European countries. Urolithiasis 41: 21–30, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Egoshi KI, Akakura K, Kodama T, Ito H: Identification of five novel SLC3A1 (rBAT) gene mutations in Japanese cystinuria. Kidney Int 57: 25–32, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Yuen YP, Lam CW, Lai CK, Tong SF, Li PS, Tam S, Kwan EY, Chan SY, Tsang WK, Chan KY, Mak WL, Cheng CW, Chan YW: Heterogeneous mutations in the SLC3A1 and SLC7A9 genes in Chinese patients with cystinuria. Kidney Int 69: 123–128, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Shigeta Y, Kanai Y, Chairoungdua A, Ahmed N, Sakamoto S, Matsuo H, Kim DK, Fujimura M, Anzai N, Mizoguchi K, Ueda T, Akakura K, Ichikawa T, Ito H, Endou H: A novel missense mutation of SLC7A9 frequent in Japanese cystinuria cases affecting the C-terminus of the transporter. Kidney Int 69: 1198–1206, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Wong KA, Mein R, Wass M, Flinter F, Pardy C, Bultitude M, Thomas K: The genetic diversity of cystinuria in a UK population of patients [published online ahead of print August 11, 2014]. BJU Int 10.1111/bju.12894 [DOI] [PubMed] [Google Scholar]
- 23.UK Registry for Rare Kidney Diseases (RaDaR): Renal RaDaR—The UK Registry for Rare Kidney Diseases 2014. Available at: https://www.renalradar.org/. Accessed October 20, 2014
- 24.Türk C, Knoll T, Petrik A, Sarica K, Skolarikos A, Straub M, Seitz C: Guidelines on Urolithiasis, 2014. Available at: http://uroweb.org/wp-content/uploads/22-Urolithiasis_LR.pdf. Accessed January 12, 2015
- 25.Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR, American Urological Assocation : Medical management of kidney stones: AUA guideline. J Urol 192: 316–324, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Soucy F, Ko R, Duvdevani M, Nott L, Denstedt JD, Razvi H: Percutaneous nephrolithotomy for staghorn calculi: a single center’s experience over 15 years. J Endourol 23: 1669–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Viprakasit DP, Sawyer MD, Herrell SD, Miller NL: Changing composition of staghorn calculi. J Urol 186: 2285–2290, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Cupisti A, Farnesi I, Armillotta N, Francesca F: Staghorn cystine stone in a 72-year-old recurrent calcium stone former. Clin Nephrol 78: 76–80, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Halperin EC, Thier SO, Rosenberg LE: The use of D-penicillamine in cystinuria: Efficacy and untoward reactions. Yale J Biol Med 54: 439–446, 1981 [PMC free article] [PubMed] [Google Scholar]
- 30.Becker G, Caring for Australians with Renal Impairment (CARI) : The CARI guidelines. Kidney stones: Cystine stones. Nephrology (Carlton) 12[Suppl 1]: S4–S10, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fattah H, Hambaroush Y, Goldfarb DS: Cystine nephrolithiasis. Transl Androl Urol 3: 228–233, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pak CY, Fuller C, Sakhaee K, Zerwekh JE, Adams BV: Management of cystine nephrolithiasis with alpha-mercaptopropionylglycine. J Urol 136: 1003–1008, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Brinkmann OA, Griehl A, Kuwertz-Bröking E, Bulla M, Hertle L: Extracorporeal shock wave lithotripsy in children. Efficacy, complications and long-term follow-up. Eur Urol 39: 591–597, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Landau EH, Shenfeld OZ, Pode D, Shapiro A, Meretyk S, Katz G, Katz R, Duvdevani M, Hardak B, Cipele H, Hidas G, Yutkin V, Gofrit ON: Extracorporeal shock wave lithotripsy in prepubertal children: 22-year experience at a single institution with a single lithotriptor. J Urol 182[Suppl]: 1835–1839, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Rare Kidney Stone Consortium: Cystinuria 2010. Available at: http://www.rarekidneystones.org/cystinuria/. Accessed January 12, 2015
- 36.Botzenhart E, Vester U, Schmidt C, Hesse A, Halber M, Wagner C, Lang F, Hoyer P, Zerres K, Eggermann T, Arbeitsgemeinschaft für Pädiatrische Nephrologie (APN) : Cystinuria in children: Distribution and frequencies of mutations in the SLC3A1 and SLC7A9 genes. Kidney Int 62: 1136–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Barbosa M, Lopes A, Mota C, Martins E, Oliveira J, Alves S, De Bonis P, Mota MC, Dias C, Rodrigues-Santos P, Fortuna AM, Quelhas D, Lacerda L, Bisceglia L, Cardoso ML: Clinical, biochemical and molecular characterization of cystinuria in a cohort of 12 patients. Clin Genet 81: 47–55, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Font MA, Feliubadaló L, Estivill X, Nunes V, Golomb E, Kreiss Y, Pras E, Bisceglia L, d’Adamo AP, Zelante L, Gasparini P, Bassi MT, George AL, Jr., Manzoni M, Riboni M, Ballabio A, Borsani G, Reig N, Fernández E, Zorzano A, Bertran J, Palacín M, International Cystinuria Consortium : Functional analysis of mutations in SLC7A9, and genotype-phenotype correlation in non-Type I cystinuria. Hum Mol Genet 10: 305–316, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.