Abstract
Background and objectives
Benefits of transvenous implantable cardioverter-defibrillators (ICDs) in prevention of sudden cardiac death among the general population are proven. However, the benefit of ICDs remains unclear in CKD. A propensity-matched analysis was conducted to examine the survival benefits of ICDs placed for primary prevention in those with CKD not on dialysis (eGFR<60 ml/min per 1.73 m2).
Design, setting, participants, & measurements
The Cleveland Clinic CKD registry was utilized to identify individuals who had an echocardiogram at the institution (between 2001 and October 2011). A propensity score of the likelihood of receiving an ICD was developed with the following variables: demographics, comorbid conditions, use of cardioprotective medications, eGFR, left ventricular ejection fraction, and ventricular arrhythmia. One-to-one greedy matching was used with 0.1 caliper width to match patients with and without an ICD. A Cox proportional hazards model was used to examine survival of matched patients with and without an ICD.
Results
This study included 1053 ICD patients and 9435 potential controls. Of 1053 ICD patients (60%), 631 were matched to the control group. During a median follow-up of 2.9 years (25th and 75th percentiles, 1.5, 4.7), 578 patients died. After adjusting for covariates, the hazard of mortality among propensity-matched patients was 0.69 (95% confidence interval [95% CI], 0.59 to 0.82) for the ICD group compared with the non-ICD group. A significant interaction was found between ICDs and eGFR (P=0.04). Presence of an ICD was associated with a lower risk of death among those with eGFRs of 45–59 ml/min per 1.73 m2 (hazard ratio [HR], 0.58; 95% CI, 0.44 to 0.77) and 30–44 ml/min per 1.73 m2 (HR, 0.65; 95% CI, 0.50 to 0.85), but not among those with eGFRs<30 ml/min per 1.73 m2 (HR, 0.98; 95% CI, 0.71 to 1.35).
Conclusions
Transvenous ICDs placed for primary prevention are associated with a survival benefit in those with stage 3 CKD, but not in those with stage 4 CKD.
Keywords: cardiovascular disease, defibrillator, CKD
Introduction
CKD is a worldwide public health problem that affects 13.1% of the American population (1). CKD is associated with increased cardiovascular mortality, particularly from sudden cardiac death (2–4). As noted in recent clinical trials, the benefit of implantable cardioverter-defibrillators (ICDs) in primary and secondary prevention of sudden cardiac death is well established in the general population with cardiovascular disease (5–8). The American College of Cardiology/American Heart Association clinical practice guidelines recommend placement of ICDs for secondary prevention in patients in which a reversible cause is not identified, and for primary prevention in patients with heart failure associated with prior myocardial infarction (ejection fraction ≤30%), cardiomyopathy (ejection fraction ≤35%), or intraventricular conduction delay (QRS≥120 milliseconds) (9–11).
Most of the clinical trials studying transvenous ICDs either excluded patients with advanced renal disease or did not report renal function details (12). Thus, evidence exploring the benefits of ICDs in those with nondialysis-dependent CKD remains scarce. Recent studies have shown that patients with an ICD and kidney disease have a higher mortality rate compared with those without kidney disease (13–16). However, the absence of control groups (comprising patients that did not receive an ICD) in most of these studies prevents us from drawing conclusions as to whether ICD therapy confers a survival benefit in patients with nondialysis-dependent CKD. Neither the American College of Cardiology/American Heart Association/Heart Rhythm Society nor the European Society of Cardiology guidelines address the particular indications of ICDs in patients with CKD. Therefore, the purpose of this study is to examine the mortality benefits of transvenous ICD placement in patients with different stages of CKD (eGFR<60 ml/min per 1.73 m2), an important question that suffers from a striking paucity of data.
Materials and Methods
Study Population
We conducted an analysis using our preexisting electronic health record (EHR)–based CKD registry. The development and validation of our EHR-based CKD registry at Cleveland Clinic were described in detail elsewhere (17). Patients who met the following criteria between January 1, 2005, and September 15, 2009, were considered for inclusion in this analysis: (1) had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider; (2) had two eGFR values <60 ml/min per 1.73 m2, calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation >90 days apart (18); and (3) for the ICD group in our current analysis, patients had an ICD implanted for primary prevention between January 1, 2001, and October 31, 2011. During the development of the CKD registry, patients aged <18 years and those who were diagnosed with ESRD needing dialysis or renal transplantation before CKD diagnosis were excluded.
Definitions and Outcome Measures
Renal Function.
To calculate eGFR, we applied the CKD-EPI equation to patients in our health system that had two outpatient serum creatinine levels between January 1, 2005, and September 15, 2009. All creatinine measurements were performed by the modified kinetic Jaffe reaction, using a Hitachi D2400 Modular Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) in our laboratory. All serum creatinine assays were standardized to isotope dilution mass spectrometry. CKD was defined according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines, as follows: stage 3 CKD (eGFR 30–59 ml/min per 1.73 m2), stage 4 CKD (eGFR 15–29 ml/min per 1.73 m2), and stage 5 CKD (eGFR<15 ml/min per 1.73 m2). We further categorized stage 3 into CKD stage 3a (eGFR 45–59 ml/min per 1.73 m2) and stage 3b (eGFR 30–44 ml/min per 1.73 m2).
ICD and Left Ventricular Ejection Fraction.
We identified patients with International Classification of Diseases, Ninth Revision, codes for ICD in the encounter diagnosis or problem list of the EHR at any time before October 31, 2011. We defined ICD by the codes V45.02 and V53.32. One of the investigators (G.N.) conducted a chart review to confirm the presence of an ICD and to obtain details about the left ventricular ejection fraction as well as the primary indication of the procedure for all patients.
Comorbid Conditions and Laboratory Parameters.
Demographic details were extracted from the EHR. Diabetes mellitus, hypertension, coronary artery disease, and other comorbid conditions were defined using prespecified criteria and validated in a previous publication (17). All comorbid conditions were defined at study entry.
Outcome Measures.
The primary outcome of interest (all-cause mortality) was ascertained from our EHRs and linkage of our CKD registry with the Social Security Death Index. Patients were followed from their date of study entry (date of second qualifying eGFR or first ICD) until October 31, 2011.
Statistical Analyses
We compared the baseline characteristics of patients with and without an ICD using t tests for continuous variables and chi-squared tests for categorical variables. We developed a propensity score of the likelihood of receiving an ICD, utilizing the following variables: age, sex, race, diabetes, hypertension, malignancy, body mass index, coronary artery disease, coronary revascularization, congestive heart failure, ventricular arrhythmia, cerebrovascular disease, eGFR, left ventricular ejection fraction, and use of renin-angiotensin system blockers, statins, and β-blockers. We used one-to-one greedy matching with 0.1 caliper width to match patients with an ICD to those without. We evaluated the resulting matched patients by graphing standardized differences of the variables included in the propensity score before and after the match. We also compared their characteristics with t tests for continuous variables and chi-squared tests for categorical ones.
We used a Kaplan–Meier curve to compare the survival of matched patients with and without ICDs at different eGFR intervals (<30 ml/min per 1.73 m2, 30–44 ml/min per 1.73 m2, and 45–59 ml/min per 1.73 m2). We also used unadjusted and adjusted Cox proportional hazard models, and we tested an interaction between the three eGFR intervals and ICDs. We fit an adjusted model including hemoglobin and albumin, which were not included in the propensity match, as well as age and ventricular arrhythmia, which were included in the propensity match but remained slightly imbalanced after matching. We tested an interaction between ICD and eGFR of 45–59 ml/min per 1.73 m2 versus eGFR of 30–44 ml/min per 1.73 m2 versus eGFR<30 ml/min per 1.73 m2. To account for the matched nature of the data, we used the covariance sandwich estimator in the Cox proportional hazards models. For the survival models, patients with no ICD had inception date at second eGFR<60 ml/min per 1.73 m2. Patients with an ICD had their inception at second eGFR<60 ml/min per 1.73 m2 or ICD placement, whichever was last. Because patients who had an ICD (before and after development of CKD) were included in the primary analysis, we performed two different sensitivity analyses. In the first sensitivity analysis, we evaluated the associations of ICDs with mortality exclusively among patients who had their ICD placed after second eGFR<60 ml/min per 1.73 m2, along with their respective controls. In this sensitivity analysis, inception for the ICD group was date of ICD placement. Similarly, we evaluated the associations of ICDs with mortality exclusively among patients who had their ICD placed before second eGFR<60 ml/min per 1.73 m2 along with their respective controls. In this sensitivity analysis, inception for the ICD group was date of second eGFR<60 ml/min per 1.73 m2. To evaluate the effect of excluding unmatched patients with an ICD from analysis, we also fit a Cox model on all participants with ICDs and all potential controls while adjusting for age, sex, race, diabetes, hypertension, malignancy, body mass index, coronary artery disease, coronary revascularization, congestive heart failure, ventricular arrhythmia, cerebrovascular disease, eGFR, left ventricular ejection fraction, hemoglobin, albumin, and use of renin-angiotensin system blockers, statins, and β-blockers. In addition, we tested the interaction between ICDs and the three eGFR groups. In a sensitivity analysis, we also fitted a similar model in the unmatched data while excluding all patients with baseline malignancy. Fourteen percent of patients were missing albumin and 7% were missing hemoglobin. Mean value imputation was used in adjusted models while including dummy indicators for missing data, and complete case analysis was also performed that yielded similar results. In a sensitivity analysis, we censored patients at the time of transitioning to dialysis to ensure that the analysis represents only nondialysis-dependent CKD. All patients who survived until September 15, 2009, (last date United States Renal Data System data were available for our CKD registry) were censored on that date for mortality analyses.
Results
Baseline Characteristics
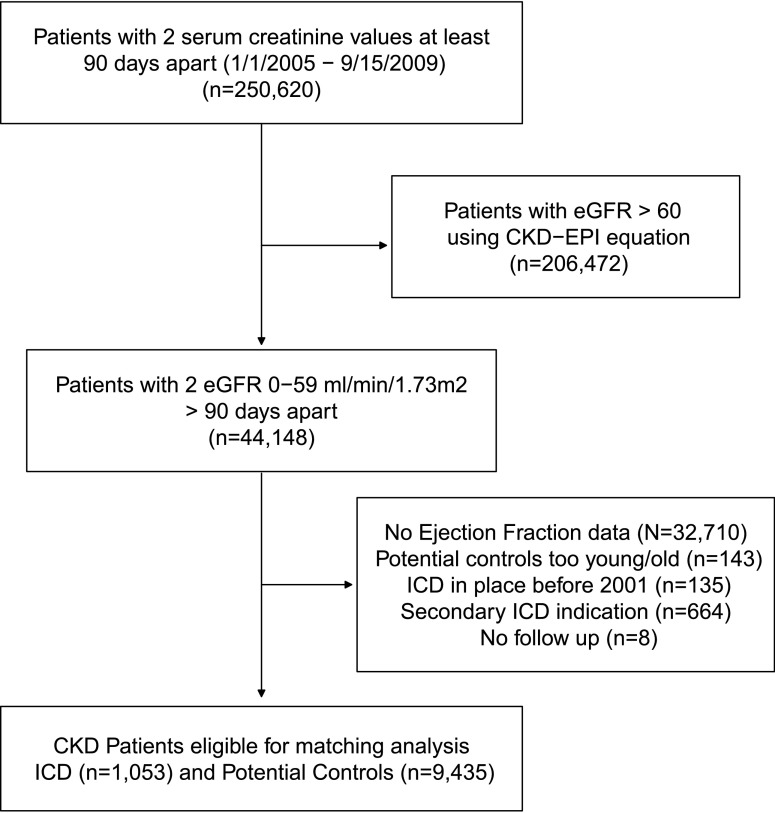
We included 1053 patients who had an ICD placed for primary prevention (Figure 1). The median time between ICD and the eGFR used in the study was 7 months, with the 25th and 75th percentiles being 1 and 21 months, respectively. We identified 9435 potential controls for those with an ICD. The overall mean age was 71.1±11.4 years. Eighteen percent of patients were black and 32% were women. Table 1 shows patient characteristics before the propensity matching. Patients with an ICD were more likely to be younger, were more likely to be men, and were more likely to have lower ejection fraction, diabetes, congestive heart failure, and coronary artery disease compared with those with no ICD (Table 1). As expected, there was a higher incidence of arrhythmia in the ICD group.
Figure 1.
Flow chart showing how patients were selected for this analysis. CKD-EPI, CKD Epidemiology Collaboration; ICD, implantable cardioverter defibrillator.
Table 1.
Patient characteristics by presence of an ICD before propensity match
| Factor | Patients (n) | Non-ICD Group (n=9435) | ICD Group (n=1053) | P Value |
|---|---|---|---|---|
| Age (yr) | 10,488 | 72.0±11.7 | 69.6±11.5 | <0.001 |
| Men | 10,488 | 4770 (50.6) | 748 (71.0) | <0.001 |
| Black race | 10,488 | 1526 (16.2) | 173 (16.4) | 0.83 |
| BMI (kg/m2) | 10,182 | 29.2±6.6 | 28.9±5.9 | 0.21 |
| BMI group (kg/m2) | 10,488 | <0.001 | ||
| <18.5 | 129 (1.4) | 6 (0.57) | ||
| 18.5–24.9 | 2370 (25.1) | 282 (26.8) | ||
| 25–29.9 | 3263 (34.6) | 368 (34.9) | ||
| ≥30 | 3372 (35.7) | 392 (37.2) | ||
| Missing | 301 (3.2) | 5 (0.47) | ||
| eGFR (ml/min per 1.73 m2) | 10,488 | 44.6±12.0 | 43.5±11.3 | 0.004 |
| CKD stage, by eGFR (ml/min per 1.73 m2) | 10,488 | <0.001 | ||
| 45–59 | 5408 (57.3) | 532 (50.5) | ||
| 30–44 | 2751 (29.2) | 374 (35.5) | ||
| 15–29 | 1081 (11.5) | 141 (13.4) | ||
| <15 | 195 (2.1) | 6 (0.57) | ||
| Diabetes | 10,488 | 2136 (22.6) | 322 (30.6) | <0.001 |
| Hypertension | 10,488 | 8041 (85.2) | 802 (76.2) | <0.001 |
| Congestive heart failure | 10,488 | 1349 (14.3) | 698 (66.3) | <0.001 |
| Coronary artery disease | 10,488 | 2875 (30.5) | 602 (57.2) | <0.001 |
| Cerebrovascular disease | 10,488 | 1165 (12.3) | 124 (11.8) | 0.59 |
| Peripheral vascular disease | 10,488 | 322 (3.4) | 54 (5.1) | 0.01 |
| Coronary revascularization | 10,488 | 590 (6.3) | 181 (17.2) | <0.001 |
| Ventricular arrhythmia | 10,488 | 41 (0.43) | 72 (6.8) | <0.001 |
| Albumin (g/dl) | 8967 | 4.0±0.52 | 4.1±0.48 | 0.001 |
| Hemoglobin (g/dl) | 9595 | 12.4±1.9 | 12.6±1.9 | <0.001 |
| Malignancy | 10,488 | 2284 (24.2) | 145 (13.8) | <0.001 |
| LVEF (%) | 10,488 | 53.6±10.8 | 24.0±8.3 | <0.001 |
| Use of ACEIs/ARBs | 10,488 | 6600 (70.0) | 989 (93.9) | <0.001 |
| Use of statins | 10,488 | 5790 (61.4) | 840 (79.8) | <0.001 |
| Use of β-blockers | 10,488 | 6673 (70.7) | 996 (94.6) | <0.001 |
| Smoking status | 10,488 | 0.72 | ||
| No | 7670 (81.3) | 855 (81.2) | ||
| Yes | 591 (6.3) | 72 (6.8) | ||
| Missing | 1174 (12.4) | 126 (12.0) | ||
| Insurance group | 10,488 | 0.84 | ||
| Private | 1671 (17.7) | 182 (17.3) | ||
| Medicare/Medicaid | 7545 (80.0) | 844 (80.2) | ||
| Missing | 219 (2.3) | 27 (2.6) |
Data are given as n (%) or means±SD unless otherwise indicated. ICD, implantable cardioverter-defibrillator; BMI: body mass index; LVEF, left ventricular ejection fraction; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Matching
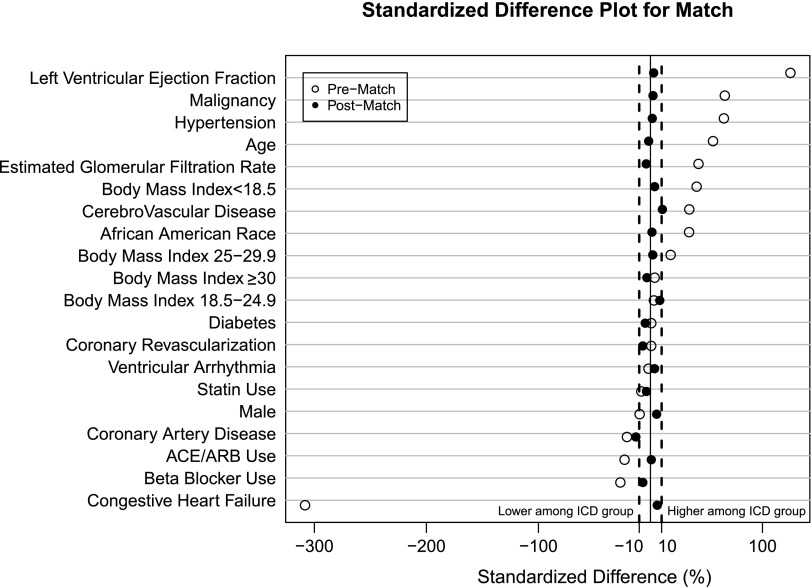
The logistic model for the propensity score had a c-statistic of 0.97. We were able to match 631 of 1053 patients (60%) with an ICD with 0.1 calipers. After the match, all variables had standardized differences <10%, except age and ventricular arrhythmia, which had a standardized difference of 12% and 11%, respectively (Figure 2). Table 2 displays the characteristics of the matched group.
Figure 2.
Standardized difference plot for match. ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICD, implantable cardioverter defibrillator.
Table 2.
Patient characteristics by presence of ICD after propensity match
| Factor | Non-ICD Group (n=631) | ICD Group (n=631) | P Value |
|---|---|---|---|
| Age | 72.0±10.8 | 70.5±11.6 | 0.02 |
| Men | 439 (69.6) | 428 (67.8) | 0.50 |
| Black race | 128 (20.3) | 111 (17.6) | 0.22 |
| BMI (kg/m2) | 28.4±6.1 | 28.9±6.0 | 0.19 |
| BMI group (kg/m2) | 0.65 | ||
| <18.5 | 6 (0.95) | 4 (0.63) | |
| 18.5–24.9 | 182 (28.8) | 173 (27.4) | |
| 25–29.9 | 233 (36.9) | 219 (34.7) | |
| ≥30 | 206 (32.6) | 231 (36.6) | |
| Missing | 4 (0.63) | 4 (0.63) | |
| eGFR (ml/min per 1.73 m2) | 42.2±11.8 | 42.8±11.6 | 0.33 |
| CKD stage, by eGFR (ml/min per 1.73 m2) | 0.11 | ||
| 45–59 | 305 (48.3) | 303 (48.0) | |
| 30–44 | 219 (34.7) | 227 (36.0) | |
| 15–29 | 91 (14.4) | 96 (15.2) | |
| <15 | 16 (2.5) | 5 (0.79) | |
| Diabetes | 186 (29.5) | 192 (30.4) | 0.71 |
| Hypertension | 486 (77.0) | 488 (77.3) | 0.89 |
| Congestive heart failure | 338 (53.6) | 347 (55.0) | 0.61 |
| Coronary artery disease | 338 (53.6) | 333 (52.8) | 0.78 |
| Cerebrovascular disease | 82 (13.0) | 90 (14.3) | 0.51 |
| Peripheral vascular disease | 33 (5.2) | 31 (4.9) | 0.80 |
| Coronary revascularization | 88 (13.9) | 91 (14.4) | 0.81 |
| Ventricular arrhythmia | 18 (2.9) | 31 (4.9) | 0.06 |
| Albumin (g/dl) | 4.0±0.50 | 4.0±0.50 | 0.04 |
| Hemoglobin (g/dl) | 12.3±1.9 | 12.5±1.9 | 0.02 |
| LVEF (%) | 26.7±9.2 | 27.2±8.2 | 0.28 |
| Malignancy | 115 (18.2) | 99 (15.7) | 0.23 |
| Use of ACEIs/ARBs | 573 (90.8) | 576 (91.3) | 0.77 |
| Use of statins | 480 (76.1) | 490 (77.7) | 0.50 |
| Use of β-blockers | 577 (91.4) | 581 (92.1) | 0.68 |
| Smoking | 0.42 | ||
| No | 488 (77.3) | 506 (80.2) | |
| Yes | 51 (8.1) | 48 (7.6) | |
| Missing | 92 (14.6) | 77 (12.2) | |
| Insurance | 0.15 | ||
| Private | 129 (20.4) | 107 (17.0) | |
| Medicare/Medicaid | 481 (76.2) | 509 (80.7) | |
| Missing | 21 (3.3) | 15 (2.4) |
Data are given as n (%) or means±SD unless otherwise indicated. ICD, implantable cardioverter-defibrillator; BMI: body mass index; LVEF, left ventricular ejection fraction; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Outcomes
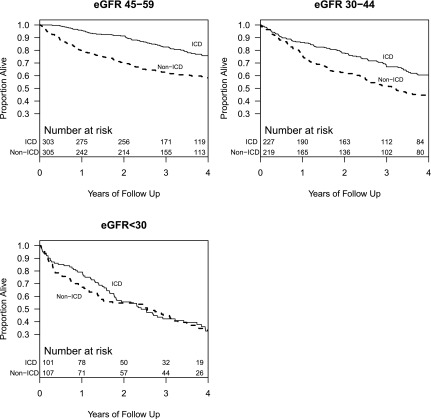
Among the 1262 matched cases and controls, there were 578 deaths during a median follow-up of 2.9 years (25th and 75th percentiles, 1.5, 4.7). Figure 3 shows a Kaplan–Meier plot of survival by ICD among matched patients with different eGFR categories. After propensity score matching, ICD was associated with significantly lower mortality among those with an eGFR<60 ml/min per 1.73 m2 in both the unadjusted and adjusted models (Table 3). In the unadjusted model, presence of an ICD was associated with a hazard ratio (HR) of 0.65 (95% confidence interval [95% CI], 0.55 to 0.77). In the adjusted model, presence of an ICD was associated with a HR of 0.69 (95% CI, 0.59 to 0.82). We found a significant interaction (P=0.04) between ICD and an eGFR of 45–59 ml/min per 1.73 m2 and an eGFR of 30–44 ml/min per 1.73 m2, in which patients with an ICD and an eGFR within these two intervals had a significantly lower hazard of mortality, with HRs of 0.58 (95% CI, 0.44 to 0.77) and 0.65 (95% CI, 0.50 to 0.85), respectively. No such association was noted among those with an eGFR<30 ml/min per 1.73 m2 (Table 3).
Figure 3.
Kaplan–Meier curves showing survival of those with and without an implantable cardioverter defibrillator for various eGFR categories. ICD, implantable cardioverter defibrillator.
Table 3.
Associations of ICD with death among propensity-matched patients
| Association | Unadjusted Model | Adjusted Modela |
|---|---|---|
| ICD (versus no ICD)—overall | 0.65 (0.55 to 0.77) | 0.69 (0.59 to 0.82) |
| ICD interaction with eGFR stage (ml/min per 1.73 m2) | ||
| 45–59 | 0.55 (0.42 to 0.73) | 0.58 (0.44 to 0.77) |
| 30–44 | 0.64 (0.49 to 0.83) | 0.65 (0.50 to 0.85) |
| <30 | 0.92 (0.67 to 1.27) | 0.98 (0.71 to 1.35) |
Data are presented as hazard ratios (95% confidence intervals). ICD, implantable cardioverter-defibrillator.
Adjusted for age, ventricular arrhythmia, hemoglobin, albumin, and indicators for missing values.
Sensitivity Analyses
In the adjusted Cox proportional hazards model that included only patients who had ICDs placed after second eGFR<60 ml/min per 1.73 m2 along with their corresponding controls (n=602), ICD was associated with lower mortality (HR, 0.73; 95% CI, 0.57 to 0.93). The interaction between ICD and eGFR stage did not reach significance (P=0.20), but the effect estimates were in the same direction as in the primary analysis (eGFR 45–59 ml/min per 1.73 m2: HR, 0.54; 95% CI, 0.34 to 0.84; eGFR 30–44 ml/min per 1.73 m2: HR, 0.70; 95% CI, 0.48 to 1.04; and eGFR<30 ml/min per 1.73 m2: HR, 0.94; 95% CI, 0.61 to 1.45).
In another adjusted Cox proportional hazards model, we included patients who had an ICD before they developed CKD (two eGFR values <60 ml/min/per 1.73 m2 90 days apart) and controls. In this subset (n=660), presence of an ICD was associated with lower mortality (HR, 0.66; 95% CI, 0.52 to 0.84; P<0.001). The interaction between ICD and eGFR stage did not reach significance (P=0.11), but the effect estimates were in the same direction as in the primary analysis (eGFR of 45–59 ml/min per 1.73 m2: HR, 0.62; 95% CI, 0.43 to 0.91; eGFR of 30–44 ml/min per 1.73 m2: HR, 0.62; 95% CI, 0.43 to 0.90; and eGFR<30 ml/min per 1.73 m2: HR, 1.14; 95% CI, 0.68 to 1.91).
In the adjusted Cox proportional hazards model that included all patients with an ICD and all potential controls without matching (n=10,488 and n=3979 deaths, respectively), we also found a significant interaction between ICD and eGFR stage (P=0.01). Presence of an ICD was associated with lower mortality among patients with an eGFR of 45–59 ml/min per 1.73 m2 (HR, 0.68; 95% CI, 0.57 to 0.82) and an eGFR of 30–44 ml/min per 1.73 m2 (HR, 0.65; 95% CI, 0.54 to 0.78), but no such association was noted among those with an eGFR<30 ml/min per 1.73 m2 (HR, 1.0; 95% CI, 0.79 to 1.25). In a sensitivity analysis including only patients with no prior history of baseline malignancy (n=8059), similar results were noted (data not shown). In a sensitivity analysis by censoring at the time of transitioning to dialysis, similar results were seen (eGFR of 45–59 ml/min per 1.73 m2: HR, 0.36; 95% CI, 0.24 to 0.52; eGFR of 30–44 ml/min per 1.73 m2: HR, 0.55; 95% CI, 0.38 to 0.77; eGFR<30 ml/min per 1.73 m2: HR, 0.85; 95% CI, 0.54 to 1.33).
Discussion
A significant proportion of patients with kidney disease die of cardiovascular disease before reaching dialysis (19). More importantly, sudden cardiac death contributes to a significant proportion of these deaths; thus, ICDs are postulated to confer survival advantage to this population. In this propensity-matched study of patients with CKD who had similar left ventricular and kidney function, we report that the presence of an ICD was associated with lower mortality in those with stage 3 CKD, but not among those with stage 4 CKD.
Patients with stages 1 and 2 CKD who receive an ICD tend to have similar survival advantage compared with those with preserved kidney function (20). On the other hand, patients with ESRD do not appear to have similar survival benefit when ICDs were implanted for primary prevention (21). However, only few studies examined the benefits of ICD placement in a nondialysis-dependent CKD population, and it seems that much of the decision-making regarding device therapy in this group is not based on clinical trial evidence. Among those with an ICD, a decrement in kidney function was associated with increased mortality (12%–16% increased mortality for each 10-ml/min per 1.73 m2 lower eGFR) (14,22). More recently, Singh et al. reported that the presence of an ICD in those with stage 4 CKD (n=108) was not associated with lower mortality (23). Another study examining the National Cardiovascular Data Registry ICD registry reported that the risk of death after primary prevention ICD placement is proportional to CKD severity (24). However, the lack of a control group of patients with CKD without an ICD precludes us from excluding the potential benefits of ICDs in this population. In a patient-level meta-analysis of seven clinical trials, Pun et al. showed that the differences in baseline eGFR decrease the survival benefits of those receiving an ICD (25). However, very few patients with an eGFR<30 ml/min per 1.73 m2 were included in this analysis, arguing for additional studies.
In our study, survival benefit was noted in those with stage 3 CKD, a category that constitutes the vast majority of nondialysis-dependent CKD. Kidney disease increases the risk of arrhythmic complications to an extent that cannot be explained by the severity of the atherosclerotic process (26,27). Several underlying pathophysiologic processes, including electrolyte imbalance, autonomic disturbances, and uremic cardiomyopathy, may explain the higher risk of sudden cardiac death (26). Although the magnitude of benefit might be lower with decline in kidney function, the observed protective associations by us and others suggest the utility of transvenous ICDs in this large segment of the nondialysis-dependent CKD population (25,28).
However, we did not observe a similar protective association of ICDs among the stage 4 CKD population. A recent pooled analysis of clinical trials that examined the benefits of ICDs noted that the presence of higher comorbidities (four versus two) was associated with higher mortality rates in those with an ICD (16). This report argued for real-world outcomes data of medically complex patients receiving ICDs. Patients with CKD (particularly with advanced kidney disease) sustain higher comorbidity burden, and as such could explain the lack of association noted in those with stage 4 CKD. We matched the ICD group based on several different comorbidities, including cardiac and kidney function, and had a reasonable number of patients with stage 4 CKD. The preponderance of nonarrhythmic deaths in those with advanced kidney disease (stage 4 CKD) and heart failure might explain the lack of association in stage 4 CKD. Deaths in our cohort were not adjudicated for arrhythmic versus other causes of death and future studies should examine such differences.
Apart from higher costs, other issues merit consideration when deciding about ICD implantation in patients with kidney disease. Procedure-related complications, particularly hematoma at the site of ICD implantation, are higher in those with kidney disease than those without (29). We recently noted that ICD-related infections among dialysis patients were associated with higher length of stay and mortality (30). Although it is unknown whether this higher risk is present in individuals with nondialysis-dependent CKD, recent studies have shown higher risk for infections in this population and this should be also be taken into consideration before ICD placement in these patients (31).
Strengths of this analysis include the large sample size (both for stage 3 and stage 4 CKD), the diverse patient population, and the availability of comprehensive data, including the cardiovascular and kidney function data for the study cohort. Previous studies lacked details related to patients who progressed to dialysis, and our sensitivity analysis findings further confirm the protective associations of ICDs in those with stage 3 CKD. We attempted to avoid the inherent bias of an observational study by using a propensity matched cohort, and the c-statistic of the logistic model for the propensity score was 0.97, suggesting the reliability of the model. However, our study is subject to other limitations, including the fact that the data are primarily from a single major health care system. The decision to place an ICD is often complex, and residual confounding might still be present despite the propensity matching. Furthermore, we lacked details about prior history of myocardial infarction, hospitalization details for heart failure and other reasons, and admissions to a nursing home or skilled nursing facility. Higher urinary protein excretion is associated with higher cardiovascular mortality. We did not have details about proteinuria for all of the study participants; hence, this was not included in the propensity matching. We were only able to match 62% of patients who received an ICD. There were still differences between groups on some variables (e.g., age and ventricular arrhythmia), which we adjusted for in the final Cox proportional hazards model. In addition, we conducted a Cox proportional hazards model that included all patients, which yielded similar results. The control group had similar left ventricular ejection fraction and comorbid conditions but did not receive ICDs, which suggests that this group could be sicker or this treatment difference could be related to provider bias. However, we included several key medical problems, including patient nutritional status details, in the analysis to account for this. We also lacked cause-of-death details to determine whether the survival benefit relates to improvements in arrhythmic deaths, which could be a topic of future investigations.
In summary, in a large cohort matched for demographics, comorbidities, and cardiac and kidney function, presence of an ICD was associated with lower mortality in those with stage 3 CKD, but not in those with stage 4 CKD. Although cumulative evidence supports the benefits of ICDs in stage 3 CKD, further clinical trials examining the benefits and other complications of ICDs in this population are warranted to support ICD placement in the stage 4 CKD population.
Disclosures
B.L.W. served on the Physician Advisory Boards for Medtronic, St. Jude Medical, Boston Scientific, and Spectranetics.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01-DK101500 to S.D.N., DK094112 to J.V.N., and 1K23-DK091363 to S.E.J.).
The creation of the Cleveland Clinic CKD registry was funded by an unrestricted grant from Amgen Inc. to the Cleveland Clinic Department of Nephrology and Hypertension Research and Education Fund.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Part of the data in this article were presented as a poster at the American Society of Nephrology annual meeting, held November 11–16, 2014, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Shades of Grey: The Conundrum of Implantable Defibrillators in Individuals with Advanced CKD,” on pages 1107–1109.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP: Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 76: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Shamseddin MK, Parfrey PS: Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat Rev Nephrol 7: 145–154, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M, Multicenter Automatic Defibrillator Implantation Trial Investigators : Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 335: 1933–1940, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators : Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877–883, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators : Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352: 225–237, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmeister JM, Estes NA, 3rd, Garlitski AC: Prevention of sudden cardiac death in patients with chronic kidney disease: Risk and benefits of the implantable cardioverter defibrillator. J Interv Card Electrophysiol 35: 227–234, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines : 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147–e239, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW: ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: A report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 61: 1318–1368, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, European Heart Rhythm Association. Heart Rhythm Society. American College of Cardiology. American Heart Association Task Force. European Society of Cardiology Committee for Practice Guidelines : ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 48: e247–e346, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Cannizzaro LA, Piccini JP, Patel UD, Hernandez AF: Device therapy in heart failure patients with chronic kidney disease. J Am Coll Cardiol 58: 889–896, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Cuculich PS, Sánchez JM, Kerzner R, Greenberg SL, Sengupta J, Chen J, Faddis MN, Gleva MJ, Smith TW, Lindsay BD: Poor prognosis for patients with chronic kidney disease despite ICD therapy for the primary prevention of sudden death. Pacing Clin Electrophysiol 30: 207–213, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Turakhia MP, Varosy PD, Lee K, Tseng ZH, Lee R, Badhwar N, Scheinman M, Lee BK, Olgin JE: Impact of renal function on survival in patients with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol 30: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Robin J, Weinberg K, Tiongson J, Carnethon M, Reddy M, Ciaccio C, Quadrini M, Hsu J, Fan J, Choi P, Kadish A, Goldberger J, Passman R: Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm 3: 1196–1201, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Steinberg BA, Al-Khatib SM, Edwards R, Han J, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Hallstrom A, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Inoue LY, Sanders GD: Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail 2: 623–629, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J, Lyons J, Simon JF, Schreiber MJ, Jr, Jain A, Nally JV, Jr: Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol 6: 40–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Makki N, Swaminathan PD, Hanmer J, Olshansky B: Do implantable cardioverter defibrillators improve survival in patients with chronic kidney disease at high risk of sudden cardiac death? A meta-analysis of observational studies. Europace 16: 55–62, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Sakhuja R, Keebler M, Lai TS, McLaughlin Gavin C, Thakur R, Bhatt DL: Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol 103: 735–741, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB, Multicenter Automatic Defibrillator Implantation Trial-II Investigators : Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 98: 485–490, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Singh SM, Wang X, Austin PC, Parekh RS, Lee DS, Ontario ICD Database Investigators : Prophylactic defibrillators in patients with severe chronic kidney disease. JAMA Intern Med 174: 995–996, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Hess PL, Hellkamp AS, Peterson ED, Sanders GD, Al-Khalidi HR, Curtis LH, Hammill BG, Pun PH, Curtis JP, Anstrom KJ, Hammill SC, Al-Khatib SM: Survival after primary prevention implantable cardioverter-defibrillator placement among patients with chronic kidney disease. Circ Arrhythm Electrophysiol 7: 793–799, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pun PH, Al-Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Hallstrom A, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Hess PL, Inoue LY, Sanders GD: Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: A meta-analysis of patient-level data from 3 randomized trials. Am J Kidney Dis 64: 32–39, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitman IR, Feldman HI, Deo R: CKD and sudden cardiac death: Epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 23: 1929–1939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulikakos D, Banerjee D, Malik M: Risk of sudden cardiac death in chronic kidney disease. J Cardiovasc Electrophysiol 25: 222–231, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Charytan DM, Reynolds MR: Do implantable defibrillators help patients with CKD? Am J Kidney Dis 64: 4–6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buiten MS, De Bie MK, Van Der Heijden AC, Rotmans JI, Bootsma M, Marc Groeneveld JH, Wolterbeek R, Rabelink TJ, Jukema JW, Schalij MJ, Van Erven L: Chronic kidney disease and implantable cardioverter defibrillator related complications: 16 years of experience. J Cardiovasc Electrophysiol 25: 998–1004, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Opelami O, Sakhuja A, Liu X, Tang WH, Schold JD, Navaneethan SD: Outcomes of infected cardiovascular implantable devices in dialysis patients. Am J Nephrol 40: 280–287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloom H, Heeke B, Leon A, Mera F, Delurgio D, Beshai J, Langberg J: Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 29: 142–145, 2006 [DOI] [PubMed] [Google Scholar]