Abstract
Background and objectives
Outcomes-based research rarely focuses on patients with ESRD caused by GN. The hypotheses were that the GN subtype would clinically discriminate patient groups and independently associate with survival after ESRD therapy initiation.
Design, setting, participants, & measurements
Data were extracted from the US Renal Data System for adult patients with incident (1996–2011) ESRD attributed to six GN subtypes: FSGS, IgA nephropathy (IgAN), membranous nephropathy, membranoproliferative glomeruonephritis, lupus nephritis (LN), and vasculitis. ESRD attributed to diabetes and autosomal dominant polycystic kidney disease served as non-GN comparators. Unadjusted and adjusted mortality hazard ratios (aHRs) with 95% confidence intervals (95% CIs) were estimated using Cox regression (reference, IgAN). Models sequentially adjusted for sociodemographic (model 2), comorbidity/laboratory (model 3), and ESRD treatment modality (model 4) variables.
Results
Among 84,301 patients with ESRD attributed to GN, the median age ranged from 39 (LN) to 66 (vasculitis) years, male sex ranged from 18% (LN) to 68% (IgAN), and black race ranged from 7% (IgAN) to 49% (LN). Patients with IgAN had the fewest comorbidities and lowest use of hemodialysis (70.1%). After a median follow-up of 2.5 (interquartile range, 1.0–4.9) years, crude mortality was lowest in IgAN (3.7 deaths/100 person years). Compared to IgAN, adjusted mortality was highest in LN (model 4 aHR=1.75; 95% CI, 1.68 to 1.83) and in diabetes (aHR=1.73; 95% CI, 1.67 to 1.79), and was also higher in all other GN subtypes (membranous nephropathy: aHR=1.23; 95% CI, 1.17 to 1.29; FSGS: aHR=1.37; 95% CI, 1.32 to 1.42; membranoproliferative GN: aHR=1.38; 95% CI, 1.31 to 1.45; vasculitis: aHR=1.51; 95% CI, 1.45 to 1.58) and in autosomal dominant polycystic kidney disease (aHR=1.22; 95% CI, 1.18 to 1.27).
Conclusions
This study exposes substantial heterogeneity across GN subtypes at ESRD therapy initiation and identifies independent associations between GN subtype and post-ESRD mortality. These survival discrepancies warrant further study, and the utility of current research practice to group GN subtypes together when evaluating ESRD outcomes should be questioned.
Keywords: glomerulonephritis, epidemiology and outcomes, end-stage renal disease, United States Renal Data System, IgA nephropathy
Introduction
GN is the third most common cause of ESRD in patients initiating dialysis or receiving a kidney transplant in the United States. In the most recent Annual Data Report of the US Renal Data System (USRDS), GN accounted for 6.3% of patients initiating ESRD therapy in 2011, trailing only diabetes (43.9%) and hypertension (27.8%), and patients with GN comprised 14.3% of the prevalent treated ESRD population (1). Treatment of patients with ESRD caused by GN incurs an estimated annual cost to Medicare alone of almost $3 billion (1). Mortality in GN increases dramatically after the onset of ESRD (2–5); however, the relative contributions from generic ESRD-related factors and from the underlying cause of GN to this finding have not formally been evaluated.
The USRDS adopts GN as one of four major cause-of-ESRD stratification variables in published reports, along with diabetes, hypertension, and cystic kidney disease. However, when juxtaposing the characteristics and outcomes of patients with GN to those with other causes of ESRD, it is important to consider that GN is not a single disease entity but rather a broad disease category comprised of histologically and clinically distinct GN subtypes. Alongside differing renal manifestations, heterogeneity across GN subtypes with respect to systemic comorbidities and mortality has been identified in non-ESRD populations (6,7). Whether these phenotypic and prognostic distinctions diverge or converge after ESRD development remains largely unknown.
We conducted this study to examine differences among GN subtypes with respect to demographic and clinical attributes at presentation to ESRD and prognosis after initiation of ESRD therapy. We posit that exploring any such differences would serve to elucidate and quantify long-term disease-specific risks, explain post-ESRD survival discrepancies between GN and non-GN patient groups, and facilitate the development of a more individualized and equitable patient care approach.
Materials and Methods
Study Population and Data Sources
All adult patients aged ≥18 years who initiated ESRD therapy with hemodialysis, peritoneal dialysis, or kidney transplantation between January 1, 1996, and December 31, 2011, were retrospectively identified from the USRDS, a national registry of almost all patients with treated ESRD. Patients with ESRD attributed to one of six common GN subtypes—FSGS, IgA nephropathy (IgAN), membranous nephropathy (MN), membranoproliferative GN (MPGN), lupus nephritis (LN), and vasculitis—were selected as the principal study cohort. These patients were identified using cause of ESRD diagnostic codes (Supplemental Appendix) obtained from Medical Evidence Reports (form CMS-2728). These reports are submitted by attending nephrologists to the Centers for Medicare and Medicaid Services within 45 days of a patient commencing ESRD therapy. Patients with ESRD attributed to diabetes (DN) and autosomal dominant polycystic kidney disease (ADPKD) were selected as non-GN comparator groups. Missing or uncertain cause of ESRD or a defined cause other than the eight of interest were the sole study exclusion criteria.
Patient Characteristics
Baseline sociodemographic and clinical data were extracted from USRDS Patient and Medical Evidence files. Age, sex, race (white, black, Asian, or other), Hispanic ethnicity (yes/no), Medicaid insurance (yes/no), and geographic region (Northeast, Midwest, South, or West) were selected as sociodemographic variables. Initial ESRD therapy modalities were defined as hemodialysis, peritoneal dialysis, or kidney transplantation, using USRDS-defined codes (Supplemental Appendix). Date of first kidney transplantation was also obtained for patients initiating ESRD therapy with dialysis. Baseline comorbidities (reported in Medical Evidence Reports to be present currently or within the last 10 years) included DN, heart failure, coronary heart disease, cerebrovascular disease, hypertension, chronic obstructive pulmonary disease, current smoking, cancer, peripheral vascular disease, and nonambulant status (unable to ambulate or transfer). Laboratory values (reported in Medical Evidence Reports as measured within 45 days before commencing ESRD therapy) included albumin, hemoglobin, and creatinine (used to calculate Modification of Diet in Renal Disease [MDRD] eGFR).
Outcomes
Death was our primary study outcome. Cause of death (reported on CMS-2746 Death Notification Forms), using collapsed USRDS-defined categories (Supplemental Appendix), was a secondary outcome. Date and cause of death were ascertained from USRDS Patient files. Patients were censored at the end of study (January 1, 2012).
Statistical Analyses
Cross-tabulation and distribution plots were used to examine unadjusted differences in baseline characteristics between groups. Categorical variables were summarized as frequencies and proportions, and continuous variables were summarized as medians and interquartile ranges or means±SDs, as appropriate. Differences in mortality were examined using time-to-event analysis. Cumulative survival curves were derived by the Kaplan–Meier method and compared using the log-rank test. Mortality hazard ratios (HRs) with 95% confidence intervals (95% CIs) were estimated using Cox proportional hazards regression, stratified by year of ESRD therapy initiation, with IgAN as the referent group. Model 1 was unadjusted, model 2 was adjusted for sociodemographic characteristics (age, sex, race, ethnicity, and Medicaid insurance), and model 3 included additional adjustment for baseline comorbidity and laboratory variables. To adjust for ESRD therapy modality at baseline and subsequent access to transplantation, we fitted a fourth model (model 4) that added to model 3 baseline modality as a fixed covariate and post-ESRD transplantation as a time-dependent covariate. This allowed patients who initiated ESRD therapy with dialysis but later received a transplant to obtain a second ESRD treatment record starting on the transplant date. Squared terms were included for all continuous variables (age and laboratory values). Proportionality was examined using plotted log (-log) survival curves.
Approximately 32% of patients had at least one missing variable. To handle these missing data, we assumed them to be missing at random and used standard multiple imputation techniques to impute up to 32 datasets (8). In addition to including all model 4 covariates, the imputation model included the event indicator and the Nelson–Aalen estimator of the cumulative marginal hazard H(T), where T is the time to event or censoring (9). Imputations were performed separately by year of ESRD therapy initiation and assumed a joint modeling approach (10). Log HR from the models applied to each imputation dataset were then combined, as described by Little and Rubin (11). As a sensitivity analysis, models were repeated using complete case analysis.
All data were analyzed using SAS version 9.4 software (SAS Institute, Cary, NC). This study was approved by an Internal Review Board of Stanford University School of Medicine.
Results
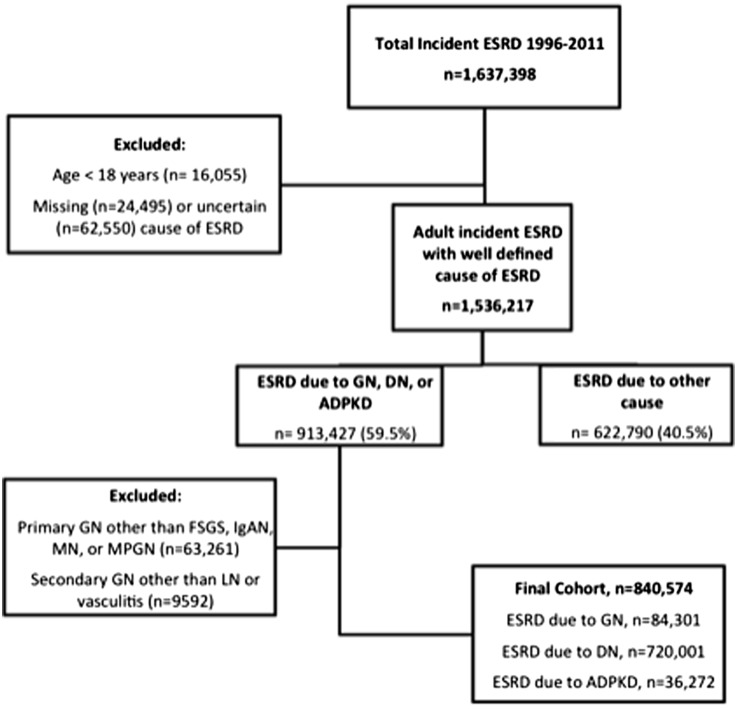
The final study population comprised 84,301 patients with ESRD attributed to six major GN subtypes: 34,330 (40.7%) with FSGS; 13,012 (15.4%) with IgAN; 7177 (8.5%) with MN; 5193 (6.2%) with MPGN; 16,463 (19.5%) with LN; and 8126 (9.6%) with vasculitis (Figure 1). In addition, 36,272 patients with ADPKD and 720,001 patients with DN were studied as external non-GN comparator groups.
Figure 1.
Flow diagram of cohort assembly. ADPKD, autosomal dominant polycystic kidney disease; DN, diabetes-related ESRD; IgAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy; MPGN, membranoproliferative GN.
Sociodemographic characteristics varied across GN subtypes (Table 1). The median age ranged from 39 (LN) to 66 years (vasculitis). There were approximately twice as many men as women within all primary GN subtypes, ranging from 60% men in MPGN to 68% men in IgAN. Sex was balanced in vasculitis (52% men), and women predominated in LN (18% were men). Black race was overrepresented in LN (48.6%) and FSGS (36.2%), Asian race was overrepresented in IgAN (15.4%), and white race was overrepresented in vasculitis (88.8%). Medicaid insurance was most common in LN (31.0%).
Table 1.
Baseline characteristics according to GN subtype
| Characteristic | Primary GN Subtypes | Secondary GN Subtypes | Non-GN Comparator Groups | |||||
|---|---|---|---|---|---|---|---|---|
| FSGS | IgAN | MN | MPGN | LN | Vasculitis | DN | ADPKD | |
| n (% among GN types) | 34,330 (40.7) | 13,012 (15.4) | 7177 (8.5) | 5193 (6.2) | 16,463 (19.5) | 8126 (9.6) | 720,001 (n/a) | 36,272 (n/a) |
| Age, median (IQR), y | 51 (38–65) | 44 (33–57) | 59 (47–71) | 53 (41–65) | 39 (29–50) | 66 (54–75) | 63 (54–72) | 54 (47–64) |
| Sex, male | 61.6 | 67.6 | 66.0 | 60.4 | 18.2 | 52.4 | 52.2 | 54.0 |
| Race | ||||||||
| White | 59.0 | 75.1 | 69.5 | 74.5 | 43.4 | 88.8 | 64.9 | 82.0 |
| Black | 36.2 | 6.6 | 26.0 | 18.1 | 48.6 | 7.5 | 27.8 | 13.1 |
| Asian | 3.5 | 15.4 | 3.0 | 4.9 | 5.8 | 1.9 | 4.4 | 3.8 |
| Other | 1.4 | 2.9 | 1.5 | 2.4 | 2.1 | 1.8 | 2.8 | 1.0 |
| Hispanic ethnicity | 8.6 | 12.4 | 10.0 | 10.6 | 16.2 | 8.9 | 17.1 | 8.6 |
| Medicaid insured | 18.4 | 13.4 | 16.5 | 20.2 | 31.0 | 11.9 | 28.3 | 12.5 |
| ESRD therapy | ||||||||
| Hemodialysis | 78.4 | 70.1 | 82.0 | 80.9 | 85.5 | 91.6 | 91.4 | 70.5 |
| Peritoneal dialysis | 14.5 | 16.8 | 12.8 | 11.6 | 10.8 | 6.5 | 7.1 | 15.6 |
| Transplant | 6.1 | 11.9 | 4.2 | 6.3 | 3.1 | 1.5 | 0.9 | 12.7 |
| Missing | 1.0 | 1.3 | 1.0 | 1.2 | 0.7 | 0.4 | 0.6 | 1.2 |
| Geographic region | ||||||||
| Northeast | 20.0 | 19.6 | 20.4 | 20.9 | 16.1 | 19.3 | 16.8 | 19.7 |
| Midwest | 23.3 | 23.0 | 23.6 | 24.7 | 18.4 | 27.2 | 20.5 | 22.9 |
| South | 39.6 | 30.3 | 37.9 | 32.9 | 44.1 | 33.2 | 39.8 | 36.1 |
| West | 16.6 | 26.6 | 17.0 | 20.5 | 20.3 | 20.0 | 20.9 | 20.2 |
| Missing | 0.6 | 0.5 | 1.2 | 1.0 | 1.1 | 0.3 | 2.0 | 1.1 |
| Comorbidities | ||||||||
| Diabetes | 13.3 | 9.3 | 15.0 | 14.9 | 8.9 | 15.9 | 88.0 | 7.5 |
| Heart failure | 14.6 | 8.3 | 18.8 | 17.9 | 15.0 | 18.2 | 38.5 | 8.4 |
| Coronary heart disease | 12.2 | 6.5 | 15.2 | 10.6 | 6.3 | 14.5 | 28.1 | 9.6 |
| Cerebrovascular event | 4.1 | 2.4 | 5.7 | 4.2 | 5.2 | 5.3 | 10.5 | 4.3 |
| Hypertension | 78.5 | 79.1 | 78.3 | 77.7 | 75.1 | 67.3 | 81.1 | 79.8 |
| COPD | 5.7 | 2.7 | 7.2 | 6.8 | 2.4 | 10.2 | 7.6 | 3.5 |
| Current smoker | 7.4 | 4.8 | 7.2 | 8.5 | 4.2 | 4.4 | 4.7 | 5.8 |
| Cancer | 4.8 | 2.8 | 6.2 | 5.7 | 1.6 | 6.0 | 3.9 | 3.6 |
| PVD | 5.1 | 2.9 | 6.2 | 5.0 | 3.5 | 7.6 | 19.8 | 3.4 |
| Nonambulant | 1.6 | 0.9 | 2.6 | 2.2 | 2.4 | 3.5 | 6.6 | 1.0 |
| Laboratory measurements | ||||||||
| Albumin, g/dl | 3.3±0.8 | 3.5±0.7 | 2.9±0.9 | 3.0±0.8 | 2.9±0.8 | 3.0±0.7 | 3.1±0.7 | 3.8±0.6 |
| Albumin missing | 23.1 | 22.5 | 22.3 | 22.3 | 23.6 | 23.2 | 25.5 | 23.4 |
| Hemoglobin, g/dl | 10.1±1.8 | 10.1±1.9 | 10.0±1.8 | 9.9±1.8 | 9.4±1.8 | 9.6±1.7 | 9.9±1.6 | 10.5±1.8 |
| Hemoglobin missing | 11.4 | 11.5 | 11.4 | 11.7 | 9.9 | 10.6 | 10.5 | 11.7 |
| Creatinine, mg/d | 8.4±4.2 | 8.4±4.0 | 7.7±3.8 | 7.4±3.7 | 7.4±3.5 | 7.5±3.6 | 6.3±2.8 | 7.6±3.3 |
| Creatinine missing | 2.0 | 1.5 | 1.6 | 1.8 | 1.4 | 1.1 | 1.2 | 1.4 |
| eGFR, ml/min per 1.73 m2 | 8.7±4.1 | 8.4±4.0 | 9.2±4.5 | 9.5±4.6 | 9.4±4.6 | 8.7±4.3 | 10.6±4.7 | 8.5±3.8 |
| eGFR missing | 2.6 | 2.1 | 2.6 | 2.9 | 2.5 | 1.7 | 2.2 | 2.0 |
Values are percentages, mean±SDs, or as otherwise indicated. IQR, interquartile range; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; eGFR, eGRF using the 4-variable Modification of Diet in Renal Disease formula; IgAN, IgA nephropathy; MN, membranous nephropathy; MPGN, membranoproliferative GN; LN, lupus nephritis; DN, diabetes-related ESRD; n/a, not applicable; ADPKD, autosomal dominant polycystic kidney disease.
Baseline reported comorbidity and laboratory characteristics differed among GN subtypes. Congestive heart failure (8.3%), cerebrovascular disease (2.4%), and peripheral vascular disease (2.9%) were least common in IgAN, whereas DN (8.9%), atherosclerotic heart disease (6.3%), and cancer (1.6%) were least common in LN. Serum albumin was highest in IgAN (3.5±0.7 g/dl) and lowest in MN (2.9±0.9 g/dl). Hemoglobin was highest in IgAN (10.1±1.9 g/dl) and lowest in LN (9.4±1.8 g/dl).
Among non-GN comparator groups, patients with ADPKD had a relatively favorable comorbidity and laboratory profile, comparable with that in IgAN. Patients with DN had the highest comorbidity burden of all; however, hemoglobin and albumin levels were higher than in some GN subtypes.
The proportion of patients receiving a preemptive kidney transplant was substantially higher in IgAN (11.9%) than in LN (3.1%) and vasculitis (1.5%). Use of peritoneal dialysis as an initial ESRD therapy was also higher in IgAN (16.8%) than in LN (10.8%) and vasculitis (6.5%).
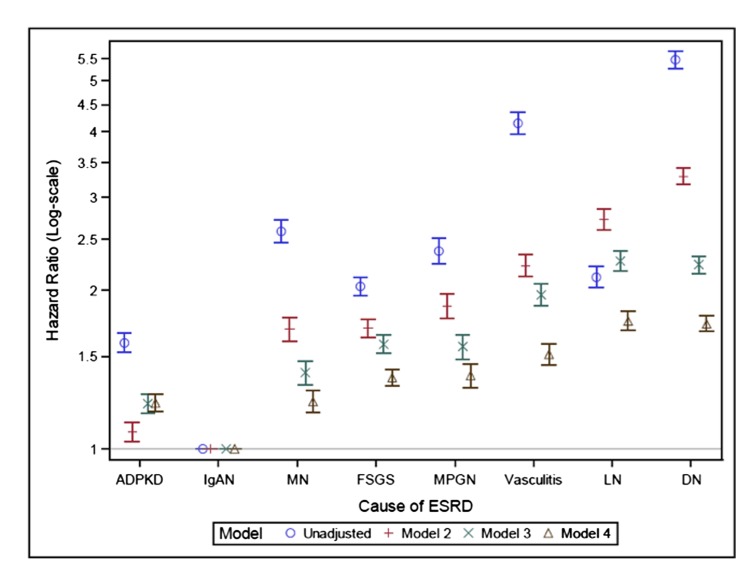
On follow-up, 84,301 patients with ESRD attributed to GN contributed 431,657 person years of observation, during which time 33,774 deaths were observed (crude mortality rate, 7.8/100 person years). Unadjusted 5-year survival ranged from 45.5% (95% CI, 44.2% to 46.7%) in vasculitis to 81.5% (95% CI, 80.7% to 82.2%) in IgAN, as illustrated in Kaplan–Meier survival plots (Figure 2). These survival curves corresponded to mortality rates ranging from 3.69 (95% CI, 3.56 to 3.83) per 100 person years in IgAN to 15.91 (95% CI, 15.45 to 16.36) per 100 person years in vasculitis (Table 2). Survival discrepancies are further reflected in unadjusted HRs for mortality (Figure 3, Supplemental Table 1), which differed by >4-fold across GN subtypes (HR=4.15; 95% CI, 3.96 to 4.35 in vasculitis). Adjustment for sociodemographic characteristics attenuated HRs in all subgroups except for LN, such that LN became the GN subtype associated with the highest sociodemographic-adjusted mortality (model 2). Additional adjustment for baseline comorbidity and laboratory characteristics (model 3) and for ESRD therapy modality, including time-varying adjustment for kidney transplantation (model 4), attenuated HRs further within all GN subtypes, as compared with IgAN. Even after accounting for these differences in the case mix, however, pronounced mortality differences across GN subtypes persisted. Compared with patients with ESRD caused by IgAN, adjusted (model 4) mortality hazards were 23% (95% CI, 17% to 29%), 37% (95% CI, 32% to 42%), 38% (95% CI, 31% to 45%), 51% (95% CI, 45% to 58%), and 75% (95% CI, 68% to 83%) higher in MN, FSGS, MPGN, vasculitis, and LN, respectively. Adjusted mortality in IgAN was also lower than in ADPKD (adjusted mortality hazard ratio [aHR]=1.22; 95% CI, 1.18 to 1.27), a disease with a generally favorable prognosis in ESRD (12,13), whereas mortality in LN (aHR=1.75; 95% CI, 1.68 to 1.83) was similar to in DN (aHR=1.73; 95% CI, 1.67 to 1.79), a disease with a particularly poor prognosis in ESRD (14).
Figure 2.
Kaplan–Meier survival curves. ADPKD, autosomal dominant polycystic kidney disease; DN, diabetes-related ESRD; IgAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy; MPGN, membranoproliferative GN.
Table 2.
Follow-up details of the study population, including unadjusted mortality rates, by GN subtype
| GN Subtype | No. of Patients (%) | Person Time at Risk, y | No. of Deaths (%) | Mortality Rate per 100 Person Years (95% Confidence Interval) |
|---|---|---|---|---|
| IgAN | 13,012 (15.4) | 76,901 | 2839 (21.8) | 3.69 (3.56 to 3.83) |
| FSGS | 34,330 (40.7) | 179,364 | 13,721 (40.0) | 7.65 (7.52 to 7.78) |
| LN | 16,463 (19.5) | 84,585 | 6741 (41.0) | 7.97 (7.78 to 8.16) |
| MPGN | 5193 (6.2) | 26,869 | 2405 (46.3) | 8.95 (8.59 to 9.31) |
| MN | 7177 (8.5) | 34,482 | 3382 (47.1) | 9.81 (9.48 to 10.14) |
| Vasculitis | 8126 (9.6) | 29,456 | 4686 (57.7) | 15.91 (15.45 to 16.36) |
IgAN, IgA nephropathy; LN, lupus nephritis; MPGN, membranoproliferative GN; MN, membranous nephropathy.
Figure 3.
Unadjusted and adjusted mortality hazard ratios. Model 1 is unadjusted; model 2 is demographic adjusted; model 3 is demographic and comorbidity adjusted; and model 4 is demographic, comorbidity, and ESRD therapy modality adjusted. ADPKD, autosomal dominant polycystic kidney disease; DN, diabetes-related ESRD; IgAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy; MPGN, membranoproliferative GN.
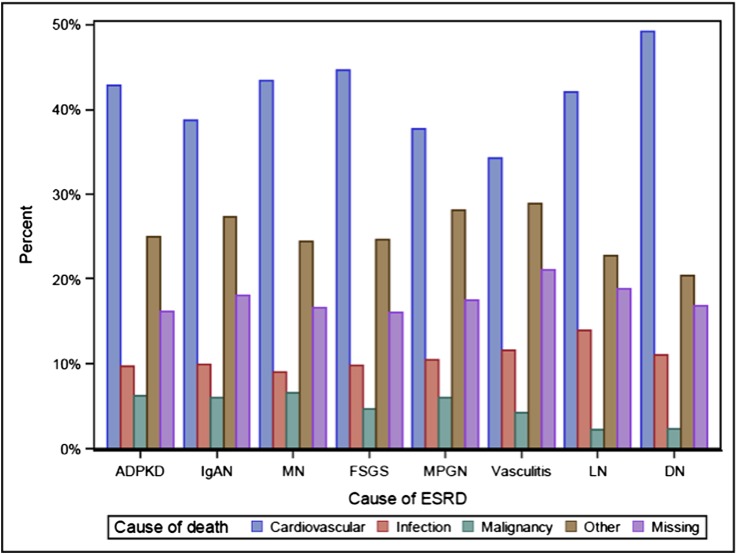
We observed some differences across GN subtypes with respect to primary cause of death (Figure 4, Supplemental Table 2). Cardiovascular disease accounted for the highest proportion of deaths within all GN subtypes, ranging from 34.2% in vasculitis to 44.6% in FSGS. The highest proportion of infection-related deaths was observed in LN (14.0%), comparing with rates of ≤10.6% among primary GN subtypes. Malignancy-related deaths were comparatively rare, ranging from 2.3% in LN to 6.5% in MN.
Figure 4.
Cause of death categories by GN subtype. ADPKD, autosomal dominant polycystic kidney disease; DN, diabetes-related ESRD; IgAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy; MPGN, membranoproliferative GN.
Sensitivity Analyses: Imputed versus Complete Data Analyses
Because of computational limitations, we were unable to generate 32 imputed datasets to reflect our 32% missing data frequency, as previously suggested (8). Instead, we generated results using eight and 15 imputed datasets. As summarized in Supplemental Figure 1 and Supplemental Table 1, complete case analyses tended to yield somewhat larger associations compared with multiply imputed analyses. Results did not materially change when increasing from eight to 15 imputed datasets, suggesting that additional efficiency was unlikely to be gained by extending imputation beyond 15 datasets.
Discussion
In this large, national, study of patients with ESRD attributed to GN who initiated dialysis or received a preemptive kidney transplant between 1996 and 2011, we identified considerable sociodemographic and clinical differences across six important GN subtypes. We also observed marked survival discrepancies that persisted even after adjustment for sociodemographic and clinical factors. Some GN subtypes (e.g., IgAN) conferred a particularly favorable prognosis, superior to that in ADPKD, whereas others (e.g., LN) displayed shortened survival, similar to in patients with ESRD caused by DN. The most obvious conclusion to draw from these findings is that combining GN subtypes into a single disease category, as is current practice in research and public health reporting, is of questionable utility; this approach fails to recognize the heterogeneity and complexity pervading this patient group.
Our study is not the first to report differential survival outcomes across GN subtypes. A single-center, retrospective study of 580 Taiwanese patients with biopsy-proven GN, not yet requiring dialysis (mean eGFR, 70.4±33.8 ml/min per 1.73 m2), reported a lower baseline comorbidity and cytotoxic treatment burden in patients with IgAN than with MN or FSGS (6). Unadjusted mortality, after a median follow-up of 5.9 years, was significantly lower in IgAN (4.6%) compared with MN (17.2%) and FSGS (14.4%); however, adjustment for between-group demographic and clinical differences was not performed, and outcomes after ESRD development were not examined. A second study of 1943 Korean patients with primary GN again demonstrated a survival advantage in IgAN compared with MN, FSGS, and MPGN (7). In the subset of patients who developed ESRD (n=257), 10-year survival risks of 85%, 80% (approximately), 61%, and 26%, respectively, were reported. Mortality comparisons accounting for case-mix differences were, again, not performed. With the exception of this subgroup analysis, most other studies examining mortality outcomes after ERSD development in GN were restricted to single GN subtypes (3,15–17) or combined subtypes into a single GN category (18,19), precluding direct comparisons across GN subtypes.
This study translates these previous findings, derived from non-ESRD, non-United States patient populations to a nationally representative cohort of United States patients with treated ESRD. It expands on prior studies by comparing outcomes not only across primary GN subtypes but also across secondary GN subtypes and non-GN related causes of ESRD. Importantly, this study also addresses the question of whether GN subtype is independently associated with post-ESRD survival or whether mortality differences are explained by differences in case mix alone. We confirm that post-ESRD survival in IgAN not only exceeds survival in other primary GN subtypes but also exceeds survival in secondary GN subtypes and ADPKD. This latter finding conflicts with prior reports suggesting inferior survival in GN compared with ADPKD. For example, a study of 44,240 Brazilian patients with treated ESRD reported a demographic-adjusted relative risk for mortality, with reference to hypertension-associated ESRD, of 0.93 (95% CI, 0.89 to 0.98) in GN compared with 0.69 (95% CI, 0.61 to 0.78) in ADPKD (18). Within the United States, 1-year mortality is reportedly 2-fold higher in primary GN than in cystic renal disease (10% versus 5%, respectively) (1). Our data explain these findings by demonstrating a spectrum of risk within GN: patients with IgAN have a survival advantage over patients with ADPKD that is counterbalanced by a survival disadvantage in other GN subtypes. This finding escapes detection when individual GN subtypes are combined together in ESRD outcomes research.
The substantially higher mortality observed in patients with LN in this study warrants further mention. High rates of infection (20–22), cardiovascular disease (23), and hospitalization (21,23) were previously described in patients with ESRD caused by LN; however, direct comparisons with other GN subtypes are largely lacking, and studies investigating mortality report conflicting findings (21,23–27). We determined that unadjusted crude mortality in LN exceeded mortality identified in many other GN subtypes, despite this being the youngest patient group with the highest proportion of patients who were black and patients who were women, factors which should have portended a favorable prognosis (28). Indeed, adjustment for sociodemographic factors uniquely increased the relative risk for mortality in LN with respect to IgAN, in contrast with the risk attenuation that was observed in all other GN subtypes (Figure 3). Further adjustment for differences in clinical characteristics, including access to kidney transplantation, reduced the HR for mortality in LN somewhat, but it remained almost 2-fold higher than in the referent group, IgAN.
Our study has several limitations. First, we cannot confirm the validity of GN subtype designations obtained from the USRDS. A previous study measured agreement between biopsy-based diagnoses and USRDS-derived diagnoses among 227 patients with biopsy-proven GN (29). Poor overall agreement was largely explained by missing (57%) and GN not histologically examined (9%) diagnoses submitted to the USRDS; positive predictive values exceeded 90% once a specific GN subtype was selected. Agreement also improved after 1995, following revisions to the Medical Evidence Report diagnostic coding system in that year. Nevertheless, we could not always distinguish primary from secondary forms of GN (e.g., primary from secondary MN), and our study findings are not necessarily applicable to nonbiopsied or misclassified patients with GN (i.e., false negatives) who may differ fundamentally from correctly classified patients. Second, as a retrospective observational study, associations between GN subtype and mortality cannot be assumed to represent causation. We could not distinguish the influence of GN-related factors (e.g., nephrotic syndrome, systemic inflammation, immunosuppressive therapy) from unmeasured or residual non-GN related factors. Some misclassification of comorbidities is likely (e.g., DN was reported in only 88% of patients with ESRD caused by DN), and detailed socioeconomic data were not available; however, we propose that misclassification of these confounding variables is likely to be nondifferential and to bias findings toward the null. At the same time, adjustment for laboratory variables and initial ESRD treatment modality may overadjust for disease mediators, particularly in those GN subtypes that typically display a more rapid and unpredictable course to ESRD (e.g., LN, vasculitis). Albumin was lowest in the GN subtypes most typically associated with nephrotic syndrome (MN and MPGN) and systemic inflammation (LN and vasculitis), whereas hemoglobin was lowest in those GN subtypes most likely to be treated with immunosuppressive therapy (LN and vasculitis), suggesting direct disease- and treatment-mediated effects. Finally, our findings apply to patients with progressive GN who survive to ESRD and should not be generalized to patients with mild, treated, or remitted GN without ESRD or to patients who die before developing ESRD.
Despite these limitations, our study has a number of strengths. We report findings derived from population-based data that are broadly applicable to all patients with ESRD attributed to GN receiving dialysis or with a functioning kidney transplant in the United States. Study investigators did not collect primary data or adjudicate outcomes, virtually eliminating investigator bias. We used sophisticated statistical techniques to overcome shortfalls inherent to observational study design, including multiple imputation methods to handle missing data and use of sequentially adjusted models to minimize confounding.
In summary, we have identified in a large, nationally representative, ESRD cohort that patients classified into individual histologic GN subtypes differ considerably from one another with respect to sociodemographic, clinical, laboratory, and ESRD therapy modality characteristics. We furthermore determined that GN subtype independently associates with survival after initiation of ESRD treatment, even after accounting for differences in case mix. We propose that GN subtype be addressed in all future studies of patients with ESRD caused by GN, to elucidate explanations for observed survival differences and to identify modifiable factors amenable to targeting in interventional trials and public health strategies.
Disclosures
Dr. Winkelmayer reports having served as an advisor or consultant, unrelated to the topic of this article, to ACUMEN, Amgen, Astra-Zeneca, Bayer, Keryx, Medtronic, Mitshubishi-Tanabe, and Rockwell Pharma. Dr. Lafayette reports having served as an advisor or consultant, unrelated to the topic of this article, to Genentech, Fibrogen, and Questcor. Drs. O’Shaughnessy and Montez-Rath have no financial disclosures to report.
Supplementary Material
Acknowledgments
Dr. O’Shaughnessy was supported by a Ben J. Lipps Research Fellowship of the ASN Foundation for Kidney Research. The Stanford Nephrology Fellowship Program was supported by grant T32-DK007357. Dr. Winkelmayer receives salary and research support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine.
The abstract from this article was presented to the American Society of Nephrology Kidney Week meeting, November 2014, Philadelphia, Pennsylvania.
The manuscript was reviewed and approved for publication by an officer of the National Institute of Diabetes and Digestive and Kidney Diseases. Data reported herein were supplied by the US Renal Data System. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the United States government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11261114/-/DCSupplemental.
See related editorial, “ESRD Outcomes and GN Subtypes,” on pages 1117–1118.
References
- 1.USRD System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 2.Knoop T, Vikse BE, Svarstad E, Leh S, Reisæter AV, Bjørneklett R: Mortality in patients with IgA nephropathy. Am J Kidney Dis 62: 883–890, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, Kim S, Chin HJ: Mortality of IgA nephropathy patients: A single center experience over 30 years. PLoS One 7: e51225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok CC, Kwok RC, Yip PS: Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 65: 2154–2160, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM: Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 27: 3248–3254, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Chou YH, Lien YC, Hu FC, Lin WC, Kao CC, Lai CF, Chiang WC, Lin SL, Tsai TJ, Wu KD, Chen YM: Clinical outcomes and predictors for ESRD and mortality in primary GN. Clin J Am Soc Nephrol 7: 1401–1408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, Kim S, Chin HJ: Mortality and renal outcome of primary glomerulonephritis in Korea: Observation in 1,943 biopsied cases. Am J Nephrol 37: 74–83, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Montez-Rath ME, Winkelmayer WC, Desai M: Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 9: 1328–1335, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White IR, Royston P: Imputing missing covariate values for the Cox model. Stat Med 28: 1982–1998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Buuren S: Flexible Imputation of Missing Data, Boca Raton, FL, Chapman and Hall/CRC Press, 2012 [Google Scholar]
- 11.Little RJA, Rubin DB: Statistical Analysis with Missing Data, 2nd Ed., Hoboken NJ, John Wiley & Sons, 2002 [Google Scholar]
- 12.Perrone RD, Ruthazer R, Terrin NC: Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis 38: 777–784, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Reule S, Sexton DJ, Solid CA, Chen SC, Collins AJ, Foley RN: ESRD from autosomal dominant polycystic kidney disease in the United States, 2001-2010. Am J Kidney Dis 64: 592–599, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroijen MA, van de Luijtgaarden MW, Noordzij M, Ravani P, Jarraya F, Collart F, Prütz KG, Fogarty DG, Leivestad T, Prischl FC, Wanner C, Dekker FW, Jager KJ, Dekkers OM: Survival in dialysis patients is different between patients with diabetes as primary renal disease and patients with diabetes as a co-morbid condition. Diabetologia 56: 1949–1957, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Komatsu H, Kikuchi M, Nakagawa H, Fukuda A, Iwakiri T, Toida T, Sato Y, Kitamura K, Fujimoto S: Long-term survival of patients with IgA nephropathy after dialysis therapy. Kidney Blood Press Res 37: 649–656, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Romeu M, Couchoud C, Delarozière JC, Burtey S, Chiche L, Harlé JR, Gondouin B, Brunet P, Berland Y, Jourde-Chiche N: Survival of patients with ANCA-associated vasculitis on chronic dialysis: data from the French REIN registry from 2002 to 2011. QJM 107: 545–555, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Tang W, Bose B, McDonald SP, Hawley CM, Badve SV, Boudville N, Brown FG, Clayton PA, Campbell SB, Peh CA, Johnson DW: The outcomes of patients with ESRD and ANCA-associated vasculitis in Australia and New Zealand. Clin J Am Soc Nephrol 8: 773–780, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista PB, Lopes AA, Costa FA: Association between attributed cause of end-stage renal disease and risk of death in Brazilian patients receiving renal replacement therapy. Ren Fail 27: 651–656, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Chang YS, Liu CJ, Wu TH, Chaou CH, Lin KC, Ou SM, Chen TJ, Chen WS, Chou CT, Tsai CY: Survival analysis in systemic lupus erythematosus patients on maintenance dialysis: A nationwide population-based study in Taiwan. Rheumatology (Oxford) 52: 166–172, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Siu YP, Leung KT, Tong MK, Kwan TH, Mok CC: Clinical outcomes of systemic lupus erythematosus patients undergoing continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 20: 2797–2802, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Huang JW, Hung KY, Yen CJ, Wu KD, Tsai TJ: Systemic lupus erythematosus and peritoneal dialysis: outcomes and infectious complications. Perit Dial Int 21: 143–147, 2001 [PubMed] [Google Scholar]
- 23.Sule S, Fivush B, Neu A, Furth S: Increased hospitalizations and death in patients with ESRD secondary to lupus. Lupus 21: 1208–1213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HA, Wang JJ, Chou CT, Chien CC, Chu CC, Sheu MJ, Lin YJ, Chen PC, Chen CH: Predictors of longterm mortality in patients with and without systemic lupus erythematosus on maintenance dialysis: A comparative study. J Rheumatol 38: 2390–2394, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Lee PT, Fang HC, Chen CL, Chiou YH, Chou KJ, Chung HM: Poor prognosis of end-stage renal disease in systemic lupus erythematosus: A cohort of Chinese patients. Lupus 12: 827–832, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Mallett A, Tang W, Clayton PA, Stevenson S, McDonald SP, Hawley CM, Badve SV, Boudville N, Brown FG, Campbell SB, Johnson DW: End-stage kidney disease due to Alport syndrome: outcomes in 296 consecutive Australia and New Zealand Dialysis and Transplant Registry cases. Nephrol Dial Transplant 29: 2277–2286, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Ward MM: Cardiovascular and cerebrovascular morbidity and mortality among women with end-stage renal disease attributable to lupus nephritis. Am J Kidney Dis 36: 516–525, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol 5: 1231–1242, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, McClellan WM: Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 5: 2046–2052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.