Abstract
Background and objectives
Metabolomics is a relatively new field of “-omics” research, focusing on high-throughput identification of small molecular weight metabolites. Diet has both acute and chronic effects on metabolic profiles; however, alterations in response to dietary sodium restriction (DSR) are completely unknown. The goal of this study was to explore changes in urine metabolites in response to DSR, as well as their association with previously reported improvements in vascular function with DSR.
Design, setting, participants, & measurements
Using stored urine samples from a 10-week randomized placebo-controlled crossover study of DSR in 17 middle-aged/older adults (six men and 11 women; mean age 62±8 years) who had moderately elevated systolic BP (130–159 mmHg) and were otherwise healthy, a liquid chromatography/mass spectrometry–based analysis of 289 metabolites was performed. This study identified metabolites that were significantly altered between the typical (153±29 mmol/d) and low (70±29 mmol/d) sodium conditions, as well as their baseline (typical sodium) association with responsiveness to previously reported improvements in vascular endothelial function (brachial artery flow-mediated dilation) and large elastic artery stiffness (aortic pulse wave velocity).
Results
Of the 289 metabolites surveyed, 10 were significantly altered (nine were upregulated and one was downregulated) during the low sodium condition, and eight of these exceeded our prespecified clinically significant threshold of a >40% change. These metabolites were involved in biologic pathways broadly related to cardiovascular risk, nitric oxide production, oxidative stress, osmotic regulation, and metabolism. One metabolite, serine, was independently (positively) associated with previously reported improvements in the primary vascular outcome of brachial artery flow-mediated dilation.
Conclusions
This proof-of-concept study provides the first evidence that DSR is a stimulus that induces significant changes in urinary metabolomic profiles. Moreover, serine was independently associated with corresponding changes in vascular endothelial function after DSR. Larger follow-up studies will be required to confirm and further elucidate the metabolic pathways that are altered in response to DSR.
Keywords: hypertension, nutrition, metabolism, endothelium
Introduction
Metabolomics is a relatively new field of “-omics” research, focusing on high-throughput identification of small molecular weight metabolites (1). Compared with other “-omics”, metabolomic profiles are more characteristic of systemic phenotypes, which is particularly useful in gaining insight into changes in both physiologic and pathophysiological function (2). Metabolomics has been used to identify novel biomarkers or profiles of disease, such as CKD (3). However, various interventions, including lifestyle interventions, also influence the metabolome, and such alterations can be interpreted similarly to changes in other physiologic phenotypes. The concept of utilizing metabolomics to identify unique changes in molecular signatures in response to interventions, particularly dietary, is highly novel, and is regarded as an important new direction in nutrition-related research (1,4). Indeed, diet has both acute and chronic effects on metabolic profiles, although the chronic effects are not currently well understood (5).
Dietary sodium restriction (DSR) is a commonly recommended lifestyle intervention to reduce the risk of cardiovascular diseases, although average sodium intake remains well above recommended levels (6). Reducing sodium intake induces benefits beyond and independent of BP lowering alone (7,8), suggesting that additional physiologic mechanisms also contribute to associated cardiovascular benefits. We recently demonstrated that DSR improves vascular endothelial function in middle-aged and older adults with moderately elevated systolic BP (SBP) via a reduction in vascular oxidative stress (8), while also reducing large elastic artery stiffness (9). However, the physiologic mechanisms associated with DSR are incompletely understood. Thus, metabolomics may be a novel tool for identifying new pathways mediating the beneficial effects of DSR on vascular function. In addition, because the degree of response to sodium restriction varies (10), metabolomics may allow for identification of unique predictors of responsiveness, which could be very useful clinically. Metabolomic alterations in response to DSR, as well the ability of metabolites to predict responsiveness to the intervention, are completely unknown.
Accordingly, we performed a post hoc analysis of a recently completed randomized placebo-controlled crossover study comparing the effect of low and typical sodium intake on vascular endothelial function and large elastic artery stiffness (8,9). Our goal was to assess urine metabolites (end products of cellular processes that can be collected noninvasively) using 24-hour urine collections and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS), and to determine their association with previously reported improvements in vascular function. We utilized an untargeted approach, which allowed for the maximum likelihood of identifying metabolites that were either upregulated or downregulated in response to DSR, or related to changes in vascular function (11). This design was hypothesis-generating by nature, because there is currently no information available regarding metabolomic changes in response to DSR. Because the crossover design of the parent study allowed for isolation of dietary sodium as the only nutritional factor altered between conditions, this study allowed the ideal setting to explore these novel questions.
Materials and Methods
The details of the parent study, a randomized placebo-controlled crossover design conducted from February 2009 to January 2012, were published previously (8,9). The study was conducted at the University of Colorado Boulder Clinical and Translational Research Center (CTRC), and the metabolomics analyses were performed at the iC42 Clinical Research and Development Center at the University of Colorado Denver Anschutz Medical Campus.
Study Participants
The inclusion and exclusion criteria were described previously (8), and are summarized in the Supplemental Materials and Methods. All study participants had a resting SBP within 130–159 mmHg, which was measured in accordance with the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (12). Participants had high normal or stage I systolic hypertension, and diastolic BP <99 mmHg, verified on a minimum of two occasions (13,14), but were otherwise free of cardiovascular disease, diabetes, kidney disease, and other clinical disorders. All procedures were approved by the University of Colorado Boulder Institutional Review Board and conformed with the Declaration of Helsinki. The nature, benefits, and risks of the study were explained to the volunteers and their written informed consent was obtained before participation.
Experimental Design and DSR
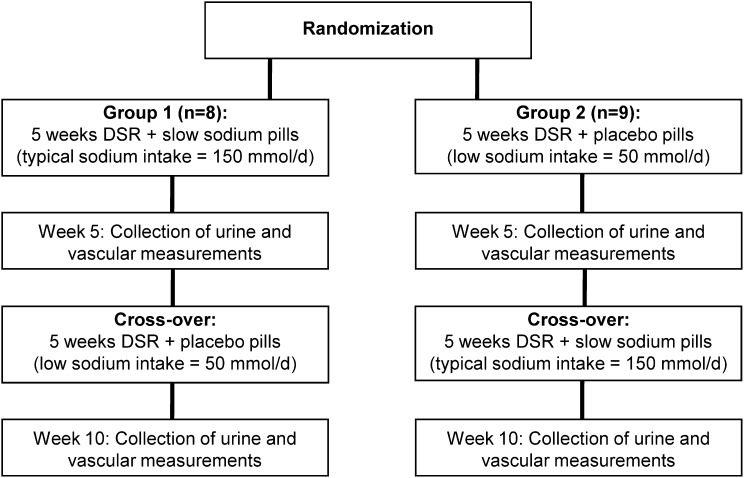
In the parent study, we used a double-blind, placebo-controlled, randomized crossover design, as described previously (8) and summarized in the schematic in Figure 1. Briefly, a low sodium intake of approximately 1500 mg/d (65 mmol/d) was compared with a typical United States sodium intake of 3600 mg/d (150 mmol/d). During the entire 10-week intervention period, participants reduced dietary sodium (target of 50 mmol/d) and were instructed to take 10 tablets spread across the day with meals. The tablets were placebo pills for 5 weeks, whereas the tablets were slow-release NaCl tablets (10 mmol [0.23 g] per tablet; HK Pharma, UK) for the other 5 weeks. The slow-release NaCl tablets aimed to return sodium intake to the approximately 150 mmol/d target. Participants were provided with comprehensive dietary education and weekly counseling by CTRC bionutritionists in order to reduce dietary sodium intake without changing caloric intake, dietary composition, or potassium intake [the results of 3-day diet record and 24-hour urinary potassium excretion analyses were all published previously (8)]. The investigators were blinded to sodium condition in the acquisition and analysis of all variables.
Figure 1.
Study design. Schematic illustrating the design of the previously published randomized placebo-controlled crossover study from which stored samples were analyzed for metabolomic profiling. DSR, dietary sodium restriction.
Vascular Function and BP
Brachial artery flow-mediated dilation (FMDBA) was assessed as a measure of vascular endothelial function, and aortic pulse wave velocity (aPWV) was used as a measure of large elastic artery stiffness, as described previously (8,9). Arterial BP was assessed noninvasively from the brachial artery as described elsewhere (8,9). This post hoc analysis aimed to test the relations between urinary metabolites and changes in vascular outcomes with DSR, as assessed with multiple linear regression.
Urine Metabolomics
Metabolic profiling involves a comprehensive measurement of the types and concentrations of metabolites in a system at a specified time (15,16). Using stored (at −80°C) 24-hour urine collection samples from the final week (i.e., week 5) of each sodium condition, we performed a LC-MS/MS–based analysis of 289 metabolites. Storage at −80°C is the recommended condition for long-term LC-MS/MS–based metabolomic analyses (17) and samples have been shown to be stable across time and freeze thaw cycles with this storage method (18). These technologies are well established in our laboratory (19–22) and utilize a positive/negative ion switching, targeted mass spectrometry–based metabolomics platform on an AB Sciex 5500 QTRAP system (AB Sciex, Concord, ON, Canada) that was established by Yuan et al. (23) and further extended and improved within our laboratory. This method utilizes compound-specific multiple reaction monitoring transitions. Internal standards were used for retention time correction and instrument performance monitoring. Further details are available in the Supplemental Materials and Methods. Urine samples were available from all 17 participants for the typical sodium condition, but were missing for two participants for the low sodium condition.
For those metabolites with a statistically significant (P<0.05) and clinically meaningful (>40%) change between sodium conditions, confirmation studies were performed by injecting pure compounds of these metabolites. This was followed by the manual integration of the peak of interest using MultiQuant 2.1 software (AB Sciex). This approach allowed for reduction of false discovery rates. Manual integration of the peak of interest was also performed for those metabolites that significantly correlated with fold change in FMDBA, aPWV, and SBP, after visual inspection for outliers. Those that remained significantly associated with these outcomes after multivariate adjustment also had confirmation studies performed. All results are presented as integrated peak areas normalized to both urinary creatinine and the internal standard.
Plasma S-Adenosyl-l-Homocysteine
Because we identified a surprising increase in urinary S-adenosyl-l-homocysteine with DSR, we further explored this finding by performing a targeted analysis to also measure plasma levels of S-adenosyl-l-homocysteine. Using stored samples from each of the sodium conditions (available on n=17 and n=16 for the low and typical sodium conditions, respectively), plasma S-adenosyl-l-homocysteine and S-adenosyl-l-methionine were measured by LC-MS/MS. Details of the methodology are provided in the Supplemental Materials and Methods.
Statistical Analyses
Clinical characteristics (Table 1), urinary metabolites, and plasma S-adenosyl-l-homocysteine/methionine were compared at the low sodium and typical sodium time points using a paired t test in SPSS 21 software. Principal component analysis (PCA) was performed using no weighting and Pareto scaling. For all metabolites, bivariate relations between urinary metabolites during the typical sodium condition and fold change in FMDBA, aPWV, and SBP were determined using the Pearson correlation coefficient. Because there were no true baseline measurements of vascular function given the cross-sectional study design, the typical sodium condition, which consisted of dietary sodium intake equivalent to baseline screening, was used as the predictor condition. Step-wise multiple linear regression was then performed using SAS 9.3 software for any metabolites (integrated peak areas normalized to the internal standard and urinary creatinine) that significantly correlated with either change in FMDBA, aPWV, or SBP with sex, age, and SBP (except for the ∆SBP model) included in the model (model 1). All data are reported as means±SDs. Statistical significance for all analyses was set at P value <0.05.
Table 1.
Select clinical characteristics
| Variable | Low Sodium | Typical Sodium | P Value |
|---|---|---|---|
| No. of participants (women/men) | 17 (6/11) | 17 (6/11) | – |
| Age (yr) | 62±8 | 62±8 | – |
| Race | – | ||
| Caucasian | 15 (88) | 15 (88) | |
| Asian | 2 (12) | 2 (12) | |
| Urine sodium (mmol/24 h) | 70±29 | 153±29 | <0.001 |
| Urine volume (ml/24 h) | 2376±844 | 2445±1001 | 0.72 |
| Modification of Diet in Renal Disease eGFR (ml/min per 1.73 m2) | 82±16 | 86±21 | 0.07 |
| Brachial artery flow-mediated dilation (%∆) | 6.0±2.3 | 3.6±1.7 | <0.001 |
| Aortic pulse wave velocity (cm/s) | 761±202 | 873±131 | 0.002 |
Data are presented as the mean±SD or n (%).
Results
Clinical Characteristics
Detailed participant characteristics, including baseline characteristics, were published previously (8,9). Select clinical characteristics from each of the two sodium conditions are shown in Table 1. The comparison of the low versus typical sodium conditions showed that participants had lower urinary sodium excretion, indicating dietary adherence, as well as greater FMDBA, and lower aPWV, with no statistically significant difference in eGFR. As a result of the crossover design and weekly counseling by CTRC bionutritionists, intake of macronutrients and micronutrients, other than sodium, were unchanged between conditions, as reported previously (8).
Urinary Metabolomics
Table 2 displays all metabolites that significantly changed between the typical and low sodium conditions, including a brief description of the metabolite’s biologic role. Ten metabolites were significantly changed with DSR, with nine increasing (from 1.3- to 2.5-fold higher) and one slightly decreasing (0.8-fold); eight of these exceeded our prespecified threshold of a clinically significant change of >40% and thus were confirmed. The results of the confirmation studies are provided in Supplemental Figure 1. Results were similar when normalized to the other internal standard (homoarginine; data not shown).
Table 2.
Urinary metabolite/creatinine ratios with a significant change after DSR
| Metabolite | Biologic Role/ Metabolic Pathway | Low Sodium (AU) | Typical Sodium (AU) | Fold Change with DSR | P Value |
|---|---|---|---|---|---|
| Methylmalonic acid | Malonic acid derivative; vital intermediate in the metabolism of fat and protein; metabolite of homocysteine | 2.23E−3±8.79E−3 | 9.46E−4±3.87E−3 | 2.4 | 0.01 |
| Succinate | Krebs cycle intermediate; donates electrons to the ETC | 1.74E−3±6.74E−3 | 7.67E−4±2.9E−3 | 2.3 | 0.01 |
| Methionine sulfoxide | Methylation; biomarker of oxidative stress | 1.78E−5±5.27E−5 | 8.07E−6±2.19E−5 | 2.2 | 0.01 |
| S-adenosyl-l-homocysteine | Intermediate precursor of homocysteine, which is associated with increased cardiovascular risk | 2.32E−5±6.12E−5 | 1.25E−5±3.27E−5 | 1.9 | 0.01 |
| Glycerophosphocholine | Choline derivative; osmolyte (counteracts urea and other macromolecules) | 2.06E−4±5.27E−4 | 1.29E−4±3.01E−4 | 1.6 | 0.03 |
| d-gluconate/-acid | Found naturally in fruit, honey, and wine; regulates acidity | 2.73E−7±7.67E−3 | 4.79E−3±6.43E−3 | 1.6 | <0.001 |
| Serine | Nonessential amino acid; in many biologic pathways | 7.73E−4±1.53E−3 | 5.16E-4±1.03E−3 | 1.5 | 0.05 |
| Asparagine | Nonessential amino acid; required for nervous system; role in synthesis of ammonia | 3.13E−4±4.96E−4 | 2.15E−4±3.70E−4 | 1.5 | <0.001 |
| Deoxyadenosine monophosphate | Derivative of ATP; monomer in DNA | 8.19E−4±6.97E−4 | 6.19E−4±5.15E−4 | 1.3 | 0.02 |
| 6-phospho-d-glucono-1,5-lactone | Intermediate in pentose phosphate pathway | 2.44E−5±1.26E−3 | 3.02E−5±1.74E−3 | 0.8 | 0.04 |
Data are presented as means±SD. Area is normalized to urinary creatinine and the internal standard (methionine-d3) and presented as arbitrary units. Deoxyadenosine monophosphate and 6-phospho-d-glucono-1,5-lactone were not confirmed, because the percent change (before the normalization to creatinine) did not exceed the prespecified clinically significant threshold of >40% change (these values are presented as integrated area). AU, arbitrary unit; DSR, dietary sodium restriction; ETC, electron transport chain.
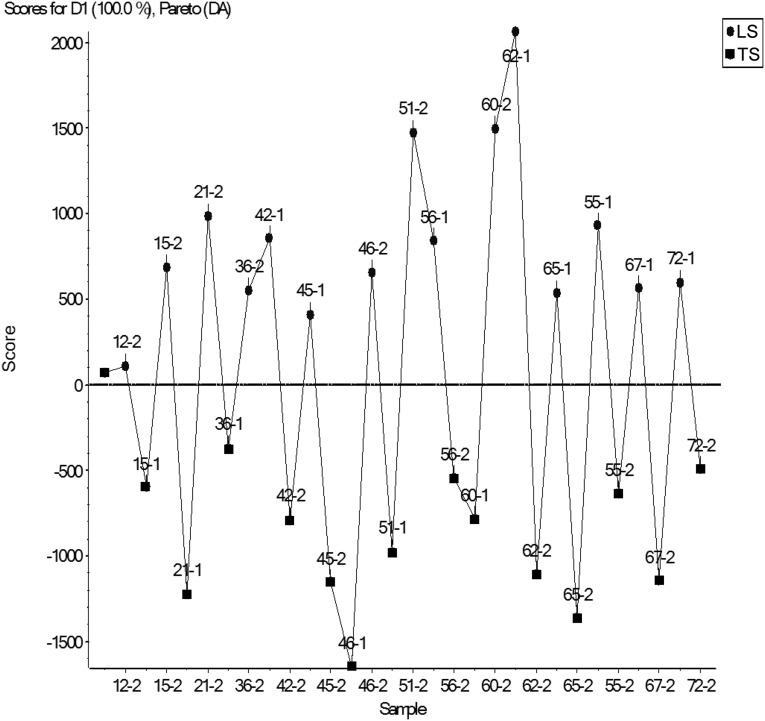
Unsupervised PCA, which considers global structure of the data, did not separate well between the groups (Supplemental Figure 2). Supervised principal component analysis–discriminant analysis (PCA-DA), which utilizes prior knowledge of sample groups to determine the variables that maximize the variation between groups and those which minimize the variation within a group did separate the two sodium conditions (Figure 2).
Figure 2.
Results of supervised principal component analysis and discriminant analysis. The results are shown by subject identification number. The Pareto algorithm was used for scaling. Ovals and squares represent the low sodium and typical sodium conditions, respectively. D1, discriminant 1; DA, discriminant analysis; LS, low sodium; TS, typical sodium.
Plasma S-Adenosyl-l-Homocysteine
Because the increase in urinary S-adenosyl-l-homocysteine with DSR was surprising, we further explored this finding by performing a targeted analysis to measure plasma levels of S-adenosyl-l-homocysteine. Unlike the urinary results, the metabolite was unchanged in the plasma (typical sodium: 28.7±16.1 nM; low sodium: 25.0±14.4 nM; P=0.51). Similarly, plasma levels of S-adenosyl-l-methionine were also unchanged (typical sodium: 110.4±41.23 nM; low sodium: 117.4±36.0 nM; P=0.61).
Regression Analyses
Serine was the sole metabolite during the typical sodium condition that was significantly associated with change in FMDBA after multivariate adjustment, as shown in Table 3. Metabolites with significant univariate correlations that did not persist after multivariate adjustment are shown in Supplemental Table 1 (these were not confirmed). In unadjusted analyses, higher levels of serine were associated with a greater increase (fold change) in FMDBA, a measure of vascular endothelial function and primary outcome of the parent study, in response to DSR. This association remained significant after adjusting for age, sex, and SBP. In adjusted analyses, none of the metabolites had a significant association with change in aPWV or SBP.
Table 3.
Association between urine metabolite and change in vascular function
| Metabolite | Dependent Variable | Model | Unstandardized Slope, Change (95% CI) | Standardized Slope, Change | P Value |
|---|---|---|---|---|---|
| Serine | ∆FMDBA | Unadjusted | 1.51E3 (6.06E2 to 2.41E3) | 0.69 | 0.003 |
| 1 | 1.59E3 (47.64 to 3.13E3) | 0.73 | 0.04 |
Metabolite was entered as the area normalized to urinary creatinine and the internal standard (methionine-d3). Model 1 was adjusted for age, sex, and systolic BP. 95% CI, 95% confidence interval; FMDBA, brachial artery flow-mediated dilation.
Discussion
These results provide the first evidence that unique metabolomic changes occur in response to DSR. Specifically, changes were detected in 10 metabolites (nine upregulated and one downregulated) during the low sodium condition, and eight of these exceeded our prespecified threshold of a >40% change to be deemed clinically meaningful. PCA-DA analysis also showed separation between the two sodium conditions. Furthermore, one of these metabolites was independently associated with the improvements previously reported in the primary vascular outcome of FMDBA (8). These findings provide proof of concept that metabolism changes in response to DSR are reflected in excreted urinary metabolites, consistent with what has been shown previously in small acute or short-term studies, including manipulation of soy intake (24), cocoa ingestion (25), and a high phytochemical diet (5).
Several metabolites were associated with biologic pathways known to be related to general cardiovascular risk. S-adenosyl-l-homocysteine is a precursor to homocysteine, an amino acid associated with increased cardiovascular risk (26). Because it was surprising that levels of S-adenosyl-l-homocysteine were increased in urine with DSR, we also evaluated levels in plasma. The lack of change in plasma levels supports that S-adenosyl-l-homocysteine excretion was increased, without any change in circulating levels. Similarly, the increase in methylmalonic acid during the low sodium condition was somewhat surprising. Methylmalonic acid is a vital intermediate in the metabolism of fat and protein and is also a metabolite of homocysteine (27). High levels can indicate vitamin B12 deficiency (28). Of note, methylmalonic acid is very efficiently excreted by the kidney, and thus urinary concentration reflects tissue depletion (29).
Serine, which was also increased with DSR, is a nonessential amino acid that is part of many biologic pathways, including methionine metabolism, sphingolipid metabolism, ammonia recycling, transcription/translation, and metabolism of serine and other small amino acids (27). It is also required to condense with homocysteine for the breakdown of the latter (27). Serine has an important role in the catalytic function of enzymes, including a residue that is phosphorylated for the activation of endothelial nitric oxide synthase and subsequent production of nitric oxide (30). Asparagine is an additional nonessential amino acid that was increased with DSR and is also involved in many biologic pathways, including roles in nervous system function and ammonia synthesis (27).
In the parent study, we found that the improvements in vascular endothelial function with DSR were mediated in part by reduced vascular oxidative stress (8). Methionine sulfoxide is considered to be a biomarker of oxidative stress (31); thus, its increase during the low sodium condition is at odds with these previous findings. However, methionine sulfoxide reductase is a regulator of antioxidant defenses and longevity in mammals (32) and was not one of the metabolites included in this untargeted analysis. Similarly, 6-phospho-d-gluco-1,5-lactone was also lower with DSR, albeit not to our predetermined clinically significant level; thus, this peak was not confirmed. 6-Phospho-d-gluco-1,5-lactone is an intermediate in the pentose phosphatase pathway, which is considered one of the main antioxidant defense regulator systems, by providing reducing power and ribose phosphate to cells (33).
In addition, several metabolites altered with DSR have roles in osmotic regulation, sodium balance, and general metabolism. The serine protease, serpin, plays a key role in sodium handling in the kidney (34). Glycerophosphocholine is a choline derivative and osmolyte that counteracts urea and other macromolecules (35). Both were increased during the low sodium condition. Finally, d-gluconate, which is found in fruit, may reflect exogenous intake (27). However, because sodium intake was manipulated with sodium added back in pill form, a difference in fruit intake between sodium conditions would not be anticipated.
Several strengths and limitations of these analyses merit discussion. The fact that the parent study was a crossover design, with sodium intake manipulated via adding it back in pill form, is an important strength that allowed for isolation of sodium as the sole manipulated dietary factor between conditions, in the exact same participants. An additional strength is the utilization of LC-MS/MS, a sensitive and quantitatively reproducible technique. The attempt to relate baseline values of metabolomic signatures with improvements in vascular function in response to DSR is a major strength of the study, providing insight into potential molecular mechanisms as well as implications for personalized medicine.
In terms of limitations, it should be noted that the population in this study comprised middle-aged and older, primarily Caucasian adults with moderately elevated SBP and the sample size was small. As such, these findings will need to be verified in a larger cohort and extended to populations other than the relatively healthy group in this study.
An additional potential limitation of this post hoc analysis is that we elected to only measure metabolites in urine and not also in plasma. Important advantages of urine include the fact that it is readily available, easily and noninvasively obtained, and less complex to process than blood, allowing for clinical translation. In addition, metabolites in urine are the end product of cellular processes, and are thus closely linked to phenotype (36). Future targeted analyses will be needed to examine changes in specific metabolites in plasma, in addition to urine.
Another limitation is that PCA-DA analysis, which showed separation between sodium conditions, is susceptible to overfitting because of the bias associated with prior knowledge of groups, particularly when smaller sample sizes such as ours are used. The PCA-DA results differed from the unsupervised PCA, which did not separate well between the groups. In addition, type I errors may have occurred in our analysis, because a Bonferroni correction was not feasible given the large number of screened metabolites. However, we minimized this possibility by performing confirmation studies injecting pure compounds of these metabolites, allowing for a reduction of false discovery rates by identifying a compound by its mass (mass spectrometry), fragmentation (multiple reaction monitoring transition), and chromatographic characteristics (retention time). In addition, all of the metabolites that changed with DSR were present in the urine of all analyzed samples from both conditions, which further limits false discovery rates.
It is possible that the effects of DSR on metabolic profiles may be short term rather than sustained. We were unable to determine whether the changes in metabolomic profiles with DSR remained stable over time; however, the limitation of using of a single sample from each sodium condition is minimized by both the crossover design and testing a 24-hour urine collection, rather than a spot urine. Finally, the changes in urinary metabolites may have been influenced by changes in BP induced by DSR, rather than by the direct effect of changes in sodium intake, as we previously reported a reduction in BP from 140±15/82±6 to 128±10/70±6 mmHg during the low sodium condition (8).
In conclusion, this proof-of-concept study provides the first evidence that DSR is a stimulus that induces significant changes in metabolomic profiles in healthy adults. Larger follow-up studies will be required to confirm and further elucidate biologic pathways that are altered in response to DSR. Because new metabolites are continually being discovered and it is not possible to include all known metabolites in a given panel (27,37), in the future, numerous other metabolites may be found to change with DSR and may be linked with improvements in physiologic function. These findings should stimulate future research related to these questions. In addition, future research may allow the identification of metabolite biomarkers that predict physiologic responsiveness to DSR among individuals. This may have important implications for other populations, such as patients with CKD, because several of the metabolites that changed with DSR have been reported to relate to CKD progression (3,26,38).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Keri Nelson, Elena Pellicer, Kate Howell, and the staff of the University of Colorado Boulder Clinical and Translational Research Center, particularly bionutritionists Kathleen Farrell and Rebecca Staley, for their technical assistance. In addition, the authors thank Nichole Reisdorph for her consultation on the manuscript.
This work was supported by awards from the National Institutes of Health (NIH) (AG013038, AG006537, and AG033994) and supported by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant #UL1 TR001082. Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11531114/-/DCSupplemental.
References
- 1.Wishart DS: Metabolomics: Applications to food science and nutrition research. Trends Food Sci Technol 19: 482–493, 2008 [Google Scholar]
- 2.Boccard J, Veuthey JL, Rudaz S: Knowledge discovery in metabolomics: An overview of MS data handling. J Sep Sci 33: 290–304, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Shah VO, Townsend RR, Feldman HI, Pappan KL, Kensicki E, Vander Jagt DL: Plasma metabolomic profiles in different stages of CKD. Clin J Am Soc Nephrol 8: 363–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B: Metabolomics in human nutrition: Opportunities and challenges. Am J Clin Nutr 82: 497–503, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Walsh MC, Brennan L, Pujos-Guillot E, Sébédio JL, Scalbert A, Fagan A, Higgins DG, Gibney MJ: Influence of acute phytochemical intake on human urinary metabolomic profiles. Am J Clin Nutr 86: 1687–1693, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV: The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the American Heart Association. Circulation 123: 1138–1143, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Frohlich ED, Susic D: Sodium and its multiorgan targets. Circulation 124: 1882–1885, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR: Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY, Seals DR: Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol 8: 1952–1959, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberger MH: Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Monteiro MS, Carvalho M, Bastos ML, Guedes de Pinho P: Metabolomics analysis for biomarker discovery: Advances and challenges. Curr Med Chem 20: 257–271, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee : Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gates PE, Tanaka H, Hiatt WR, Seals DR: Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 44: 35–41, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA: Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: Role of arterial stiffness. J Am Coll Cardiol 38: 506–513, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Christians U, Albuisson J, Klawitter J, Klawitter J: The role of metabolomics in the study of kidney diseases and in the development of diagnostic tools. In: Biomarkers of Kidney Disease, edited by Edelstein C, San Diego, CA, Elsevier, 2010 [Google Scholar]
- 16.Christians U, Schmitz V, Klawitter J, Klawitter J: Proteo-metabolomic strategies in the future of drug development. In: Analytical Techniques for Clinical Chemistry, edited by Caroli S, Zaray G, New York, John Wiley and Sons, Inc., 2012 [Google Scholar]
- 17.Dunn WB, Lin W, Broadhurst D, Begley P, Brown M, Zelena E, Vaughan AA, Halsall A, Harding N, Knowles JD, Francis-McIntyre S, Tseng A, Ellis DI, O’Hagan S, Aarons G, Benjamin B, Chew-Graham S, Moseley C, Potter P, Winder CL, Potts C, Thornton P, McWhirter C, Zubair M, Pan M, Burns A, Cruickshank JK, Jayson GC, Purandare N, Wu FC, Finn JD, Haselden JN, Nicholls AW, Wilson ID, Goodacre R, Kell DB: Molecular phenotyping of a UK population: Defining the human serum metabolome. Metabolomics 11: 9–26, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gika HG, Theodoridis GA, Wilson ID: Liquid chromatography and ultra-performance liquid chromatography-mass spectrometry fingerprinting of human urine: Sample stability under different handling and storage conditions for metabonomics studies. J Chromatogr A 1189: 314–322, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Klawitter J, Bendrick-Peart J, Rudolph B, Beckey V, Klawitter J, Haschke M, Rivard C, Chan L, Leibfritz D, Christians U, Schmitz V: Urine metabolites reflect time-dependent effects of cyclosporine and sirolimus on rat kidney function. Chem Res Toxicol 22: 118–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klawitter J, Klawitter J, Gurshtein J, Corby K, Fong S, Tagliaferri M, Quattrochi L, Cohen I, Shtivelman E, Christians U: Bezielle (BZL101)-induced oxidative stress damage followed by redistribution of metabolic fluxes in breast cancer cells: A combined proteomic and metabolomic study. Int J Cancer 129: 2945–2957, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Klawitter J, Gottschalk S, Hainz C, Leibfritz D, Christians U, Serkova NJ: Immunosuppressant neurotoxicity in rat brain models: Oxidative stress and cellular metabolism. Chem Res Toxicol 23: 608–619, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klawitter J, Kominsky DJ, Brown JL, Klawitter J, Christians U, Leibfritz D, Melo JV, Eckhardt SG, Serkova NJ: Metabolic characteristics of imatinib resistance in chronic myeloid leukaemia cells. Br J Pharmacol 158: 588–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan M, Breitkopf SB, Yang X, Asara JM: A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 7: 872–881, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solanky KS, Bailey NJ, Beckwith-Hall BM, Davis A, Bingham S, Holmes E, Nicholson JK, Cassidy A: Application of biofluid 1H nuclear magnetic resonance-based metabonomic techniques for the analysis of the biochemical effects of dietary isoflavones on human plasma profile. Anal Biochem 323: 197–204, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Llorach R, Urpi-Sarda M, Jauregui O, Monagas M, Andres-Lacueva C: An LC-MS-based metabolomics approach for exploring urinary metabolome modifications after cocoa consumption. J Proteome Res 8: 5060–5068, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Wald DS, Law M, Morris JK: Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 325: 1202, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A: HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res 41: D801–D807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennypacker LC, Allen RH, Kelly JP, Matthews LM, Grigsby J, Kaye K, Lindenbaum J, Stabler SP: High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc 40: 1197–1204, 1992 [PubMed] [Google Scholar]
- 29.Flatley JE, Garner CM, Al-Turki M, Manning NJ, Olpin SE, Barker ME, Powers HJ: Determinants of urinary methylmalonic acid concentration in an elderly population in the United Kingdom. Am J Clin Nutr 95: 686–693, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM: Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Mashima R, Nakanishi-Ueda T, Yamamoto Y: Simultaneous determination of methionine sulfoxide and methionine in blood plasma using gas chromatography-mass spectrometry. Anal Biochem 313: 28–33, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER: Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 98: 12920–12925, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D: The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med 53: 421–436, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Kitamura K, Tomita K: Regulation of renal sodium handling through the interaction between serine proteases and serine protease inhibitors. Clin Exp Nephrol 14: 405–410, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Zablocki K, Miller SP, Garcia-Perez A, Burg MB: Accumulation of glycerophosphocholine (GPC) by renal cells: Osmotic regulation of GPC:choline phosphodiesterase. Proc Natl Acad Sci U S A 88: 7820–7824, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang A, Sun H, Wu X, Wang X: Urine metabolomics. Clin Chim Acta 414: 65–69, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS: The human serum metabolome. PLoS ONE 6: e16957, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, Momose A, Toki N, Sato H, Nakayama M, Hozawa A, Tsuji I, Ito S, Soga T, Abe T: Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 33: 944–952, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.