Abstract
Background and objectives
Several studies have reported beneficial cardiovascular effects of marine n-3 polyunsaturated fatty acids. To date, no large studies have investigated the potential benefits of marine n-3 polyunsaturated fatty acids in recipients of renal transplants.
Design, setting, participants, & measurements
In this observational cohort study of 1990 Norwegian recipients of renal transplants transplanted between 1999 and 2011, associations between marine n-3 polyunsaturated fatty acid levels and mortality were investigated by stratified analysis and multivariable Cox proportional hazard regression analysis adjusting for traditional and transplant-specific mortality risk factors. Marine n-3 polyunsaturated fatty acid levels in plasma phospholipids were measured by gas chromatography in a stable phase 10 weeks after transplantation.
Results
There were 406 deaths (20.4%) during a median follow-up period of 6.8 years. Mortality rates were lower in patients with high marine n-3 polyunsaturated fatty acid levels (≥7.95 weight percentage) compared with low levels (<7.95 weight percentage) for all age categories (pooled mortality rate ratio estimate, 0.69; 95% confidence interval, 0.57 to 0.85). When divided into quartiles according to marine n-3 polyunsaturated fatty acid levels, patients in the upper quartile compared with the lower quartile had a 56% lower risk of death (adjusted hazard ratio, 0.44; 95% confidence interval, 0.26 to 0.75) using multivariable Cox proportional hazard regression analysis. There was a lower hazard ratio for death from cardiovascular disease with high levels of marine n-3 polyunsaturated fatty acid and a lower hazard ratio for death from infectious disease with high levels of the marine n-3 polyunsaturated fatty acid eicosapentaenoic acid, whereas there was no association between total or individual marine n-3 polyunsaturated fatty acid levels and cancer mortality.
Conclusions
Higher plasma phospholipid marine n-3 polyunsaturated fatty acid levels were independently associated with better patient survival.
Keywords: ω3 fatty acids, transplantation, survival, mortality
Introduction
Dietary factors, like the essential marine n-3 polyunsaturated fatty acids (PUFAs), may exert potent biologic effects (1). Cross-cultural epidemiologic studies in the 1970s reported a strong negative association between fish consumption and the incidence of cardiovascular disease (CVD) (2). Several beneficial metabolic effects of marine n-3 PUFAs have been reported, including lipid modulation (3), plaque stabilization (4), reduced BP (5), less artery calcification (6), and improved endothelial (7) and myocardial function (8). Furthermore, anti-inflammatory (9), antiarrhythmic (10), antiatherosclerotic (11), and antithrombotic effects (12) have been reported, which may influence the incidence of CVD and mortality rates. However, recent prospective cohort studies and randomized, controlled trials (RCTs) have shown mixed results, with only moderate beneficial or even neutral effects on major cardiovascular event and mortality rates (13–15).
In patients with ESRD, favorable effects of marine n-3 PUFAs on cardiovascular morbidity and mortality have been shown (16,17). Although renal transplantation reduces the risk of CVD in patients with ESRD, CVD remains the leading cause of death in recipients of renal transplants (RTRs) (18).
No cohort study and only small RCTs have assessed the effects of marine n-3 PUFAs in RTRs, with insufficient data to evaluate the effects on mortality (19–21). Because intake of marine n-3 PUFAs in Scandinavia is generally high, with considerable variation between individuals (22,23), our cohort is ideal for epidemiologic studies of associations between marine n-3 PUFA levels and mortality in RTRs. The aim of this study was to assess whether plasma phospholipid levels of marine n-3 PUFAs, which derive from consumption of fish, seafood, or marine n-3 PUFA supplements, were associated with overall and cause-specific mortality in RTRs.
Materials and Methods
Study Design and Population
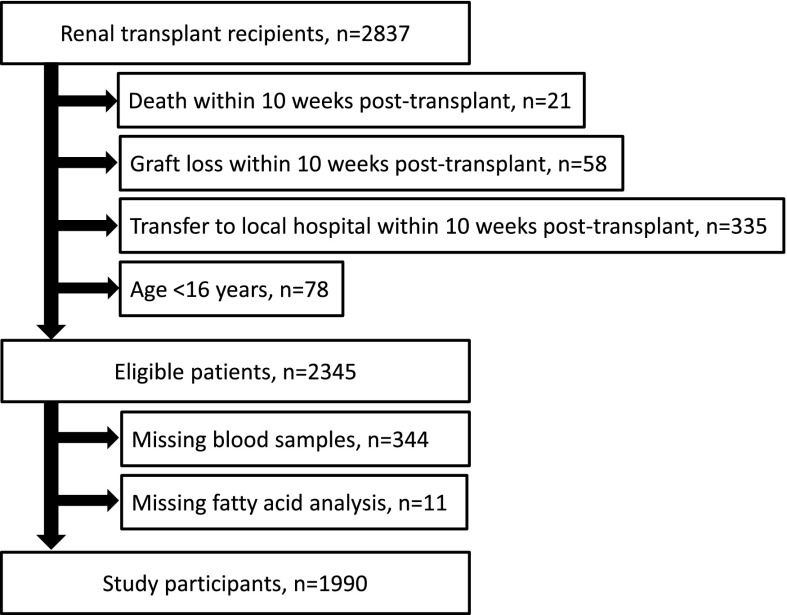
In Norway, all organ transplantations are performed at Oslo University Hospital, Rikshospitalet. From a population of approximately 5 million inhabitants, 2978 renal transplantations were performed in 2837 patients with ESRD between September 30, 1999 and October 13, 2011. Patients under the age of 16 years old (n=78) and patients who were transferred to their local hospitals (n=335), suffered graft loss (n=58), or died (n=21) within the first 10 weeks post-transplant were not eligible for participation in the study (Figure 1). Most patients attended our outpatient clinic for the first 10 weeks after renal transplantation. Informed consent was obtained from 2001 of 2345 eligible patients, in whom blood samples were drawn, measurements were taken, and clinical information was recorded at a final appointment 10 weeks post-transplant. From eligible patients, 344 patients did not attend the final appointment because of reduced capacity at our laboratory during 2007 and 2008. They were not included in the study because of the lack of laboratory and clinical data. In 11 patients, the amount of plasma was too small for individual fatty acids to be adequately analyzed. The remaining 1990 patients were included in this study.
Figure 1.
Flowchart of the inclusion of study participants.
Data Collection and Registry
In study participants, blood samples were drawn in the fasting state at the clinical appointment 10 weeks post-transplant. Some samples were analyzed at a central biochemical department, and laboratory test results were entered uniformly into a database. Other blood samples were immediately frozen, stored at −80°C, and later on sent to The Lipid Research Center, Aalborg University Hospital for analysis of plasma phospholipid fatty acid composition by gas chromatography.
End point data were collected from The Norwegian Renal Registry. The registry is on the basis of annual reports from all nephrologists working in Norway and includes all patients on RRT. Mortality is continuously registered. Surviving patients were censored on February 1, 2014. Mortality end points were defined according to the European Renal Association–European Dialysis and Transplant Association causes of death and included the broad categories cancer, infectious disease, and cardiovascular mortality (from the latter category, death from stroke, myocardial infarction [MI], and sudden cardiac death [SCD]).
In a random selection of 200 patients, all variables included in the Cox models were checked for consistency between data continuously registered at The Norwegian Renal Registry and data retrieved from medical records. There was excellent correspondence of data, with the exception of smoking status. After reviewing the medical records, correcting smoking status data, and obtaining missing data of all variables in 1990 study participants, there was 0.6% missing data on smoking status and <0.5% for other variables, and for most variables, there were no missing data. In total, there were only 14 study participants (0.7%) with missing data. They were not included in the multivariable Cox regression analysis. Baseline characteristics of patients with missing data did not differ significantly from patients without missing data. Description of the standard treatment protocol, clinical characteristics of adult RTRs not included in the study, and fatty acid analysis are described in Supplemental Appendix. In short, individual fatty acids were identified by gas chromatography and quantitated as the weight percentage (wt%) of total fatty acids. In this study, marine n-3 PUFAs were defined as the sum of plasma phospholipid levels of three individual marine n-3 PUFAs: eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA).
Statistical Analyses
Differences in baseline characteristics by quartiles of marine n-3 PUFAs according to plasma phospholipid levels were evaluated using the Mantel–Haenszel test of linear trend for categorical data, the nonparametric Kruskal–Wallis test for time in dialysis, and linear regression for other continuous data. The associations between total and individual marine n-3 PUFAs and mortality end points were studied by stratified and Cox proportional hazard regression analyses. A stratified analysis adjusting for recipient age was used for estimation of mortality rates. Identification of confounders and fitting of Cox models and graphing are described in Supplemental Appendix. In short, recipient age was identified as a strong confounder and an effect-measure modifier for marine n-3 PUFAs; hence, a product term was included in the survival analysis. Two Cox models were fitted. In addition to recipient age and a product term of recipient age and marine n-3 PUFA level, model 1 included the following traditional and transplant-specific mortality risk factors: sex, eGFR according to the Modification of Diet in Renal Disease formula, time in dialysis before transplantation, preemptive transplantation (no dialysis treatment before transplantation), choice of calcineurin inhibitor (treatments with cyclosporin A or tacrolimus were included as two separate categorical variables), smoking status (current smoker, former smoker, or lifelong nonsmoker as a categorical variable), body mass index, albumin and total plasma cholesterol concentrations, number of antihypertensive drugs, diabetes mellitus before transplantation, pretransplant coronary artery, and cerebrovascular and peripheral vascular disease. Model 2 added n-6 PUFA levels as a covariate. A review of the rationale for the two models is found in Supplemental Appendix. Model assumptions were checked by inspection of the log-log survival time plots and formal hypothesis tests (Schoenfeld residuals). A two-sided P value of <0.05 was considered statistically significant. PASW Statistics, version 17.0 (IBM, New York, NY) and STATA, version 13.0 (Stata Corp, College Station, TX) were used for the statistical analysis.
The study was approved by the Regional Committees for Medical and Health Research Ethics in Norway and performed in accordance with the Declaration of Helsinki (https://clinicaltrials.gov/ no. NCT02017990). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Results
Baseline characteristics of the study participants grouped according to marine n-3 PUFA levels are shown in Table 1. The median level of marine n-3 PUFAs in plasma phospholipids was 7.95 wt%. Patients with high marine n-3 PUFA levels (≥7.95 wt%) were older than patients with lower levels (<7.95 wt%). From 2007, most patients under the age of 50 years old were treated with tacrolimus, whereas older patients received cyclosporin A. When adjusted for age and transplant era, neither choice of calcineurin inhibitor nor eGFR differed between high and low levels of marine n-3 PUFAs. We found a negative association between marine n-3 PUFA levels and both current smoking and n-6 PUFA levels, even after adjustments for age and transplant era, and a trend toward less living donor transplantations and lower prevalence of diabetes mellitus with high marine n-3 PUFA levels. Adult RTRs not included the study were older (mean age of 55.1 years old) than the study participants (mean age of 51.6 years old). Because there were more patients not included in the study after 2007, the groups differed with regards to choice of calcineurin inhibitors (Supplemental Table 1). When adjusting for age, other baseline characteristics for the two groups were similar.
Table 1.
Baseline characteristics of the study participants according to levels of marine n-3 polyunsaturated fatty acids
| Variables | All Patients | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value (Trend) |
|---|---|---|---|---|---|---|
| Marine n-3 PUFA level, wt% | 1.35–23.87 | ≤6.20 | 6.21–7.94 | 7.95–10.02 | ≥10.03 | |
| No. of patients | 1990 | 499 | 499 | 495 | 497 | |
| Recipient age, yr | 51.6 (14.6) | 43.3 (14.0) | 51.6 (14.1) | 54.2 (13.3) | 57.3 (13.1) | <0.001 |
| Sex (men), % | 66.9 | 69.0 | 64.8 | 65.6 | 68.3 | 0.90 |
| Tacrolimus, % | 23.1 | 35.5 | 22.2 | 19.4 | 15.3 | <0.001 |
| Cyclosporin A, % | 74.2 | 11.9 | 17.3 | 15.9 | 16.0 | 0.13 |
| Cerebrovascular disease, % | 5.2 | 4.2 | 5.8 | 6.0 | 4.8 | 0.66 |
| Peripheral vascular disease, % | 8.0 | 6.2 | 9.3 | 8.2 | 8.2 | 0.37 |
| Diabetes mellitus, % | 18.2 | 23.5 | 21.1 | 15.3 | 13.0 | <0.001 |
| Current smoker, % | 16.0 | 23.5 | 18.0 | 12.9 | 9.4 | <0.001 |
| Former smoker, % | 36.2 | 30.2 | 37.7 | 36.5 | 40.4 | 0.002 |
| No. of antihypertensive drugs, % | 0.22 | |||||
| None or one | 54.4 | 56.3 | 55.1 | 53.5 | 52.7 | |
| Two or three | 41.2 | 39.0 | 41.6 | 41.9 | 42.3 | |
| Four or more | 4.4 | 4.6 | 3.2 | 4.6 | 5.0 | |
| Body mass index, kg/m2 | 24.8 (3.9) | 24.6 (4.2) | 24.7 (3.9) | 25.0 (3.7) | 24.9 (3.5) | 0.09 |
| Total cholesterol, mg/dl | 244.4 (59.0) | 238.2 (60.6) | 243.2 (56.4) | 249.8 (59.1) | 246.7 (59.5) | 0.01 |
| Albumin, g/dl | 4.00 (0.37) | 4.06 (0.40) | 3.98 (0.36) | 3.98 (0.34) | 3.98 (0.35) | 0.001 |
| Time in dialysis, mo | 9 (0–19) | 8 (0–18) | 9 (1–20) | 8 (0–18) | 9 (1–19) | 0.21 |
| Preemptive transplantation, % | 25.2 | 27.0 | 23.9 | 25.6 | 24.2 | 0.41 |
| First renal transplantation, % | 90.3 | 88.2 | 88.8 | 92.0 | 92.2 | 0.01 |
| eGFR, ml/min per 1.73 m2 | 56.9 (18.8) | 64.5 (20.4) | 56.5 (18.3) | 54.2 (17.3) | 52.6 (16.9) | <0.001 |
| Delayed graft function, % | 6.3 | 6.8 | 6.6 | 8.6 | 3.6 | 0.26 |
| Early rejection episode, % | 27.7 | 27.8 | 29.7 | 27.3 | 26.3 | 0.44 |
| Donor age, yr | 47.2 (16.1) | 44.2 (15.7) | 46.1 (16.7) | 49.3 (15.3) | 49.5 (16.1) | <0.001 |
| Living donor transplantation, % | 37.8 | 42.1 | 33.2 | 41.4 | 34.7 | 0.08 |
| No. of HLA mismatches, % | 0.23 | |||||
| None or one | 15.7 | 20.5 | 11.5 | 14.9 | 16.0 | |
| Two | 23.4 | 20.3 | 24.1 | 25.6 | 23.2 | |
| Three | 32.9 | 31.2 | 34.6 | 32.2 | 33.5 | |
| Four or more | 28.0 | 28.0 | 29.8 | 27.4 | 27.1 |
Baseline characteristics of the study population according to marine n-3 polyunsaturated fatty acid (PUFA) levels defined as the sum of plasma phospholipid eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid levels in weight percentage (wt%) of total plasma phospholipid fatty acids. Results are presented as proportions for categorical data, medians and interquartile ranges for time in dialysis, and means and SDs for other continuous data. Differences in baseline characteristics were evaluated using the Mantel–Haenszel test of linear trend for categorical data, the Kruskal–Wallis test for time in dialysis, and linear regression for other continuous data. Pretransplant diabetes mellitus, coronary artery, cerebrovascular and peripheral vascular disease were recorded before first renal transplantation. Recipient and donor age, deceased or living donor, time in dialysis, number of HLA mismatches, and smoking status were recorded at the time of transplantation. Choice of calcineurin inhibitor, number of antihypertensive drugs, delayed graft function, early rejection episodes, body mass index, eGFR using the Modification of Diet in Renal Disease formula, total cholesterol, and albumin values were recorded at a clinical appointment 10 weeks post-transplant.
During the study period, the total number of deaths was 406 (20.4%). In 164 patients, death was caused by CVD (40.4% of deaths). There were 95 deaths by cancer (23.4%) and 101 deaths by infectious disease (24.9%). The median follow-up time for study participants was 6.8 years.
The crude analysis showed a small positive association between levels of marine n-3 PUFAs and mortality. However, in an age-stratified analysis reducing the confounding effect of recipient age, the mortality rate was lower in patients with marine n-3 PUFA levels at or above the median value of 7.95 wt% compared with the patients with lower marine n-3 PUFA levels for all age categories (Figure 2, Table 2). The pooled estimate for the mortality rate ratio was 0.69 (95% confidence interval [95% CI], 0.57 to 0.85). Consistent with this finding, we found a negative association between marine n-3 PUFA levels and mortality in multivariable-adjusted Cox proportional hazard regression analysis using both models 1 and 2. The association between marine n-3 PUFA levels and mortality was similar in periods with high and low inclusion rates (Supplemental Table 2), and adjustment for transplant eras had no effect on results.
Figure 2.
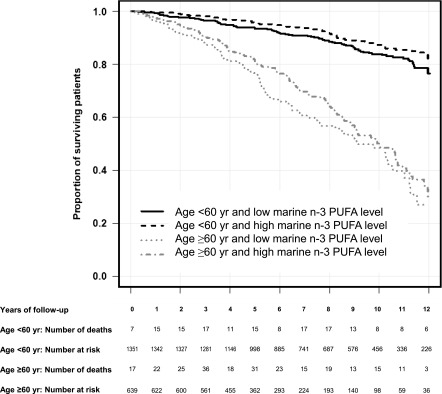
Kaplan–Meier survival curve in recipients of renal transplants. Shown are the proportions of surviving patients grouped according to age (age <60 and ≥60 years old) and marine n-3 polyunsaturated fatty acid (PUFA) levels (the sum of eicosapentaenoic, docosapentaenoic, and docosahexaenoic acid levels in weight percentage [wt%] of total plasma phospholipid fatty acids <7.95 wt% [low] and ≥7.95 wt% [high]).
Table 2.
Mortality rates by marine n-3 polyunsaturated fatty acid levels and age category
| Age Category, yr | ||||||
|---|---|---|---|---|---|---|
| 16–44 | 45–59 | 60–80 | ||||
| Marine n-3 PUFA level | High | Low | High | Low | High | Low |
| Mortality rate, patients per 1000 person-yr | 4.1 | 10.1 | 16.6 | 26.7 | 59.2 | 69.0 |
| Mortality rate difference | −6.0 | −10.1 | −9.8 | |||
| Mortality rate ratio (95% confidence interval) | 0.41 (0.17 to 0.88) | 0.62 (0.40 to 0.85) | 0.86 (0.63 to 1.05) | |||
| Pooled estimate (95% confidence interval) | 0.69 (0.57 to 0.85) | 0.69 (0.57 to 0.85) | 0.69 (0.57 to 0.85) | |||
The mortality rate according marine n-3 polyunsaturated fatty acid (PUFA) levels (the sum of eicosapentaenoic, docosapentaenoic, and docosahexaenoic acid levels in weight percentage [wt%] of total plasma phospholipid fatty acids; ≥7.95 wt% [high] and <7.95 wt% [low]) in different age groups. Mortality rate ratios for each age category were obtained by dividing the mortality rate of patients with high marine n-3 PUFA levels by the mortality rate of patients with low levels.
We found lower levels of n-6 PUFAs with higher levels of marine n-3 PUFAs (Supplemental Table 3). There was no interaction between marine n-3 PUFA and n-6 PUFA levels. The mean ratio of n-6 PUFA to n-3 PUFA was 4.75. When using marine n-6 PUFA to n-3 PUFA ratio (Supplemental Table 4) or arachidonic acid (AA) to EPA ratio as the primary exposure, we found similar results to using marine n-3 PUFA levels alone. Although there were differences in hazard ratio (HR) estimates between models 1 and 2, similar trends were found for all causes of death with both models (Table 3).
Table 3.
Estimated mortality risk according to quartiles of marine n-3 polyunsaturated fatty acid levels using multivariable Cox proportional hazard regression analysis
| Cox Models | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||||
| wt% | ≤6.20 | 6.21–7.94 | 7.95–10.02 | ≥10.03 | ||||||
| No. of patients | 495 | 498 | 492 | 491 | ||||||
| Mortality | HR | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value |
| All cause | 1.0 | 0.82 | 0.60 to 1.12 | 0.22 | 0.59 | 0.40 to 0.86 | 0.01 | 0.44 | 0.26 to 0.75 | 0.003 |
| Cardiovascular | 1.0 | 0.56 | 0.34 to 0.91 | 0.02 | 0.36 | 0.20 to 0.65 | 0.001 | 0.18 | 0.08 to 0.42 | <0.001 |
| MI | 1.0 | 0.55 | 0.14 to 2.08 | 0.38 | 0.63 | 0.14 to 2.83 | 0.55 | 0.51 | 0.07 to 3.76 | 0.51 |
| SCD | 1.0 | 0.58 | 0.29 to 1.16 | 0.12 | 0.31 | 0.13 to 0.73 | 0.01 | 0.08 | 0.02 to 0.35 | 0.001 |
| Stroke | 1.0 | 0.50 | 0.16 to 1.54 | 0.22 | 0.30 | 0.08 to 1.20 | 0.09 | 0.14 | 0.02 to 1.09 | 0.06 |
| Infectious disease | 1.0 | 0.78 | 0.41 to 1.46 | 0.43 | 0.47 | 0.21 to 1.04 | 0.06 | 0.51 | 0.18 to 1.50 | 0.22 |
| Cancer | 1.0 | 1.47 | 0.71 to 3.06 | 0.31 | 1.30 | 0.56 to 3.04 | 0.54 | 1.37 | 0.45 to 4.18 | 0.58 |
| Model 2 | ||||||||||
| wt% | ≤6.20 | 6.21–7.94 | 7.95–10.02 | ≥10.03 | ||||||
| No. of patients | 495 | 498 | 492 | 491 | ||||||
| Mortality | HR | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value |
| All cause | 1.0 | 0.77 | 0.55 to 1.06 | 0.11 | 0.48 | 0.32 to 0.73 | 0.001 | 0.33 | 0.19 to 0.58 | <0.001 |
| Cardiovascular | 1.0 | 0.51 | 0.31 to 0.85 | 0.01 | 0.29 | 0.15 to 0.55 | <0.001 | 0.12 | 0.05 to 0.30 | <0.001 |
| MI | 1.0 | 0.70 | 0.17 to 2.85 | 0.61 | 1.00 | 0.17 to 6.04 | >0.99 | 0.62 | 0.06 to 6.13 | 0.68 |
| SCD | 1.0 | 0.55 | 0.27 to 1.12 | 0.10 | 0.29 | 0.11 to 0.73 | 0.01 | 0.07 | 0.02 to 0.32 | 0.001 |
| Stroke | 1.0 | 0.48 | 0.15 to 1.54 | 0.22 | 0.26 | 0.06 to 1.12 | 0.07 | 0.08 | 0.01 to 0.70 | 0.02 |
| Infectious disease | 1.0 | 0.70 | 0.37 to 1.34 | 0.28 | 0.36 | 0.15 to 0.84 | 0.02 | 0.39 | 0.13 to 1.19 | 0.10 |
| Cancer | 1.0 | 1.42 | 0.67 to 3.05 | 0.36 | 1.15 | 0.45 to 2.93 | 0.77 | 1.08 | 0.32 to 3.59 | 0.90 |
The estimated risk of total and cause-specific mortality using multivariable-adjusted Cox proportional hazard regression models 1 and 2. Results are presented as multivariable-adjusted hazard ratios for developing mortality end points relative to the lower quartile of marine n-3 polyunsaturated fatty acid (the sum of eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid in weight percentage [wt%] of total plasma phospholipid fatty acids) levels. In addition to marine n-3 polyunsaturated fatty acids levels, model 1 included the following variables: recipient age, a product term of recipient age and marine n-3 polyunsaturated fatty acid levels, sex, eGFR using the Modification of Diet in Renal Disease formula, time in dialysis before transplantation, preemptive transplantation, body mass index, number of antihypertensive drugs, diabetes mellitus, coronary artery, cerebrovascular and peripheral vascular disease, and albumin and total plasma cholesterol concentrations. Model 2 also includes n-6 polyunsaturated fatty acids levels as a covariate. HR, hazard ratio; 95% CI, 95% confidence interval; MI, myocardial infarction; SCD, sudden cardiac death.
When grouped according to marine n-3 PUFA levels comparing the upper with the lower quartile, there was a 56% lower risk of death (multivariable-adjusted HR, 0.44; 95% CI, 0.26 to 0.75) using model 1 (Table 3) and 67% lower risk of death (multivariable-adjusted HR, 0.33; 95% CI, 0.19 to 0.58) using model 2 (Figure 3, Table 3). Similar results were found for EPA and DHA alone (Supplemental Table 5), whereas no association was found between DPA levels and mortality. The risk of death from CVD was markedly lower in patients with high marine n-3 PUFA levels compared with low levels. In particular, death from stroke and SCD showed a strong negative association with marine n-3 PUFA levels (Table 3). Patients with high levels of EPA were also less likely to die from infectious disease and equally associated with nonsepticemia infectious disease mortality as death from septicemia (Supplemental Table 5). No association was found between marine n-3 PUFA levels and death by cancer.
Figure 3.
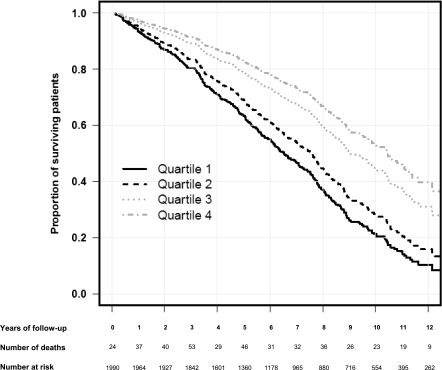
Estimated survival probability curve in recipients of renal transplants in multivariable–adjusted Cox proportional hazard regression model 2. Shown are the survival probabilities of patients belonging to quartiles 1–4 according to marine n-3 polyunsaturated fatty acid (PUFA) levels (the sum of eicosapentaenoic, docosapentaenoic, and docosahexaenoic acid levels in weight percentage [wt%] of total plasma phospholipid fatty acids) after adjustment for the following variables: recipient age, sex, n-6 PUFA levels, eGFR using the Modification of Diet in Renal Disease formula, time in dialysis before transplantation, preemptive transplantation, body mass index, number of antihypertensive drugs, diabetes mellitus, coronary artery, cerebrovascular and peripheral vascular diseases, and albumin and total plasma cholesterol concentrations. Quartile 1, marine n-3 PUFA ≤6.20 wt%; quartile 2, marine n-3 PUFA =6.21–7.94 wt%; quartile 3, n-3 PUFA =7.95–10.02 wt%; quartile 4, marine n-3 PUFA ≥10.03 wt%.
In 908 of 984 patients (92.2%) with functional renal grafts 5 years post-transplant, we looked at the decline in renal function over time according to marine n-3 PUFA levels. Creatinine values increased more in patients with low marine n-3 PUFA levels than in patients with higher marine n-3 PUFA levels (Table 4).
Table 4.
Change in renal function within the first 5 years after transplantation according to levels of marine n-3 polyunsaturated fatty acids
| Serum Creatinine | All patients | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value (Trend) |
|---|---|---|---|---|---|---|
| Marine n-3 PUFA level, wt% | 1.35–23.87 | ≤6.20 | 6.21–7.94 | 7.95–10.02 | ≥10.03 | |
| No. of patients | 908 | 196 | 216 | 234 | 262 | |
| Serum creatinine at 10 wk post-transplant, mg/dl | 1.33 (0.42) | 1.27 (0.37) | 1.34 (0.39) | 1.36 (0.47) | 1.34 (0.41) | 0.10 |
| Serum creatinine at 5 yr post-transplant, mg/dl | 1.43 (0.56) | 1.48 (0.63) | 1.42 (0.57) | 1.41 (0.55) | 1.42 (0.53) | 0.26 |
| Change between 10 wk and 5 yr, mg/dl | 0.10 (0.50) | 0.21 (0.55) | 0.08 (0.49) | 0.06 (0.48) | 0.08 (0.46) | 0.01 |
Change in renal function in the first 5 years post-transplant in study participants with functional renal grafts transplanted between 1999 and 2008 according to marine n-3 polyunsaturated fatty acid (PUFA) levels defined as the sum of plasma phospholipid eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid levels in weight percentage (wt%) of total plasma phospholipid fatty acids. Results are presented as means and SDs for serum creatinine values. Statistic trend was evaluated by linear regression analysis.
Discussion
The major finding of our study was an independent and negative association between plasma phospholipid marine n-3 PUFA levels and overall and cardiovascular mortality after renal transplantation. Deaths from infectious disease were strongly associated with low levels of EPA.
Three separate meta-analyses of RCTs focusing on the effect of marine n-3 PUFA supplementation after renal transplantation have reported a significant reduction in triglyceride levels, a minor reduction of diastolic BP, and slightly increased concentrations of HDL cholesterol, with possible beneficial effect on patient survival (19–21). However, effects on mortality rates could not be evaluated because of a low number of events (only seven deaths in 846 patients) and short follow-up (19,21). The much higher number of patients and longer follow-up time in our study provide a possibility to evaluate mortality.
CVD is prevalent in RTRs (18). Along with traditional CVD risk factors, post-transplant immunosuppressive treatment (24), pretransplant uremia (25), and renal graft function (18) constitute important risk factors for CVD in RTRs. Favorable effects of marine n-3 PUFA supplementation have been shown in diabetic nephropathy and IgA nephritis (26,27), and renal functions were better preserved with marine n-3 PUFA supplementation in patients with a history of MI (28). We found a steeper decline in renal function during the first 5 years after transplantation in patients with low levels of marine n-3 PUFAs, indicating potential renoprotective effects of marine n-3 PUFAs. It is possible that this could have influenced subsequent mortality.
Beneficial effects of marine n-3 PUFAs have also been reported in patients with ESRD. Whether these effects are applicable to RTRs is unclear. In patients who are uremic and treated with hemodialysis, high intake of marine n-3 PUFAs was associated with a lower risk of SCD (17), and an RCT found a lower cumulative incidence of MI after marine n-3 PUFA supplementation (16). In RTRs, there have been reports of improved renal function with marine n-3 PUFA supplementation (29). Most studies, however, reported no effect (19–21).
The assumption that increased intake of marine n-3 PUFAs significantly reduces the risk of cardiovascular morbidity and mortality has been challenged in recent meta-analyses of RCTs (13–15,30–32). The divergent results of early and recent studies may have several explanations, including more patients on optimal antihypertensive, antithrombotic, and statin treatment (33) and an increased background consumption of fish and seafood (34). In this study of a CVD high-risk population, even with optimal conventional treatment, we found an association between high marine n-3 PUFA levels and a lower risk of CVD-related death in RTRs.
A recent meta-analysis of prospective cohort studies found a negative association between cerebrovascular disease incidence and fish consumption (35). Although hampered by a low event rate, our findings indicate negative association between marine n-3 PUFA levels and death from stroke (Table 3).
Impaired glucose metabolism increases the risk of CVD, serious infections, and mortality after renal transplantation (18). Because reduction of total dietary fat in the Western diet during the last decades has led to increased consumption of carbohydrates, with possibly negative health effects (36), there is now more emphasis on the beneficial effects of PUFAs and the composition of various nutrients. High levels of marine n-3 PUFAs were associated with a lower prevalence of diabetes mellitus at the time of transplantation, despite a higher age group. A meta-analysis of RCTs reported a positive effect of marine n-3 PUFA supplementation on insulin sensitivity (37), possibly preventing or delaying onset of diabetes. A large RCT in patients with diabetes found no effect on cardiovascular mortality with low-dose marine n-3 PUFA supplementation (38).
In patients with septicemia, reduced mortality rates have been found with both a high-dose marine n-3 PUFA (>0.1 g/kg per day) supplementation (39) and an inflammation-modulating diet consisting of marine n-3 PUFAs, borage oil, and antioxidants (40). Our study indicates that moderate to high levels of EPA are associated with a lower risk of death from infectious disease in RTRs. EPA competes with the proinflammatory n-6 PUFA AA as substrate in the cyclooxygenase pathway and may, therefore, limit the inflammatory response (9).
A typical Western diet has a high content of n-6 PUFA relative to marine n-3 PUFA or the n-3 PUFA α-linolenic acid found in some green plants, rapeseed, and nuts (41). A study performed in the United States reported a mean n-6:n-3 PUFA ratio of 10:1 (42). In contrast, the mean n-6:n-3 PUFA ratio was 4.75 in this Norwegian population, and 42% of the study participants met with dietary recommendations of an n-6:n-3 PUFA ratio of 4:1 or less.
DPA, an elongation metabolite of EPA, may exert similar effects as EPA (43). We found no association between DPA levels and mortality. The difference between high and low levels of DPA was lower than for EPA and DHA and did not correlate well with either EPA or DHA level, indicating that DPA was a less-sensitive parameter of fish intake than EPA and DHA.
We found no association between marine n-3 PUFA levels and overall or type-specific cancer mortality. This finding is consistent with most studies reporting neutral effects from marine n-3 supplementation in patients with cancer (44). The potential influence of competing risk on cancer mortality is discussed in Supplemental Appendix.
The dietary consumption of fish and seafood varies between regions. In Norway, the average marine n-3 PUFA intake is more than five times higher than in the United States (34). The median value for patients belonging to the upper marine n-3 PUFA quintile in the Cardiovascular Health Study performed in the United States (45) was within the lower quartile in this study. A positive association between fish consumption or marine n-3 PUFA levels and survival has been reported in populations with high, moderate, and low consumption of fish and seafood, indicating beneficial effects, regardless of level (45–51).
Major limitations in our study include lack of dietary data to adjust for the full matrix of nutrients and the fact that we performed only a single measurement of plasma phospholipid marine n-3 PUFA levels 10 weeks post-transplant. This ignores temporal changes in fatty acid levels, and hence, we assume that a single measurement of plasma marine n-3 PUFAs was a unique correlate with mortality. Although an obvious limitation, a recent report from a Norwegian population showed a highly significant coherence in plasma phospholipid levels for all marine n-3 PUFAs measured 3 years apart, and plasma marine n-3 PUFA levels correlated well with marine food intake (52). Nonetheless, repeated measurements of plasma fatty acids would have been ideal to check for consistency in dietary habits and exclusion of measurement errors.
Several lifestyle factors may be associated with intake of fish and act as confounders not adjusted for in the analyses. We did not register information on education level, socioeconomic status, or physical activity. A recent Nordic study showed a positive association between the intake of fish and education level, physical activity, and various dietary components considered healthy (22). We have no data on the consumption of marine n-3 supplements versus fish consumption, and we performed no measurements of mercury or other contaminants found in fish. We have data on the number but not the type of antihypertensive drugs. We also lack data on antidiabetic treatment and statin use. Because the majority of patients in this Norwegian study were white, the results may not be applicable in other ethnic groups or regions with different dietary intake of marine n-3 PUFAs. We found an inverse association between marine n-3 PUFA levels and current smoking and diabetes mellitus before transplantation. Although adjustments were made in the Cox regression analysis, the effect of these confounders may not have been fully corrected. Competing risk may influence on cause-specific mortality HRs. Finally, it should be noted that the observational study design does not allow causal inference.
This study also has several strengths. Most importantly, individual plasma phospholipid fatty acids were measured by gas chromatography, which in contrast to dietary questionnaires, correlates very well with actual marine n-3 PUFA intake (53). Moreover, a very low percentage of missing data, quality-assured data, a relatively long follow-up period, a large number of events, a well defined and large study population with a high inclusion rate from a single center, and several traditional and transplant-specific mortality risk factors included as confounders in the Cox models constitute strengths of this study.
In summary, this observational cohort study found lower overall and cardiovascular mortality risk after renal transplantation with high plasma phospholipid marine n-3 PUFA levels, indicating that RTRs may benefit from marine n-3 PUFA supplementation. Future RCTs focusing on the effects of marine n-3 PUFAs on traditional and transplant-specific mortality risk factors with adequate sample sizes, follow-up periods, and dosages of marine n-3 PUFA supplements are warranted in renal transplantation. Hopefully, the ongoing Omega-3 Fatty Acids in Renal Transplantation Trial (ClinicalTrials.gov no. NCT01744067) can provide important information.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank coworkers Els Breistein, Kirsten Lund, and May Ellen Lauritzen at The Renal Physiology Laboratory at Oslo University Hospital, Rikshospitalet for performing the baseline patient workup in this study. We thank coworkers Rikke Bülow Eschen, Annette Andreassen, Birthe H. Thomsen, and Inge Aardestrup at The Lipid Research Laboratory, Aalborg University Hospital for analyzing plasma phospholipid fatty acids and Dr. Stein Bergan at Oslo University Hospital, Rikshospitalet for laboratory analyses. We also thank Dr. Torbjørn Leivestad at The Norwegian Renal Registry, Oslo University Hospital, Rikshospitalet for provision of data and Dr. James Eide Macpherson, Oslo University Hospital for collegial advice and revision of the manuscript. We also thank the funding sources and the study participants.
I.A.E. received research funding from South-Eastern Norway Regional Health Authority, Gidske and Peter Jacob Sørensen Research Fund, The Norwegian National Association for Kidney Patients and Transplant Recipients Research Fund, Nathalia and Knut Juul Christensen Research Fund, Signe and Albert Bergsmarken Research Fund, and Gertrude and Jack Nelsons Research Fund.
The funding organizations had no role in design of the study, data collection, data analysis, interpretation, manuscript preparation, or decision to submit.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11931214/-/DCSupplemental.
References
- 1.De Caterina R: n-3 fatty acids in cardiovascular disease. N Engl J Med 364: 2439–2450, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Kromann N, Green A: Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Acta Med Scand 208: 401–406, 1980 [PubMed] [Google Scholar]
- 3.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ: Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 197: 12–24, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF: Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 361: 477–485, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ: Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J Hypertens 20: 1493–1499, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Sekikawa A, Miura K, Lee S, Fujiyoshi A, Edmundowicz D, Kadowaki T, Evans RW, Kadowaki S, Sutton-Tyrrell K, Okamura T, Bertolet M, Masaki KH, Nakamura Y, Barinas-Mitchell EJ, Willcox BJ, Kadota A, Seto TB, Maegawa H, Kuller LH, Ueshima H, ERA JUMP Study Group : Long chain n-3 polyunsaturated fatty acids and incidence rate of coronary artery calcification in Japanese men in Japan and white men in the USA: Population based prospective cohort study. Heart 100: 569–573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D: Effect of omega-3 fatty acids supplementation on endothelial function: A meta-analysis of randomized controlled trials. Atherosclerosis 221: 536–543, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Masson S, Marchioli R, Mozaffarian D, Bernasconi R, Milani V, Dragani L, Tacconi M, Marfisi RM, Borgese L, Cirrincione V, Febo O, Nicolis E, Maggioni AP, Tognoni G, Tavazzi L, Latini R: Plasma n-3 polyunsaturated fatty acids in chronic heart failure in the GISSI-Heart Failure Trial: Relation with fish intake, circulating biomarkers, and mortality. Am Heart J 165: 208–215, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Calder PC: n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83[Suppl]: 1505S–1519S, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Christensen JH: Omega-3 polyunsaturated Fatty acids and heart rate variability. Front Physiol 2: 84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H: The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 130: 554–562, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Eritsland J, Arnesen H, Seljeflot I, Kierulf P: Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagul Fibrinolysis 6: 17–22, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B: Omega 3 Fatty acids and cardiovascular outcomes: Systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 5: 808–818, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Casula M, Soranna D, Catapano AL, Corrao G: Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler Suppl 14: 243–251, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS: Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 308: 1024–1033, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Svensson M, Schmidt EB, Jørgensen KA, Christensen JH, OPACH Study Group : N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol 1: 780–786, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Friedman AN, Yu Z, Tabbey R, Denski C, Tamez H, Wenger J, Thadhani R, Li Y, Watkins BA: Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int 83: 1130–1135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, Kasiske BL, PORT Investigators : Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant 10: 338–353, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Lim AK, Manley KJ, Roberts MA, Fraenkel MB: Fish oil treatment for kidney transplant recipients: A meta-analysis of randomized controlled trials. Transplantation 83: 831–838, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Tatsioni A, Chung M, Sun Y, Kupelnick B, Lichtenstein AH, Perrone R, Chew P, Lau J, Bonis PA: Effects of fish oil supplementation on kidney transplantation: A systematic review and meta-analysis of randomized, controlled trials. J Am Soc Nephrol 16: 2462–2470, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bonis PA, Chung M, Tatsioni A, Sun Y, Kupelnick B, Lichtenstein A, Perrone R, Chew P, DeVine D, Lau J: Effects of omega-3 fatty acids on organ transplantation. Evid Rep Technol Assess (Summ) 115: 1–11, 2005 [PMC free article] [PubMed] [Google Scholar]
- 22.Wennberg M, Tornevi A, Johansson I, Hörnell A, Norberg M, Bergdahl IA: Diet and lifestyle factors associated with fish consumption in men and women: A study of whether gender differences can result in gender-specific confounding. Nutr J 11: 101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roswall N, Olsen A, Boll K, Christensen J, Halkjær J, Sørensen TI, Dahm CC, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Cottet V, Teucher B, Kaaks R, Boeing H, von Ruesten A, Trichopoulou A, Oikonomou E, Vasilopoulou E, Pala V, Sacerdote C, Mattiello A, Masala G, Peeters PH, Bueno-de-Mesquita HB, Engeset D, Skeie G, Asli LA, Amiano P, Jakszyn P, Ardanaz E, Huerta JM, Quirós JR, Molina-Montes E, Nilsson LM, Johansson I, Wirfält E, Drake I, Mulligan AA, Khaw KT, Romaguera D, Vergnaud AC, Key T, Riboli E, Tjønneland A: Consumption of predefined ‘Nordic’ dietary items in ten European countries - an investigation in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Public Health Nutr 17: 2650–2659, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojo AO: Cardiovascular complications after renal transplantation and their prevention. Transplantation 82: 603–611, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donadio JV, Jr., Larson TS, Bergstralh EJ, Grande JP: A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol 12: 791–799, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Rossing P, Hansen BV, Nielsen FS, Myrup B, Hølmer G, Parving HH: Fish oil in diabetic nephropathy. Diabetes Care 19: 1214–1219, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Hoogeveen EK, Geleijnse JM, Kromhout D, Stijnen T, Gemen EF, Kusters R, Giltay EJ: Effect of omega-3 fatty acids on kidney function after myocardial infarction: The Alpha Omega Trial. Clin J Am Soc Nephrol 9: 1676–1683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Heide JJ, Bilo HJ, Donker JM, Wilmink JM, Tegzess AM: Effect of dietary fish oil on renal function and rejection in cyclosporine-treated recipients of renal transplants. N Engl J Med 329: 769–773, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE, Riemersma RA, Ebrahim SB, Davey Smith G: Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 332: 752–760, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F: Long chain omega-3 fatty acids and cardiovascular disease: A systematic review. Br J Nutr 107[Suppl 2]: S201–S213, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Wu JH: Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58: 2047–2067, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H: Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur Heart J 33: 436–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D, Global Burden of Diseases Nutrition and Chronic Diseases Expert Group NutriCoDE : Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 348: g2272, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB, Franco OH: Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 345: e6698, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D, Barber R, Huynh C, Dicker D, Templin T, Wolock TM, Ozgoren AA, Abd-Allah F, Abera SF, Abubakar I, Achoki T, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Agardh EE, Akena D, Alasfoor D, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Al Kahbouri MJ, Alla F, Allen PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzmán N, Amankwaa AA, Amare AT, Amini H, Ammar W, Antonio CA, Anwari P, Arnlöv J, Arsenijevic VS, Artaman A, Asad MM, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Basu A, Basu S, Beardsley J, Bedi N, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhutta Z, Bin Abdulhak A, Blore JD, Basara BB, Bose D, Breitborde N, Cárdenas R, Castañeda-Orjuela CA, Castro RE, Catalá-López F, Cavlin A, Chang JC, Che X, Christophi CA, Chugh SS, Cirillo M, Colquhoun SM, Cooper LT, Cooper C, da Costa Leite I, Dandona L, Dandona R, Davis A, Dayama A, Degenhardt L, De Leo D, del Pozo-Cruz B, Deribe K, Dessalegn M, deVeber GA, Dharmaratne SD, Dilmen U, Ding EL, Dorrington RE, Driscoll TR, Ermakov SP, Esteghamati A, Faraon EJ, Farzadfar F, Felicio MM, Fereshtehnejad SM, de Lima GM, Forouzanfar MH, França EB, Gaffikin L, Gambashidze K, Gankpé FG, Garcia AC, Geleijnse JM, Gibney KB, Giroud M, Glaser EL, Goginashvili K, Gona P, González-Castell D, Goto A, Gouda HN, Gugnani HC, Gupta R, Gupta R, Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Havmoeller R, Hay SI, Pi IB, Hoek HW, Hosgood HD, Hoy DG, Husseini A, Idrisov BT, Innos K, Inoue M, Jacobsen KH, Jahangir E, Jee SH, Jensen PN, Jha V, Jiang G, Jonas JB, Juel K, Kabagambe EK, Kan H, Karam NE, Karch A, Karema CK, Kaul A, Kawakami N, Kazanjan K, Kazi DS, Kemp AH, Kengne AP, Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Knibbs L, Kokubo Y, Kosen S, Defo BK, Kulkarni C, Kulkarni VS, Kumar GA, Kumar K, Kumar RB, Kwan G, Lai T, Lalloo R, Lam H, Lansingh VC, Larsson A, Lee JT, Leigh J, Leinsalu M, Leung R, Li X, Li Y, Li Y, Liang J, Liang X, Lim SS, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, London SJ, Lotufo PA, Ma J, Ma S, Machado VM, Mainoo NK, Majdan M, Mapoma CC, Marcenes W, Marzan MB, Mason-Jones AJ, Mehndiratta MM, Mejia-Rodriguez F, Memish ZA, Mendoza W, Miller TR, Mills EJ, Mokdad AH, Mola GL, Monasta L, de la Cruz Monis J, Hernandez JC, Moore AR, Moradi-Lakeh M, Mori R, Mueller UO, Mukaigawara M, Naheed A, Naidoo KS, Nand D, Nangia V, Nash D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nieuwenhuijsen MJ, Nisar MI, Nolte S, Norheim OF, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C, Park JH, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira DM, Pesudovs K, Petzold M, Poenaru D, Polanczyk GV, Polinder S, Pope D, Pourmalek F, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, ur Rahman S, Raju M, Rana SM, Refaat A, Ronfani L, Roy N, Pimienta TG, Sahraian MA, Salomon JA, Sampson U, Santos IS, Sawhney M, Sayinzoga F, Schneider IJ, Schumacher A, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shakh-Nazarova M, Sheikhbahaei S, Shibuya K, Shin HH, Shiue I, Sigfusdottir ID, Silberberg DH, Silva AP, Singh JA, Skirbekk V, Sliwa K, Soshnikov SS, Sposato LA, Sreeramareddy CT, Stroumpoulis K, Sturua L, Sykes BL, Tabb KM, Talongwa RT, Tan F, Teixeira CM, Tenkorang EY, Terkawi AS, Thorne-Lyman AL, Tirschwell DL, Towbin JA, Tran BX, Tsilimbaris M, Uchendu US, Ukwaja KN, Undurraga EA, Uzun SB, Vallely AJ, van Gool CH, Vasankari TJ, Vavilala MS, Venketasubramanian N, Villalpando S, Violante FS, Vlassov VV, Vos T, Waller S, Wang H, Wang L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman R, Wilkinson JD, Woldeyohannes SM, Wong JQ, Wordofa MA, Xu G, Yang YC, Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Jin KY, El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD, Naghavi M, Murray CJ, Lozano R: Global, regional, and national levels and causes of maternal mortality during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 980–1004, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JH, Cahill LE, Mozaffarian D: Effect of fish oil on circulating adiponectin: A systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 98: 2451–2459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S, ORIGIN Trial Investigators : n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 367: 309–318, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Heller AR, Rössler S, Litz RJ, Stehr SN, Heller SC, Koch R, Koch T: Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med 34: 972–979, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Pontes-Arruda A, Demichele S, Seth A, Singer P: The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of outcome data. JPEN J Parenter Enteral Nutr 32: 596–605, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Simopoulos AP: Essential fatty acids in health and chronic disease. Am J Clin Nutr 70[Suppl]: 560S–569S, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD: Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 71[Suppl]: 179S–188S, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Kaur G, Cameron-Smith D, Garg M, Sinclair AJ: Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog Lipid Res 50: 28–34, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Zhang YF, Gao HF, Hou AJ, Zhou YH: Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: A meta-analysis of randomized controlled trials. BMC Public Health 14: 204, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS: Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann Intern Med 158: 515–525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S, JPHC Study Group : Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 113: 195–202, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Nagata C, Takatsuka N, Shimizu H: Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol 156: 824–831, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Kromhout D, Bosschieter EB, de Lezenne Coulander C: The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 312: 1205–1209, 1985 [DOI] [PubMed] [Google Scholar]
- 49.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J: Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 346: 1113–1118, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB: Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 336: 1046–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D: Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: The Zutphen study. Eur Heart J 29: 2024–2030, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Lindberg M, Midthjell K, Bjerve KS: Long-term tracking of plasma phospholipid fatty acid concentrations and their correlation with the dietary intake of marine foods in newly diagnosed diabetic patients: Results from a follow-up of the HUNT Study, Norway. Br J Nutr 109: 1123–1134, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Hodson L, Skeaff CM, Fielding BA: Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47: 348–380, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.