Abstract
The current event-related potential study investigated the modulation effects of different emotion regulation strategies on electrocortical responses. When watching negative or neutral pictures, participants were instructed to perform three tasks: cognitive reappraisal, expressive suppression and passive viewing. We found that negative pictures elicited a larger late positive potential (LPP) than neutral pictures. Moreover, processes involved in strategy also had an effect on LPP amplitude, which was indicated by a larger LPP in reappraisal compared with suppression and viewing tasks when neutral pictures were presented. After the influence of processes on LPP was excluded, results showed that reappraisal effectively decreased the emotion-enhanced LPP than suppression and viewing. The difference in regulatory effect may be determined by the underlying processing mechanism. A larger frontal-central component, N2, was observed in suppression than reappraisal and viewing, which suggested that it involved the processes focusing on behavioral response. While the larger LPP found in reappraisal implicated that it recruited cognitive processes focusing on the picture meaning.
Keywords: Cognitive reappraisal, Expressive suppression, Regulatory effect, Underlying processing, LPP, N2
Introduction
Emotion regulation is a basic ability for one’s personal and social life, and closely relates to mental health. Difficulty in emotion regulation is found in mood disorders who usually fail to regulate their emotional responses and tend to avoid the emotional situations (Phillips et al. 2008). A variety of emotion regulation strategies are adopted in our daily life. However, their regulatory effects are quite different. The current study compared the regulatory effects of two common strategies, cognitive reappraisal and expressive suppression with event-related potentials (ERPs). We examined the effects of the two strategies on electrocortical responses evoked by negative pictures. Moreover, we also investigated their underlying processes that might result in the regulatory effects.
Reappraisal is an often adopted strategy in which people interpret the meaning of emotional stimuli in an alternative way, such as understanding the emotional stimuli in a neutral meaning. As a consequence, the multiple emotional responses in subjective feelings, physiological activities and behaviors could be changed (Gross and Thompson 2007). Reappraisal has been proved to be positively correlated to mental health and considered as an adaptive strategy (Gross and John 2003; Gross and Levenson 1993). Another strategy investigated in the present study is suppression, in which people inhibit the emotional expressive behaviors including facial expressions and gestures in social situations (Gross 1998). Although it was consider that reappraisal is an better regulatory effect than suppression, mood disorders such as depressive individuals usually adopt suppression to behaviorally regulate their emotions (Ehring et al. 2010; Campbell-Sills et al. 2006) or brooding and inhibiting thoughts to cognitively regulate their emotions (Bornas et al. 2014).
Reappraisal and suppression are found to have different effects on multiple emotional responses. Suppression can inhibit one’s facial expressions and other emotional behaviors, but its effect on reducing the emotional feelings is limited. In contrast, reappraisal is effective both in inhibiting the emotional behaviors and decreasing subjective feelings (Gross 1998; Gross and Levenson 1997). Besides, suppression needs physiological cost to inhibit emotional facial expressions, which is reflected by increased physiological activations, such as skin conductance, heart rate, sympathetic activation of cardiovascular system, etc. (Gross 1998; Gross and Levenson 1997). Moreover, it has been demonstrated that reappraisal can significantly decrease the activation level of emotion evaluation regions including amygdala and insula (Ochsner et al. 2004; Schaefer et al. 2002; Beauregard et al. 2001; Phan et al. 2005). But this effect is not found in suppression (Goldin et al. 2008).
Different regulatory effects may result from the distinct processes involved in the two strategies. Given emotion generation is a course unfolding with time, Gross has proposed that reappraisal is an antecedent-focused strategy while suppression is a response-focused strategy (Gross and Thompson 2007). Reappraisal focuses on the cognitive steps which transform information into the percepts with emotional significance. The goal is to alter the emotional meaning of the situation. Suppression targets the late stage when emotional response tendencies have been initiated and are going to be implemented. It aims to directly control emotional behaviors. Neuroimaging studies have found that reappraisal and suppression activated different regions in prefrontal cortex. Reappraisal activates dorsal anterior cingulate cortex (dACC), dorsal medial prefrontal (dMPFC) and medial orbito-frontal cortex (mOFC) (Ochsner et al. 2004; Lévesque et al. 2003). The ACC is found to be related to the processing of emotional conflict (Botvinick et al. 2004; Etkin et al. 2006), dMPFC is involved in affect empathy (Balconi and Bortolotti 2013) and MOFC is associated with cognitive control (Ridderinkhof et al. 2004). It suggests that these processes may be recruited by reappraisal. Whereas suppression recruits dorsal fronto-median prefrontal cortex (FMPFC) for voluntary inhibition of action (Kühn et al. 2009, 2011). Goldin further found that reappraisal activated related regions in an early period (0–4.5 s) while suppression in a late period (10.5–15 s) (Goldin et al. 2008). It supported that these two strategies target different stages in emotion generation.
Besides behavioral and neuroimaging method, EEG and event-related potentials (ERPs) are also widely used in investigating emotion regulation. For instance, an EEG study proposed that reappraisal in MBCT (Mindfulness-Based Cognitive Therapy) ministered an excitatory mechanism to regulate emotions through modulations upon α and γ power (Schoenberg and Speckens 2014). In ERP studies, the late positive potential (LPP) is used as an index of emotional response which indicates automatic attention and elaborated processing of emotional stimuli. A larger LPP is steadily found to be elicited 300 ms after exposure to pleasant or unpleasant stimuli than neutral ones (Cuthbert et al. 2000). Moreover, the amplitude of LPP is sensitive to emotional arousal, which is larger when high arousal stimuli were presented than low arousal stimuli (Schupp et al. 2000). Emotion regulation studies have evidenced that the amplitude of LPP elicited by negative stimuli can be modulated by reappraisal. When participants are instructed to down-regulate the feelings on emotional pictures by reappraising their meanings in a neutral way, the amplitude of LPP is significantly reduced compared with passive viewing (Moser et al. 2006; Krompinger et al. 2008; Hajcak and Nieuwenhuis 2006; Hajcak et al. 2006).
However, a usually neglected factor in prior ERP studies is that the changing of LPP amplitude in emotion regulation may not only indicate emotional response, but also reflect a variety of cognitive processes. It is reported that LPP complex is sensitive to some top-down processes (Gevins et al. 1996; Johnson and Donchin 1985) including information retrieval from working memory (García-Larrea and Cézanne-Bert 1998), internal attention switching (Rushworth et al. 2005; Swainson et al. 2006), evaluation of stimulus meaning (Shigeto et al. 2011) and conflict processing (Baetens et al. 2011). In emotion regulation, both the emotional response and processes are changed compared with passive viewing. Therefore, it can be reasonably speculated that the amplitude of LPP in emotion regulation could be influenced by reappraisal-relevant processes as well as emotion.
Given the different underlying processing mechanisms of reappraisal and suppression, we would predict that they have different effects on LPP amplitude. To separate the strategy-influenced LPP from the emotion-enhanced LPP, negative and neutral pictures were presented in the current study. The difference waves of LPPs between negative and neutral picture conditions in reappraisal, suppression and viewing tasks were attained. The difference wave in each task indexes the emotional response after regulating by strategy. We predicted that negative pictures would elicit larger LPPs than neutral pictures. Moreover, processes involved in different strategies might also influence the amplitudes of LPP. The regulatory effects of reappraisal and suppression can be revealed by comparing the difference waves in different tasks.
Methods
Ethics statement
All participants were provided written informed consent in accordance with the Declaration of Helsinki. The ethics committee of the Institute of Psychology, Chinese Academy of Sciences approved the participant-recruitment procedure and the methodology of this study.
Participants
Thirty-four female university students (mean age = 22 years, range = 19–25 years) participated in the experiment for payment. All participants reported normal or correct-to-normal vision and no history of neurological or psychiatric disorders. They were also told that they could quit whenever they feel uncomfortable when the unpleasant stimuli were presented in the experiment.
Materials and design
The experiment consisted of 120 negative pictures and 120 neutral pictures from the International Affective Picture System (IAPS) (Lang et al. 2008). The valence of negative pictures (M = 2.88, SD = 0.94) was lower than neutral pictures (M = 5.62, SD = 0.98). Additionally, the arousal of negative pictures (M = 5.70, SD = 0.84) was higher than neutral pictures (M = 3.64, SD = 1.07). Another set of 240 pictures (e.g., scenes and objects) was used to help participants relax after each trial.
Participants were instructed to perform three tasks about these IAPS pictures. In reappraisal task, participants were asked to reappraise the upcoming picture according to the preceding description. For example, the negative picture Fig. 1 showed “A man is pointing a gun against a woman’s head” should be reappraised in a neutral meaning “The government filmed a riot public advertisement” presented in the preceding screen. All neutral pictures were described in a neutral meaning. According to Foti and Hajcak (2008), the presentation of neutral description can help participants reappraise the negative pictures and decrease negative responses. Furthermore, it can exclude the confounding strategies that may be involved in the reappraisal task, such as detachment and attention shift (Foti and Hajcak 2008). In suppression task, participants were required to inhibit any expressive behaviors like frowning and curling lip elicited by negative pictures. When neutral pictures were presented, participants were also asked to relax and control any emotional expressive behaviors. In passive viewing task, participants watched pictures and didn’t have to inhibit or exaggerate their responses. Therefore, six conditions were constructed in our study, reappraisal-neutral, reappraisal-negative, suppression-neutral, suppression-negative, viewing-neutral, and viewing-negative. Throughout the experiment, participants’ facial behaviors were monitored by a camera, and feedbacks were given to them in the practice period. Participants were well trained before the experiment. Neutral and negative IAPS pictures were evenly assigned into three tasks with their emotional valences and arousals matched across tasks.
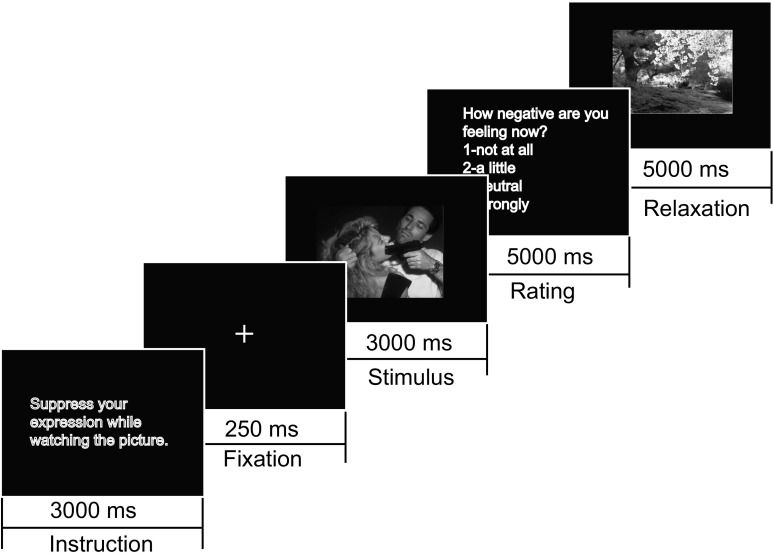
Fig. 1.
An example trial of presentation. The instruction was presented for 3000 ms. After the 250 ms fixation, the target picture (either negative or neutral) was presented for 3000 ms. Then a negative feeling rating was required to be answered with keyboard in 5000 ms. At last, the relaxing pictures was presented for 5000 ms
Procedure
Participants were seated in a comfortable distance (60–80 cm) from the monitor. In each trial (as shown in Fig. 1), an instructed sentence was presented (in reappraisal task a sentence with neutral description was provided) on the screen for 3000 ms. Participants were required to perform reappraisal, suppression or viewing task according to these instructions. After the fixation (“+”) for 250 ms, the target picture was presented on the screen for 3000 ms. Participants needed to keep watching the picture according to the instruction. After the target picture disappeared, participants rated their intensity of negative feelings with a scale from1 (not negative) to 5 (extremely negative) in 5000 ms. Followed was the intertrial interval of 5000 ms, in which a relaxing picture was presented to help participants restore their emotions. Then the next trial began.
Eight runs were conducted, including pseudorandomized 240 trials from six conditions (40 trails for each). Each run lasted for about 6 min and participants can take a break after it. Before the experimental procedure, all participants performed 60 practice trials to get familiar with the tasks. The target and relaxing pictures used in the practice trials were from internet and not overlapped with that used in experiment trials.
Facial behavior recording and encoding
A black camera recorded the continuous facial expressions from the forehead to the mouth of the participants, which was positioned upon the monitor. The facial expressions were rated by two coders who were professional at coding facial gestures with Facial Action Coding System (Ekman et al. 2002). Coders were blind to the experimental materials and design, and they were required to rating the negative-expressive behaviors on a scale from 1 to 5 (1 = none, 5 = extremely strong).
Electroencephalogram (EEG) recording and preprocessing
Continuous EEG recordings were collected by an elastic cap equipped with 64 Ag/AgCI electrodes with SynAmps amplifiers II (Neuroscan Inc.) based on 10/20 system. GND on the forehead of the cap served as ground. Left mastoid served as online reference. Vertical electro-oculogram (EOG) generated from eye-blinks was recorded with two electrodes placed 2 cm below and above the left eye. Horizontal EOG was recorded with two electrodes placed on bilateral external canthuses. The EEG signals were digitized at a rate of 500 HZ with a band-pass from 0.01 to 100 Hz. The impedances of all the electrodes were kept below 10 kΩ.
The raw EEG data were processed with NeuroScan 4.3. Ocular artifacts were removed from the EEG signals using a regression procedure implemented in the Neuroscan software (Semlitsch et al. 1986). All of the EEG data were filtered by a band pass of 0.1–30 Hz and re-referenced to the algebraic average of two mastoids. EEG data were segmented to 3500 ms epochs time-locked to each target picture, starting 500 ms prior to the onset. And the epochs were baseline-corrected 500 ms before the onset of pictures. Trials with excessive physiological artifacts exceeding the amplitude of ±100 μV were excluded from further processing. More than 35 trials for each type of picture in each strategy were remained for each participant. Data from four of the participants were excluded from final analysis because over 30 % of their EEG data were rejected.
To reduce the spatial dimensions, electrodes were divided according to three level, anteriority (anterior, middle, posterior), laterality (superior, inferior) and hemisphere (left, right) (Dien and Santuzzi’s 2005). As in previous studies (Foti and Hajcak 2008), the hemisphere difference is usually found to be not obvious, we divided the electrodes only in the dimensions of anteriority and laterality. Thus six clusters were created in our study: anterior–superior cluster (F1, F2, FC1, FC2, F3, F4, FC3, FC4, FZ, FCZ), anterior-inferior cluster (F5, F6, FC5, FC6, F7, F8, FT7, FT8), middle-superior cluster (C1, C2, CP1 CP2, C3, C4, CP3, CP4, CZ, CPZ), middle-inferior cluster (C5, C6, CP5, CP6, T7, T8, TP7, TP8), posterior-superior cluster (P1, P2, PO1, PO2, P3, P4, PO3, PO4, PZ, POZ), and posterior-inferior cluster (P5, P6, PO5, PO6, P7, P8, PO7, PO8). LPP amplitude in each condition for each participant was scored by averaging the amplitudes of electrodes within each cluster in two windows, 400–1000 ms for the early period and 1000–3000 ms for the late period.
In order to evaluate the effects of emotion, task, and electrodes on LPP, a 2 (emotion: neutral, negative) × 3 (task: viewing, suppression, reappraisal) × 3 (anteriority: anterior, middle, posterior) × 2 (laterality: superior, inferior) repeated-measures ANOVA was conducted in the early and the late periods.
We tested the emotion effect, i.e., whether negative pictures evoked larger amplitude of LPP than neutral pictures. We also examined if three tasks elicited different amplitudes of LPP for neutral and negative pictures. If the amplitudes of LPP were different among three tasks when neutral pictures were presented, it might suggest that reappraisal, suppression and viewing involved different processes. Therefore, when negative pictures were presented, the LPP amplitude cannot be directly used to indicate the emotional response as the regulatory effects because it was influenced both by the negative pictures and the processes involved in strategies. Instead, the difference wave, which was obtained by subtracting LPP amplitude evoked by neutral pictures from that by negative pictures in each task, was used as the index of regulatory effect.
Besides LPP, frontal-central N2, an early component, was used to differentiate the underlying processing of reappraisal, suppression and viewing tasks in both neutral and negative pictures. It was scored as the averaged amplitudes from frontal-central sites (F1, F2, FC1, FC2, C1, C2, F3, F4, FC3, FC4, FZ, FCZ, C3, C4, CZ) in 200–300 ms after stimulus picture onset in each condition for each participants. The N2 effect was examined in a 2 (emotion: neutral, negative) × 3 (task: reappraisal, suppression, viewing) repeated-measures ANOVA.
All the behavioral and ERP data were statistically tested using SPSS (Version 13.0) General Linear Model software. Greenhouse-Geisser correction was applied if appropriate; p values were adjusted with the Bonferroni correction for multiple comparisons.
Results
Behavioral data
Self-reported feelings
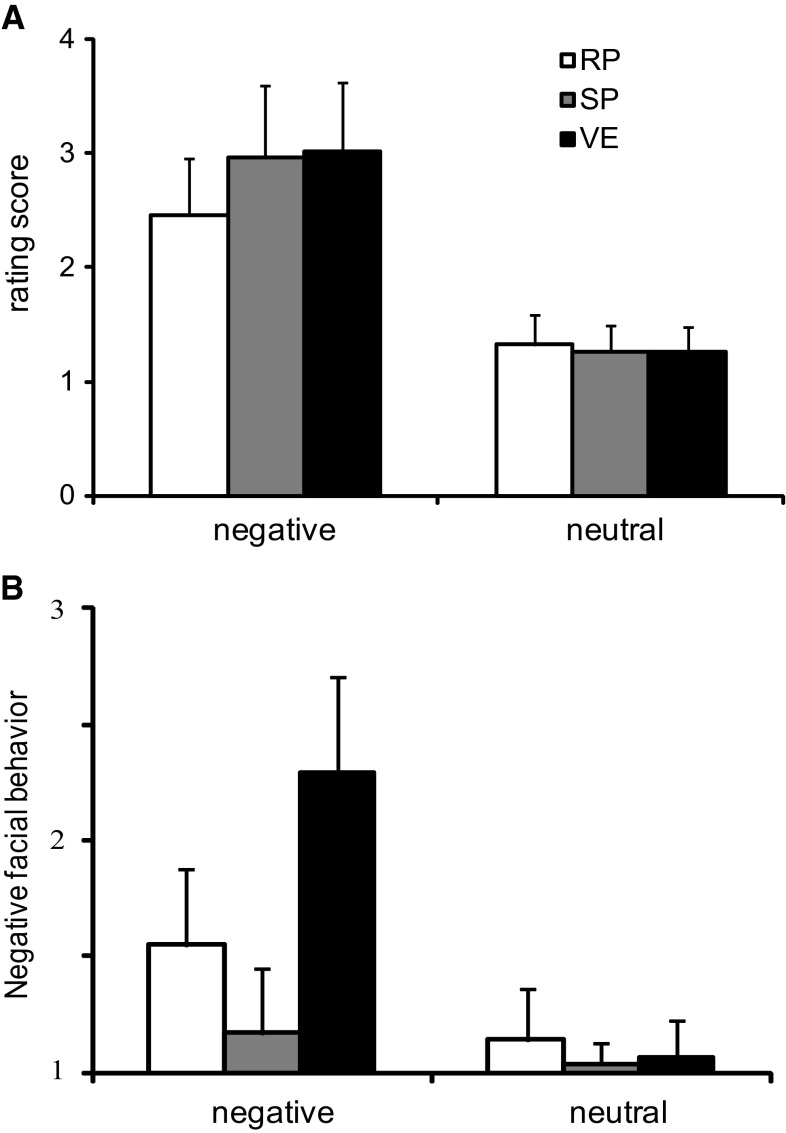
As shown in Fig. 2a, the rating scores revealed that negative pictures were rated as less negative in reappraisal task than suppression and viewing tasks. The 2 (emotion: neutral, negative) × 3 (task: reappraisal, suppression, viewing) repeated-measures ANOVA showed that the main effect of emotion was significant, F(1, 29) = 417.64, p < 0.001, in which negative pictures elicited more negative feeling than neutral pictures. The main effect of task was significant, F(2, 58) = 33.61, p < 0.001, participants experienced less negative in reappraisal task than other two tasks. Moreover, the interaction between emotion and task was significant, F(2, 58) = 80.62, p < 0.001, the negative feelings evoked in three tasks were different in negative picture condition, F(2, 58) = 64.73, p < 0.001, but not in neutral picture condition, F(2, 58) = 2.61, p = 0.08. When negative pictures were presented, participants felt less negative in reappraisal task (Mean = 2.46, SD = 0.49) than viewing (Mean = 2.96, SD = 0.63; p < 0.01) and suppression (Mean = 3.02, SD = 0.60; p < 0.01) tasks. There was no difference between suppression and viewing tasks (p = 0.79). These results suggested that reappraisal was more effective than suppression in down-regulating negative feelings elicited by negative pictures.
Fig. 2.
The rating scores of negative feeling and facial behaviors. a Participants experienced more negative feeling in negative picture condition than neutral picture condition. Reappraisal (RP) effectively decreased negative feeling elicited by negative pictures compared to suppression (SP) and viewing (VE). b Negative pictures induced more negative facial behaviors than neutral pictures in all tasks. The negative behaviors were less in SP than RP and VE, and were also less in RP than VE. The error bars indicated the standard derivations (SDs) of rating scores in six conditions in both of a and b
Expressive behaviors
The rating scores of the video data from two coders were analyzed and shown in Fig. 2b. The inter-coder reliability was adequate (Kappa = 0.76). The 2 (emotion) × 3 (task) repeated-measures ANOVA found a significant effect of emotion, F(1, 29) = 188.29, p < 0.001, negative pictures induced more negative facial behaviors than neutral pictures. The main effect of task was significant, F(2, 58) = 74.97, p < 0.001. And its interaction with emotion was also significant, F(2, 58) = 74.37, p < 0.001. In neutral picture condition, the simple main effect of task on facial behavior only reached marginally significant, F(2, 58) = 3.04, p = 0.06. But it was significant in negative picture condition, F(2, 58) = 95.03, p < 0.001. The negative facial behaviors were significantly reduced in suppression compared with passive viewing, t(29) = 12.51, p < 0.001, and reappraisal, t(29) = 4.71, p < 0.001, which indicated that participants inhibited the expressions successfully. At the same time, reappraisal also attenuated the negative facial behaviors compared to passive viewing, t(29) = 9.62, p < 0.001, which might result from the successful regulation of emotion.
ERP data
N2: 200–300 ms
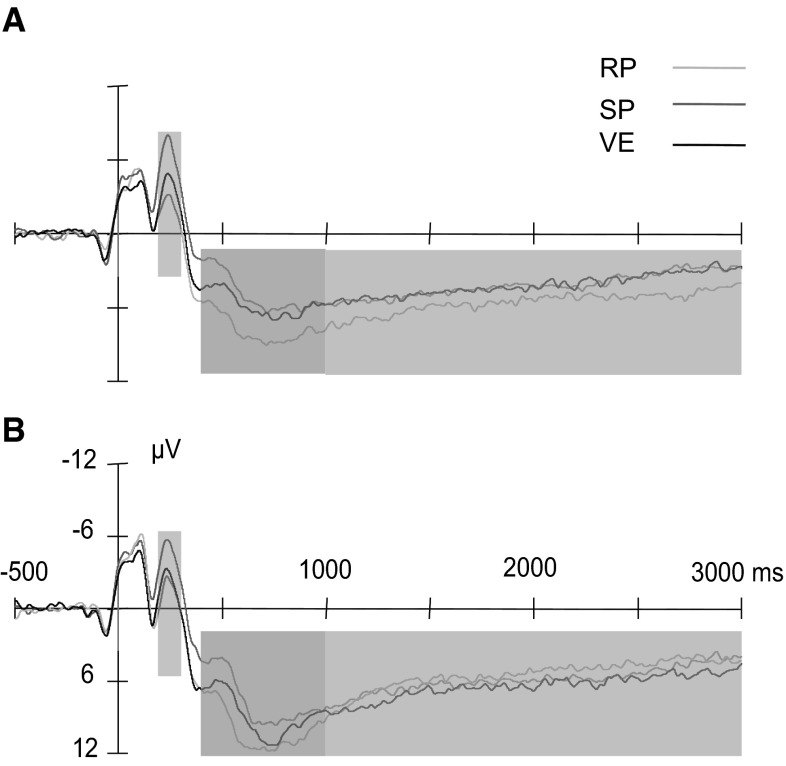
As shown in Fig. 3, the amplitudes of N2 were different among reappraisal, suppression and viewing, F(2, 58) = 30.70, p < 0.001. Suppression elicited a larger anterior-central N2 than reappraisal and viewing. Negative pictures also elicited a larger amplitude of N2 than neutral pictures, F(1, 29) = 14.09, p < 0.001. Moreover, the interaction between task and emotion was observed, F(2, 58) = 5.67, p < 0.01. When neutral pictures were presented, N2 amplitudes were different among tasks, F(2, 58) = 44.19, p < 0.001. Suppression elicited larger amplitude of N2 than reappraisal, t(29) = 9.98, p < 0.001, and viewing, t(29) = 5.19, p < 0.001, (see Fig. 3a). And N2 amplitude was also larger in viewing than reappraisal, t(29) = 3.85, p < 0.01. When negative pictures were presented, the task effect was also significant, F(2, 58) = 9.79, p < 0.001. Suppression elicited significantly larger amplitude of N2 than reappraisal, t(29) = 3.70, p < 0.01, and viewing, t(29) = 4.85, p < 0.001. While reappraisal and viewing were not different from each other in N2 amplitude (p > 0.05) (see Fig. 3b). These results may reflect different underlying processes in suppression compared with reappraisal and passive viewing.
Fig. 3.
Grand average N2 and LPP for the neutral and negative picture conditions in different tasks at CZ. a Grand average waveforms in reappraisal (RP), suppression (SP) and viewing (VE) tasks in neutral picture condition. SP enhanced N2 amplitude in 200–300 ms (indicated by grey box) compared with RP and VE. RP elicited more positive LPP than SP and VE in 400–1000 ms (indicated by yellow box) and 1000–3000 ms (indicated by blue box). b Grand average waveforms of three tasks in negative picture condition. As in neutral picture condition, larger N2 (200–300 ms) was found in SP than RP and VE. In 400–1000 ms, RP elicited larger LPP than SP and VE, and this effect disappeared in 1000–3000 ms. (Color figure online)
LPP: 400–1000 ms
In the early period of LPP, the amplitudes in reappraisal, suppression and viewing were different from each other when neutral and negative pictures were presented (see Fig. 4). The 2 (emotion) × 3 (task) × 3 (anteriority) × 2 (laterality) ANOVA revealed the significant effects of emotion, F(1, 29) = 56.07, p < 0.001, and task, F(2, 58) = 31.82, p < 0.001. The results also showed a four-way interaction among emotion, task, and two electrode factors, F(4, 116) = 3.077, p < 0.05. And the task effect was interacted with electrode in a three way interaction (strategy by anteriority by laterality), F(4, 116) = 12.12, p < 0.001. Thus we conducted the 2 (emotion) × 3 (task) × 2 (laterality) repeated-measures ANOVAs separately in anterior, middle and posterior electrodes.
Fig. 4.
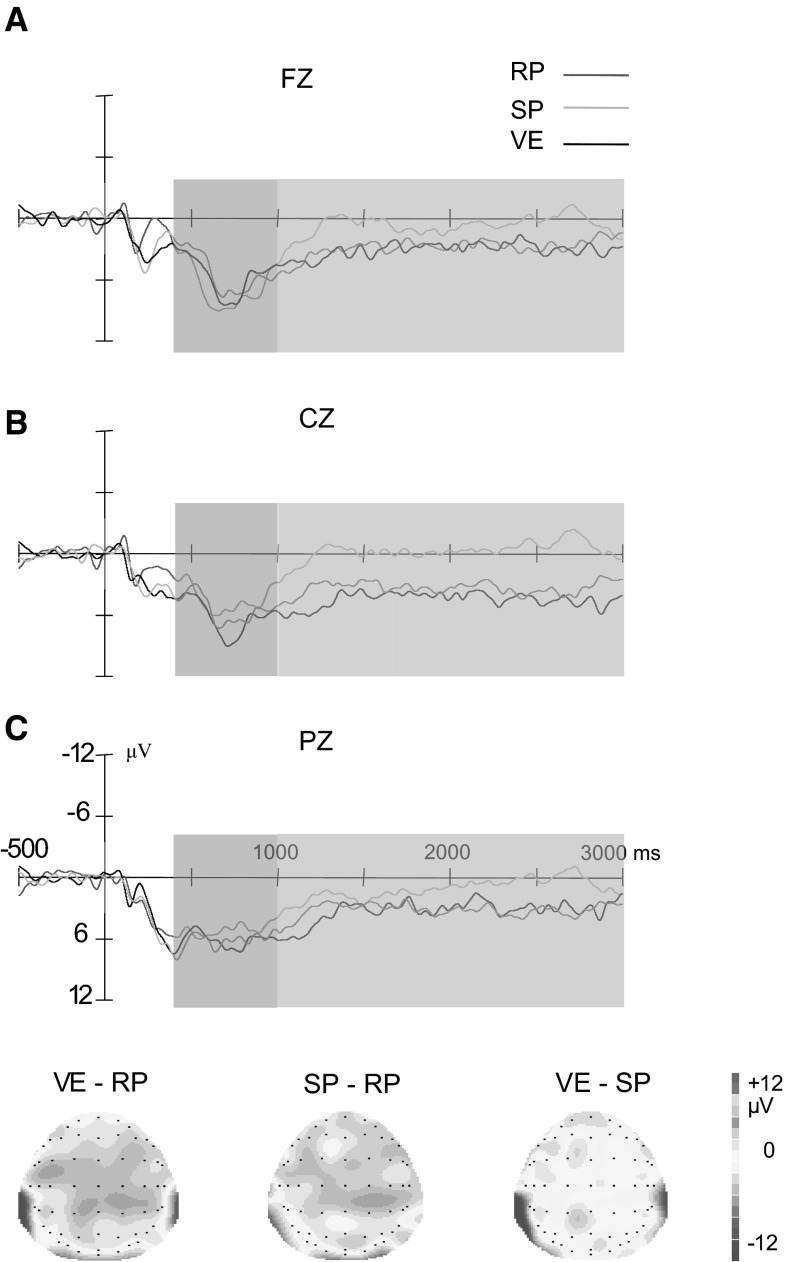
Grand average difference waves of tasks at FZ, CZ and PZ respectively. The difference wave of reappraisal (RP) was significantly smaller than suppression (SP) and viewing (VE) in 1000–3000 ms (indicated by the blue box) at FZ and CZ, while the difference wave of SP was not different from VE. Topographies showed the average amplitude differences for the contrast of VE-RP, SP-RP and VE-SP in the time window of 1000–3000 ms. (Color figure online)
The results of ANOVAs were shown in Table 1. Significant main effect of emotion was observed at all anterior, middle and posterior electrodes. The negative pictures elicited larger LPPs than neutral pictures. The significant effect of task was also observed at all electrodes. Multiple comparisons revealed that reappraisal elicited larger LPPs than suppression (anterior: t(29) = 6.54, p < 0.001; middle: t(29) = 7.39, p < 0.001; posterior: t(29) = 4.92, p < 0.001) and viewing (anterior: t(29) = 6.33, p < 0.001; middle: t(29) = 3.68, p < 0.01; posterior: t(29) = 1.07, p > 0.05). No difference was found between suppression and viewing at all electrodes (ps >0.1). These results indicated that besides negative pictures, reappraisal also increased the amplitude of LPP compared to suppression and viewing. The interaction of emotion and task was not significant, F(2, 58) = 0.64, p = 0.53, it revealed that in both of neutral and negative picture conditions, tasks influenced the amplitudes of LPP differently (see Fig. 3).
Table 1.
F-values of LPP effect obtained from repeated-measures ANOVAs conducted in anterior, middle and posterior electrodes in 400–1000 and 1000–3000 ms respectively
| df | Anterior | Middle | Posterior | |
|---|---|---|---|---|
| 400–1000 ms | ||||
| Emotion | 1.29 | 43.12*** | 49.40*** | 52.30*** |
| Task | 2.58 | 29.48*** | 26.15*** | 19.29*** |
| Emotion × L | 1.29 | 38.20*** | 39.18*** | 40.34*** |
| Superior | 1.29 | 50.80*** | 56.07*** | 59.56*** |
| Inferior | 1.29 | 31.31*** | 36.33*** | 39.98*** |
| Task × L | 2.58 | 22.93*** | 19.40*** | 12.56*** |
| Superior | 2.58 | 32.11*** | 26.79*** | 21.79*** |
| Inferior | 2.58 | 20.21*** | 20.57*** | 14.94*** |
| 1000–3000 ms | ||||
| Emotion | 1.29 | 3.58 | 7.14* | 5.78* |
| Task | 2.58 | 5.74** | 0.70 | 10.30*** |
| Emotion × task | 2.58 | 7.45*** | 9.45*** | 0.99 |
| Neutral | 2.58 | 12.25*** | 7.17** | |
| Negative | 2.58 | 1.14 | 2.53 | |
L laterality
* p < 0.05; ** p < 0.01; *** p < 0.001
LPP: 1000–3000 ms
As in the early period, a 2 (emotion) × 3 (task) × 3 (anteriority) × 2 (laterality) repeated-measures ANOVA was conducted. The results showed the significant main effect of emotion, F(1, 29) = 7.03, p < 0.05, but not for task, F(2, 58) = 2.51, p = 0.09. The significant two-way (emotion by task, F(2, 58) = 6.92, p < 0.05) and three-way interactions (emotion by task by anteriority, F(4, 116) = 3.00, p < 0.05) were also found. Further analyses were conducted on 2 (emotion) × 3 (task) repeated-measures ANOVAs at anterior, middle and posterior electrodes respectively.
As shown in Table 1, the main effect of emotion was observed at middle and posterior electrodes, the negative pictures elicited larger LPPs than neutral pictures. We also observed the significant task effect at anterior and posterior electrodes. The interaction of emotion and task was significant at anterior and middle electrodes. When negative pictures were presented, the amplitudes of LPP in reappraisal, suppression and viewing tasks were not different from each other (see Fig. 3b). But when neutral pictures were presented, LPP amplitudes in three tasks were significantly different. As shown in Fig. 3a, reappraisal elicited larger LPPs than suppression (anterior: t(29) = 4.48, p < 0.01; middle: t(29) = 3.55, p < 0.01) and viewing (anterior: t(29) = 4.35, p < 0.01; middle: t(29) = 3.09, p < 0.01). No difference was found between suppression and viewing (ps >.05).
Difference wave
To investigate the regulatory effect of reappraisal and suppression, the difference waves (DWs) between negative picture and neutral picture conditions for each task were obtained. They were taken as the emotion-enhanced LPP after regulation with reappraisal and suppression. As shown in Fig. 4, the DW of reappraisal was steadily smaller than the DWs of suppression and viewing from 1000 to 3000 ms. And the DW of suppression was not different from viewing.
To compare the regulatory effects in three tasks, we examined the DW amplitudes of tasks with a 3 (DW of task: reappraisal, suppression, and viewing) × 3 (anteriority) × 2 (laterality) repeated-measures ANOVA in the early (400–1000 ms) and late (1000–3000 ms) periods respectively. Moreover, to investigate whether strategies down-regulate the emotion-enhanced LPP to a neutral level, we compared the DW amplitude of each task with baseline (zero). To this end, a 2 (DW: DW of reappraisal, suppression or viewing; baseline) × 3 (anteriority) × 2 (laterality) repeated-measures ANOVA was conducted for each task in the early and late periods.
In 400–1000 ms, the statistical results of comparisons among tasks were shown in Table 2 (left). The main effect of DW was not significant, no difference was found in the DW amplitudes of reappraisal, suppression and viewing. Moreover, DW interacted with anteriority significantly. In anterior and posterior electrodes, DW amplitudes of three tasks were not different from each other. In middle electrodes, reappraisal had significantly smaller DW than viewing, t(29) = 2.82, p < 0.05, but the difference between reappraisal and suppression was not found, and also the difference between suppression and viewing, ps >0.05.
Table 2.
F-values of DW effect for the comparisons among reappraisal (RP), suppression (SP) and viewing (VE) tasks (left columns) and the comparisons between each task and baseline (right columns) in 400–1000 and 1000–3000 ms respectively
| Comparisons among tasks | Comparisons with zero | |||||
|---|---|---|---|---|---|---|
| df | F | df | F(RP) | F(SP) | F(VE) | |
| 400–1000 ms | ||||||
| DW | 2.58 | 1.22 | 1.29 | 9.29* | 60.63*** | 11.67** |
| DW × A | 4.116 | 4.99** | 2.58 | 3.79* | 8.58** | 2.48 |
| Anterior | 2.58 | 2.45 | 1.29 | 21.70*** | – | – |
| Middle | 2.58 | 4.91* | 1.29 | 1.25 | – | – |
| Posterior | 2.58 | 0.01 | 1.29 | 4.84* | – | – |
| DW × L | 2.58 | 0.39 | 1.29 | 3.42 | 15.26** | 6.76* |
| Superior | 2.58 | – | 1.29 | – | – | 15.04** |
| Inferior | 2.58 | – | 1.29 | – | – | 6.88* |
| DW × A × L | 4.116 | 1.40 | 2.58 | 0.40 | 3.48* | 0.58 |
| Anterior | 2.58 | – | 1.29 | – | 95.63*** | – |
| Middle | 2.58 | – | 1.29 | – | 39.40*** | – |
| Posterior | 2.58 | – | 1.29 | – | 14.06** | – |
| 1000–3000 ms | ||||||
| DW | 2.58 | 6.93** | 1.29 | 0.03 | 13.91** | 11.67** |
| DW × A | 4.116 | 3.01* | 2.58 | 1.91 | 3.36* | 2.48 |
| Anterior | 2.58 | – | 1.29 | – | – | – |
| Middle | 2.58 | – | 1.29 | – | – | – |
| Posterior | 2.58 | – | 1.29 | – | – | – |
| DW × L | 2.58 | 2.34 | 1.29 | 1.22 | 6.67* | 6.76* |
| Superior | 2.58 | – | 1.29 | – | 15.04** | |
| Inferior | 2.58 | – | 1.29 | – | 6.88* | |
| DW × A × L | 4.116 | 1.75 | 2.58 | 0.80 | 3.58* | 0.58 |
| Anterior | 2.58 | – | 1.29 | 15.92*** | – | |
| Middle | 2.58 | – | 1.29 | 13.07** | – | |
| Posterior | 2.58 | – | 1.29 | 8.90** | – | |
A anteriority, L laterality
* p < 0.05; ** p < 0.01; *** p < 0.001
The comparisons of tasks with baseline were shown in Table 2 (right). It revealed that the DW of reappraisal was larger than baseline, and this effect was found in anterior and posterior electrodes, ps <0.05. It suggested that an obvious emotional effect in reappraisal task. For suppression, the DW effect was also found to be larger than baseline, and it had a three way interaction with anteriority and laterality. In anterior and middle electrodes, the DW effect was significant in both of superior and inferior sites, ps <0.0001. In posterior electrodes, the DW effect was found only in superior sites, p < 0.0001.
In 1000–3000 ms, it was found that DWs of three tasks were different from each other. This task effect significantly interacted with anteriority, which was observed at anterior and middle electrodes, ps <0.01, but not at posterior electrodes. Multiple comparisons further revealed that DWs of reappraisal were smaller than suppression (anterior: t(29) = 3.66, p < 0.01; middle: t(29) = 3.54, p < 0.01) and viewing (anterior: t(29) = 3.02, p < 0.01; middle: t(29) = 4.10, p < 0.001). The DW of suppression was not different from passive viewing (ps >0.05). These results showed that reappraisal decreased the emotion-enhanced LPP compared with passive viewing. And suppression did not show any regulatory effect.
The comparisons of DW amplitudes of three tasks with baseline revealed that in reappraisal task, the DW amplitude was not different from baseline which indicated that reappraisal effectively regulated the negative response and decreased it to a neutral level (see Fig. 4). In suppression task, we found the DW was larger than baseline and its interaction with anteriority and laterality was significant. Further simple effect analysis revealed that the difference was exist in anterior, middle and posterior electrodes, and also in superior and inferior electrodes (ps <0.05). In viewing task, we also found the larger DW than baseline. These results suggested a conspicuous emotion-enhanced effect in suppression and viewing tasks.
Overall, analysis on DWs of tasks suggested that reappraisal had a regulatory effect on the emotion-enhanced LPP compared with suppression and passive viewing. And this effect mainly found in the late period (1000–3000 ms).
Discussion
In the current study, we directly compared the regulatory effects of two frequently used emotion regulation strategies, cognitive reappraisal and expressive suppression on electrocortical response, LPP. The results indicated that when negative pictures were presented, LPP amplitude could be enhanced by both of the emotional significance of the pictures and the processes involved in reappraisal. To obtain the emotion-independent LPPs which were able to indicate the regulatory effects of strategies, the LPPs increased by processes involved in strategies were excluded from original LPPs. And as the enhanced LPP amplitudes in neutral picture condition were only influenced by strategy processes, they were subtracted from LPP amplitudes in negative picture condition (i.e., difference waves). The difference waves indicated the emotion-enhanced LPP in strategies. We found that reappraisal significantly decreased emotion-enhanced LPP compared with suppression, and this effect was observed mainly in the late period (1000–3000 ms). The difference in regulatory effect between reappraisal and suppression might result from their involved processes.
As in the literature of ERP studies on emotion regulation, LPP indicates electrocortical response evoked by emotional stimuli, and the decrease of its amplitude reflects the attenuated emotional response. In the present study, a larger LPP was observed when negative picture were presented than neutral picture. If no effective regulation strategy was applied, the influence of picture emotion could last for the whole duration of picture presentation. On the other hand, when regulatory strategies are applied, processes involved in strategy modulated the amplitude of LPP differently. We found a larger LPP in reappraisal than suppression and viewing when neutral pictures were presented. Therefore, our results revealed that when regulating emotions with reappraisal, the LPP amplitude can be influenced both by cognitive processes involved in strategy and the emotion evoked by negative pictures.
Different LPP amplitudes among strategies in neutral pictures indicated the different involved processes of reappraisal and suppression. Previous studies have found that LPP amplitude can be increased by a set of cognitive processes including attention switching, working memory operation, meaning evaluation, etc. (Gevins et al. 1996; Johnson and Donchin 1985; García-Larrea and Cézanne-Bert 1998; Rushworth et al. 2005; Shigeto et al. 2011). Consistent with these findings, we found that LPP amplitude was larger in reappraisal than suppression and viewing tasks. Especially when neutral pictures were presented, strategy processing was the only factor to influence the amplitude of LPP in three tasks. In reappraisal task in our experiment, participants were instructed to reinterpret the meaning of picture, they mainly focused on cognitive processing including re-evaluating the original meaning in a new way.
In contrast, no difference in LPP amplitudes was found between suppression and viewing, which suggested that suppression might don’t involve the similar processes as reappraisal that could influence LPP. But suppression was found to enhance the N2 amplitude compared with reappraisal and viewing. A larger frontal-central N2 was observed in suppression both in neutral and negative picture conditions than reappraisal and viewing. In previous studies, N2 is reported to reflect an active inhibition of prepotent motor responses (Jackson et al. 1999) which is generated from dorsolateral and ventral lateral prefrontal regions (Liddle et al. 2001). It is often observed in NoGo trials in which participants are asked to withhold their responses compared with that in Go trials in which participants need to implement their responses (Pfefferbaum et al. 1985). Therefore, the larger N2 found in suppression may suggest that participants control the facial behaviors evoked by negative pictures. However, recent studies have proposed that N2 is related with an alternative function, conflict monitoring (Cavanagh and Shackman 2014). For instance, Donkers and van Boxtel (2004) found that N2 was not different between no-go trials and Go trials (responding with maximal force), but it was larger in the 20 % go signals than 50 % go signals, suggesting that N2 was not influenced by response inhibition but a response conflict monitoring. Botvinick et al. (2001) has advanced in his conflict monitoring theory that N2 amplitude can be modulated when different and incompatible representations were activated at the same time. In our study, when negative pictures were presented participants’ negative facial expressions were frequent and prepotent behavioral responses. But in suppression task, the required correct response was inhibiting their facial behaviors and keeping calm face. The activation and processing of these two incompatible response elicited conflict or competition and enhanced N2 (Botvinick et al. 2001).
The different processing mechanisms underlying reappraisal and suppression may result in their different regulatory effects on emotion. The behavioral data directly supported the excellent effect of reappraisal as it decreased both emotional experience and facial behaviors while suppression only attenuated the latter one. After excluding the influence of processes relevant LPP, the ERP results were also evidenced the regulatory effect of reappraisal on emotion-enhanced LPP compared with suppression.
When neutral pictures were presented, no emotion regulation was needed across tasks. And reappraisal task resulted in more positive LPP than suppression and viewing for its cognitive processes. When negative pictures were presented, reappraisal task didn’t reduce the LPP amplitude comparing with suppression and viewing. Instead, it had more positive LPP than other two tasks in the early period (400–1000 ms). It is speculated that reappraisal did not effectively regulate the emotion-enhanced LPP. Instead its involved processes increased the LPP amplitude. It resulted in a larger accumulated LPP in reappraisal task than suppression and viewing tasks. While in the late period (1000–3000 ms), reappraisal effectively reduced the emotion-enhanced LPP but the processing-enhanced LPP was intact. So its accumulated LPP lowered down to the same amplitude as in suppression and viewing tasks.
To further demonstrate the regulatory effect, the difference wave analysis was conducted in this study. We found no difference between suppression and viewing in the whole period of picture presentation, and the amplitudes of difference wave were significantly larger than zero, which suggested that suppression was not an effective strategy to reduce the emotional electrocortical responses. In contrast, reappraisal showed a smaller difference wave than other two tasks which suggested that it had less negative response 1000 ms after the onset of pictures. Moreover, its difference wave was close to zero, which further confirmed that reappraisal successfully eliminated the emotion-enhanced responses.
Our results support the emotion regulation model which proposed that different strategies target different stages and aspects in emotion generation (Gross and Thompson, 2007). Suppression involve processes focusing on responses, i.e., detecting the conflict between different facial responses representations and inhibiting emotional facial behaviors. They don’t relate to the emotional meaning of negative pictures. In contrast, reappraisal is an antecedent-focused strategy. Participants mainly target the meaning of negative picture. It is proposed that to neutralize the meaning of negative pictures, participants need to monitor the conflict between top-down neutral reappraisal and bottom-up emotional evaluations (Ochsner et al. 2002), and switch the emotional meaning of the negative pictures to neutral meaning, thus the emotional responses could be attenuated. These processes all target the emotional content of the stimuli and change the trajectory of the emotional processing, but not avoid the emotional information. As has been demonstrated by Bebko et al. (2011), more attention to the emotional aspects was the first step for successfully regulation (Bebko et al. 2011).
In clinical practice, a larger LPP on negative stimuli is found in mood disorders such as attachment anxieties (Zilber et al. 2007), PTSD children (Pollak et al. 2001) than controls. It is explained that mood disorders tend to use the strategy of suppression than reappraisal in emotional situations (Campbell-Sills et al. 2006; Swart et al. 2009). As suppression cannot modulate the negative feelings and neural responses, their negative emotions are reasonably higher than healthy people. These negative emotions may impair their mental functioning and social performances in the long run (Keltner and Kring 1998). To help them successfully deal with the emotional problems, introducing an effective emotion regulation strategy to them is necessary. For an instance, as a widely recommended therapy, cognitive behavioral therapy (CBT) indicates that symptoms in mood disorders are rooted in the maladaptive core beliefs stored in long term memory, thus clinical interventions should help patients eliminate these beliefs and reappraise emotional situations in a correct way (Butler et al. 2006). More importantly, besides the online regulation, reappraisal has been found to have an extended regulatory effect (Walter et al. 2009), which suggests the great significance of reappraisal in psychotherapy.
Conclusion
A direct comparison between cognitive reappraisal and expressive suppression was conducted in the current ERP experiment. Their different regulatory effects on emotional feelings, behaviors and electrocortical responses and potential processes were investigated. The results indicated that reappraisal was effective in reducing multiple responses including negative feelings, behaviors and emotion-enhanced LPP; while suppression only reduced negative facial behaviors. The difference in regulatory effect may result from their different underlying processing mechanisms. It was found that reappraisal elicited LPP that may related with cognitive processing on stimulus meaning while suppression elicited N2 that may be associated with processing on behavioral responses.
Acknowledgments
This research was supported by the Natural Science Foundation of China (31070989), Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (CNKOPYB0909). We gratefully thank Aishi Jiang for help on data analysis and Xiaohong Yang for discussion on paper writing.
References
- Baetens K, der Cruyssen LV, Achtziger A, Vandekerckhove M, Van Overwalle F. N400 and LPP in spontaneous trait inferences. Brain Res. 2011;1418:83–92. doi: 10.1016/j.brainres.2011.08.067. [DOI] [PubMed] [Google Scholar]
- Balconi M, Bortolotti A. Emotional face recognition, empathic trait (BEES), and cortical contribution in response to positive and negative cues. The effect of rTMS on dorsal medial prefrontal cortex. Cogn Neurodyn. 2013;7:13–21. doi: 10.1007/s11571-012-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko GM, Franconeri SL, Ochsner KN, Chiao JY. Look before you regulate: differential perceptual strategies underlying expressive suppression and cognitive reappraisal. Emotion. 2011;11(4):732–742. doi: 10.1037/a0024009. [DOI] [PubMed] [Google Scholar]
- Bornas X, Fiol-Veny A, Balle M, Morillas-Romero A, Tortella-Feliu M. Long range temporal correlations in EEG oscillations of subclinically depressed individuals: their association with brooding and suppression. Cogn Neurodyn. 2014;9(1):53–62. doi: 10.1007/s11571-014-9313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CC, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Butler A, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Acceptability and suppression of negative emotion in anxiety and mood disorders. Emotion. 2006;6(4):587–595. doi: 10.1037/1528-3542.6.4.587. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J Physiol Paris. 2014 doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley N, Birbaumer MM, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52(2):95–111. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dien J, Santuzzi AM. Application of repeated measures ANOVA to high-density ERP datasets: a review and tutorial. In: Handy TC, editor. Event-related potentials: A methods handbook. Cambridge: The MIT Press; 2005. pp. 57–82. [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no–go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnülle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010;10(4):563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W, Hager JC. Facial action coding system. Salt Lake City: Network Information Research Corporation; 2002. [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral cingulate cortex in modulating activity in the amydala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Cézanne-Bert G. P3, positive slow wave and working memory load: a study on the functional correlates of slow wave activity. Electroencephalogr Clin Neurophysiol. 1998;108(3):260–273. doi: 10.1016/S0168-5597(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Le J, Leong H, Bennett J, et al. High resolution evoked potential imaging of the cortical dynamics of human working memory. Electroencephalogr Clin Neurophysiol. 1996;98(4):327–348. doi: 10.1016/0013-4694(96)00288-X. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74(1):224–237. doi: 10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion suppression: physiology, self-report, and expressive behavior. J Pers Soc Psychol. 1993;64(6):970–986. doi: 10.1037/0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106(1):95–103. doi: 10.1037/0021-843X.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: The Guilford Press; 2007. pp. 03–24. [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6(4):291–297. doi: 10.3758/CABN.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser J, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6(3):517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Jackson GM, Roberts M. The selection and suppression of action: ERP correlates of executive control in humans. NeuroReport. 1999;10(4):861–865. doi: 10.1097/00001756-199903170-00035. [DOI] [PubMed] [Google Scholar]
- Johnson R, Donchin E. Second thoughts: multiple p300 s elicited by a single stimulus. Psychophysiology. 1985;22(2):182–194. doi: 10.1111/j.1469-8986.1985.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Keltner D, Kring AM. Emotion, social function, and psychopathology. Rev Gen Psychol. 1998;2(3):320–342. doi: 10.1037/1089-2680.2.3.320. [DOI] [Google Scholar]
- Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8(1):132–137. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- Kühn S, Haggard P, Brass M. Intentional inhibition: how the “veto-area” exerts control. Hum Brain Mapp. 2009;30(9):2834–2843. doi: 10.1002/hbm.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J, Brass M. “Keep calm and carry on”: structural correlates of expressive suppression of emotions. PLoS One. 2011;6(1):e16569. doi: 10.1371/journal.pone.0016569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (2008) International affective picture system (IAPS): affective ratings of pictures and instruction manual (Technical Report No. A-6). University of Florida, Gainesville, FL
- Lévesque J, Eugène F, Joanette Y, Paquette V. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53(6):502–510. doi: 10.1016/S0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. An event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J, Hajcak G, Bukay E, Simons R. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43(3):292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: a fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60(5):423–434. doi: 10.1016/0013-4694(85)91017-X. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829–857. doi: 10.1038/mp.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reaction to facial displays of emotion. Psychophysiology. 2001;38(2):267–274. doi: 10.1111/1469-8986.3820267. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Passingham RE, Nobre AC. Components of attentional set-switching. Exp Psychol. 2005;52(2):83–98. doi: 10.1027/1618-3169.52.2.83. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, et al. Modulation of amygdalar activity by the conscious regulation of negative emotion. J Cogn Neurosci. 2002;14(6):913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Schoenberg PLA, Speckens AEM. Multi-dimensional modulations of α and γ cortical dynamics following mindfulness-based cognitive therapy in Major Depressive Disorder. Cogn Neurodyn. 2014 doi: 10.1007/s11571-014-9308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, et al. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(2):257–261. doi: 10.1111/1469-8986.3720257. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shigeto H, Ishiguro J, Nittono H. Effects of visual stimulus complexity on event-related brain potentials and viewing duration in a free-viewing task. Neurosci Lett. 2011;497(2):85–89. doi: 10.1016/j.neulet.2011.04.035. [DOI] [PubMed] [Google Scholar]
- Swainson R, Jackson SR, Jackson GM. Using advance information in dynamic cognitive control: an ERP study of task-switching. Brain Res. 2006;1105(1):61–72. doi: 10.1016/j.brainres.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, Aleman A. Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS One. 2009;4(6):e5751. doi: 10.1371/journal.pone.0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, et al. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4(8):e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber A, Goldstein A, Mikulincer M. Adult attachment orientations and the processing of emotional pictures-ERP correlates. Pers Individ Differ. 2007;43(7):1898–1907. doi: 10.1016/j.paid.2007.06.015. [DOI] [Google Scholar]