Abstract
Aims
In this study, we compared the glucose-lowering effectiveness of insulin analogues and their combination according to baseline glycemic status in patients with type 2 diabetes (T2D) from the A1chieve® study conducted in Korea.
Methods
This sub-analysis from the A1chieve® study was a 24-week prospective, multicenter, non-interventional, open-labelled study. Of the 4058 patients, 3074 patients who had their HbA1c level measured at baseline were included in this sub-analysis. We classified patients into three groups according to baseline HbA1c levels: group I (HbA1c < 7.5%), group II (7.5% ≤ HbA1c < 9.0%) and group III (HbA1c ≥ 9.0%).
Results
Patients in group I showed no significant HbA1c reduction with any insulin regimens (detemir, aspart, detemir and aspart or biphasic aspart 30 (Novo Nordisk A/S, DK-2880 Bagsværd, Denmark) after 24 weeks of treatment. In group II, although HbA1c was decreased for all insulin regimens, there was no difference in mean HbA1c reduction among the four insulin regimens. In patients with a high baseline HbA1c level (group III), mean HbA1c reduction was the greatest in patients on a basal-bolus regimen (detemir and aspart, −3.50%) and lowest in patients on a bolus regimen (aspart, −1.81%; p < 0.001).
Conclusion
For optimal glycaemic control, a basal-bolus regimen may be adequate for Korean patients with poorly controlled T2D (HbA1c ≥ 9.0%).
What's known
In the A1chieve® study, treatment with insulin analogues was associated with marked improvements in glycemic, blood pressure and lipid control without increasing hypoglycemic rates or body weight.
In the A1chieve® study conducted in Korea, treatment with insulin analogues showed reductions in HbA1c, fasting plasma glucose, and postprandial plasma glucose. In addition, the overall quality of life score was improved while no major hypoglycemic episodes were observed.
What's new
No significant HbA1c reduction was observed with any insulin regimens in Korean patients with relatively well controlled type 2 diabetes (T2D; HbA1c < 7.5%).
For optimal glycemic control, a basal-bolus regimen may be adequate for Korean patients with poorly controlled T2D (HbA1c ≥ 9.0%).
Introduction
To determine if insulin analogues are beneficial when treating patients with type 2 diabetes (T2D), the A1chieve® study was conducted as a 6-month prospective, multinational (28 countries), open-labelled, observational study. The study enrolled 66,726 patients with T2D, both insulin and non-insulin users who were started on detemir, aspart or biphasic aspart 30. The study results showed that insulin analogue therapy was associated with marked improvements in glycemic, blood pressure and lipid control without increasing hypoglycaemic rates or body weight (1). In the A1chieve® study conducted in Korea, the treatment with insulin analogues showed beneficial 24-week reductions in HbA1c, fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) (−1.6 ± 2.2%, −2.5 ± 4.7 and −4.0 ± 6.4 mmol/l, respectively). In addition, the overall quality of life score was improved, while no major hypoglycaemic episodes were observed and the rate of minor hypoglycaemic episodes marginally decreased (2).
Although the A1chieve® study in Korea demonstrated the benefits of insulin analogues, individualised recommendations regarding the optimal approach to insulin analogue therapy was not provided, including types of insulin formulation [basal, rapid-acting (henceforth bolus), basal and bolus and biphasic insulin] and insulin regimen (starting doses, number of injections). In addition, few reports exist regarding the characteristics of Korean patients with T2D who respond adequately to insulin analogue therapy (3)–(5).
Therefore, in this sub-analysis from the A1chieve® study, we compared the glucose-lowering effectiveness of insulin analogues and their combination according to baseline glycemic status in Korean patients with T2D.
Patients and methods
Patients and study design
The study population and design were described in a previous report (2). Briefly, Korean patients with T2D, including those who were started on biphasic aspart 30, detemir or aspart within the 4 weeks prior to the initiation of the study were eligible to participate in the study. Patients with a hypersensitivity to the study products or women who were pregnant, breast feeding or had the intention of becoming pregnant within the next 6 months were excluded from the A1chieve® study. The cessation of study insulin was at the discretion of the patients’ physician, who also determined all subsequent treatments (aspart, biphasic aspart 30, or detemir) according to standard protocol. Patients were allowed to withdraw from the study at any time. The protocol was reviewed and approved by independent Institutional Review Boards in the study sites and all participants gave written informed consent before any trial-related activity. The study was performed in accordance with the Declaration of Helsinki and the guidelines for Good Pharmacoepidemiology Practices. A1chieve® was registered at http://Clinicaltrial.gov with the identifier NCT00869908.
The A1chieve® study in Korea was a 24-week prospective, multicentre (104 sites in Korea), non-interventional, real clinical practice setting and open-labelled study. Data were collected at baseline, interim visit (approximately 12 weeks after the baseline visit) and final visit (approximately 24 weeks after the baseline visit). During the study period, the primary end-point was serious adverse drug reactions including major hypoglycaemic events, and secondary study end-points were effectiveness and safety. The secondary effectiveness end-points included changes in FPG, PPG after breakfast, HbA1c and lipid profile from baseline to interim and final visit. The safety end-points were as follows: change in number of hypoglycaemic events and nocturnal hypoglycaemic events in the last 4 weeks before the interim and final visits compared with the last 4 weeks before baseline visit and the number of adverse drug reactions. A hypoglycaemic event was defined either as symptoms of hypoglycaemia that resolved with oral carbohydrate intake, glucagon and intravenous glucose or any symptomatic or asymptomatic plasma glucose < 3.1 mmol/l. A nocturnal hypoglycaemic event was defined as an individualised symptomatic event that occurred while the patient was asleep.
Analysis design
Based on the aims of this study, we first classified patients into three groups according to baseline HbA1c levels: group I (HbA1c < 7.5%), group II (7.5% ≤ HbA1c < 9.0%) and group III (≥ 9.0%). Secondly, we subclassified each group into four subgroups according to type or regimen of insulin analogues: subgroup I used a basal regimen (detemir), subgroup II used a bolus regimen (aspart), subgroup III used a basal-bolus regimen (detemir and aspart) and subgroup IV used a biphasic regimen (biphasic aspart 30).
Statistical analyses
Data were expressed as mean ± standard deviation (SD) or as proportions. The comparison of effectiveness end-points between HbA1c levels was performed using ANOVA with repeated measures. The mean improvement from baseline HbA1c and corresponding 95% confidence interval (CI) was calculated and compared between treatment groups using ANOVA. The association between the effect of treatment group and degree of hyperglycaemia was represented by n (%) at different levels of HbA1c at the end of trial. The number of hypoglycaemic episodes was represented by n (%) and was further classified as major, minor or nocturnal. Comparison of hypoglycaemic episodes between the categories was performed using the χ2 test. All data were analysed by Novo Nordisk using sas (Version 9.1.3, COGNIZANT TECHNOLOGY SOLUTIONS, Mumbai, India) and p-values < 0.05 were considered statistically significant.
Results
Baseline characteristics according to baseline HbA1c levels
Of the 4058 patients who were exposed to the selected insulin at least once and constituted the full analysis set (FAS), 3074 patients had their HbA1c level measured at the baseline and final visit and 2952 patients (72.7% of FAS) who used one of four insulin analogue regimens were eligible for analysis (Figure 1).
Figure 1.
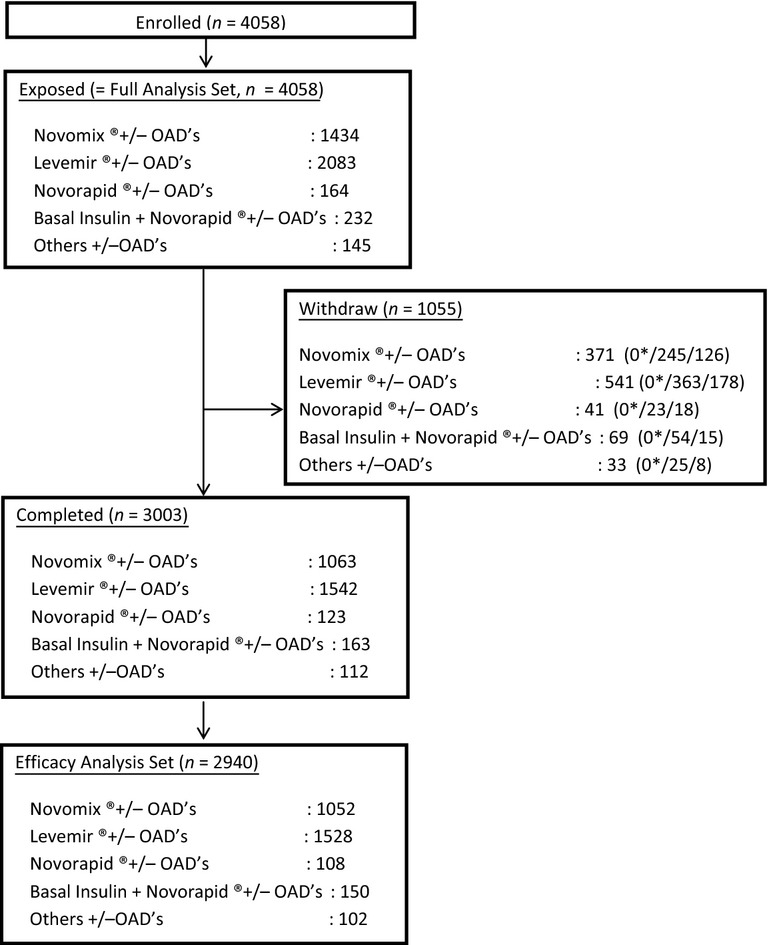
Flow diagram of study
Baseline characteristics of the study patients according to baseline HbA1 levels are shown in Table 1. Patients were allocated to group I (HbA1c < 7.5%, n = 302, 173 males, 129 females), group II (7.5% ≤ HbA1c < 9.0%, n = 877, 449 males, 428 females) or group III (≥ 9.0%, n = 1895, 1049 males, 846 females). The duration of diabetes was significantly longer in group II (10.0 years, 11.4 years and 9.3 years in groups I, II and III, respectively; p < 0.001). In addition, body mass index (BMI) was statistically different between groups (24.0, 24.6 and 24.2 kg/m2, groups I, II and III, respectively; p = 0.016).
Table 1.
Baseline clinical and biochemical characteristics of the study patients
| HbA1c | Group I < 7.5% | Group II 7.5–9.0% | Group III ≥ 9.0% | p-value |
|---|---|---|---|---|
| Number | 302 | 877 | 1895 | |
| Male (%) | 173 (57.3) | 449 (51.2) | 1049 (55.1) | 0.069* |
| Age (years) | 58.1 (13.3) | 58.2 (11.7) | 56.2 (13.5) | < 0.001† |
| Weight (kg) | 63.9 (11.2) | 64.6 (11.0) | 64.1 (11.9) | 0.581† |
| BMI (kg/m2) | 24.0 (3.6) | 24.6 (3.5) | 24.2 (3.7) | 0.016† |
| Diabetes duration (years) | 10.0 (8.0) | 11.4 (7.7) | 9.3 (7.7) | < 0.001† |
| HbA1c (mmol/mol) | 51.3 (5.4) | 66.9 (4.6) | 96.3 (18.3) | < 0.001† |
| FPG (mmol/l), before | ||||
| Breakfast | 8.3 (3.4) | 8.9 (3.1) | 11.8 (4.7) | < 0.001† |
| Lunch | 11.6 (5.1) | 10.3 (3.4) | 13.3 (5.6) | < 0.001† |
| Dinner | 10.7 (4.2) | 11.9 (4.6) | 13.8 (5.5) | < 0.001† |
| PPG2 (mmol/l) after | ||||
| Breakfast | 12.3 (4.3) | 13.8 (4.7) | 16.9 (6.0) | < 0.001† |
| Lunch | 12.0 (5.1) | 13.7 (4.2) | 16.5 (6.0) | < 0.001† |
| Dinner | 12.0 (4.1) | 13.4 (4.3) | 14.2 (4.7) | 0.019† |
| Prior OADs (%) | ||||
| Metformin | 164 (54.3) | 622 (70.9) | 1345 (71.0) | < 0.001* |
| Sulfonylureas | 129 (42.7) | 499 (56.9) | 960 (50.7) | < 0.001* |
| Glinide | 52 (17.2) | 134 (15.3) | 228 (12.0) | < 0.001* |
| Thiazolidinediones | 18 (6.0) | 52 (5.9) | 132 (7.0) | 0.086* |
| DPP-4 inhibitors | 12 (4.0) | 32 (3.6) | 85 (4.5) | 0.045* |
| α-glucosidase inhibitor | 65 (21.5) | 261 (29.8) | 484 (25.5) | 0.030* |
Data are expressed as frequency (%) and the p-value estimated based on the χ2 test.
Data are expressed as mean (SD) and the p-value estimated based on one way ANOVA. BMI, body mass index; FPG, fasting plasma glucose; PPG2, postprandial glucose 2 h; OAD, oral antihyperglycaemic drug; DPP, dipeptidyl peptidase; NS, not significant.
Glucose-lowering effectiveness according to baseline HbA1c levels
In a previous A1chieve® study report in Korea (2), HbA1c decreased from 9.7% at baseline to 8.1% at the 24-week end-point, resulting in a significant reduction of 1.6 ± 2.2% (p < 0.001). In addition, the proportion of patients who achieved the target HbA1c level of < 7.0% increased from 4.8% at baseline to 18.1% at the 12-week interim and 22.7% at the 24-week end-point. In terms of type and regimen of insulin analogues, mean HbA1c reduction was the greatest in patients on a basal-bolus regimen (levemir and aspart, 2.2 ± 2.5%; p < 0.001) and lowest in patients on a bolus regimen (aspart, 0.7 ± 2.3%; p = 0.036).
In the first step of this study analysis, we classified patients into three groups according to baseline HbA1c levels. Table 2 shows the glucose-lowering effectiveness of insulin analogues according to baseline HbA1c levels. Baseline mean HbA1c levels were 6.8%, 8.3% and 11.0% in group I (HbA1c < 7.5%), II (7.5 ≤ HbA1c < 9.0%) and III (HbA1c > 9.0%), respectively. For Korean patients with relatively well controlled (group I, HbA1c < 7.5%) and poorly controlled (group II, 7.5% ≤ HbA1c < 9.0%) glucose status, physicians prescribed predominantly the basal regimen (57.2% in group I and 54.6% in group II). In group I, no significant HbA1c change was observed in any insulin regimen after 24 weeks of treatment. In all group II subgroups, the mean HbA1c was decreased. The mean HbA1c reduction was greatest in patients with a basal-bolus regimen and lowest in patients with a bolus regimen (aspart). However, there were no statistical differences in mean HbA1c reduction among the four subgroups. In terms of target HbA1c achievement, the proportion of patients achieving HbA1c < 6.5% and < 7.0% was the greatest in patients with a basal-bolus regimen (11.3%) and bolus regimen (28.6%), respectively. In patients with a very poorly controlled glucose status (group III, HbA1c > 9.0%), Korean physicians preferred both basal (46.3%) and biphasic (40.4%) insulin regimens. In group III, mean HbA1c reduction was the greatest in patients with a basal-bolus regimen (−3.50%) and lowest in patients with a bolus regimen (−1.81%; p < 0.001).
Table 2.
Glucose-lowering effectiveness according to type or regimen of insulin analogues
| Basal regimen (n = 1531) | Bolus regimen (n = 112) | Basal-bolus regimen (n = 187) | Biphasic regimen (n = 1122) | p-value | |
|---|---|---|---|---|---|
| Group I (n) | 173 | 19 | 20 | 80 | |
| Baseline A1c (mmol/mol) | 51.7 (5.3) | 50.9 (5.1) | 48.4 (7.3) | 51.5 (5.3) | 0.084* |
| A1c change (mmol/mol, 95% CI) | 1.0 (−0.9, 3.0) | 2.3 (−2.9, 7.4) | −0.2 (−7.0, 6.7) | 4.6 (0.4, 8.9) | 0.058* |
| A1c < 48 (mmol/mol) | 31 (17.9) | 2 (10.5) | 1 (5.0) | 8 (10.0) | 0.28† |
| A1c < 53 (mmol/mol) | 51 (29.5) | 7 (36.8) | 6 (30.0) | 19 (23.8) | 0.27† |
| A1c < 58 (mmol/mol) | 73 (42.2) | 10 (52.6) | 7 (35.0) | 27 (33.8) | 0.26† |
| Group II (n) | 479 | 35 | 53 | 275 | |
| Baseline A1c (mmol/mol) | 66.7 (4.6) | 65.6 (5.2) | 66.8 (4.9) | 67.3 (4.5) | 0.12* |
| A1c change (mmol/mol, 95% CI) | −5.7 (−7.0, −4.4) | −0.5 (−10.4, 9.4) | −10.0 (−14.1, −6.0) | −5.8 (−7.4, −4.2) | 0.11* |
| A1c < 48 (mmol/mol) | 12 (2.5) | 2 (5.7) | 6 (11.3) | 9 (3.3) | 0.022† |
| A1c < 53 (mmol/mol) | 56 (11.7) | 10 (28.6) | 12 (22.6) | 35 (12.7) | 0.049† |
| A1c < 58 (mmol/mol) | 129 (26.9) | 12 (34.3) | 22 (41.5) | 75 (27.3) | 0.16† |
| Group III (n) | 879 | 58 | 114 | 767 | |
| Baseline A1c (mmol/mol) | 94.1 (16.7) | 93.8 (13.2) | 102.0 (20.7) | 98.0 (19.4) | < 0.001* |
| A1c change (mmol/mol, 95% CI) | −26.2 (−28.4, −24.1) | −19.8 (−31.1, −8.6) | −38.2 (−46.1, −30.3) | −26.4 (−29.0, −23.8) | < 0.001* |
| A1c < 48 (mmol/mol) | 40 (4.6) | 4 (6.9) | 10 (8.8) | 37 (4.8) | 0.23† |
| A1c < 53 (mmol/mol) | 85 (9.7) | 5 (8.6) | 13 (11.4) | 66 (8.6) | 0.61† |
| A1c < 58 (mmol/mol) | 137 (15.6) | 10 (17.2) | 22 (19.3) | 111 (14.5) | 0.37† |
Data are expressed as mean (SD), mean (95% CI), or frequency (%).
Data are expressed as mean (SD) and the p-value estimated based on one way ANOVA.
Data are expressed as frequency (%) and the p-value estimated based on the chi-square test.
With respect to the effectiveness of insulin analogues based on baseline HbA1c levels, the glucose-lowering effectiveness (−0.01% to 0.42% reduction in HbA1c level) was minimal or equivalent in group I (HbA1c < 7.5%, mean HbA1c level of 6.8%). In addition, the percentage of patients reaching a target HbA1c level of < 7.0% was not different among the four insulin regimens (23.8–36.8%). In the poorly controlled T2D group II patients (7.5% ≤ HbA1c ≤ 9.0%, mean HbA1c level of 8.3%), the HbA1c reduction effectiveness was perceivable (−0.04% to −0.92% reduction in HbA1c level). The percentage of patients reaching a target HbA1c level of < 7.0% was significantly higher in the subgroup using bolus (28.6%) and basal-bolus (22.6%) regimens (p = 0.049). In the very poorly controlled T2D group III patients (HbA1c > 9.0%, mean HbA1c level of 11.0%), the HbA1c reduction effectiveness was pronounced (−1.81% to −3.50% reduction in HbA1c level). The percentage of patients reaching the target HbA1c level of < 7.0% was not significantly different among the four subgroups (8.6–11.4%). Despite statistical insignificance, the basal-bolus regimen showed the highest percentage of patients reaching the target HbA1c level of < 7.0% (11.4%). Regarding the use of basal-bolus regimen in clinical practice, except for the patients achieving the HbA1c level of < 7.0% in group II, higher HbA1c reduction effectiveness and higher percentages of Korean patients that achieved target HbA1c levels < 6.5% or < 7.0% were found in the poorly or very poorly controlled T2D groups II and III, respectively.
Hypoglycaemic events and body weight change according to baseline HbA1c levels
Although hypoglycaemic events were similar across the different insulin regimens in group I, hypoglycaemic events were most frequently observed in patients with basal-bolus regimen in group II (17.0%) and group III (14.0%; both p < 0.001). In terms of body weight change, treatment with basal-bolus or biphasic regimens showed greater weight gain compared with other insulin modalities in group III (p = 0.036); however, differences in body weight change were not observed in group I and II according to different insulin regimens. Next, we determined the best insulin modality allows patients to meet their glycemic goals while avoiding the risk of hypoglycaemia according to different insulin regimens. The percentage of patients reaching a target HbA1c level of < 7.0% without hypoglycaemia was greater in patients on a bolus regimen in group II (25.7%). In group III, although statistically significant, the achievement of glycemic goals without hypoglycaemia was similar across the insulin regimens (Table 3).
Table 3.
Safety issues according to type or regimen of insulin analogues
| Basal regimen (n = 1531) | Bolus regimen (n = 112) | Basal-bolus regimen (n = 187) | Biphasic regimen (n = 1122) | p-value | |
|---|---|---|---|---|---|
| Group I (n) | 173 | 19 | 20 | 80 | |
| Any hypoglycaemia (%)* | 7 (4.1) | 2 (10.5) | 1 (5.0) | 5 (6.3) | 0.11† |
| Body weight at baseline (kg) | 64.3 (11.4) | 67.6 (10.0) | 61.9 (11.6) | 62.5 (11.1) | 0.43‡ |
| Body weight change (kg) | −0.2 (2.0) | 1.1 (2.9) | −0.5 (1.2) | 0.0 (3.3) | 0.28‡ |
| A1c < 53 mmol/mol without any hypoglycaemia (%) | 48 (27.8) | 6 (31.6) | 6 (30.0) | 17 (21.3) | < 0.001† |
| A1c < 58 mmol/mol without any hypoglycaemia (%) | 69 (39.9) | 9 (47.4) | 7 (35.0) | 24 (30.0) | < 0.001† |
| Group II (n) | 479 | 35 | 53 | 275 | |
| Any hypoglycaemia (%)* | 26 (5.4) | 2 (5.7) | 9 (17.0) | 29 (10.6) | < 0.001† |
| Body weight at baseline (kg) | 64.5 (10.6) | 67.2 (15.1) | 64.9 (12.4) | 65.4 (11.5) | 0.53‡ |
| Body weight change (kg) | 0.1 (2.6) | 0.3 (2.8) | 1.4 (2.3) | 0.3 (2.9) | 0.08‡ |
| A1c < 53 mmol/mol without any hypoglycaemia (%) | 51 (10.7) | 9 (25.7) | 8 (15.1) | 31 (11.3) | < 0.001† |
| A1c < 58 mmol/mol without any hypoglycaemia (%) | 118 (24.6) | 11 (31.4) | 15 (28.3) | 63 (22.9) | < 0.001† |
| Group III (n) | 879 | 58 | 114 | 767 | |
| Any hypoglycaemia (%)* | 45 (5.1) | 6 (10.3) | 16 (14.0) | 67 (8.7) | < 0.001† |
| Body weight at baseline (kg) | 63.9 (11.6) | 63.8 (8.9) | 63.0 (10.7) | 64.3 (11.9) | 0.80‡ |
| Body weight change (kg) | 0.6 (3.0) | 0.7 (2.4) | 1.0 (3.6) | 1.3 (3.4) | 0.036‡ |
| A1c < 53 mmol/mol without any hypoglycaemia (%) | 77 (8.8) | 4 (6.9) | 9 (7.9) | 60 (7.8) | < 0.001† |
| A1c < 58 mmol/mol without any hypoglycaemia (%) | 123 (14.0) | 8 (13.8) | 17 (14.9) | 100 (13.0) | < 0.001† |
Data are expressed as mean (SD) or frequency (%).
Hypoglycaemic events were assessed at last visit.
Data are expressed as frequency (%) and the p-value estimated based on the χ2 test.
Data are expressed as mean (SD) and the p-value estimated based on one way ANOVA.
Discussion
Despite little controversy regarding the need for optimal glycemic control in insulin therapy for patients with poorly controlled diabetes (6–8), which insulin therapy treatment modality allows patients to best meet and maintain individualised glycemic goals while avoiding the risk of hypoglycaemia remains unclear (9). Furthermore, most studies recommend the insulin therapy to be based on HbA1c levels (6–8). In terms of its non-randomised, non-interventional study design, the present study has both strengths and limitations that could be complemented by further investigations. Although randomised clinical trials are considered the gold standard, the non-interventional study design evaluates effectiveness and optimal regimens in real clinical practice (4,10). Based on a previous report (2), treatment with insulin analogues (aspart, biphasic aspart 30, detemir, or detemir and aspart) reduced HbA1c, FPG and PPG levels during the 24-week of treatment period (1.6 ± 2.2%, 2.5 ± 4.7 and 4.0 ± 6.4 mmol/l, respectively).
To date, scientific reports investigating optimal approaches to treatment with insulin analogues and comparing their glucose-lowering effectiveness in real practice have been lacking in Korean patients with T2D. Therefore, we investigated current decision-making on the initiation of insulin analogues based on baseline HbA1c levels and the effectiveness of insulin regimens based on reductions in HbA1c, as well as the proportion of patients reaching target HbA1c < 7.0%. By understanding daily practice settings, we hope to suggest the optimal insulin analogue-based glycemic control in Korean patients with T2D.
Regarding current decisions on the initiation of insulin analogues, approximately 88.5% of patients in this observational study initiated insulin analogue therapy with HbA1c levels greater than 7.5% and a disease duration of approximately 10.0 years. According to the consensus statements of the American Diabetes Association and the European Association for the Study of Diabetes (11), insulin initiation is recommended when FPG levels are above 250 mg/dl, random glucose levels are above 300 mg/dl, or HbA1c is above 10.0%. However, insulin could also be considered whenever HbA1c is above 8.5% and patients are already receiving a treatment to achieve a more effective control. Considering the status of glycemic control determined by HbA1c levels, current decisions on the initiation of insulin analogues are within limits of the consensus reached by the Korean medical practitioners.
Because the underlying pathophysiological nature of T2D involves initially increased insulin resistance and decreased insulin secretion with ongoing progressive deterioration in pancreatic β-cell function and resulting in pancreatic islet exhaustion, which corresponds clinically with deteriorating hyperglycaemia (12–14), early initiation of insulin therapy might be considered the optimal approach. In addition, because of T2D characteristics in the Korean population where secretory dysfunction of pancreatic β-cells is the major underlying pathophysiology for the development and aggravation of hyperglycaemia (12,15,16), an insulin regimen advocating control of postprandial hyperglycaemia on an individual basis might be an important area of study in the Korean population (5).
This study had several limitations. First, this study was performed on Korean subjects and thus, determining the glucose-lowering effectiveness of insulin analogues and their combination in other ethnicities or study populations is necessary. Second, we did not consider the effect of other confounders potentially affecting glucose-lowering effectiveness and adverse effects of different insulin regimens, including age, gender, BMI, duration of diabetes and concomitant oral hypoglycaemic agents.
In summary, this observational study provides important information on how pharmaceutical insulin therapies perform in real clinical practice. Physicians might decide to start insulin therapy in patients with T2D if the HbA1c level is greater than 7.5%. Based on our results, we suggest that a basal-bolus regimen might be adquate in Korean patients with poorly controlled T2D (HbA1c > 9.0%). A further large-scale, randomised, interventional study should be performed to clarify the effectiveness and safety of a basal-bolus regimen in Korean patients with T2D.
Acknowledgments
This study for data mining and statistical analysis was sponsored by Novo Nordisk Pharma Korea and Novo Nordisk International Operations.
Author contributions
Y.-C. Hwang analysed and interpreted the data, contributed to the discussion, and wrote the manuscript. B.-W. Lee designed the study, analysed and interpreted the data, and reviewed/edited the manuscript. J. G. Kang, K. J. Ahn, B. S. Cha and S.-H. Ihm contributed to the discussion and reviewed the manuscript. S. Lee and M. Kim collected the data.
References
- 1.Home P, Naggar NE, Khamseh M, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352–63. doi: 10.1016/j.diabres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Yoo HJPK, Park KS, Ahn KJ, et al. Safety and efficacy of modern insulin products. Diabetes Metab J. 2013;37:181–9. doi: 10.4093/dmj.2013.37.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011;65:1076–84. doi: 10.1111/j.1742-1241.2011.02755.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee YH, Lee BW, Kwon HJ, Kang ES, Cha BS, Lee HC. Higher morning to evening ratio in total dose of twice-daily biphasic insulin analog might be effective in achieving glucose control in patients with poorly controlled type 2 diabetes. Diabetes Technol Ther. 2012;14:508–14. doi: 10.1089/dia.2011.0208. [DOI] [PubMed] [Google Scholar]
- 5.Choe EY, Lee YH, Lee BW, Kang ES, Cha BS, Lee HC. Glycemic effects of once-a-day rapid-acting insulin analogue addition on a basal insulin analogue in Korean patients with poorly controlled type 2 diabetes mellitus. Diabetes Metab J. 2012;36:230–6. doi: 10.4093/dmj.2012.36.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–59. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 8.Ko SH, Kim SR, Kim DJ, et al. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011;35:431–6. doi: 10.4093/dmj.2011.35.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tibaldi J, Rakel RE. Why, when and how to initiate insulin therapy in patients with type 2 diabetes. Int J Clin Pract. 2007;61:633–44. doi: 10.1111/j.1742-1241.2007.01309.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah SN, Litwak L, Haddad J, Chakkarwar PN, Hajjaji I. The A1chieve study: a 60 000-person, global, prospective, observational study of basal, meal-time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract. 2010;88(Suppl. 1):S11–6. doi: 10.1016/S0168-8227(10)70003-6. [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011;35:107–16. doi: 10.4093/dmj.2011.35.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groop LC, Pelkonen R, Koskimies S, Bottazzo GF, Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 1986;9:129–33. doi: 10.2337/diacare.9.2.129. [DOI] [PubMed] [Google Scholar]
- 14.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–81. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee BW, Kang HW, Heo JS, et al. Insulin secretory defect plays a major role in the development of diabetes in patients with distal pancreatectomy. Metabolism. 2006;55:135–41. doi: 10.1016/j.metabol.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of korean type 2 diabetes mellitus. Metabolism. 2001;50:590–3. doi: 10.1053/meta.2001.22558. [DOI] [PubMed] [Google Scholar]


