Abstract
The Gallyas method is a silver impregnation technique that is essential in the field of neuropathology because of its high sensitivity for the detection of argentophilic inclusion bodies in the central nervous system. In Japan, the Gallyas method has improved and is widely used as the “modified Gallyas method”. However, this method is not popularly used in general pathology laboratories because of the need for special reagents, several staining processes, and skilled techniques. The objective of the current study was to provide a simplified Gallyas method. We omitted the lanthanum nitrate step from the staining process and verified the adequacy in comparison with the original method as well as immunohistochemistry, using specimens from patients of Alzheimer's disease, argyrophilic grain disease, multiple system atrophy, Pick's disease, and Lewy body disease. The simplified method provided good staining to all the structures in archival tissues, compared with the modified Gallyas method in a significantly shorter staining time. The lanthanum nitrate step can be omitted from the modified Gallyas method, resulting in reduction in the number of reagents required and shortening of the staining time.
Keywords: argyrophilic grains, glial cytoplasmic inclusion, lanthanum nitrate hexahydrate, neurofibrillary tangles, simplification technique
Introduction
The modified Gallyas method is a silver impregnation technique which showed high sensitivity for argyrophilic structure, including neurofibrillary tangles (NFTs) in Alzheimer disease (AD), argyrophilic grains in argyrophilic grain diseases (AGD) and cytoplasmic inclusion bodies in multiple system atrophy (MSA). This staining method was first invented by Gallyas1 in 1971 for anatomical study and subsequently applied by Braak2 to neuropathological specimens. Uchihara et al.3 reported that potassium permanganate is useful as a pretreatment and dramatically improved the detection sensitivity. This is widely used as the current modified Gallyas method in facilities dedicated to neuropathology. However, this method has some limitations: the reagents used in the processes are special, there are many staining processes, and skilled techniques are required for the staining. Therefore, general pathologists who do not specialize in neuropathology hesitate to use the modified Gallyas method.
In the present study, we reviewed the staining processes involved in the modified Gallyas method step by step and found that skipping lanthanum nitrate hexahydrate (La(NO3)3·6H2O, hereinafter referred to as lanthanum nitrate) may not alter the staining quality.
Methods
Materials
The archival specimens from our brain banks4,5 included 4 cases of AD, 2 cases of corticobasal degeneration (CBD), 3 cases of AGD, 4 cases of Pick's disease, 10 cases of multiple system atrophy (MSA) and 3 cases of Lewy body disease (LBD). In addition to the method described below, eleven cases of progressive supranuclear palsy (PSP) were selected and additionally fixed in formalin for 3 weeks, 4 weeks, 5 months, and 1 year before embedding, in order to check the influence of staining period.
Staining methods
Fixation
The specimens were obtained at autopsy and fixed in 15 to 20% of neutral buffered formalin (hereinafter referred to as formalin, Wako Pure Chemical Industries, Osaka, Japan) for 7–13 days, or in 4% paraformaldehyde (PFA, Wako Pure Chemical Industries) two overnight before embedded in paraffin. After the fixation, the specimens were delipidated, dehydrated in increasing stepwise concentrations of alcohol, and embedded in paraffin using a closed-type automatic embedding machine (ETP, Sakura Finetek Japan, Tokyo, Japan). The paraffin sections were cut at a thickness of 6 μm, mounted on uncoated glass slides (FF-002, Matsunami Glass, Japan), and dried overnight in an incubator at 40°C.
The simplified Gallyas method omitting lanthanum nitrate
The specimens were stained with the modified Gallyas method (Table 1) and our simplified Gallyas method (Table 2) simultaneously for comparison. The most appropriate time after preparation of the developer solution, temperature of the developer solution and reaction time was also proved for each condition at least in two different occasions without adjusting room temperature. Three independent neuropathologists checked the staining results.
Table 1.
The modified Gallyas method
| Procedural step | Time (minutes) |
|---|---|
| (1) Potassium permanganate solution (0.3%) | 10 |
| (2) Oxalic acid solution (1%) | 1 |
| (3) Wash with water | 5 |
| (4) Distilled water | |
| (5) Lanthanum nitrate solution (0.4%) | 40 |
| (6) Wash in distilled water (3 times) | 1 (each) |
| (7) Alkali silver iodide solution | 1 |
| (8) Wash in 0.5% aqueous acetic acid (3 times) | 1 (each) |
| (9) Physical developer solution | 18–20 |
| (10) Wash in 0.5% aqueous acetic acid (3 times) | 1 (each) |
| (11) Gold chloride solution (0.5%) | 5 |
| (12) Lightly wash with water, distilled water | |
| (13) Aqueous solution of sodium thiosulfate (1%) | 1 |
| (14) Wash with water, distilled water | 1 |
| (15) Counter stain (0.1% nuclear fast red) | 3 |
| (16) Dehydrate in alcohol, clear with xylene, mount in Entellan new® | |
| Total time required | 94 |
Table 2.
The simplified method
| Procedural step | Time (minutes) | |
|---|---|---|
| (1) Potassium permanganate solution (0.3%) | 10 | |
| (2) Lightly wash with water | ||
| (3) Oxalic acid solution (1%) | 1 | |
| (4) Wash with water | 5 | |
| (5) Distilled water | ||
| (6) Alkali silver iodide solution | 1 | aqueous solution of silver nitrate and stir until clear. Add distilled water to a final volume of 50 mL. |
| (7) Wash in 0.5% aqueous acetic acid (3 times) | Each 1 | |
| (8) Physical developer solution | 16–18 | The developer is always prepared immediately before use by mixing 2 solutions. Both stock solutions are stable and can be stored for 2–3 months in a brown bottle. |
| Solution A: Dissolve sequentially in 1000 mL distilled water: 2 g silver nitrate, 2 g ammonium nitrate, 10 g tungstosilicic acid, and 5.1 mL of a 35% aqueous solution of formaldehyde. | ||
| Solution B: 50 g of anhydrous sodium carbonate in 1000 mL distilled water. | ||
| (9) Wash in 0.5% aqueous acetic acid (3 times) | Each 1 | |
| (10) Gold chloride solution (0.5%) | 5 | |
| (11) Lightly wash with water | ||
| (12) Aqueous solution of sodium thiosulfate (1%) | 1 | |
| (13) Wash with water, distilled water | 1 | |
| (14) Counter stain (0.1% nuclear fast red) | 3 | |
| (15) Dehydrate in alcohol, clear with xylene, mount in Entellan new® | ||
| Total time required | 48 |
Comparison with immunohistochemical staining
The most appropriate protocol of the simplified Gallyas method determined above was compared with immunohistochemical stainings with the antibodies raised against -phosphorylated tau (AT8, 1:2000; Innogenetics, Gent, Belgium), three repeat tau (RD3, 1:5000; Upstate, Lake Placid, NY, USA), four repeat tau (RD4, 1:200; Millipore, Billerica, MA, USA), ubiquitin (polyclonal, 1:200; Dako, Carpinteria, CA, USA) and alpha-synuclein (Pser#64, Pser#64, 1:500, a generous gift from T. Iwatsubo, University of Tokyo). The staining was performed using an automated immunostaining machine (Discovery XT; Ventana [Roche, Tokyo, Japan]).
Results
Efficacy in omitting lanthanum nitrate
Both the Gallyas method and the simplified Gallyas method visualized appropriate argentophilic structures constantly. Ghost tangles were more clearly visualized with the simplified Gallyas method than the Gallyas method (Fig. 1). When the simplified Gallyas method was compared with the modified Gallyas method, both methods allowed the successful characterization of abnormal structures in each degenerative disease without significant differences in their staining efficacy (including sensitivity and non-specific background reaction).
Fig. 1.
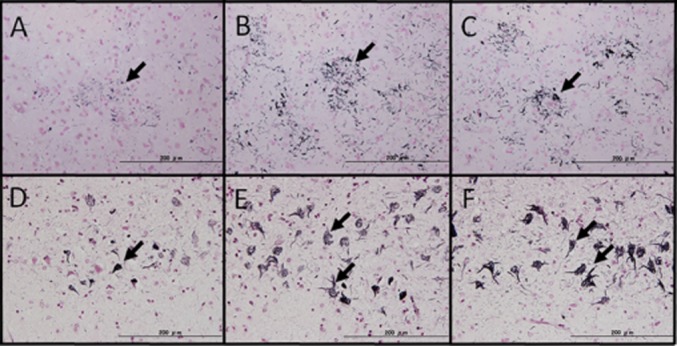
Suitability of omission of the lanthanum nitrate step (representative cases of corticobasal degeneration in A–C and Alzheimer's disease in D–F). (A):The original Gallyas method. Astrocytic plaque, characteristic of corticobasal degeneration were observed, although the sensitivity was slightly lower (black arrow). (B): The modified Gallyas method. The image shows positive staining with better sensitivity than that of the original. (C): The simplified Gallyas method. The image shows positive staining that was not inferior to that of the modified Gallyas method. Additionally, non-specific background reactivity was not observed. (D): The original method. NFTs and neuropil threads accepted that they are stained with black (black arrow). (E): The modified Gallyas method. The image shows positive staining with better sensitivity than the original, and the absence of non-specific reactions. (F): The simplified Gallyas method. The image shows detection sensitivity that was not inferior to that of the modified Gallyas method, and non-specific background reactivity was not observed. (Scale bar = 200 μm).
Each incubation period of the developer solution before the staining and the start and end of positive argentophilic reaction after dipping (minutes) were as follows: 13, 10, 17; 40, 8, 15; and 60, 6, 15 (Fig. 2). Longer incubation period results in shorter reaction time and higher background. Incubation period shorter than 60 minutes was determined to be appropriate.
Fig. 2.
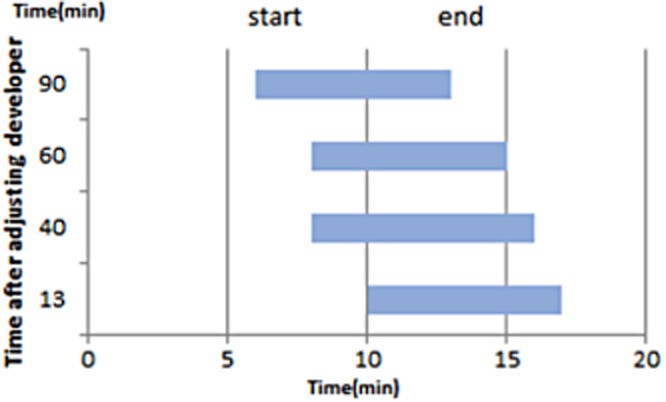
Influence of time after preparation of the developer solution on the staining efficacy. When the reaction was started 13 min after preparation of the reaction solution, black staining began gradually at 10 min and ended 17 min after the start of staining. When the reaction solution was 40 min old, staining started at 7 min and ended 17 min after the start. When the reaction solution was 60 min old, black staining started at 7 min but ended 15 min after the start. When the reaction solution was 90 min old, staining started at 6 min but ended 13 min after the start. A trend for black staining to start and end earlier in older reaction solutions was observed and occurred in parallel to deterioration of the solution.
To determine the best solution temperature for the simplified Gallyas method, the staining was performed in 15°C, 23°C and 45°C. The staining in 15°C obtained a sufficient silver reaction around 16–18 minutes with good staining efficacy, in 23°C, 9 minutes with considerable background and in 45°C, the sections were detached from the glass slides.
About fixation of the specimens, PFA fixation caused a non-specific background reaction involving red blood cells and blood vessels that was not observed with formalin fixation. However, this background staining did not cause a problem in diagnosis because the abnormal structures in each disease were absent of background staining (Fig. 3). Both the modified Gallyas and the simplified Gallyas method showed good sensitivity without non-specific staining of blood vessels or red blood cells regardless of the fixation period.
Fig. 3.
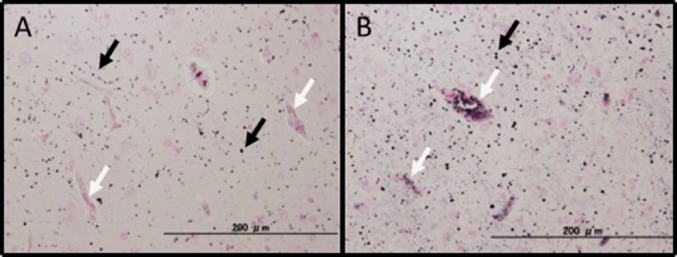
Comparison between formalin and PFA fixation solutions (representative case: dementia with grains). (A): Formalin fixation. Argyrophilic granules were observed in numbers (black arrows). Non-specific reactions were not observed in peripheral blood vessels, red blood cells, etc. (B): PFA fixation. Although non-specific reactions were observed in peripheral blood vessels and red blood cells, these non-specific reactions did not influence the silver reaction itself (white arrows).
Comparison between the simplified Gallyas method and immunohistochemical stainings
In AD, both the simplified Gallyas method and modified Gallyas method picked up AT8- immunoreactive structures appropriately (Fig. 4). In CBD and AGD both the method visualized RD4- immunoreactive structures specifically (Figs. 5). In Pick's disease, both the staining did not detect RD3-immunoreactive Pick boides as already reported. The simplified Gallyas method detected AT8 and RD4- immunoreactive argyrophilic grains appropriately. In MSA, psyn#64- immunoreactive neuronal and glial cytoplasmic inclusions were appropriately stained, but in LBD, Lewy bodies were negative for both the method.
Fig. 4.
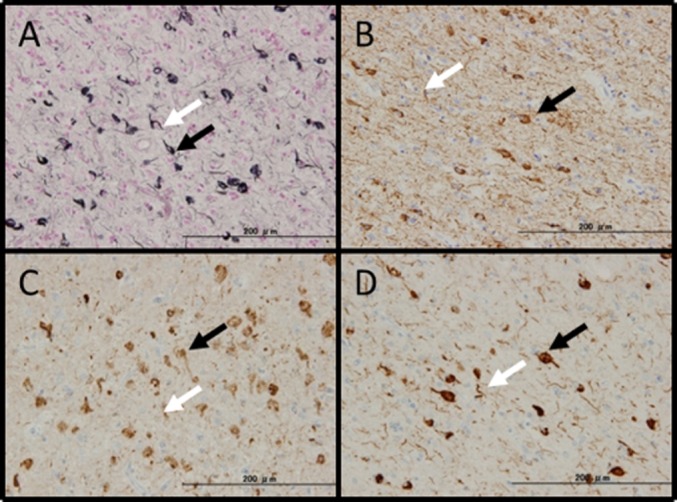
Alzheimer's disease. (A): The simplified Gallyas method. Many NFTs including tangles (black arrow) and threads (white arrow) were observed. The specimen was AT8-positive (B), RD3-positive (C), and RD4-positive (D).
Fig. 5.
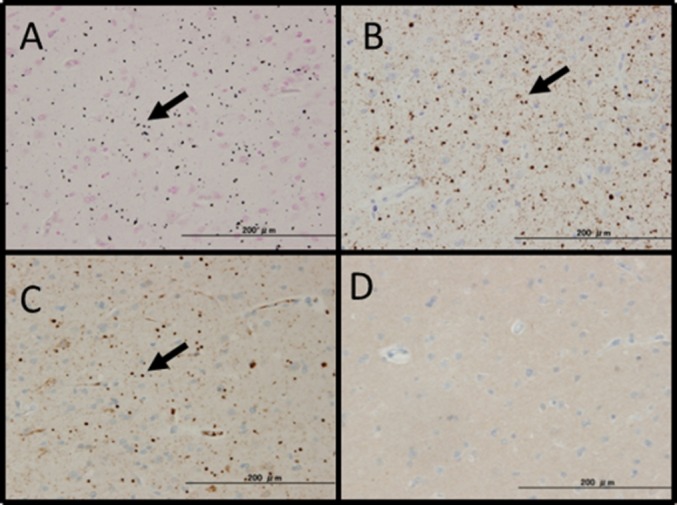
Dementia with grains. (A): The simplified Gallyas method. Many argyrophilic grains (granules and fusiform structures) (black arrow) were observed. The specimen was AT8-positive (B), RD4-positive (C), and RD3-negative (D).
Discussion
The present study demonstrated that the lanthanum nitrate step can be skipped from the modified Gallyas method.
The modified Gallyas method is established after two modifications. Gallyas1 originally inhibited non-specific reactions with the process of periodic acid, which consisted of (1) 5% periodic acid, (2) alkaline silver iodide, and (3) a reducing agent. The effects of periodic acid are described in detail by Gallyas.6 Uchihara et al.7 proposed changes to this Gallyas method by adding a step of potassium permanganate-oxalic before the step of lanthanum, dramatically improved the staining efficacy and has been now widely employed as modified Gallyas method in Japan.8–11
The potassium permanganate-oxalic acid method is a bleaching method12 in which melanin pigments are bleached to show their presence. Potassium permanganate is also used in general silver impregnation13,14 because it is a strong oxidant and can reduce the background staining by destroying many functional groups in tissues. Although lanthanum nitrate was used for reducing background staining in the Gallyas method, it was used to exert a mordant action in the modified Gallyas method. Although lanthanum nitrate is mainly used in the field of electron microscopy for observing minute structures such as the intercellular junction, it is an unfamiliar reagent in general pathological laboratories.
According to Gallyas, silver iodide binds to degenerated neurofibrils under alkaline conditions in the first reaction. In the second reaction, iodine atoms are released leaving behind silver particles due to the action of the reducing agent. The silver particles can then be visualized after photographic development with a physical developer in the third reaction.1 The important step in this reaction system is the binding of silver iodide to degenerated neurofibrils under alkaline conditions. Therefore, lanthanum nitrate is unlikely to have a direct role in this reaction system. In addition, lanthanum nitrate used for electron microscopy is presumed to play the role of “an electron-dense marker that does not react with biological components”.15 Lanthanum nitrate was used in the modified Gallyas method for its weak oxidative effects. Therefore, any reagent with the same activity can replace lanthanum nitrate (personal communication with Professor H. Braak, Clinical Neuroanatomy, Ulm University). An oxidation reaction occurs twice in the modified Gallyas method. Hence, potassium permanganate treatment is sufficient for reducing background staining.
Our study also confirmed that longer formalin fixation did not influence the results of the simplified Gallyas method and weaker PFA fixation resulted in more non-specific reactions. Excessive methylene bridges formed by formalin are considered to be less influential.16
In conclusion, our simplified Gallyas method, skipping lanthanum nitrate is easy for performance and will promote the use of this staining in the laboratories for general pathology.
Acknowledgments
This research was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan and an Intramural Research Grant (22-7) for Neurological and Psychiatric Disorders of NCNP. We thank Drs. H. Hatsuta, T. Adachi, S. Funabe, and M. Sugiyama for helpful discussions and Mr. N. Aikyo, Ms. Y. Kimura, Ms. M. Harada, Ms. N. Naoi, and Ms. Saishoji for technical support.
References
- 1.Gallyas F. Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung. 1971;19:1–8. [PubMed] [Google Scholar]
- 2.Braak H, Braak E, Ohm T, Bohl J. Silver impregnation of Alzheimer's neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol. 1988;63:197–200. doi: 10.3109/10520298809107184. [DOI] [PubMed] [Google Scholar]
- 3.Uchihara T, Kondo H, Kosaka K, Tsukagoshi H. Selective loss of nigral neurons in Alzheimer's disease: a morphometric study. Acta Neuropathol. 1992;83:271–276. doi: 10.1007/BF00296789. Pathology and Clinical Medicine. 1994; 12:163–8. [DOI] [PubMed] [Google Scholar]
- 4.Adachi T, Saito Y, Hatsuta H, et al. Neuropathological asymmetry in argyrophilic grain disease. J Neuropathol Exp Neurol. 2010;69:737–744. doi: 10.1097/NEN.0b013e3181e5ae5c. [DOI] [PubMed] [Google Scholar]
- 5.Arima K. Brain Banking in Japan: current situation and future prospect. Neuropathology. 2010;30:309. [Google Scholar]
- 6.Gallyas F. Physicochemical mechanisms of histological silver staining and their utilization for rendering individual silver methods selective and reliable. Biotech Histochem. 2008;83:221–238. doi: 10.1080/10520290802538543. [DOI] [PubMed] [Google Scholar]
- 7.Uchihara T, Kondo H, Kosaka K. Alzheimer-type pathology in melanin-bleached sections of substantianigra. J Neurol. 1995;242:485–489. doi: 10.1007/BF00867417. [DOI] [PubMed] [Google Scholar]
- 8.Uchihara T, Nakamura A, Yamazaki M, Mori O, Ikeda K, Tsuchiya K. Different conformation of neuronal tau deposits distinguished by double immunofluorescence with AT8 and thiazin red combined with Gallyas method. Acta Neuropathol. 2001;102:462–466. doi: 10.1007/s004010100401. [DOI] [PubMed] [Google Scholar]
- 9.Uchihara T, Tsuchiya K, Nakamura A, Akiyama H. Silver staining profiles distinguish Pick bodies from neurofibrillary tangles of Alzheimer type: comparison between Gallyas and Campbell-Switzer methods. Acta Neuropathol. 2005;109:483–489. doi: 10.1007/s00401-005-0988-6. [DOI] [PubMed] [Google Scholar]
- 10.Uchihara T, Nakamura A, Mochizuki Y, et al. Silver stainings distinguish Lewy bodies and glial cytoplasmic inclusions: comparison between Gallyas-Braak and Campbell-Switzer methods. Acta Neuropathol. 2005;110:255–260. doi: 10.1007/s00401-005-1044-2. [DOI] [PubMed] [Google Scholar]
- 11.Arai N, Oda M. A variety of glial pathological structues by the modified Gallyas-Braak method. Neuropathology. 1996;16:133–138. [Google Scholar]
- 12.Lillie RD. Trichoxanthin, the yellow granular pigment of guinea pig hair follicles and hairs. J Histochem Cytochem. 1957;5:346–353. doi: 10.1177/5.4.346. [DOI] [PubMed] [Google Scholar]
- 13.Perdrau JR. The silver reduction method for the demonstration of connective tissue fiber. J Pathol. 1921;24:117. [Google Scholar]
- 14.Kitoh T, Matsushita M. A new staining method of astrocytes for paraffin section. Acta Neuropathol. 1980;49:67–69. doi: 10.1007/BF00692222. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto A, Higashi N. An Actual Electron Microscopy Study (in Japanese) 2nd edn. Tokyo: Kindai; 1982. The Marker Introduction Method for Ultrastructure Fixation; pp. 123–125. [Google Scholar]
- 16.Namimatsu S, Sugisaki Y, Tsutiya S. A New Antigen Retrieval Method (6) (in Japanese with English abstract) Nichiidai Igaku Zasshi. 2010;6:178–184. [Google Scholar]


