Abstract
A large-arena bioassay is used to examine sex differences in spatiotemporal patterns of bed bug Cimex lectularius L. behavioural responses to either a human host or CO2 gas. After release in the centre of the arena, 90% of newly-fed bed bugs move to hiding places in the corners within 24 h. They require 3 days to settle down completely in the arena, with generally low activity levels and the absence of responses to human stimuli for 5 days. After 8–9 days, persistent responses can be recorded. Sex differences are observed, in which females are more active during establishment, respond faster after feeding, expose themselves more than males during the daytime, and respond more strongly to the host signal. The number of bed bugs that rest in harbourages is found to vary significantly according to light setting and sex. Both sexes stay inside harbourages more in daylight compared with night, and males hide more than females during the daytime but not during the night. The spatial distribution of the bed bugs is also found to change with the presence of CO2, and peak aggregation around the odour source is observed after 24 min. Both male and female bed bugs move from hiding places or the border of the arena toward the centre where CO2 is released. Peak responses are always highest during the night. Bed bug behaviour and behaviour-regulating features are discussed in the context of control methods.
Keywords: Attraction, bed bug, Cimex lectularius, host location, olfaction, trap
Introduction
Bed bugs (Cimex lectularius L., Hemiptera; Cimicidae) are closely connected to their host. Each nymphal stage must have a blood meal to proceed in its life cycle, and adults need blood to successfully produce offspring (Usinger, 1966; Reinhardt & Siva-Jothy, 2007). During feeding, the bed bugs increase their mortality risk by exposing themselves to the host when leaving their concealed and safe harbourages. The time spent on or around the host is therefore minimized, and the bed bugs remain hidden from humans for most of their lives. This cryptic way of living is one of the major challenges in bed bug control because it reduces the chances of bed bug detection and makes the efficient application of killing agents difficult. Bed bugs are currently increasing worldwide (Davies et al., 2012). Improved control strategies are needed to overcome pesticide resistance (Romero et al., 2007; Zhu et al., 2010; Kilpinen et al., 2011; Tawatsin et al., 2011) and cope with the bed bugs' concealed biology (Weeks et al., 2010; Koganemaru & Miller, 2013). Increased knowledge about bed bug behaviour is necessary to find potential bed bug weaknesses that can be exploited to develop efficient integrated pest management solutions successfully.
Although generally considered to be solitary, bed bug behaviour consists of subsocial elements, such as aggregation and alarm signals (Levinson et al., 1974; Siljander et al., 2008; Olson et al., 2009). The bugs also benefit from nesting activity, in which high-density accumulations reduce water loss (Benoit et al., 2007). Odours from blood secretion, faeces and exuviae combine with residues from the alarm signals to produce volatile cues that, in conjunction with tactile stimuli, allow bed bugs to aggregate in their nests after feeding on their host (Siljander et al., 2008; Olson et al., 2009; Domingue et al., 2010). Bed bug host location behaviour also depends on olfactory cues (Anderson et al., 2009; Suchy & Lewis, 2011; Harraca et al., 2012; Singh et al., 2012) and the bed bug's life history appears to be governed by an assembly of volatiles that, in conjunction with heat (Anderson et al., 2009; Reis & Miller, 2011; Singh et al., 2012), regulate movement and resting in the population.
Awareness of the complexity of olfactory signals (Bruce et al., 2005; Logan & Birkett, 2007; Lazzari, 2009) and increased knowledge of olfactory-mediated bed bug behaviour (Siljander, 2006; Weeks et al., 2010) may be utilized as tools to improve the efficiency of control methods (Benoit et al., 2009). In many other pest situations, semiochemicals are used for early detection, push–pull strategies, mass trapping, luring and killing strategies, luring and infecting strategies, and mating disruption (Agelopoulos et al., 1999; El-Sayed et al., 2006, 2009; Cook et al., 2007; Witzgall et al., 2010). In this regard, the spatiotemporal patterns of bed bug questing, together with descriptions of life stages and sex-specific responses to different semiochemicals, need to be considered to time and direct treatments appropriately.
Some disparities in bed bug activity and movement are known across sexes and between life stages (Pfiester et al., 2009; Domingue et al., 2010; Romero et al., 2010; Weeks et al., 2011) but sex differences in host perception have not been investigated, although this is considered an important applied aspect of semiochemical control (El-Sayed et al., 2006).
Laboratory behavioural studies are integral parts of the development of semiochemical control methods, and bioassays should allow the insects to act in a nearly natural way. This is difficult to achieve with bed bugs, however, because removal from stock cultures, handling and transfer to the bioassay all clearly interfere with the concealed biology of the insect and may consequently influence their responses. Behavioural studies describing bed bug activity are often performed in Petri dishes or in small-scale experimental arenas where behaviour is measured during a limited time span (Olson et al., 2009; Pfiester et al., 2009; Romero et al., 2010; Weeks et al., 2011, 2013; Harraca et al., 2012). These experimental set-ups are valuable tools for identifying basic behavioural elements, although a low spatiotemporal scale may make field application of the results more difficult. Larger-scale arena trials, which more closely mimic a natural indoor bed bug pest situation, provide information about the attraction potential of CO2 and chemical lures (Anderson et al., 2009; Wang et al., 2009, 2013; Singh et al., 2012, 2013).
To describe fully natural bed bug responses, all regulating factors need to be considered, and a bioassay should allow bed bugs to behave as naturally as possible. In the present study, a large-scale arena bioassay is described, which is used to identify elements that are necessary to produce quantifiable behavioural responses to host signals. Sex and day–night differences in responses are described, and the effect of time subsequent to feeding on the level of responsiveness is investigated. Using the arena bioassay, detailed measures of the bed bugs' response profiles are provided when stimulated by volatiles from a human. Responses to a pure CO2 point source are also investigated to describe its effects on spatial distribution in the arena.
Materials and methods
Insects
The bed bug stock culture was collected in 2009. The initial population consisted of 40 adult specimens that originated from a hotel in Oslo, Norway. The cultures were maintained in 140-mL polyethylene boxes (height 7 cm, inner diameter 5 cm) that contained folded paper towels to provide harbourages and allow the bed bugs to move, mate and lay eggs. All of the cultures were maintained under an LD 15 : 9 h light cycle at 20–22 °C and 50–60% relative humidity. The stock cultures were fed both artificially and on rodents (Aak & Rukke, 2014) until the experiments were performed in 2012.
Bed bug arena
The experimental arena (Fig. 1) was placed in a 15-m2 room (3 × 5 m2) without windows. The room was air-conditioned and maintained at a mean ± SE temperature of 22.3 ± 0.1 °C and a relative humidity of 31.7 ± 3.3%. The room was only furnished with a chair and desk, and the walls and ceiling were white or grey to minimize visual cues. All of the light sources were positioned directly above the arena and consisted of a set of eight Plexiglas tubes (diameter 1.2 cm, length 110 cm), each with 96 light-emitting diode (LED) lights positioned 1 cm apart (8 W, Northlight LED; Clas Ohlson, Norway) and four infrared (IR) lamps (Ecoline IR illuminator, TV6700; ELFA, Norway). The eight tubes were placed in a grid fashion and the 768 LED lights were directed toward the arena. The four IR lamps were directed toward the ceiling. This lighting set-up provided shadow-free and even light conditions in the arena to facilitate night and day observations of the bed bugs. Half of the Plexiglas tubes were coated with red plastic foil. Day was simulated by turning all of the light sources on. Night was simulated by turning on only the red tubes and IR lights. The light cycle was maintained at LD 15 : 9 h to keep the conditions similar to those commonly found in a bedroom and synchronized with the rearing facilities. The dark cycle began at 15.00 h and lasted until 00.00 h. A day- and night-vision Internet Protocol camera (Vivotek, FD 8361; Multicom, Norway) was used to record a 1200 × 1600 pixel video with five frames per second. Sharp night videos were obtained using the built-in IR-cut filter and iris technology. vivotek, version 1.00 (Multicom) was used as recording software.
Fig. 1.
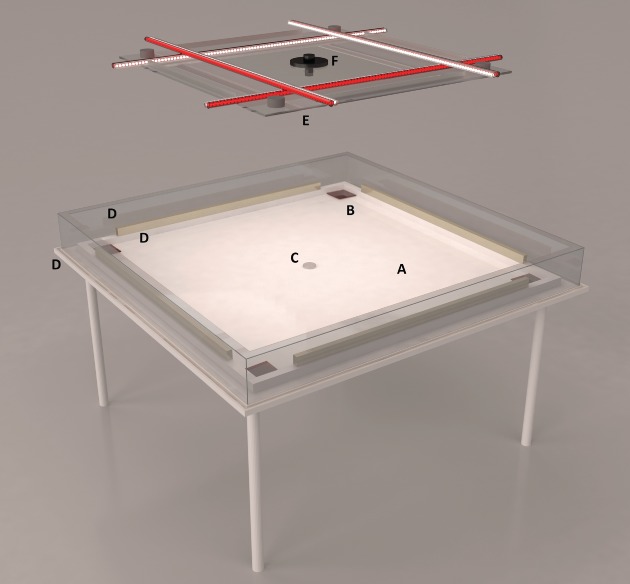
Three-dimensional rendering of the bed bug behavioural assay arena. (A) Area of movement. (B) Harbourages. (C) CO2 release point. (D) Safety barriers (polished plastic wall with brass weights, Plexiglas wall, insect glue-coated overhang and mineral oil-filled duct). (E) White, red and infrared light sources. (F) Vivotech Internet Protocol camera.
The bed bug arena was placed 90 cm above the floor. The arena base was constructed from a 2-cm thick white plastic plate (Polyoksymetylen, copolymer: 30 kg m−2; Plastkompaniet A/S, Norway) that measured 150 × 150 cm2. A thick Plexiglas wall (height 15 cm, width 2 mm), with a 2-cm insect glue-coated overhang, surrounded the arena. As an additional safety measure to prevent bed bug escape, the wall was bordered by a duct filled with mineral oil. An inner frame constructed from polished hard plastic defined the borders of the area where the bed bugs could move (130 × 130 cm2). This frame was held in place by heavy brass rods that also weighed down and kept in place one white piece of paper (134-white seamless background paper, 175 g m−2; Fotoexperten, Norway) that covered the arena floor and provided grip for the moving bed bugs. In six experimental series, the arena was used in its entirety. In two experimental series, the inner part of the arena was split in two halves (130 × 65 cm2) by an additional plastic wall. Harbourages, made from transparent dark-red Plexiglas (10 × 10 cm2) (Plexiglas, GS268; Plastkompaniet A/S), were positioned in each of the corners of the arena. The Plexiglas harbourage had a corner that faced the centre of the arena, elevated approximately 3 mm to allow the bed bugs to move underneath them to hide.
Experimental protocol
Fifth-instar nymphs were obtained from the stock culture and fed on rodents until fully engorged. This provided newly-emerged adults after approximately 2 weeks. Males and females were kept together in this period and mating was assumed to occur. The emerged adults were sexed by registration of the genitalia and offered a rodent blood meal. Immediately after feeding, equal numbers of fully engorged male and female bed bugs were transferred to the experimental room and released in the arena. The room was left empty on the initial day to allow the bed bugs to establish themselves without interference. During the experimental series, human stimulation was performed by one individual who entered the room and sat in a chair next to the bed bug arena or by CO2 that was presented to the bed bugs by placing a small Petri dish (diameter 55 mm) containing a mean ± SE of 17.1 ± 0.2 g dry ice (sublimation rate of 327 ± 7 mg min−1) in the middle of the arena. The centre of the complete arena was used as release point in both split- and mixed population (described below). Each of the stimulations lasted 30 min. As a control for human stimulation, the room was used with no humans in it. As a control for CO2 stimulation, an empty Petri dish was used. Experimental bed bugs were only used in one 14-day series and were killed by freezing upon completion of the experiment.
To describe the response of the bed bugs to their natural human host, five males and five females were initially used in the arena (mixed population). From the second day of experimentation, a human entered the room every third hour from 07.00 to 22.00 h to provide a total of six stimulations per day (Table 1). Three stimulations occurred during the day and three occurred at night. This experiment lasted for 14 days and was repeated three times. As a control, three additional 14-day experimental series were performed with no stimulation in the room.
Table 1.
Experimental set-up of the day/night regimen for the study of bed bug (Cimex lectularius) behaviour, with methods and periods of stimulation in the test arena in mixed and split populations
| Mixed population |
Split population |
||||
|---|---|---|---|---|---|
| Stimuli | Human (Days 2–14) | Control (Days 2–14) | Human (Days 2–4) | Human (Days 5–9) | CO2/empty Petri disha (Days 10–14) |
| 00.00–07.00 h | ○ | ○ | ○ | ○ | ○ |
| 07.00–07.30 h | • | ○ | ○ | ○ | ○ |
| 07.30–08.00 h | ○ | ○ | ○ | ○ | ○ |
| 08.00–10.00 h | ○ | ○ | ○ | ○ | ○ |
| 10.00–10.30 h | • | ○ | • | • | ▪ |
| 10.30–11.00 h | ○ | ○ | ○ | ○ | ○ |
| 11.00–13.00 h | ○ | ○ | ○ | ○ | ○ |
| 13.00–13.30 h | • | ○ | ○ | • | ⓪ |
| 13.30–14.00 h | ○ | ○ | ○ | ○ | ○ |
| 14.00–16.00 h | ○ | ○ | ○ | ○ | ○ |
| 16.00–16.30 h | • | ○ | ○ | • | ▪ |
| 16.30–17.00 h | ○ | ○ | ○ | ○ | ○ |
| 17.00–19.00 h | ○ | ○ | ○ | ○ | ○ |
| 19.00–19.30 h | • | ○ | ○ | • | ⓪ |
| 19.30–20.00 h | ○ | ○ | ○ | ○ | ○ |
| 20.00–22.00 h | ○ | ○ | ○ | ○ | ○ |
| 22.00–22.30 h | • | ○ | ○ | ○ | ○ |
| 22.30–23.00 h | ○ | ○ | ○ | ○ | ○ |
| 23.00–24.00 h | ○ | ○ | ○ | ○ | ○ |
| Repetitions | 14 days | 14 days | 3 days | 5 days | 5 days |
| n = 3 | n = 3 | n = 2 | n = 2 | n = 2 | |
The order of CO2/empty Petri dish was alternated daily.
White represents day (white, red and infrared lights on) and grey shading represents night (only red and infrared lights on).
○, no stimuli; •, human presence; ⓪, empty Petri dish; ▪, Petri dish with dried ice to release CO2.
To investigate sex differences, the bed bug arena was split into two halves. Ten males were released in one half and 10 females were released in the other half (split population). From the second day of experimentation until day 4, host-initiated activity was not expected, and the bed bugs were stimulated only once by a human in the daylight (Table 1). Subsequently, from days 5 to 9 in the experimental series, a human entered the room every third hour from 10.00 to 19.00 h to provide a total of four stimulations per day, 3 h apart. Two stimulations occurred during the day and two stimulations occurred during the night. From days 10 to 14, the four daily human stimulations were replaced by either CO2 or an empty Petri dish (i.e. control) (Table 1). The daily order of CO2 stimulation and the control were alternated to achieve an overall balance between which treatment being introduced first during both the day and night. The split population experiment was repeated twice.
Video analysis and quantification of behaviour
In all of the experiments, the bed bugs and their activity in the arena were video-recorded continuously. When analysing the video files, behaviour was scored for 1 min every 6 min. This gave a total of 10 min of recording every hour and a total of 5 min of recording during stimulation. During each 1 min of recording, the total number of bed bugs that occupied the open spaces of the arena, the number of bed bugs that rested in their harbourages and the cumulative number of bed bug individuals that moved during the 1-min period of observation were counted. In the split series, the spatial distribution of the bed bugs was investigated when the CO2 or empty control Petri dish was presented as a point source. Changes in the bed bugs' positions were analyzed by dividing the entire arena into an 8 × 8 square grid. The number of individuals positioned in each of the 64 squares (16 × 16 cm2) was counted in the freeze frame at the beginning of each minute of recording (i.e. 6 min before stimulation, every sixth minute during stimulation and 18 min after stimulation).
Statistical analysis
Data were analyzed using sigmaplot, version 12 (Systat Software, San Jose, California). Data were checked for normality and multiple comparisons were performed using analysis of variance (anova). Pairwise comparisons were performed using t-tests or paired t-tests. P < 0.05 was considered statistically significant. Differences between multiple comparisons were assessed using Tukey's test. If the tests of normality failed, then the nonparametric Mann–Whitney test, Mann–Whitney rank sum test or Kruskal–Wallis anova were used.
Results
General activity
No response to human stimulation was observed during the initial period of observation, which was considered as a period of acclimation in the arena and excluded from further analysis. A generally higher level of activity was observed among females compared with males, and the split populations were two- to three-fold more active than the mixed populations during the initial 3 days (Fig. 2). Almost all of the bed bugs (90.0 ± 4.9%) moved from the open spaces of the arena where they were released to the hiding places in the corners within 24 h.
Fig. 2.
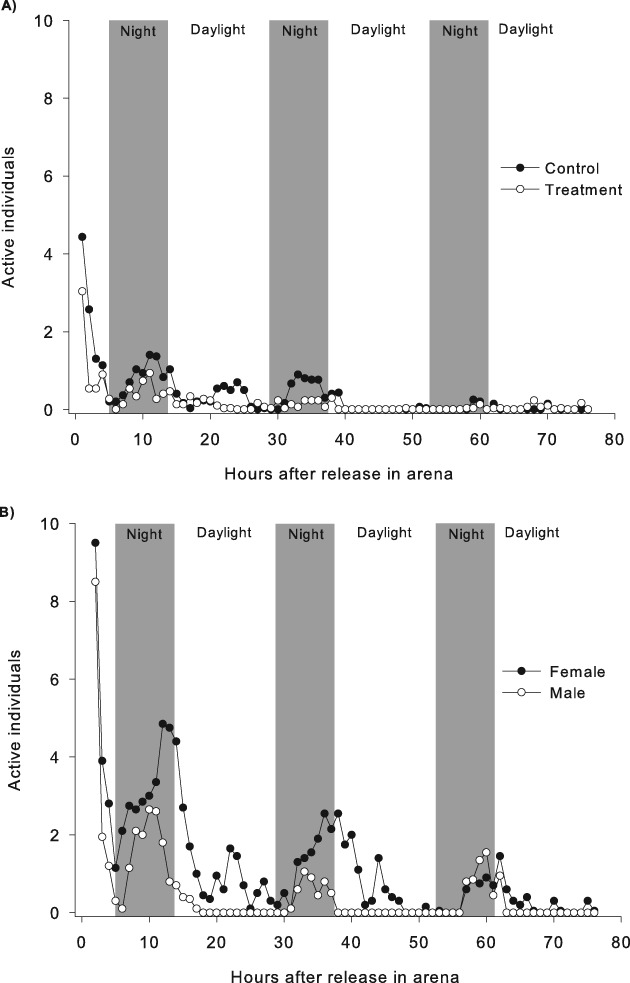
Cimex lectularius activity for 3 days after release in the arena. A mixed population (A) of five male and five female bed bugs and a split population (B) of 10 male in one half of the arena and 10 females in the other.
Overall activity in the control series with no stimulation was low. A mean ± SE of 0.02 ± 0.01 individuals moved during the 10-min observation period per hour in daylight and 0.62 ± 0.05 individuals moved at night. The bed bugs were active according to the light regimen, and distinct activity peaks could be observed during the night (Fig. 3A) when the maximum number of individuals that moved within a 1-min observation period was five out of 10. Overall activity during the human stimulation series was significantly higher than in the control series, in which 0.82 ± 0.07 individuals moved in daylight (Mann–Whitney rank sum test: t = 85 075, P < 0.001) and 1.43 ± 0.13 individuals moved at night (Mann–Whitney rank sum test: t = 74 870, P < 0.001). In the stimulation series, spikes of activity related to human presence could be observed during both the night and day (Fig. 3B). Maximum activity was reflected by the movement of all 10 individuals within a 1-min period of observation. This occurred occasionally at night, either during human stimulation or immediately after the presence of a host in the room.
Fig. 3.
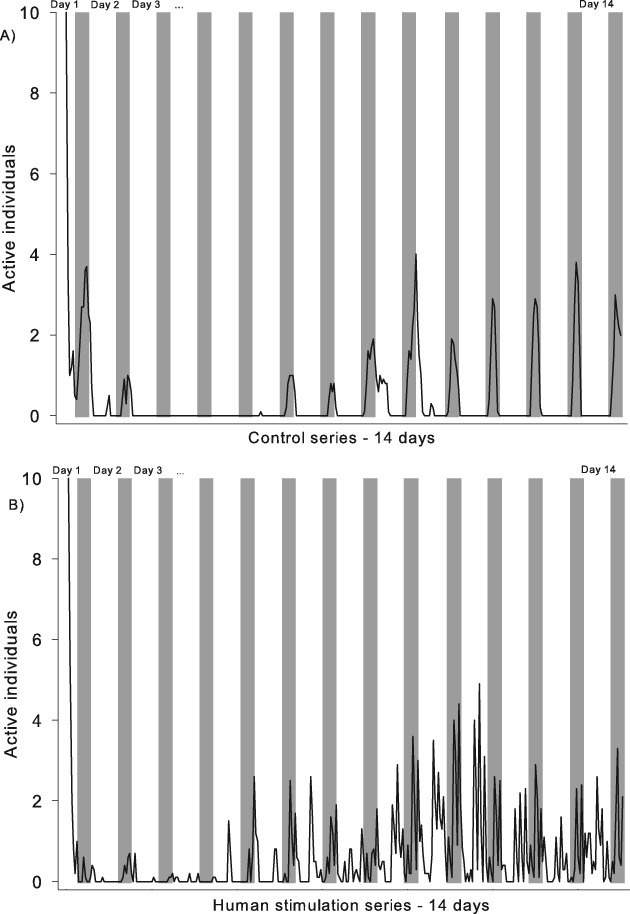
Example of typical recordings of Cimex lectularius activity in a mixed population of five males and five females during a 14-day experimental period. (A) Control series without stimuli. (B) Human stimulation series with spikes of activity related to human presence. The grey background indicates night, and the white background indicates lights on.
Responses to host signals
A marked change in activity occurred in the mixed populations on day/night 9 in response to a human presence (Fig. 4A, B). At night, no difference was found between the control and a human presence from days 4 to 8 (paired t-test: t = 0.78, P = 0.480), whereas activity was significantly higher with a human presence from day 9 onward (paired t-test: t = 12.84, P < 0.001; Fig. 4A). During daylight, activity in the control series was close to 0, and a human presence significantly increased activity during both periods (paired t-test: days 4–8, t = 3.53, P = 0.012; days 9–13, t = 6.69, P = 0.003; Fig. 4B). In the series in which males and females were separated, the level of female activity during human stimulation gradually increased until day 8, whereas male activity was low. Females always moved more than males but only significantly more on day 8 (Mann–Whitney rank sum test: t = 773.5, P < 0.001; Fig. 4C) and day 9 (Mann–Whitney rank sum test: t = 740.5, P = 0.002; Fig. 4C). When the human presence was replaced with CO2, this sex difference in responsiveness persisted, although males appeared to gradually increase their activity throughout the last 10 days of the experiments to a level similar to that of females. A significant sex difference in response to CO2 was found only on day 10 (Mann–Whitney rank sum test: t = 700.5, P < 0.020; Fig. 4C).
Fig. 4.
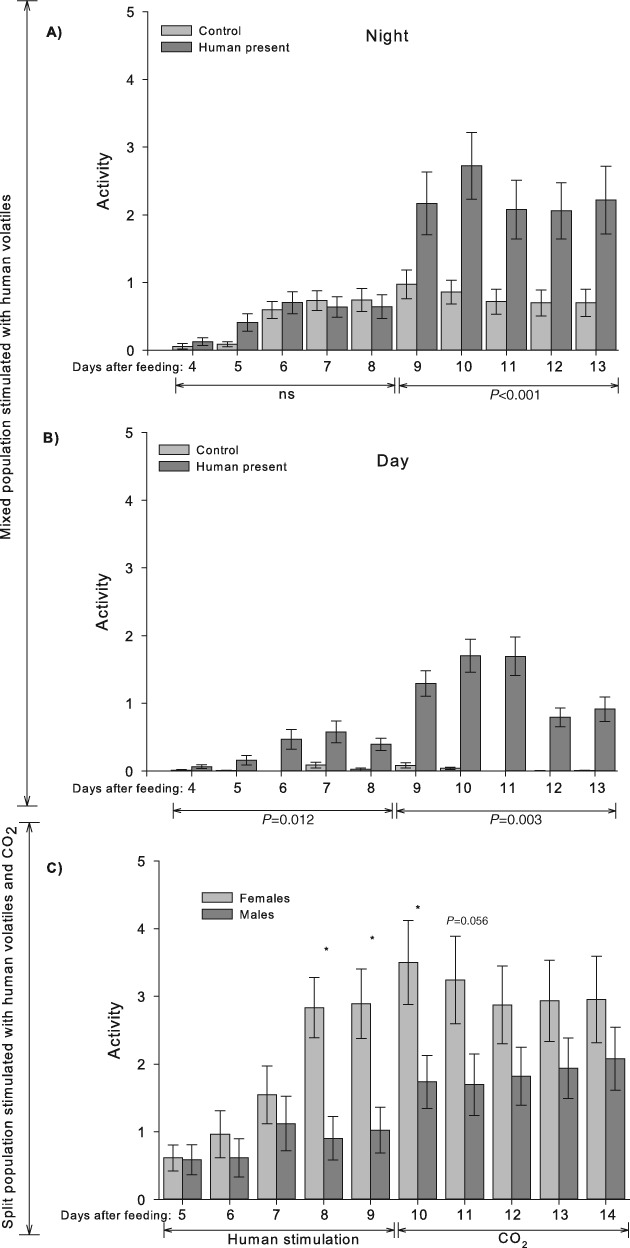
Activity (mean ± SE) of Cimex lectularius during human stimulation of mixed populations of five males and five females (A) at night and (B) in daylight and (C) during human stimulation from days 5 to 9 and CO2 stimulation from days 10 to 14 of populations with 10 males and 10 females in the each half of the arena.
When the stimulation events were divided into 6-min intervals, a distinct response was observed that began when the host entered the room and when dry ice was placed in the arena. Activity increased continuously until the stimulus was removed and then gradually decreased during the next hour. Peak responses were always the highest during the night. In the mixed population, the response profile appeared to be similar, regardless of night or day, although it was less prominent from days 4 to 8 compared with the interval from days 9 to 13 (Fig. 5A, B). In the split series, only one of five tests exhibited a significant sex difference in response to CO2, and the data were pooled across sexes to create a response profile (Fig. 5C). CO2 stimulation appeared to be similar to a human odour source with regard to the temporal change in activity, although CO2 stimulation produced more responders. Night stimulation in the split series occurred during the first half of the night, and a general increase in activity was observed in both the control and stimulation conditions during this period (Fig. 5C).
Fig. 5.
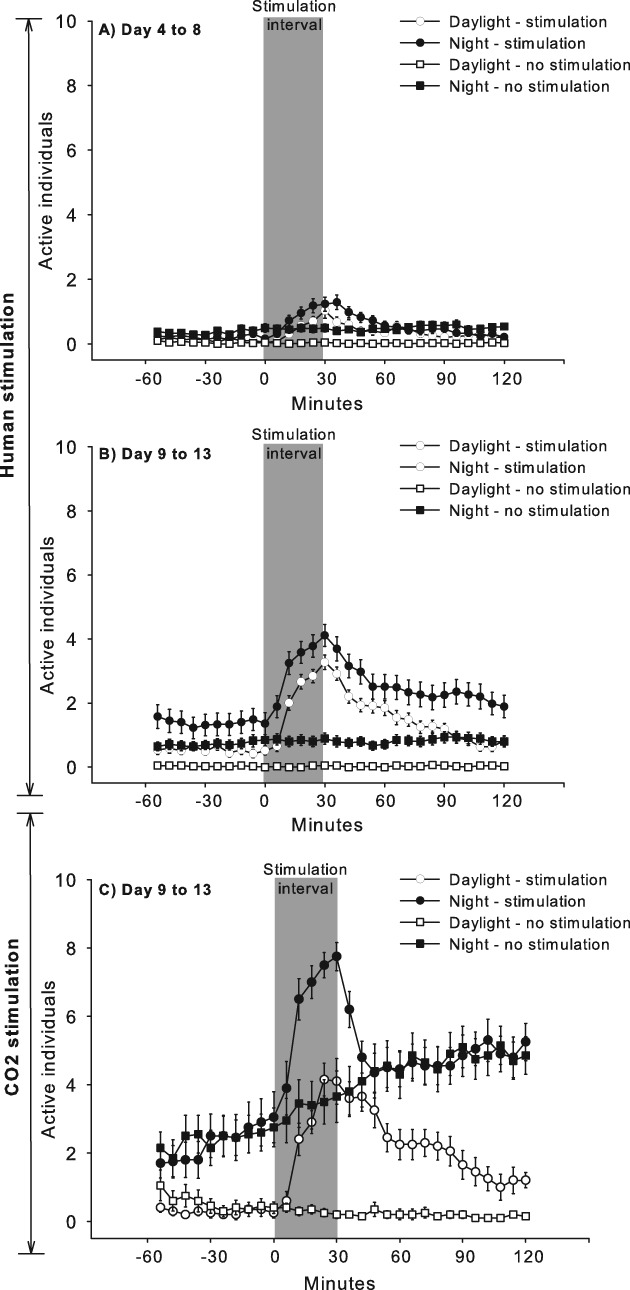
Mean ± SE number of active Cimex lectularius during stimulation (grey area) with (A) humans on day 4–8 after feeding, (B) humans on day 9–13 after feeding and (C) CO2 on day 9–13 after feeding.
Spatial distribution
Six minutes before stimulation, the number of bed bugs that rested in the four squares that contained a harbourage varied significantly according to the light setting and sex (Kruskal–Wallis anova on ranks: H = 36.092, d.f. = 3, P < 0.001). Tukey's post-hoc test revealed that both sexes positioned themselves more in the harbourages in the daylight compared with the night, and males hid more than females during the daytime. The same trend was observed during the night, although significant differences were not detected (Fig. 6). The spatial distribution of the bed bugs also changed with the presence of CO2, and peak aggregation in the four centre squares that surrounded the odour source was observed after 24 min (Fig. 7). Compared with the control series, a significant increase in the number of individuals that resided in the centre squares was observed after 6 min (Mann–Whitney rank sum test: t = 515.5, P = 0.002, or P < 0.001 for all tests after 6 min; Fig. 7). Both males and females moved from the harbourages or border of the arena toward the centre, whereas the control treatments maintained the initial distribution. During peak aggregation at 24 min, females comprised 63% of the animals during the daytime and 44% during the night. The maximum number of bed bugs observed around the CO2 source during a single stimulation was 12 of 20 individuals. After removal of the CO2 source, the bed bugs again successively dispersed from the centre of the arena.
Fig. 6.
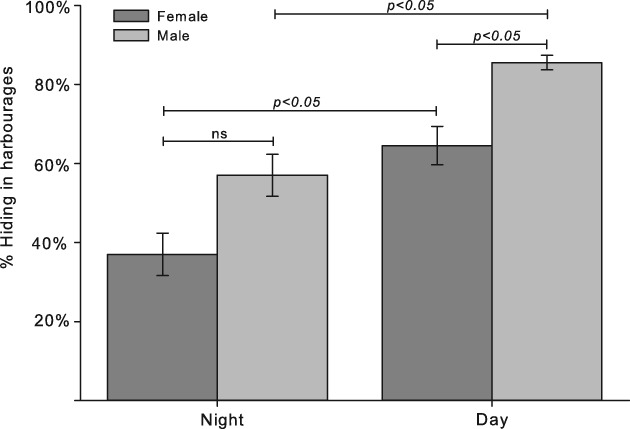
Percentage (mean ± SE) of 10 male and 10 female Cimex lectularius that hid in the corner harbourages of the arena during the day and night 6 min before stimulation.
Fig. 7.
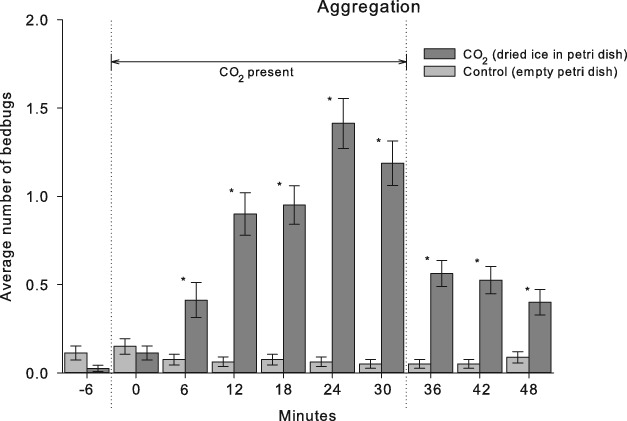
Aggregation of 20 Cimex lectularius (mean ± SE) at different times around an odour source of dry ice (solid CO2) in a Petri dish placed in the middle of the arena. The Petri dish was removed at after 30 min. An empty Petri dish was used in the control treatment. *P < 0.05 significant difference in aggregation between stimulation and control.
Discussion
The behaviour observed in the bed bug arena is consistent with bed bug habits that are described previously from both field and laboratory studies. Activity peaks occur during the night (Romero et al., 2010), with a lack of activity after feeding (Usinger, 1966; Reis & Miller, 2011), modulated movement with the presence of a host signal (Anderson et al., 2009; Harraca et al., 2012) and only a low level of spontaneous movement in daylight (Romero et al., 2010). These behavioural responses are in agreement with bed bug biology in an urban setting. The large arena used in the present study and the use of a 14-day behavioural series appear to mimic natural conditions and may represent a tool for studies that seek to improve field applications in pest control.
The present study finds sex differences in host signal responses. Females expose themselves more than males during the day, respond more strongly to the host signal and respond earlier in terms of the time subsequent to feeding. Female egg production will sequester proteins and other elements needed for metabolic processes (Boggs, 2009) and male sperm may be produced and remain viable for a longer time without high metabolic cost. The recorded day-to-day change in the level of responsiveness agrees well with such a nutritional demand scenario because the onset of female activity directed toward a host appears after resource-draining egg production occurs (after 4–8 days; Usinger, 1966). The males' gradual increase in activity may be linked to a more fixed metabolic rate. Fitness profit connected to dispersal strategies is an additional explanation of the sex differences. Bed bugs are known to walk long distances in urban environments, and this behaviour occurs mostly in adults (Wang et al., 2010). The sex ratio among dispersing individuals is partially described (Pfiester et al., 2009; Domingue et al., 2010), although the efficient colonization of new habitats by single individuals clearly requires a fertile and inseminated female. Together with the potential location of new habitats, the location of new harbourages for offspring around the present host may be an additional benefit. The distribution of eggs at several locations may be a part of a bet-hedging strategy among females to reduce the total risk of exposure and offspring mortality (Hopper, 1999). Males, by contrast, have no opportunity to recolonize new areas alone and must do this indirectly by mating with females. The chances of finding a new host and a female at the same time may be so low that their optimum strategy is to await mating opportunities in the established nest.
The observed response patterns have implications for the field application of killing agents. Because females lay eggs, they are the main target for control and should be the focus of population suppression and eradication (El-Sayed et al., 2006). Most females in the present study remain rather inactive for 5–7 days, even with stimuli present. Such a low level of activity is problematic in terms of exposing bed bugs to killing agents outside their resting places. Because of the passivity of bed bugs immediately after feeding (Usinger, 1966), a reasonable strategy will be to leave rooms empty for some days before the application of pesticides or desiccant dusts. This may prevent bed bugs from remaining hidden when the killing agents are most potent. It is not expected that natural populations are as synchronized in feeding, as in the present study, and frequent and almost continuous feeding and egg laying may occur within a population (Reinhardt et al., 2010; Pereira et al., 2013). Therefore, if artificial host signals are to be utilized efficiently in pest management, then the time subsequent to the last feeding and overall hunger state of the population needs to be considered. Waiting at least 5 days before applying killing agents may increase the blood lust of a larger proportion of the population to spur the activity of as many individuals during as few stimulation events as possible. Shortening the passive time of the bed bugs might also be possible by raising room temperatures to increase metabolism, the need for a host and questing activity during artificial stimulation (Reinhardt et al., 2010). Such approaches must be weighed against an increase in egg production and potential hunger-mediated dispersal to adjacent rooms or out of apartments (Wang et al., 2010).
Regardless of when treatment is performed or how it is connected to the feeding habits of the resident population, an effect of CO2 on the activity level of the bed bugs is reported in the present study. Relatively inexpensive and simple activators, such as CO2 released from dry ice, regulated tanks or sugar-yeast solutions, can thus be utilized to improve control (Singh et al., 2013). CO2 is also used to activate bed bugs to make them move across treated substrates (Wang et al., 2013). The present study shows that this single component may be sufficient to manipulate their behaviour to lure them out from their harbourages. The similar response profiles to humans and CO2 also indicate that CO2 is the major component of the host signal. The addition of other attractive host compounds (Anderson et al., 2009; Harraca et al., 2012) may further improve such effects. Ideally, all behaviourally active host compounds should be included in the odour replica to lure as many bed bugs as possible into questing and exposing themselves. Currently, full activation can only be achieved with a natural host (i.e. humans), although using natural bait is ethically questionable because many killing agents are toxic to humans, and even residual pesticides may have a negative effect on the health of the residents (Mostafalou & Abdollahi, 2013). This means that, during treatment with potential harmful substances, an artificial host signal is needed to activate the bed bugs to ensure contact with the killing agents. Host signals also appear to more strongly influence egg-laying females, which are an important focus of control efforts.
To identify the full host signal, more behavioural studies are needed, and bioassays that are capable of quantifying bed bug responses should be further developed. Interestingly, in the present study, the relative response in terms of activated animals compared with the control condition is largest with the lights on. In daylight, the stimulation produces a peak response with a more than 10-fold increase in activity, whereas activity is only four-fold higher at night. Significant differences between a human presence and control conditions are also detected earlier (i.e. on days 4–8) only when the lights are on. In terms of measuring differences in odour-released activity, experiments may be performed in the daylight to distinguish small responses from general night-time movements in the arena. Both sexes also hide more when experiencing daylight. This facilitates the detection of activity when they leave their harbourages. This rather surprising result may be influenced by the use of habituated laboratory animals that normally feed in the daylight. However, the clear interactions between sex, light setting and time subsequent to feeding should be considered when designing bed bug bioassays.
Arenas, such as the one described in the present study, appear to be powerful tools for understanding the dynamics of the questing and nesting activity of bed bugs. The current arena contains hiding places. Because the arena is not cleaned between the experimental series, the harbourages likely contain attractant and arresting odours. The human odours are presented in a natural way by simply entering the room, and this triggers a fairly strong and persistent bed bug response. This allows quantification and comparisons with artificial cues. The bed bugs are also found to locate the point source of CO2 quite efficiently when positioned in the centre of the arena, showing that this single component is sufficient to allow spatial orientation and host location in both daylight and darkness. No indication is observed of directional movement towards the host that was positioned outside of the arena during stimulation. In addition to providing knowledge for improving control methods, such studies can be used to test, evaluate and improve the efficiency of traps and lures that are intended to monitor or suppress bed bug populations. The observed behaviour also indicates responses and orientation mechanisms in bed bugs that differ from the more rapid responses and distinct movement patterns found during optomotor anemotaxis in flying insects (Carde & Willis, 2008). The use of more detailed video tracking systems might provide valuable quantification of the behavioural mechanisms that lead to source location in crawling insects that manoeuvre in darkness. Although only representing an incremental step in determining bed bug chemical ecology and the dynamics of questing and nesting activity, the observations made in the present study may contribute to improvements in control strategies. Further experimental approaches, combined with field studies, are clearly needed to understand fully the dynamics of a growing bed bug population. Properly revealing the behaviour-regulating features of bed bugs may allow the development of more efficient control methods.
Acknowledgments
Pest control technician Thomas Riise provided us with the initial bed bug stock culture. Masters of Architecture Lina Grundstrøm and Frederic Bachman made the three-dimensional photo rendering of the bed bug arena. Special thanks are extended to senior engineers Magne Føllesdal and Eivind Arntzen for expert advice and help with building and designing the bed bug arena. We also extend our gratitude to Professor Tone Birkemoe who provided valuable comments on the manuscript.
References
- Aak A, Rukke BA. Bed bugs, their blood sources and life history parameters: a comparison of artificial and natural feeding. Medical and Veterinary Entomology. 2014;28:50–59. doi: 10.1111/mve.12015. [DOI] [PubMed] [Google Scholar]
- Agelopoulos N, Birkett MA, Hick AJ, et al. Exploiting semiochemicals in insect control. Pesticide Science. 1999;55:225–235. [Google Scholar]
- Anderson JF, Ferrandino FJ, McKnight S, et al. A carbon dioxide, heat and chemical lure trap for the bedbug, Cimex lectularius. Medical and Veterinary Entomology. 2009;23:99–105. doi: 10.1111/j.1365-2915.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Del Grosso NA, Yoder JA, Denlinger DL. Resistance to dehydration between bouts of blood feeding in the bed bug, Cimex lectularius, is enhanced by water conservation, aggregation, and quiescence. American Journal of Tropical Medicine and Hygiene. 2007;76:987–993. [PubMed] [Google Scholar]
- Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. Journal of Medical Entomology. 2009;46:572–579. doi: 10.1603/033.046.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology. 2009;23:27–37. [Google Scholar]
- Bruce TJA, Wadhams LJ, Woodcock CM. Insect host location: a volatile situation. Trends in Plant Science. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Carde RT, Willis MA. Navigational strategies used by insects to find distant, wind-borne sources of odor. Journal of Chemical Ecology. 2008;34:854–866. doi: 10.1007/s10886-008-9484-5. [DOI] [PubMed] [Google Scholar]
- Cook SM, Khan ZR, Pickett JA. The use of push-pull strategies in integrated pest management. Annual Review of Entomology. 2007;52:375–400. doi: 10.1146/annurev.ento.52.110405.091407. [DOI] [PubMed] [Google Scholar]
- Davies TGE, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Medical and Veterinary Entomology. 2012;26:241–254. doi: 10.1111/j.1365-2915.2011.01006.x. [DOI] [PubMed] [Google Scholar]
- Domingue MJ, Kramer M, Feldlaufer MF. Sexual dimorphism of arrestment and gregariousness in the bed bug (Cimex lectularius) in response to cuticular extracts from nymphal exuviae. Physiological Entomology. 2010;35:203–213. [Google Scholar]
- El-Sayed AM, Suckling DM, Wearing CH, Byers JA. Potential of mass trapping for long-term pest management and eradication of invasive species. Journal of Economic Entomology. 2006;99:1550–1564. doi: 10.1603/0022-0493-99.5.1550. [DOI] [PubMed] [Google Scholar]
- El-Sayed AM, Suckling DM, Byers JA, et al. Potential of ‘Lure and Kill’ in long-term pest management and eradication of invasive species. Journal of Economic Entomology. 2009;102:815–835. doi: 10.1603/029.102.0301. [DOI] [PubMed] [Google Scholar]
- Harraca V, Ryne C, Birgersson G, Ignell R. Smelling your way to food: can bed bugs use our odour? Journal of Experimental Biology. 2012;215:623–629. doi: 10.1242/jeb.065748. [DOI] [PubMed] [Google Scholar]
- Hopper KR. Risk-spreading and bet-hedging in insect population biology. Annual Review of Entomology. 1999;44:535–560. doi: 10.1146/annurev.ento.44.1.535. [DOI] [PubMed] [Google Scholar]
- Kilpinen O, Kristensen M, Jensen KMV. Resistance differences between chlorpyrifos and synthetic pyrethroids in Cimex lectularius population from Denmark. Parasitology Research. 2011;109:1461–1464. doi: 10.1007/s00436-011-2423-3. [DOI] [PubMed] [Google Scholar]
- Koganemaru R, Miller DM. The bed bug problem: past, present, and future control methods. Pesticide Biochemistry and Physiology. 2013;106:177–189. [Google Scholar]
- Lazzari CR. Orientation towards hosts in haematophagous insects: an integrative perspective. In: Simpson SJ, Casas J, editors. Advances in Insect Physiology, Physiology of Human and Animal Disease Vectors. Vol. 37. San Diego, California: Elsevier Academic Press Inc; 2009. pp. 1–58. [Google Scholar]
- Levinson HZ, Levinson AR, Müller B, Steinbrecht RA. Structure of sensilla, olfactory perception, and behaviour of the bedbug, Cimex lectularius, in response to its alarm pheromone. Journal of Insect Physiology. 1974;20:1231–1248. doi: 10.1016/0022-1910(74)90229-7. [DOI] [PubMed] [Google Scholar]
- Logan JG, Birkett MA. Semiochemicals for biting fly control: their identification and exploitation. Pest Management Science. 2007;63:647–657. doi: 10.1002/ps.1408. [DOI] [PubMed] [Google Scholar]
- Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicology and Applied Pharmacology. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Olson JF, Moon RD, Kells SA. Off-host aggregation behavior and sensory basis of arrestment by Cimex lectularius (Heteroptera: Cimicidae) Journal of Insect Physiology. 2009;55:580–587. doi: 10.1016/j.jinsphys.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Pereira RM, Taylor AS, Lehnert MP, Koehler PG. Potential population growth and harmful effects on humans from bed bug populations exposed to different feeding regimes. Medical and Veterinary Entomology. 2013;27:148–155. doi: 10.1111/j.1365-2915.2012.01057.x. [DOI] [PubMed] [Google Scholar]
- Pfiester M, Koehler PG, Pereira RM. Effect of population structure and size on aggregation behavior of Cimex lectularius (Hemiptera: Cimicidae) Journal of Medical Entomology. 2009;46:1015–1020. doi: 10.1603/033.046.0506. [DOI] [PubMed] [Google Scholar]
- Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae) Annual Review of Entomology. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- Reinhardt K, Isaac D, Naylor R. Estimating the feeding rate of the bedbug Cimex lectularius in an infested room: an inexpensive method and a case study. Medical and Veterinary Entomology. 2010;24:46–54. doi: 10.1111/j.1365-2915.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- Reis MD, Miller DM. Host searching and aggregation activity of recently fed and unfed bed bugs (Cimex lectularius L.) Insects. 2011;2:186–194. doi: 10.3390/insects2020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? Journal of Medical Entomology. 2007;44:175–178. doi: 10.1603/0022-2585(2007)44[175:IRITBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Romero A, Potter MF, Haynes KF. Circadian rhythm of spontaneous locomotor activity in the bed bug, Cimex lectularius L. Journal of Insect Physiology. 2010;56:1516–1522. doi: 10.1016/j.jinsphys.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Siljander E. Foraging and communication ecology of bed bugs, Cimex lectularius L. (Hemiptera: Cimicidae) American Entomologist. 2006;52:116–117. [Google Scholar]
- Siljander E, Gries R, Khaskin G, Gries G. Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. Journal of Chemical Ecology. 2008;34:708–718. doi: 10.1007/s10886-008-9446-y. [DOI] [PubMed] [Google Scholar]
- Singh N, Wang CL, Cooper R, Liu C. Interactions among carbon dioxide, heat, and chemical lures in attracting the bedbug, Cimex lectularius L. (Hemiptera: Cimicidae) Psyche: A Journal of Entomology. 2012;2012:1–9. [Google Scholar]
- Singh N, Wang CL, Cooper R. Effect of trap design, chemical lure, carbon dioxide release rate, and source of carbon dioxide on efficacy of bed bug monitors. Journal of Economic Entomology. 2013;106:1802–1811. doi: 10.1603/ec13075. [DOI] [PubMed] [Google Scholar]
- Suchy JT, Lewis VR. Host-seeking behavior in the bed bug, Cimex lectularius. Insects. 2011;2:22–35. doi: 10.3390/insects2010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae) Journal of Medical Entomology. 2011;48:1023–1030. doi: 10.1603/me11003. [DOI] [PubMed] [Google Scholar]
- Usinger RL. Monograph of Cimicidae. College Park, Maryland: The Thomas Say Foundation; 1966. [Google Scholar]
- Wang CL, Gibb T, Bennett GW, McKnight S. Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. Journal of Economic Entomology. 2009;102:1580–1585. doi: 10.1603/029.102.0423. [DOI] [PubMed] [Google Scholar]
- Wang CL, Saltzmann K, Chin E, et al. Characteristics of Cimex lectularius (Hemiptera: Cimicidae), infestation and dispersal in a high-rise apartment building. Journal of Economic Entomology. 2010;103:172–177. doi: 10.1603/ec09230. [DOI] [PubMed] [Google Scholar]
- Wang CL, Singh N, Cooper R, et al. Evaluation of an insecticide dust band treatment method for controlling bed bugs. Journal of Economic Entomology. 2013;106:347–352. doi: 10.1603/ec12259. [DOI] [PubMed] [Google Scholar]
- Weeks ENI, Birkett MA, Cameron MM, et al. Semiochemicals of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae), and their potential for use in monitoring and control. Pest Management Science. 2010;67:10–20. doi: 10.1002/ps.2024. [DOI] [PubMed] [Google Scholar]
- Weeks ENI, Logan JG, Gezan SA, et al. A bioassay for studying behavioural responses of the common bed bug, Cimex lectularius (Hemiptera: Cimicidae) to bed bug-derived volatiles. Bulletin of Entomological Research. 2011;101:1–8. doi: 10.1017/S0007485309990599. [DOI] [PubMed] [Google Scholar]
- Weeks ENI, Logan JG, Birkett MA, et al. Tracking bed bugs (Cimex lectularius): a study of the effect of physiological and extrinsic factors on the response to bed bug-derived volatiles. Journal of Experimental Biology. 2013;216:460–469. doi: 10.1242/jeb.074930. [DOI] [PubMed] [Google Scholar]
- Witzgall P, Kirsch P, Cork A. Sex pheromones and their impact on pest management. Journal of Chemical Ecology. 2010;36:80–100. doi: 10.1007/s10886-009-9737-y. [DOI] [PubMed] [Google Scholar]
- Zhu F, Wigginton J, Romero A, et al. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex Lectularius (Hemiptera: Cimicidae), populations in the United States. Archives of Insect Biochemistry and Physiology. 2010;73:245–257. doi: 10.1002/arch.20355. [DOI] [PubMed] [Google Scholar]


