Abstract
Purpose. Neutrophil-dominated airway inflammation is a key feature of progressive lung damage in cystic fibrosis (CF). Thus, reducing airway inflammation is a major goal to prevent lung damage in CF. However, current anti-inflammatory drugs have shown several limits. PI3Kγ plays a pivotal role in leukocyte recruitment and activation; in the present study we determined the effects of genetic deletion and pharmacologic inhibition of PI3Kγ on airway inflammation and structural lung damage in a mouse model of CF lung disease. Methods. βENaC overexpressing mice (βENaC-Tg) were backcrossed with PI3Kγ-deficient (PI3Kγ KO) mice. Tissue damage was assessed by histology and morphometry and inflammatory cell number was evaluated in bronchoalveolar lavage fluid (BALF). Furthermore, we assessed the effect of a specific PI3Kγ inhibitor (AS-605240) on inflammatory cell number in BALF. Results. Genetic deletion of PI3Kγ decreased neutrophil numbers in BALF of PI3Kγ KO/βENaC-Tg mice, and this was associated with reduced emphysematous changes. Treatment with the PI3Kγ inhibitor AS-605240 decreased the number of neutrophils in BALF of βENaC-Tg mice, reproducing the effect observed with genetic deletion of the enzyme. Conclusions. These results demonstrate the biological efficacy of both genetic deletion and pharmacological inhibition of PI3Kγ in reducing chronic neutrophilic inflammation in CF-like lung disease in vivo.
1. Introduction
Cystic fibrosis (CF), the most common genetic disease in Caucasian populations, results from mutations in a single gene encoding for 1480 residues transmembrane glycoprotein, the cystic fibrosis transmembrane conductance regulator (CFTR), that regulates cAMP-mediated chloride conductance at the apical surface of secretory epithelia [1, 2]. Impaired CFTR-mediated secretion of Cl- and bicarbonate results in dehydration and acidification of the airway surface liquid, which in turn causes impaired mucociliary clearance and bacterial killing. These defects trigger a progressive lung disease characterized by airway mucus obstruction, chronic neutrophilic inflammation, bacterial infection, and structural lung damage that remains the major cause of morbidity and mortality in patients with CF [3].
A growing number of in vitro and in vivo studies support the notion that chronic neutrophilic inflammation with the release of damaging neutrophil products, such as neutrophil elastase, constitutes a key risk factor in early structural lung damage and lung function decline in CF [4–6]. Neutrophilic airway inflammation is augmented after onset of chronic bacterial infection with Pseudomonas aeruginosa and other pathogens. In this context, the inflammatory response in the CF lung is nonresolving and self-perpetuating, and a vicious cycle of neutrophilic inflammation, noxious mediator release, and overwhelmed defenses amplifies inflammation, perpetuates infection and contributes to irreversible lung damage and disease progression [7–9]. Therefore, anti-inflammatory therapy, combined with antibiotic therapy, appears crucial to prevent chronic lung damage. However, traditional therapeutic strategies, as well as more recently studied anti-inflammatory drugs, have shown several limitations and limited clinical benefit [8–10]. Clearly, novel approaches have to be undertaken to provide effective anti-inflammatory therapy to CF patients. One possibility is to interfere with leukocyte trafficking into CF airways. Trafficking of leukocytes is controlled by chemotactic factors which bind to heterotrimeric G-protein-coupled receptors (GPCR) and trigger a complex set of signaling pathways inside the cell involving the generation of second messengers like phosphoinositides. Phosphoinositides are substrates of the phosphoinositide 3-kinases (PI3Ks), enzymes that catalyze the phosphorylation of the phosphatidylinositol at the 3rd position of the inositol ring. PI3Ks modulate a wide number of cellular functions such as proliferation and survival, cytoskeletal remodeling, and membrane trafficking and represent important mediators in the signaling cascade leading to the initiation of the inflammatory response [11–14]. PI3Ks can be divided in three classes (I, II, and III) based on their biochemical properties. Leukocytes express all four known isoforms of class I PI3Ks, namely, PI3K α, β, δ, and γ [14]; nonetheless PI3Kγ plays a fundamental role in leukocyte migration and function by acting as a chemokine sensor and regulating neutrophil oxidative burst, T cell proliferation, and mast degranulation. We therefore hypothesized that PI3Kγ plays a pivotal role in mediating leukocyte recruitment and activation and may thus represent a potential target for anti-inflammatory treatment to reduce neutrophilic airway inflammation and lung damage in CF. To test this hypothesis, we used transgenic mice with airway-specific overexpression of the epithelial Na+ channel (ENaC) and determined the effects of genetic deletion and pharmacologic inhibition of PI3Kγ [15–17].
2. Materials and Methods
2.1. Mice
PI3Kγ WT/βENaC-Tg (βENaC-Tg) [15–18] and PI3Kγ-deficient (PI3Kγ KO, Harlan, Italy) mice on the C57BL/6 background were intercrossed to generate βENaC-Tg/PI3Kγ KO mice. All experiments were performed in 7- to 8-week-old adult mice. βENaC-Tg, PI3Kγ KO, PI3Kγ KO/βENaC-Tg, and wild-type (PI3Kγ WT) mice were housed in a pathogen-free animal facility at the Istituto per la Ricerca e la Cura del Cancro, University of Turin, in accordance with the Institutional Animal Welfare Guidelines and Italian legislation. The animal study protocols were reviewed and approved by the Institutional Animal Ethics Committee of the Istituto per la Ricerca e la Cura del Cancro, University of Turin, Turin, Italy, and performed according to the Institutional Animal Welfare Guidelines and Italian legislation.
2.2. Assessment of Inflammatory Cells in Bronchoalveolar Lavage
Inflammatory cell numbers were assessed in the broncoalveolar fluid (BALF) of PI3Kγ WT mice and of PI3Kγ WT/βENaC-Tg, PI3Kγ KO, and PI3Kγ KO/βENaC-Tg mice. Briefly, mice from each genotype were sacrificed and BALF was then collected by lavaging lungs in situ with 3 × 1-mL volumes of PBS. After centrifugation of the BALFs, cell pellets, in 500 μL of RPMI medium, were deposited onto glass slides using a Cytospin Cytocentrifuge. Slides were then stained using the Diff-Quick system (MICROPTIC S.L., Spain) and a differential cell count was performed as previously described [19]. In addition, BALF inflammatory cells were also analyzed in mice treated with the PI3Kγ inhibitor AS-605240 [5-(quinoxalin-6-ylmethylidene)-1,3-thiazolidine-2,4-dione] (Sigma, Germany). PI3Kγ WT and PI3Kγ WT/βENaC-Tg mice were treated once daily for 3 days with the AS-605240 by intraperitoneal injection of 10 mg/kg of the drug or vehicle (0.5% carboxymethyl cellulose, 0.25% Tween) alone.
2.3. Lung Histology and Morphometry
Animals of each group were sacrificed under anaesthesia with pentobarbital (60 mg/Kg) and the lungs fixed intratracheally with buffered formalin (5%) at a constant pressure of 20 cm H2O. Lung volume (V) was measured by water displacement according to Scherle [20]. Sagittal sections of each pair of lungs were cut and stained with haematoxylin/eosin. The slides were coded to prevent bias. Morphometric evaluations included determination of the average interalveolar distance (mean linear intercept: Lm) [21] and internal surface area (ISA) estimated by the Lm method at postfixation lung volume by the formula 4V/Lm, where V is the postfixation lung volume [22]. For the determination of the Lm for each pair of lungs, 40 histological fields were evaluated both vertically and horizontally. The development of goblet cell metaplasia was evaluated by periodic acid-Schiff reaction (PAS) according to standard histological protocols [23]. The total number of cells, as well as the percentage of PAS-positive cells, was determined. The number of cells in airways that demonstrated PAS staining was determined by examining eight intrapulmonary airways per section and counting at least 3,000 cells/section. Data were reported as the percentage of positive cells per total cells.
2.4. Statistical Analysis
Statistical analyses were performed using one-way analysis of variance. Survival curves were compared using Kaplan-Meier log rank analysis. P < 0.05 was considered statistically significant and “n” represents the number of mice in each experimental group. Data are expressed as mean ± SD.
3. Results
3.1. Genetic Deletion of PI3Kγ Reduces Neutrophilic Airway Inflammation and Mortality in βENaC-Tg Mice
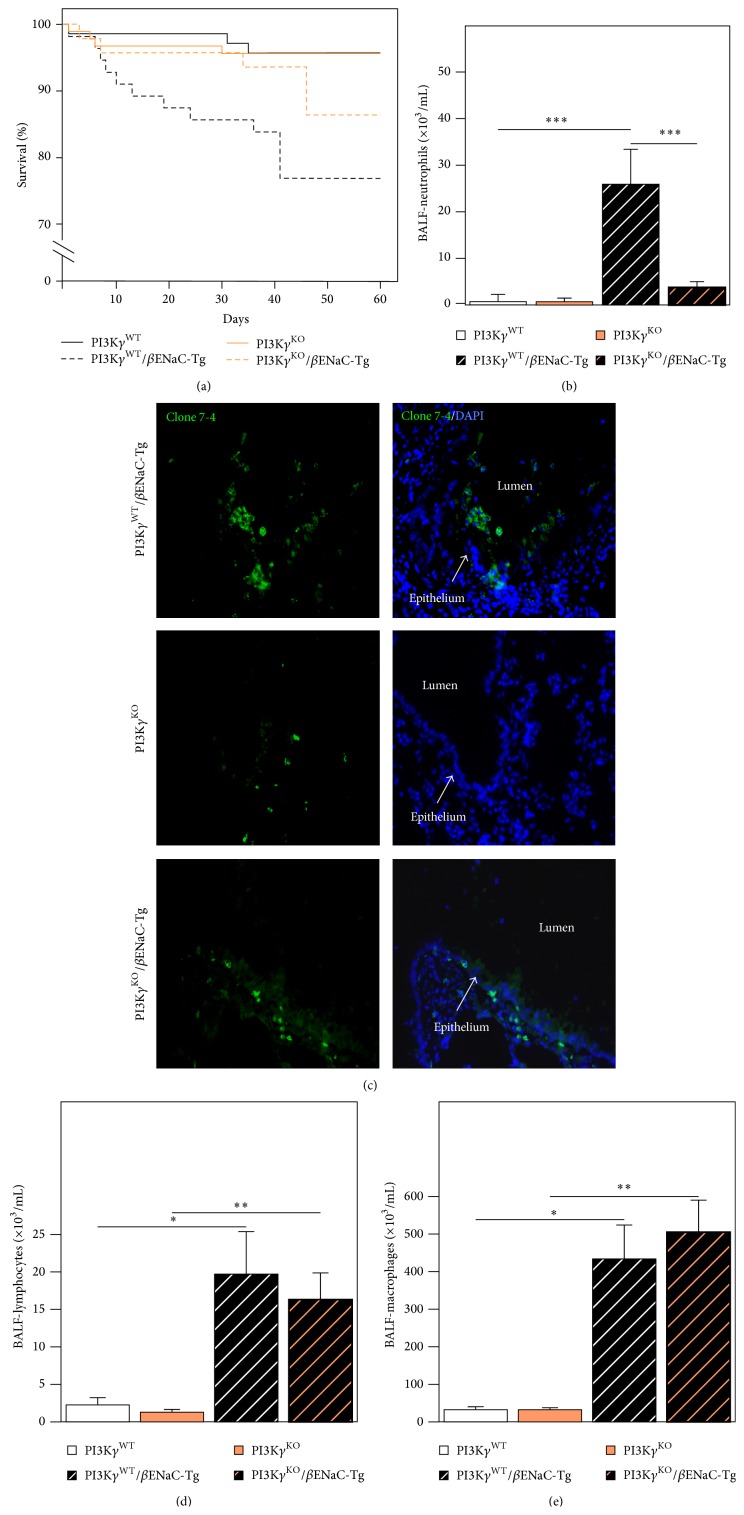
As observed in previous studies, βENaC-Tg (PI3Kγ WT/βENaC-Tg; Figure 1(a)) mice on the C56BL/6J background exhibited a spontaneous mortality of ~23% [18, 24]. Deletion of PI3Kγ had no effect on survival in wild-type mice; however, in the presence of the βENaC transgene (PI3Kγ KO/βENaC-Tg), PI3Kγ loss significantly reduced the mortality by ~50%, since at 60 days the survival rate is more than 85% (P < 0.05, Figure 1(a)).
Figure 1.
Effect of genetic deletion of PI3Kγ on mortality and airway inflammation in βENaC-Tg mice. (a) Survival curves for the different groups of mice studied (P < 0.05). (b) Neutrophil numbers were assessed in BALF of PI3Kγ WT, PI3Kγ WT/βENaC-Tg, PI3Kγ KO, and PI3Kγ KO/βENaC-Tg mice. Neutrophils are expressed as cell numbers per mL of BALF (n = 10 mice for each group). Comparison between the different groups was performed by one-way analysis of variance. *** P < 0.001 PI3Kγ WT versus PI3Kγ WT/βENaC-Tg and *** P < 0.001 PI3Kγ WT/βENaC-Tg versus PI3Kγ KO/βENaC-Tg. (c) Immunofluorescent detection of neutrophils in lung tissues of PI3Kγ WT/βENaC-Tg, PI3Kγ KO, and PI3Kγ KO/βENaC-Tg mice. Neutrophils were stained by using monoclonal rat antibodies to neutrophils (clone 7/4, Acris) and nuclei with DAPI. (d) Lymphocyte numbers were assessed in BALF of PI3Kγ WT, PI3Kγ WT/βENaC-Tg, PI3Kγ KO, and PI3Kγ KO/βENaC-Tg mice. Lymphocytes are expressed as number of cells per mL of BALF (n = 10 mice for each group) * P < 0.05 PI3Kγ WT versus PI3Kγ WT/βENaC-Tg and ** P < 0.01 PI3Kγ KO versus PI3Kγ KO/βENaC-Tg. (e) Macrophage numbers were assessed in BALF of PI3Kγ WT, PI3Kγ WT/βENaC-Tg, PI3Kγ KO, and PI3Kγ KO/βENaC-Tg mice. Macrophages are expressed as number of cells per mL of BALF (n = 10 mice for each group). * P < 0.05 PI3Kγ WT versus PI3Kγ WT/βENaC-Tg and ** P < 0.01 PI3Kγ KO versus PI3Kγ KO/βENaC-Tg.
To determine the effect of genetic deletion of PI3Kγ on airway inflammation, we compared inflammatory cell numbers in BAL fluid from surviving PI3Kγ WT/βENaC-Tg and PI3Kγ KO/βENaC-Tg mice. As expected, in homozygous PI3Kγ WT and PI3Kγ KO control mice, neutrophils were rarely detected in the BALF (Figure 1(b)) as well as in the airways lumen (Figure 1(c)). The number of neutrophils, in BALF and in the airways lumen, was markedly elevated in PI3Kγ WT/βENaC-Tg mice (Figures 1(b) and 1(c)). On the contrary, the absence of PI3Kγ expression in PI3Kγ KO/βENaC-Tg mice led to a large reduction of neutrophil recruitment into the lung if compared to PI3Kγ WT/βENaC-Tg mice (Figure 1(b)). Nonetheless, deletion of PI3Kγ did not affect macrophage and lymphocyte recruitment as no differences were detected between PI3Kγ KO/βENaC-Tg and PI3Kγ WT/βENaC-Tg mice in BALF (Figures 1(d) and 1(e)).
3.2. Genetic Deletion of PI3Kγ Reduces Structural Lung Damage in βENaC-Tg Mice
Chronic inflammation, in PI3Kγ WT/βENaC-Tg mice, triggers emphysema with distal airspace enlargement and alveolar destruction resulting in reduced lung tissue density and increased lung compliance [6, 17, 19]. To assess the protective effects of the genetic deletion of PI3Kγ on emphysema-like changes in PI3Kγ KO/βENaC-Tg mice, we determined the averaged interalveolar distance (mean linear intercept, Lm) and the internal surface area (ISA) estimated by the Lm method at postfixation lung volume. ISA and Lm were not altered in the lungs of controls PI3Kγ WT and PI3Kγ KO mice (Figures 2(a) and 2(b)), and morphological analysis showed a well-fixed normal parenchyma with normal airways (data not shown). As expected from previous studies [6, 17, 19], PI3Kγ WT/βENaC-Tg mice lungs showed significant emphysematous changes (Figures 2(a)–2(c)) while the genetic deletion of PI3Kγ in PI3Kγ KO/βENaC-Tg mice resulted in a significant reduction of the degree of emphysema, as assessed by both morphometric analyses (ISA: P < 0.0002 versus PI3Kγ WT/βENaC-Tg mice; Lm: P < 0.0003 versus PI3Kγ WT/βENaC-Tg mice; Figures 2(a) and 2(b)) and morphology (Figure 2(c)).
Figure 2.
Genetic deletion of PI3Kγ decreases emphysema in βENaC-Tg mice. Mouse lungs were fixed in 4% formalin and embedded in paraffin and 5 µm sections were stained with hematoxylin/eosin; assessment of emphysema included the internal surface area (ISA) at postfixation lung volume and the morphometric assessment of the average inter-alveolar distance (mean linear intercept: Lm). (a) ISA and (b) Lm from 8-week-old wild type (PI3Kγ WT), βENaC-Tg, PI3Kγ KO, and PI3Kγ KO/βENaC-Tg mice (n = 8–10 mice for each group). Comparison among groups was performed using one-way analysis of variance. (a) * P < 0.05 PI3Kγ WT versus PI3Kγ WT/βENaC-Tg, ** P < 0.01 PI3Kγ KO versus PI3Kγ WT/βENaC-Tg, *** P < 0.001 PI3Kγ WT/βENaC-Tg versus PI3Kγ KO/βENaC-Tg; (b) ** P < 0.01 PI3Kγ WT versus PI3Kγ WT/βENaC-Tg, and P < 0.01 PI3Kγ KO versus PI3Kγ WT/βENaC-Tg, *** P < 0.001 PI3Kγ WT/βENaC-Tg versus PI3Kγ KO/βENaC-Tg mice. (c) Representative histological sections from the lung of 8-week-old βENaC-Tg mousee (left) showing evident areas of emphysema and PI3Kγ KO/βENaC-Tg (right) mouse showing a focal areas of mild emphysema. Haematoxylin and eosin stain. Original magnification ×40.
In addition to neutrophilic inflammation, goblet cell metaplasia and mucus obstruction were a common feature of the airways of adult PI3Kγ WT/βENaC-Tg mice [19]. Since neutrophil products, such as neutrophil elastase, have been implicated in goblet cell metaplasia and mucin hypersecretion in CF [25, 26], we assessed the effects of genetic deletion of PI3Kγ on goblet cell metaplasia. Goblet cells were not observed in PI3Kγ WT and PI3Kγ KO mice; in PI3Kγ KO/βENaC-Tg mice, the goblet cell metaplasia appeared reduced compared to PI3Kγ WT/βENaC-Tg mice; however, this difference was not statistically significant, based on the variability and the number of mice included in our studies (data not shown).
3.3. Pharmacological Inhibition of PI3Kγ Reduces Neutrophilic Airway Inflammation in βENaC-Tg Mice
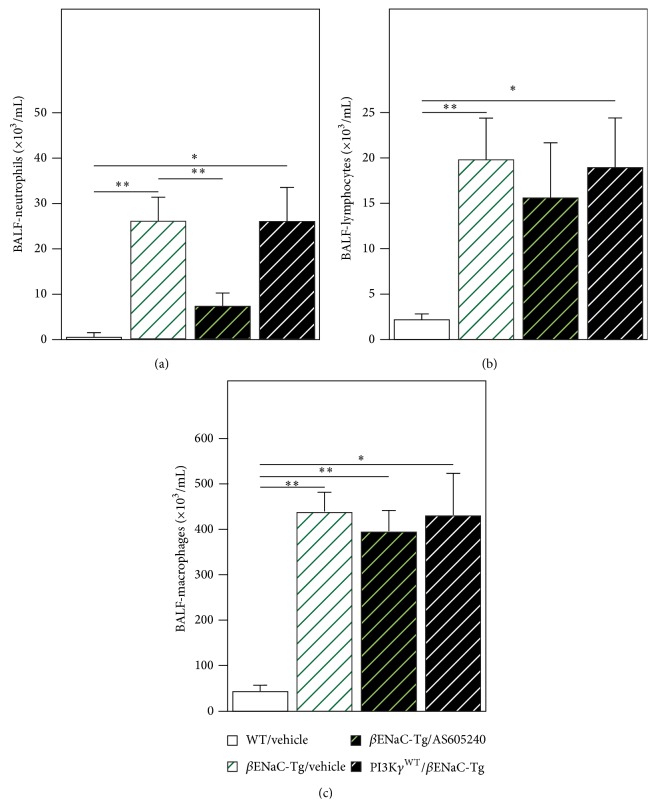
Next we tested effects of pharmacological inhibition of PI3Kγ by using the inhibitor AS-605240 on airway inflammation in βENaC-Tg mice. Treatment of βENaC-Tg mice with AS-605240 but not with vehicle alone reduced neutrophil infiltrates in BALF of βENaC-Tg mice (Figure 3(a)). In contrast, as observed in PI3Kγ KO/βENaC-Tg mice, the PI3Kγ inhibitor had no effect on the recruitment of macrophages or lymphocytes into the lung (Figures 3(b) and 3(c)).
Figure 3.
Pharmacological inhibition of PI3Kγ decreases neutrophilic airway inflammation in βENaC-Tg mice. Neutrophils (a), lymphocytes (b), and macrophages (c) numbers were determined in BAL fluid of control (WT/Vehicle) and βENaC-Tg mice untreated or treated with the PI3Kγ inhibitor AS-605240 (βENaC-Tg/AS-605240) or with vehicle (βENaC-Tg/Vehicle). Cells are expressed as number per mL of BAL fluid. (a) ** P < 0.01 WT/Vehicle versus βENaC-Tg/Vehicle, ** P < 0.01 βENaC-Tg/Vehicle versus βENaC-Tg/AS-605240 and * P < 0.05 WT/Vehicle versus PI3Kγ WT/βENaC-Tg. (b) ** P < 0.01 WT/Vehicle versus βENaC-Tg/Vehicle and * P < 0.05 WT/Vehicle versus PI3Kγ WT/βENaC-Tg. (c). ** P < 0.01 WT/Vehicle versus βENaC-Tg/Vehicle, ** P < 0.01 WT/Vehicle versus βENaC-Tg/AS-605240, and * P < 0.05 WT/Vehicle versus PI3Kγ WT/βENaC-Tg.
4. Discussion
Progressive lung disease is the major cause of morbidity and mortality in CF and is characterized by chronic airway infection and associated airway inflammation leading to irreversible lung destruction and early death [1–3]. Accumulating evidences suggest that CFTR dysfunction impairs mucociliary clearance and bacterial killing as crucial innate defense mechanisms of the lung leading to chronic bacterial infection and nonresolving inflammation in CF airways [3]. The main feature of airway inflammation in CF is a persistent influx of neutrophils that release a variety of oxidants and granule-associated enzymes, thus contributing to the development of lung injury and to the chronicity of pulmonary infection [7–9]. Repeated episodes of exacerbation of chronic infection and inflammation occur during the natural history of the disease, further increasing the structural damage in the CF lung [27, 28]. Therefore, anti-inflammatory therapy, combined with antibiotic therapy, offers a rational approach to prevent chronic lung damage. However, current anti-inflammatory drugs have shown several limits. The use of oral corticosteroids has been limited by severe adverse effects and studies using inhaled corticosteroids in CF have not been particularly successful [8, 9]. In addition, nonsteroidal anti-inflammatory drugs, such as ibuprofen, although revealing beneficial effects in younger CF patients [29], are difficult to dose and thus are not widely used [30]. Likewise, a phase 3 study of the LTB4 receptor antagonist BIIL 284 had been stopped due to adverse effects in the treatment group [31]. An alternative approach to decrease chronic inflammation is to use a more targeted anti-inflammatory therapy directed at reducing neutrophil trafficking in the CF lung. In this context, class I PI3K member, PI3Kγ, has been demonstrated to play a pivotal role in mediating leukocyte recruitment and activation into sites of inflammation [11]. Therefore PI3Kγ may represent an innovative and appropriate target to interfere with the excessive neutrophil-mediated inflammation and damage in CF. Of note, recently developed small-molecule PI3Kγ inhibitors were shown to be effective in suppressing joint inflammation in mouse models of rheumatoid arthritis [32]. In the present study we evaluated the effects of genetic deletion and pharmacologic inhibition of PI3Kγ in the βENaC-Tg mouse as a model of CF lung disease [15, 16, 33]. Such model phenocopies the airway surface dehydration and mucociliary dysfunction characteristic of CF airways. βENaC-Tg mice develop spontaneous CF-like lung disease with early onset goblet cell metaplasia and airway mucus obstruction, reduced bacterial clearance, and chronic neutrophilic inflammation triggering emphysema-like structural lung damage [15, 17, 34, 35]. Genetic deletion of PI3Kγ resulted in decreased neutrophil numbers in BALF of PI3Kγ KO/βENaC-Tg mice, and reduced neutrophilia was associated with reduced emphysematous changes in these mice. Taken together, these data support an important role of PI3Kγ for transmigration of neutrophils from the blood into the airway lumen and a crucial role of neutrophilic airway inflammation in the in vivo pathogenesis of lung damage. Several leukocyte-derived proteases including neutrophil elastase have been shown to cause emphysema in mice [36–38]. Furthermore, previous studies demonstrated that overexpression of several proinflammatory mediators in genetically modified mice induces an imbalance in the pulmonary protease/antiprotease system and emphysema in these mice [39, 40]. Thus, it is likely that neutrophil-dominated chronic pulmonary inflammation and the disruption of protease/antiprotease balance contribute to the development of emphysema in PI3Kγ WT/βENaC-Tg mice. Neutrophil elastase (NE) is the major product of activated neutrophils and has been implicated in the pathogenesis of key features of CF lung disease, such as chronic airway inflammation, mucus hypersecretion, goblet cell metaplasia, and structural damage [41–47]. We hypothesize that deletion of PI3Kγ decreases lung damage through the reduction of neutrophilic inflammation and neutrophil-associated active elastase. Consistently, a recent study demonstrated that NE activity is increased at the surface of airway neutrophils in PI3Kγ WT/βENaC-Tg mice and patients with CF [6] and that genetic deletion of NE results in a significant reduction of emphysema-like changes in PI3Kγ WT/βENaC-Tg mice, suggesting that NE is implicated in emphysema associated with chronic neutrophilic airway inflammation in vivo.
Recently, selective PI3Kγ inhibitors have been developed and investigated in different mouse models of chronic inflammation [48–51]. Therefore, we evaluated the efficacy of the PI3Kγ inhibitor AS-605240 on airway inflammation in βENaC-Tg mice; we decided to use AS-605240 for its well characterized in vivo profile of efficacy and selectivity, indicated by the so far largest number of reports of pharmacological PI3Kγ inhibition in mice [48–53]. We showed that treatment with the PI3Kγ inhibitor decreased the number of neutrophils in BALF of βENaC-Tg mice, thus reproducing the effect observed with the genetic deletion of PI3Kγ. Several technical problems limit the assessment of the increased PI3Kγ activity in βENaC mice; however, the findings that PI3Kγ WT/βENaC-Tg inflamed lungs have more leukocytes than PI3Kγ KO/βENaC-Tg controls are an indirect indication of increased PI3Kγ activity in these mice. Taken together, our data demonstrate the biological efficacy of both genetic deletion and pharmacological inhibition of PI3Kγ in reducing chronic neutrophilic inflammation in CF-like lung disease in vivo.
Whereas blockade of PI3Kγ activity by small-molecule inhibitors may represent a valid approach to modulate excessive leukocyte accumulation in inflamed tissues where leukocyte recruitment is correlated with disease progression, on the other hand increased susceptibility to infection might be a potential side effect of the use of these molecules. In this context, a previous study [54] showed that either gene deletion or pharmacologic inhibition of PI3Kγ in mice infected with S. pneumoniae caused an impaired exudate macrophage recruitment associated with a reduced lung pneumococcal clearance and an impaired resolution/repair process, leading to progressive pneumococcal pneumonia. Thus, whereas pharmacological inhibition of PI3Kγ, eventually in association with antibacterial treatment, may be a viable strategy to inhibit chronic inflammation and limit lung damage in stable CF lung disease, it might have adverse effects on host defense in acute infections when high bacterial burden occurs. In view of a clinical application of PI3Kγ inhibitors, target validation will be an important future aspect to discriminate between specific effects of the drug and potential side effects.
5. Conclusions
Neutrophil-dominated airway inflammation has been implicated as a key feature of progressive lung damage in CF. Thus, reducing airway inflammation is a major goal to prevent lung damage and maintain lung function in CF. Current therapeutic strategies that aim to reduce chronic neutrophilic inflammation in the airways of CF patients have been largely unsuccessful. This study shows that genetic deletion and pharmacological inhibition of PI3Kγ decrease neutrophilic airway inflammation and structural lung damage in a mouse model of CF lung disease. These results provide insight into the molecular mechanisms of chronic airway inflammation and suggest a novel treatment strategy to reduce inflammation and lung damage in patients with CF and potentially other neutrophilic airway diseases. Further studies with emerging PI3Kγ inhibitors [49–51] are required to confirm the efficacy of these molecules and exclude their potentially adverse effects on host defense.
Acknowledgments
This work was supported by a research grant from Italian Cystic Fibrosis Research Foundation (Project FFC #20/2009).
Disclosure
Emilio Hirsch and Virginia De Rose are co-senior authors.
Conflict of Interests
Emilio Hirsch has equity ownership in Kither Biotech S.r.l. which is developing products related to the research being reported. Marcus Mall is inventor of a patent filled by the University of North Carolina and related to βENaC transgenic mice. All other authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Maria Galluzzo and Elisa Ciraolo contributed equally to this work. Gerd Doring is deceased.
References
- 1.Ratjen F., Döring G. Cystic fibrosis. The Lancet. 2003;361(9358):681–689. doi: 10.1016/s0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan B. P., Freedman S. D. Cystic fibrosis. The Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 3.Mall M. A., Hartl D. CFTR: cystic fibrosis and beyond. European Respiratory Journal. 2014;44(4):1042–1054. doi: 10.1183/09031936.00228013. [DOI] [PubMed] [Google Scholar]
- 4.Sly P. D., Gangell C. L., Chen L., et al. Risk factors for bronchiectasis in children with cystic fibrosis. The New England Journal of Medicine. 2013;368(21):1963–1970. doi: 10.1056/nejmoa1301725. [DOI] [PubMed] [Google Scholar]
- 5.Sagel S. D., Wagner B. D., Anthony M. M., Emmett P., Zemanick E. T. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2012;186(9):857–865. doi: 10.1164/rccm.201203-0507oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehrig S., Duerr J., Weitnauer M., et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosislike lung disease. American Journal of Respiratory and Critical Care Medicine. 2014;189(9):1082–1092. doi: 10.1164/rccm.201311-1932oc. [DOI] [PubMed] [Google Scholar]
- 7.de Rose V. Mechanisms and markers of airway inflammation in cystic fibrosis. European Respiratory Journal. 2002;19(2):333–340. doi: 10.1183/09031936.02.00229202. [DOI] [PubMed] [Google Scholar]
- 8.Chmiel J. F., Konstan M. W. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clinics in Chest Medicine. 2007;28(2):331–346. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Cymberknoh M., Kerem E., Ferkol T., Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68(12):1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 10.Prescott W. A., Jr., Johnson G. E. Anti-inflammatory therapies for cystic fibrosis: past, present, and future. Pharmacotherapy. 2005;25(4):555–573. doi: 10.1592/phco.25.4.555.61025. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch E., Katanaev V. L., Garlanda C., et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1052. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch E., Ciraolo E., Ghigo A., Costa C. Taming the PI3K team to hold inflammation and cancer at bay. Pharmacology and Therapeutics. 2008;118(2):192–205. doi: 10.1016/j.pharmthera.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Ghigo A., Damilano F., Braccini L., Hirsch E. PI3K inhibition in inflammation: toward tailored therapies for specific diseases. BioEssays. 2010;32(3):185–196. doi: 10.1002/bies.200900150. [DOI] [PubMed] [Google Scholar]
- 14.Ghigo A., Morello F., Perino A., Hirsch E. Phosphoinositides I: Enzymes of Synthesis and Degradation. Vol. 58. Dordrecht, The Netherlands: Springer; 2012. Phosphoinositide 3-kinases in health and disease; pp. 183–213. (Subcellular Biochemistry). [DOI] [PubMed] [Google Scholar]
- 15.Mall M., Grubb B. R., Harkema J. R., O'Neal W. K., Boucher R. C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nature Medicine. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z., Duerr J., Johannesson B., et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. Journal of Cystic Fibrosis. 2011;10(supplement 2):S172–S182. doi: 10.1016/s1569-1993(11)60021-0. [DOI] [PubMed] [Google Scholar]
- 17.Wielpütz M. O., Eichinger M., Zhou Z., et al. In vivo monitoring of cystic fibrosis-like lung disease in mice by volumetric computed tomography. European Respiratory Journal. 2011;38(5):1060–1070. doi: 10.1183/09031936.00149810. [DOI] [PubMed] [Google Scholar]
- 18.Johannesson B., Hirtz S., Schatterny J., Schultz C., Mall M. A. CFTR regulates early pathogenesis of chronic obstructive lung disease in βenac-overexpressing mice. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0044059.e44059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mall M. A., Harkema J. R., Trojanek J. B., et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. The American Journal of Respiratory and Critical Care Medicine. 2008;177(7):730–742. doi: 10.1164/rccm.200708-1233oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26(1):57–60. [PubMed] [Google Scholar]
- 21.Thurlbeck W. M. Measurement of pulmonary emphysema. The American Review of Respiratory Disease. 1967;95(5):752–764. doi: 10.1164/arrd.1967.95.5.752. [DOI] [PubMed] [Google Scholar]
- 22.Thurlbeck W. M. The internal surface area of nonemphysematous lungs. American Review of Respiratory Disease. 1967;95(5):765–773. doi: 10.1164/arrd.1967.95.5.765. [DOI] [PubMed] [Google Scholar]
- 23.Atzori L., Lucattelli M., Scotton C. J., et al. Absence of proteinase-activated receptor-1 signaling in mice confers protection from fMLP-induced goblet cell metaplasia. The American Journal of Respiratory Cell and Molecular Biology. 2009;41(6):680–687. doi: 10.1165/rcmb.2007-0386oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livraghi A., Grubb B. R., Hudson E. J., et al. Airway and lung pathology due to mucosal surface dehydration in β-epithelial Na+ channel-overexpressing mice: role of TNF-α and IL-4Rα signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. Journal of Immunology. 2009;182(7):4357–4367. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voynow J. A., Fischer B. M., Malarkey D. E., et al. Neutrophil elastase induces mucus cell metaplasia in mouse lung. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2004;287(6):L1293–L1302. doi: 10.1152/ajplung.00140.2004. [DOI] [PubMed] [Google Scholar]
- 26.Voynow J. A., Young L. R., Wang Y., Horger T., Rose M. C., Fischer B. M. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 1999;276(5):L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 27.Goss C. H., Burns J. L. Exacerbations in cystic fibrosis·1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konstan M. W., Morgan W. J., Butler S. M., et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. Journal of Pediatrics. 2007;151(2):134.e1–139.e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Konstan M. W., Byard P. J., Hoppel C. L., Davis P. B. Effect of high-dose ibuprofen in patients with cystic fibrosis. New England Journal of Medicine. 1995;332(13):848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 30.Konstan M. W. Ibuprofen therapy for cystic fibrosis lung disease: revisited. Current Opinion in Pulmonary Medicine. 2008;14(6):567–573. doi: 10.1097/mcp.0b013e32831311e8. [DOI] [PubMed] [Google Scholar]
- 31.Konstan M. W., Döring G., Heltshe S. L., et al. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. Journal of Cystic Fibrosis. 2014;13(2):148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camps M., Ruckle T., Ji H., Ardissone V. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nature Medicine. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 33.Mall M. A., Graeber S. Y., Stahl M., Zhou-Suckow Z. Early cystic fibrosis lung disease: role of airway surface dehydration and lessons from preventive rehydration therapies in mice. International Journal of Biochemistry and Cell Biology. 2014;52:174–179. doi: 10.1016/j.biocel.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Mall M. A., Button B., Johannesson B., et al. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice. The Journal of Biological Chemistry. 2010;285(35):26945–26955. doi: 10.1074/jbc.m110.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trojanek J. B., Cobos-Correa A., Diemer S., et al. Airway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12-dependent emphysema. The American Journal of Respiratory Cell and Molecular Biology. 2014;51(5):709–720. doi: 10.1165/rcmb.2013-0407oc. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro S. D., Goldstein N. M., Houghton A. M., Kobayashi D. K., Kelley D., Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. American Journal of Pathology. 2003;163(6):2329–2335. doi: 10.1016/s0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churg A., Wright J. L. Proteases and emphysema. Current Opinion in Pulmonary Medicine. 2005;11(2):153–159. doi: 10.1097/01.mcp.0000149592.51761.e3. [DOI] [PubMed] [Google Scholar]
- 38.Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 39.Fujita M., Shannon J. M., Irvin C. G., et al. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2001;280(1):L39–L49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- 40.Zheng T., Zhu Z., Wang Z., et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. Journal of Clinical Investigation. 2000;106(9):1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voynow J. A., Fischer B. M., Zheng S. Proteases and cystic fibrosis. International Journal of Biochemistry and Cell Biology. 2008;40(6-7):1238–1245. doi: 10.1016/j.biocel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owen C. A. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2008;3(2):253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pham C. T. N. Neutrophil serine proteases: specific regulators of inflammation. Nature Reviews Immunology. 2006;6(7):541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 44.Lee W. L., Downey G. P. Leukocyte elastase: physiological functions and role in acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2001;164(5):896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- 45.Fahy J. V., Dickey B. F. Airway mucus function and dysfunction. The New England Journal of Medicine. 2010;363(23):2233–2247. doi: 10.1056/nejmra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson R. L., Burns J. L., Ramsey B. W. Pathophysiology and management of pulmonary infections in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505so. [DOI] [PubMed] [Google Scholar]
- 47.Taggart C. C., Greene C. M., Carroll T. P., O'Neill S. J., McElvaney N. G. Elastolytic proteases: inflammation resolution and dysregulation in chronic infective lung disease. American Journal of Respiratory and Critical Care Medicine. 2005;171(10):1070–1076. doi: 10.1164/rccm.200407-881pp. [DOI] [PubMed] [Google Scholar]
- 48.Venable J. D., Ameriks M. K., Blevitt J. M., Thurmond R. L., Fung-Leung W.-P. Phosphoinositide 3-kinase gamma (PI3Kγ) inhibitors for the treatment of inflammation and autoimmune disease. Recent Patents on Inflammation and Allergy Drug Discovery. 2010;4(1):1–15. doi: 10.2174/187221310789895603. [DOI] [PubMed] [Google Scholar]
- 49.Foster J. G., Blunt M. D., Carter E., Ward S. G. Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacological Reviews. 2012;64(4):1027–1054. doi: 10.1124/pr.110.004051. [DOI] [PubMed] [Google Scholar]
- 50.Markman B., Tao J. J., Scaltriti M. PI3K pathway inhibitors: better not left alone. Current Pharmaceutical Design. 2013;19(5):895–906. doi: 10.2174/138161213804547213. [DOI] [PubMed] [Google Scholar]
- 51.Cushing T. D., Metz D. P., Whittington D. A., McGee L. R. PI3Kdelta and PI3Kgamma as targets for autoimmune and inflammatory diseases. Journal of Medicinal Chemistry. 2012;55(20):8559–8581. doi: 10.1021/jm300847w. [DOI] [PubMed] [Google Scholar]
- 52.Fougerat A., Gayral S., Gourdy P., et al. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation. 2008;117(10):1310–1317. doi: 10.1161/circulationaha.107.720466. [DOI] [PubMed] [Google Scholar]
- 53.Ghigo A., Perino A., Mehel H., et al. Phosphoinositide 3-kinase gamma protects against catecholamine-induced ventricular arrhythmia through protein kinase A-mediated regulation of distinct phosphodiesterases. Circulation. 2012;126(17):2073–2083. doi: 10.1161/circulationaha.112.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maus U. A., Backi M., Winter C., et al. Importance of phosphoinositide 3-kinase γ in the host defense against pneumococcal infection. American Journal of Respiratory and Critical Care Medicine. 2007;175(9):958–966. doi: 10.1164/rccm.200610-1533oc. [DOI] [PubMed] [Google Scholar]