Abstract
High quality gamete production in males and females requires the pituitary gonadotropin follicle stimulating hormone (FSH). In this report a novel chemical class of small molecule inhibitors of FSH receptor (FSHR) is described. ADX61623, a negative allosteric modulator (NAM), increased the affinity of interaction between 125I-hFSH and human FSHR (hFSHR) five fold. This form of FSHR occupied simultaneously by FSH and ADX61623 was inactive for cAMP and progesterone production in primary cultures of rat granulosa cells. In contrast, ADX61623 did not block estrogen production. This demonstrates for the first time, biased antagonism at the FSHR. To determine if ADX61623 blocked FSH induction of follicle development in vivo, a bioassay to measure follicular development and oocyte production in immature female rats was validated. ADX61623 was not completely effective in blocking FSH induced follicular development in vivo at doses up to 100 mg/kg as oocyte production and ovarian weight gain were only moderately reduced. These data illustrate that FSHR couples to multiple signaling pathways in vivo. Suppression of one pool of FSHR uncouples Gαs and cAMP production, and decreases progesterone production. Occupancy of another pool of FSHR sensitizes granulosa cells to FSH induced estradiol production. Therefore, ADX61623 is a useful tool to investigate further the mechanism of the FSHR signaling dichotomy. This may lead to a greater understanding of the signaling infrastructure which enables estrogen biosynthesis and may prove useful in treating estrogen dependent disease.
Keywords: Follicle stimulating hormone, Receptor, Small molecule
1. Introduction
High quality gamete production in males and females requires the pituitary gonadotropin follicle stimulating hormone (FSH). The discovery of such regulatory mechanisms has made possible new opportunities for the development of effective treatment of various disorders related to the reproductive process.
Current pharmaceutical usage of gonadotropins including FSH and the related luteinizing hormone (LH) and human chorionic gonadotropin (hCG) is primarily for follicle maturation and induced ovulation respectively in assisted reproductive technologies (Perez et al., 2000). Current therapies include recombinant gonadotropins or gonadotropin purified from human urine (Brinsden et al., 2000; Coelingh Bennink et al., 1998; Williams et al., 2003).
Due to their specific action on ovarian tissue without impacting peripheral and central tissues, FSH receptor (FSHR) antagonists could potentially represent a novel non-steroidal approach for contraception. Interestingly, female FSHβ gene knock-out mice are infertile because of a block in folliculogenesis (Kumar et al., 1997). Likewise, women with resistant ovary syndrome due to non-functional FSH receptors are infertile (Aittomaki et al., 1995), reinforcing the hypothesis that an antagonist of FSH receptor would act to limit proliferation of follicular granulosa cells in the ovary, therefore acting as a contraceptive.
FSH is comprised of two dissimilar (α,β) subunits, and each chain is decorated with 1–2 complex carbohydrate glycans (Fox et al., 2001). The FSHR is a plasma membrane receptor that is a member of the large family of G-protein coupled receptors (GPCR), and FSH binding leads to activation of the adenyl cyclase system and elevation of intracellular levels of the second messenger cAMP (Conti, 2002). It has a large extracellular domain of 350 amino acids and a predicted rhodopsin like transmembrane domain structure of roughly an additional 300 amino acids (Sprengel et al., 1990).
The crystal structure of FSH and of the FSHR:FSH complex has revealed extensive contacts made by many key residues that are essential for high affinity interaction between FSH and its receptor (Fan and Hendrickson, 2005). These extensive contacts suggest that ab initio design of small molecule antagonists of FSH binding could be difficult to achieve. Despite this reasoning, high throughput screening has led to identification of small molecule antagonists which inhibit FSH binding, and may act at the level of the extra-cellular domain (Arey et al., 2008). In one report, where the FSHR antagonist was a competitive inhibitor of FSH binding this compound blocked ovulation but caused inflammation of the ovaries at 100 mg/kg and decreased body weight, uterus weight and ovarian weight in immature and adult cycling rats. Further work has not been reported of progress developing this small molecule towards a contraceptive (Arey et al., 2008).
Small molecules which have FSHR agonist activities have also been reported but none are commercially available. Since they do not inhibit FSH binding these agonists are likely interacting with the transmembrane domains, and not the extracellular domain suggesting an allosteric mechanism of agonism (Arey, 2008; Arey et al., 2008; Guo et al., 2004a,b,c; Maclean et al., 2004; Palmer et al., 2005; Yanofsky et al., 2006). Chemical modifications of some of those small molecule allosteric agonists of FSHR can be converted to completely antagonize the FSH-induced signal in different cell lines, making them negative allosteric modulators (NAM) (Arey et al., 2008; van Straten et al., 2005). This tetrahydroquinolone derivative antagonist which did not compete for FSH binding blocked FSH induced cAMP production. Likewise a thiazolidonone FSHR allosteric agonist can be modified so that it loses FSHR agonistic activity while gaining the ability to block FSH induced cAMP and aromatase activity. Those data suggest that a range of activities may be achievable with a single small molecule core structure (Arey et al., 2008). In this report, properties of an FSHR NAM derived from a novel chemical class is delineated. Furthermore this FSHR NAM can block one differentiation phenotype of the granulosa cell measured as progesterone production yet not block another arm measured as estrogen production. This observation clearly establishes multiple arms of the FSHR signaling complex and provides evidence for biased antagonism at this receptor.
2. Materials and methods
2.1. Hormone preparation and small molecules
Recombinant human FSH (Gonal F) was provided by Merck Serono or purchased from Cell Science (Canton, MA, USA). Human pituitary FSH was purified as previously described (Fox et al., 2001). Highly purified human urinary FSH was purchased from Cytoshop (Rehovot, Israel). The FSHR NAM ADX61623 was prepared by Addex Pharmaceuticals S.A. (Geneva, Switzerland), Patent WO2008117175 and has a MW around 320. A previously described (van Straten et al., 2005) reference FSHR NAM (ADX49626) was synthesized by Addex Pharmaceuticals S.A. (Geneva, Switzerland).
2.2. Preparation of cell lines for library screen
The cDNA encoding the human follicle stimulating hormone receptor (hFSHR) (accession number M95489, NCBI Nucleotide database browser) was subcloned into an expression vector containing also the hygromycin resistance gene. Transfection of this vector into HEK293 cells with PolyFect reagent (Qiagen, Valencia, CA) according to supplier’s protocol, and hygromycin treatment allowed selection of antibiotic resistant cells which had integrated stably one or more copies of the plasmid. Positive cellular clones expressing hFSHR were identified in a functional assay measuring cAMP production in cells following stimulation by addition of purified human follicle stimulating hormone (hFSH).
2.3. Intracellular cAMP measurement assay for library screen
The determination of the cAMP accumulation was performed using an HTRF assay (Trinquet et al., 2006). Briefly, cells were incubated for 3 min in the presence of increasing concentrations of NAM (from 1 nM to 60 μM) and then 30 min in the presence of 1 ng/ml of hFSH, which in this assay system corresponds to the EC80, a concentration that gives 80% of the maximal response of the agonist. Likewise, 10-point concentration–response curves of FSH were tested in the absence or in the presence of increasing concentrations of NAM in order to detect a rightward-shift of the concentration–response curve of the agonist (revealed by a increase in the EC50) and a decrease of its maximal efficacy (characteristic of negative allosteric modulation). Cells were then lysed by adding the HTRF assay components, the europium cryptate-labeled anti-cAMP antibody, and the XL665-labeled cAMP analog, previously diluted in a HEPES buffer (50 mM, pH 7.0) containing 0.8 M potassium fluoride, 0.2% (w/v) BSA, and 1% (v/v) Triton X-100 (a percentage of detergent that ensures complete cell lysis). The assay was then incubated for 1 h at room temperature, and HTRF signal was measured after excitation at 337 nm, and dual emission at 620 and 665 nm, using a RubyStar fluorimeter (BMG Labtechnologies). Moreover, the fluorescence ratio of an appropriate range of known concentrations of cAMP standards was also included on each assay plate to produce a standard cAMP curve. Providing that the fluorescence ratio of the cAMP inhibited by the compound falls in the linear part of the cAMP standard curve (i.e. where a change in fluorescence ratio is proportional to change in cAMP concentration) this allows the exact concentration of cAMP inhibited by the compounds to be calculated. The assay signal was therefore expressed as the percentage of signal stimulation.
2.4. 125I-hFSH binding assay
Binding and internalization of 125I-hFSH was measured using HEK293 cells stably expressing the human FSH receptor as previously described (Cohen et al., 2003). The radiolabeled hFSH was prepared using human urinary FSH (Cytoshop, Rehovot, Israel). Cell surface binding was distinguished from internalized hormone as previously described (Cohen et al., 2003). Binding isotherms were based on surface bound 125I-hFSH and were analyzed using the software LIGAND (Munson and Rodbard, 1980).
2.5. In vitro bioassay of FSH
All animal studies were approved by the Wadsworth Center Institutional Animal Care and Use Committee (Protocols 06-407 and 08-393). Wistar or Sprague Dawley rats (Taconic Farms, Germantown, NY) were provided with standard pellet diet and water ad libitum.
The in vitro bioassay for FSH was conducted as previously described with some modifications (Liu et al., 1981). Immature (21 ± 3 days old) female rats were injected s.c. with 1 mg diethylstilbesterol (DES) in sesame oil. DES was solubilized in sesame oil (10 mg/ml) in a sonicating water bath. Animals were injected once a day for four days with 100 μl of DES (10 mg/ml), in the scruff of the neck. On the 5th day animals were sacrificed and the ovaries and part of the uterus dissected, and placed in Modified Eagles Media (MEM) with 10% fetal bovine serum (FBS). In some experiments DMEM/F12 with 10% chicken serum was used. Collected tissues were kept at 37 °C until the ovaries were freed from the bursa, trimmed and placed into fresh medium. Granulosa cells were harvested by puncturing the ovaries with 27 gauge needles in warm MEM, containing 500 nM testosterone, chicken serum, gentamycin and HEPES (0.01 M). Only two ovaries were processed at a time, keeping all other tissues at 37 °C 5% CO2. When all tissues were processed, cells and ovaries were taken up into 10 ml pipettes and triturated into a 50 ml conical tube at room temperature. The triturated cells were collected allowing the ovary shells and large particles to sediment by gravity. An equivalent of cells harvested from two ovaries was plated in a 24 well plate. This is roughly about 300,000 cells per well. Cells were incubated overnight in 37 °C incubator under a water saturated atmosphere of 5% CO2. On the following day culture medium was aspirated and 0.8 ml fresh MEM with supplements was added to the wells.
Test substances were added to each of four well replicates 1 h prior to addition of the FSH challenge dose. Test substances were dissolved in dimethylsulfoxide (DMSO), to a final concentration of 5 mM. This stock was then used to make final dilutions of compound in media such that the final concentration of DMSO was 0.6%.
Granulosa cells were pretreated with test compounds for 1 h prior to the addition of recombinant hFSH (Gonal F; Merck Serono) or pituitary hFSH as indicated. After addition of hormone, the cells were cultured for 48 h or 72 h as indicated in the graph legends. Media was collected, heated to 100 °C for 10 min, and cleared at 2500 × g. The clear supernatant was frozen until assayed for progesterone and estradiol.
To determine if ADX61623 also inhibited FSH induced estradiol synthesis, rat granulosa cells were prepared as before, but testosterone (0.5 μM) was included in the media as the substrate for the P450 aromatase. ADX61623 was added to granulosa cells for 1 h prior to addition of 50 or 100 ng/ml pituitary hFSH. The production of estradiol is maximum at 96 h, whereas progesterone production begins to decline after 72 h in this bioassay (Liu et al., 1981). In order to compare both estradiol and progesterone in the same samples, media was collected at 72 h, heated to 100 °C for 10 min, spun at 1250 × g and the clear supernatant was frozen until assayed. Progesterone and estradiol were assayed using the primary antisera GDN338 and GDN244, respectively provided by Dr. Gordon Niswender, Colorado State University. The antisera were used at a final concentration of 1:12,500. The tracers used were from Perkin Elmer, NET-381 Progesterone-1,2,6,7-3H and NET-317250UC Estradiol-2,4,6,7-3H. Twenty thousand counts were added per tube. Samples were diluted and 100 μl was tested, and the final volume incubated overnight at 4 °C was 0.5 ml. The buffer used throughout was PBS with 0.1% gelatin, pH 7.5. After overnight incubation 0.5 ml of a charcoal dextran solution (0.62 g charcoal, 0.0625 g dextran T70 per 100 ml PBS with gelatin) was added to each tube and allowed to incubate for 10 min. The tubes were spun at 2500 × g for 30 min to separate antibody-bound from charcoal-bound progesterone or estradiol. Samples were poured off into scintillation vials, 20 ml of Aquasol was added per vial and then the samples were analyzed using a Packard scintillation counter. Data were analyzed with the program NIHRIA and graphs prepared with Graphpad Prism software. Each compound was tested in its own 24 well plate. We have noted variation in the absolute amount of steroid between plates thus the results from the two compounds are not directly comparable and no relative potency estimates were calculated. Experiments were repeated four times. The error bars which represent SEM may not be visible when the error bar is smaller than the symbol.
2.6. cAMP measurement in primary cultures of rat granulosa cells
When primary cultures of granulosa cells were used, they were prepared as described for the progesterone and estradiol experiments described above. Forty-eight h after plating, cells were pre-treated with 250 μl serum-free medium (SFM) containing 1 mM isobutylmethylxanthine (IBMX) in the presence of a final concentration of 30 μM ADX61623 or vehicle control (DMSO) for 1 h. The cells were then stimulated with 300 ng FSH (final volume = 300 μl/well) for 1 h at 37 °C in a 5% CO2 -humidified incubator. Two additional controls were included; cells treated with SFM + FSH + IBMX and cells treated with DMSO + FSH − IBMX. The culture medium was removed and replaced with 300 μl fresh SFM. Plates were then frozen/thawed four times, an equal volume (300 μl) of 100% EtOH was added, and the medium was clarified by centrifugation at 13,000 × g for 10 min at room temperature. Supernatants were stored at −80 °C. cAMP accumulation was determined by radioimmunoassay (RIA) as previously described (Nechamen and Dias, 2000).
2.7. In vivo bioassay of FSH
This assay measures the ability of FSH to recruit and mature follicles for ovulation. All procedures were approved by the Wadsworth Center Animal Care and Use Committee, protocol 08-407. To determine a dose profile of FSH, immature female rats 21–22 days of age were sorted into experimental groups. The rats were injected in the scruff of the neck twice per day (0900 and 1600 h) for 2 days with pituitary hFSH (0, 0.075, 0.25, 0.75, 2.5 μg per injection) to determine the minimal effective dose. Frequency of dosing was evaluated with a dose of 1 μg FSH given once, twice, three times and four times over the two day period. On the second day, hCG (20 IU) was administered with the first dose of FSH. When inhibitors were tested, they were administered beginning at day −1. The vehicle for hCG and FSH was PBS. The vehicle for ADX61623 was PEG400. Animals were anesthetized for all injections with isoflurane (Abbot Lab., United Kingdom) vaporizer (Parkland Scientific, Coral Springs, FL) according to the manufacturers instructions.
Sixteen h following treatment with hCG rats were anesthetized followed by cervical dislocation. The ovaries and oviducts were collected and placed in Eagles MEM with 0.1% BSA and 10% chicken serum. The oviducts were teased from the ovary proper and the ovaries trimmed of fat and the ovarian bursa. Ampullae were pierced releasing the clutch of oocytes. The oocytes clutch was then transferred to a microdrop of media (100 μl). To this microdrop was added 10 μl of 10 mg/ml of sheep hyaluronidase (500 U/mg in PBS). After 10 min at 37 °C, the oocytes were readily visible and easily counted. The ovaries were weighed individually.
2.8. Data analysis
The concentration–response curves of representative compounds in the presence of EC80 of FSH receptor agonist were generated using the Prism Graph-Pad program (Graph Pad Software Inc., San Diego, USA). The curves were fitted to a four-parameter logistic equation (Y = Bottom + (Top − Bottom)/(1 + 10(Log IC50−X) × Hill Slope)) allowing determination of IC50 values. Each curve was performed using duplicate sample per data point and 10 concentrations. The concentration–response curves of a selective FSH receptor agonist in the absence or in the presence of representative compounds were also generated using Prism Graphpad Prism program (Graphpad Software Inc., San Diego, USA). The curves were fitted to a four-parameter logistic equation (Y = Bottom + (Top − Bottom)/(1 + 10(Log EC50−X) × Hill Slope)) allowing determination of EC50 values of the selective FSH receptor agonist. Each curve was performed using duplicate sample per data point and 10 concentrations.
Animal experiments were analyzed by a one-way analysis of variance performed using Prism software. Post tests were performed using Neuman–Keuls multiple range comparisons.
3. Results
3.1. Effect of FSHR NAMs on a recombinant cell line expressing hFSHR
In an aim of identifying new small molecule negative allosteric modulators of FSHR, Addex Pharmaceuticals S.A. corporate library was screened using an HEK cell line expressing the human FSHR. The screening protocol monitored fluorescence in each well at baseline and changes in fluorescence after the addition of either test vehicle or test compound (10 μM final nominal concentration). After 10 min of pre-incubation, a sub-maximally effective concentration (EC80) of FSH was added. Potential NAMs were defined as compounds that blocked an FSH-induced increase in signal without having an effect on their own (data not shown). Those compounds were then re-confirmed using 10 point concentration–response protocol on HEK-cells expressing only the hFSHR and in a functional assay measuring cAMP production following stimulation of FSHR by an FSH EC80. Fig. 1A shows ADX61623, a representative example of the benzamide series identified using this screening paradigm. This compound partially (average efficacy = 55% inhibition of FSH EC80) inhibited FSH-induced cAMP accumulation with an EC50 value of 0.43 (CL 0.32, 0.57) μM (Fig. 1B). Although compounds which fully block the FSH effect in this screen have since been identified (data not shown), ADX61623 was further characterized because it was fully active in the rat granulosa cell assay as shown below.
Fig. 1.
(A) Molecular structure of ADX61623. (B) Effect of ADX61623 on production of cAMP induced by an EC80 of hFSH on HEK cells expressing hFSHR. (C) Schild-Plot experiment of ADX61623. Measurement of cAMP production by HEK cells expressing hFSHR. Experiments are representative of replicate experiments.
Whereas the average ADX61623 IC50 for FSH = 0.7 ± 0.2 μM the compound is completely selective versus hTSH receptor at concentration up to 30 μM, while retaining some activity towards the hLH receptor (IC50 = 3.4 ± 0.8 μM, average efficacy = 48% inhibition of LH EC80, data not shown). Detailed in vitro pharmacological studies showed that this compound induced a rightward-shift of the FSH concentration–response curve with a partial decrease in the FSH maximal response (Fig. 1C). Finally, this compound inhibits the activation of the receptor in a fully reversible manner.
3.2. Effect of ADX61623 on rat granulosa cell response to FSH
The rat and human FSHR share considerable homology in the transmembrane domains. In order to ascertain that ADX61623 acted similarly at the rat FSHR, it was first investigated if FSH-induced cAMP production was inhibited in rat granulosa cells. Indeed ADX61623 at a 30 μM concentration, significantly blocked FSH induced cAMP production in rat granulosa cells (Fig. 2).
Fig. 2.

Inhibition of FSH induced cAMP production in rat granulosa cells by ADX61623. Cells were cultured in serum free media (SFM) as described in Section 2. A challenge dose of 300 ng/300 μl of hFSH (27 nM) for 1 h at 37 °C was used against an inhibitor dose of 30 μM. Results are representative of duplicate experiments except in this experiment presented an additional control that did not contain the phosphodiesterase inhibitor isobutylmethylxanthine (0.1 mM) was included.
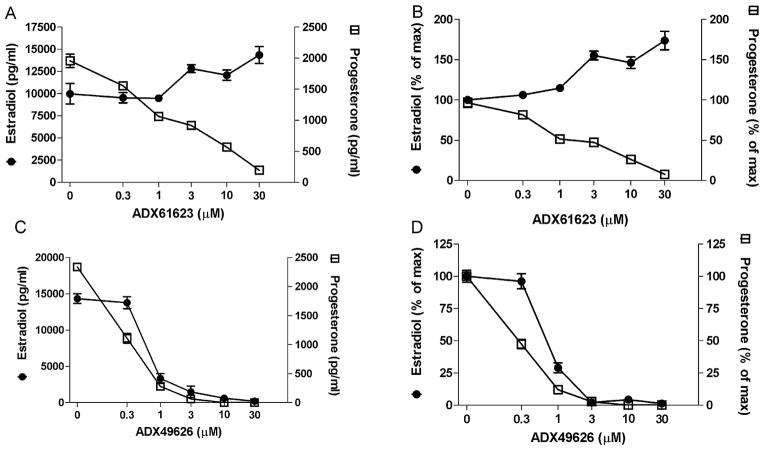
As expected, ADX61623 fully inhibited hFSH stimulated progesterone production in a concentration related manner (Fig. 3A and B). The IC50 for ADX61623 was 2.9 ± 0.3 μM similar to the one measured in recombinant systems. In contrast, ADX61623 did not inhibit estradiol production and at doses higher than 1 μM caused a concentration-dependent increase (Fig. 3A and B). There was no effect of ADX61623 on either progesterone or estradiol production when it was added in the absence of FSH. In several experiments a deflection of estradiol production was observed at 1 μM ADX61623 followed by a rise in estradiol production (data not shown).
Fig. 3.
Specificity of biased antagonism of ADX61623. (A) ADX61623 blocked FSH (50 ng/ml) induced progesterone (72 h) production in rat granulosa cells but estradiol production (72 h) was not abated. (B) Data in (A) expressed as percent of control. (C) Another FSHR NAM blocked both progesterone and estradiol production. (D) Data in (C) expressed as percent of control. Data are represented as means ± SEM. The disparate effect on estradiol production was observed in four experiments.
In order to ascertain if a lack of commensurate inhibition of FSH-induced estradiol production was unique to ADX61623, another FSHR NAM which was previously reported to have an IC50 value of 540 nM to inhibit FSH-stimulated cAMP production in a stable rat granulosa cell line (Compound 10) (van Straten et al., 2005) called ADX49626 in this study), was tested. In freshly dissociated rat granulosa cells, this FSHR NAM blocked both FSH-stimulated progesterone and estradiol production with IC50 values of 50 and 200 nM, respectively (Fig. 3C and D). Therefore ADX61623 possesses a unique property of restricted or biased antagonism at the FSHR.
3.3. Effect of FSHR NAM ADX61623 on 125I-hFSH binding to human FSHR in HEK293 cells
Previous studies have demonstrated that conformational changes in the FSHR occur upon agonist binding (Schmidt et al., 2001). Therefore it was of interest to determine if the inhibition of FSH induced progesterone production was due to a long range conformational change induced by ADX61623 which prevented the binding of 125I-hFSH to its receptor. An initial experiment in rat granulosa cell cultures suggested that FSHR NAM increased cell surface binding of radiolabeled FSH (data not shown). The total specific binding in this system is very low (400–600 cpm) so that accurate assessment of this parameter was not possible. Therefore, a cell based binding assay was used where HEK293 cells were stably transfected with human FSHR and ADX61623 was added for 30 min prior to addition of radiolabeled FSH.
The addition of ADX61623 caused a dose related increase in cell surface 125I-hFSH binding to human FSH receptors expressed in HEK293 cells (Fig. 4A). Remarkably, internalization of the FSHR proceeded despite that treatment with ADX61623 dampened G-protein activation. The amount of internalized radiolabeled FSH was similar for all doses of ADX61623 suggesting that increased binding was not due to a decreased internalization rate. Moreover this effect was also observed for another FSHR NAM (ADX49626) (Fig. 4B), indicating that the increased binding of FSH is a general property of negative allosteric modulation. Finally this finding confirmed the allosteric nature of the functional inhibition induced by this compound as there is no displacement of radiolabeled FSH but rather an opposite effect.
Fig. 4.
(A) ADX61623 enhances 125I-hFSH binding to human FSHR without affecting internalization (cell associated) of radiolabeled hFSH in HEK293 cells. (B) Both ADX61623 and another FSHR NAM ADX49626 enhance 125I-hFSH binding to human FSHR without affecting internalization (cell associated) of radiolabeled hFSH in HEK293 cells. (C) The effect of ADX61623 on the interaction between 125I-hFSH binding to the human FSHR expressed on HEK293 cells. The results are representative of two repetitions and data are expressed as SEM.
To determine if this effect was due to an increase in the number of receptors or the affinity of the receptor ligand interaction, HEK293 FSHR cells were incubated with 30 μM ADX61623 in the presence of 125I-hFSH and increasing doses of unlabelled hFSH (Fig. 4C). The analysis of the cell surface binding data revealed that ADX61623 increased the affinity of interaction between 125I-hFSH and human FSHR (hFSHR) five fold (5.02 ± 0.18; n = 2) with no change in receptor number.
3.4. Assessment of in vivo activity of ADX61623
The pharmacokinetic characteristics of ADX61623 evidenced sustained dose dependent exposure in plasma following s.c. administration (data not shown). Therefore, it was desirable to determine if this compound would block FSH induced oocyte production in vivo. In order to test this hypothesis, it was necessary to determine the minimal effective dose of FSH required for consistent oocyte production. Wistar rats were treated with 0, 0.075, 0.25, 0.75 and 2.5 μg of pituitary hFSH twice a day for two days. The fourth dose of FSH was administered together with hCG 20 IU to induce ovulation of FSH matured follicles. Consistent oocyte production was only achieved with 2.5 μg of pituitary hFSH (Fig. 5). Considerable variation was observed in the total number of oocytes per animal per dose. In the control groups which did not receive FSH, two animals ovulated in response to hCG. However, in the 0.075 μg hFSH group no animals ovulated and there was a significant difference between 0.075 v.2.5 μg dose (P < 0.05) and the 0.075 and 0.75 dose (P < 0.1). FSH also induced an increase in ovarian weight (Fig. 5). There was a significant difference between the 0.075, and 0.25 doses when compared to 0.75 and 2.5 doses of FSH (P < 0.05). There was no significant difference between the saline controls, 0.075 and 0.25 doses.
Fig. 5.

Effect of dose of FSH treatment on ovarian response. Treatment times were in the morning or afternoon of each day. All treatments received 20 IU hCG on the afternoon of day two. Ovarian weights of individual ovaries of immature female rats were determined following treatments with increasing doses of hFSH. Oocytes ovulated after hFSH treatments of Wistar Hanover immature 21–23 days rats were determined as described in Section 2. A preliminary experiment informed the full dose response range of this assay which was carried out with four animals per dose. Data are represented as the SEM of eight data points.
To determine the effect of frequency of dosing, animals were injected once, twice, three times or four times with FSH. This was equivalent to 0, 1, 2, 3 or 4 μg pituitary hFSH total per animal. All animals were treated with hCG on the fourth injection. Although oocyte production could be induced with two injections of pituitary FSH (2 μg total), the result between animals was inconsistent, and there was no significant difference compared to controls with two treatments (Fig. 6). In contrast, three doses of hFSH (3 μg total) produced a significant number of oocytes (P < 0.05) compared to controls. There was an additional benefit of a fourth treatment as well (4 μg; P < 0.05). Ovarian weights of animals treated with hFSH covaried with number of treatments (Fig. 6). There was a difference in ovarian weights even when animals were treated only once with hFSH (P < 0.05). Treatment frequency greater than twice did not significantly affect ovarian weight gain. Based on these results a frequency of four injections of FSH (1 μg each) was used in subsequent experiments.
Fig. 6.

Effect of frequency of FSH treatment on oocyte production and ovarian weight gain. In this single experiment, female 21 day rats were treated with 1 μg of pituitary hFSH 0, 1, 2, 3 or 4 times over a two day period. Treatment times were in the morning or afternoon of each day. All treatments received 20 IU hCG on the afternoon of day two. Five animals were used at 0, 4 and 10 animals were used at 1, 2 and 3 dose frequency in this single experiment. Ovarian weight of individual ovaries and oocytes ovulated after hFSH treatments were determined as described in Section 2.
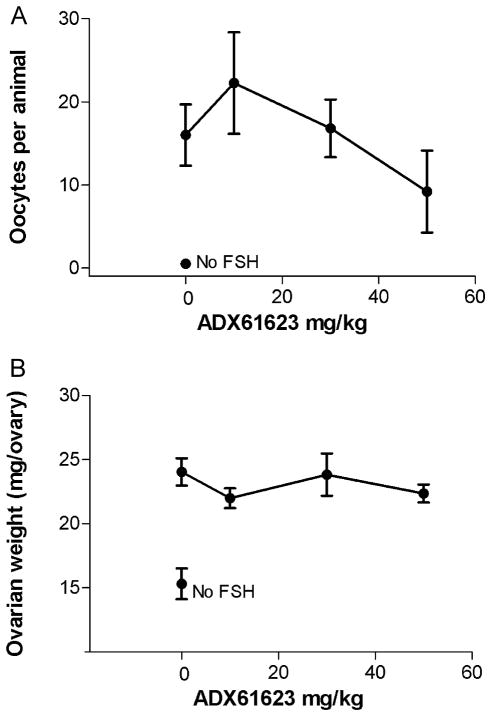
The FSHR NAM ADX61623 was tested in the in vivo bioassay at doses up to 50 mg/kg administered s.c. Oocyte recovery from the ampulla was very low in two animals (0, 1) out of 10 treated with the 50 mg/kg dose (Fig. 7A). In one animal out of 4 not treated with FSH, 2 oocytes were recovered from an ampulla. At all other doses of ADX61623, all animals had oocytes recovered in the ampullae. Since pooled oocyte counts were recorded in this experiment, a level of significance was not demonstrated at the predetermined significance level P = 0.05. No significant decreases in ovarian weight gain were observed with any dose of ADX61623 tested (Fig. 7B). As expected there was a significant increase in ovarian weight when animals were treated with FSH compared to no FSH treatment. Body weights were similar (~43–51 g).
Fig. 7.
(A) Oocytes recovered in ampullae of rats treated with 4 μg of pituitary FSH and ovulated with 20 IU hCG. ADX61623 was tested at 10, 30 and 50 mg/kg and five animals were used per dose. No significant differences were observed. The data are represented as SEM and each point is the mean of ten observations, except for the 10 mg dose and the no FSH dose, where four animals are reported. The experiment was repeated where the highest dose was extended to 100 mg/kg without effect on oocyte production. (B) Ovarian weights of rats treated with 4 μg pituitary hFSH and ovulated with 20 IU hCG.
4. Discussion
In the present study one representative compound ADX61623 which was identified through a high throughput screening campaign of a new chemical class of small molecules is described and fully characterized as a negative allosteric modulator of FSHR. The results show that ADX61623 is a potent FSHR NAM, which possesses some LHR activity and is not active on TSH receptors, two closely related receptors of the glycoprotein hormone receptors (Dias et al., 2002). Interestingly, while this compound partially blocks the signal induced by an EC80 of FSH in recombinant cells expressing the hFSHR, it completely blocks progesterone production induced by this agonist in rat granulosa cells. This difference in efficacy depending on the cell system and/or assay used has been described previously for other allosteric modulators. It is probably the results of different receptor reserves that might be much higher in recombinant systems over-expressing the hFSHR, while it is probably much lower in native cells such as rat granulosa cells, resulting in different degrees of apparent antagonism (Ehlert, 2005). The concept of receptor reserve relates to the concentration of receptor that is required to elicit a full response (Clark and Menon, 1976).
These differences in apparent antagonism were modeled by deriving an operational model of allosteric modulation (Ehlert, 2005; Kenakin, 2005; Leach et al., 2007) from the classical operational model of agonism (Black and Leff, 1983). In this model a very important parameter, called τ was introduced, which incorporates the intrinsic efficacy of orthosteric and allosteric ligands, the total density of receptors and the efficiency of stimulus–response coupling. To investigate the influence of receptor reserve on apparent antagonism, the effects of an allosteric drug were simulated varying the degree of receptor reserve by setting different values of τ. The results demonstrated that increasing concentrations of the allosteric inhibitor induced a high decrease in the maximal response of the agonist concentration–response curve when there is little receptor reserve, which resulted in partial blockade with an intermediate or high level of receptor reserve (Ehlert, 2005).
The discovery that ADX61623 failed to block FSH-induced estradiol biosynthesis was unexpected. In this regard it differed from a previously published FSHR NAM which blocked both progesterone and estradiol production. In ovarian granulosa cells and testicular Sertoli cells synthesis of the steroidogenic acute regulatory protein (StAR), the rate limiting enzyme in the process of progesterone steroidogenesis, is initiated by the cAMP/protein kinase A (PKA) signaling pathway (Stocco et al., 2005; Tremblay et al., 2002). Although FSH induction of the gene that encodes the P450 aromatase (Cyp19) which converts androgen to estrogen is considered to be primarily a cAMP/PKA mediated event, the PI3K/protein kinase B (PKB) pathway has also been identified as an effector of FSH action in granulosa cells (Zeleznik et al., 2003) and Sertoli cells (McDonald et al., 2006). In the female, the successful maturation of a preovulatory follicle involves FSH induced granulosa cell proliferation and differentiation (Ulloa-Aguirre et al., 2007). It has been dogma that FSH stimulates estrogen production in granulosa cells by inducing aromatase expression through activation of the cAMP/PKA pathway. These data challenge the paradigm that one signaling pathway engenders both progesterone and estrogen biosynthesis by granulosa cells: Thus the small molecule negative allosteric modulator (NAM) of FSHR ADX61623 completely blocked cAMP production but not estradiol production.
It was not anticipated that an FSHR NAM would increase FSH binding to the FSH receptor. This suggests that the FSH receptor exists in a metastable state, which in an inactive state can be stabilized for binding. For years, it has been well documented that FSH deglycosylated at asparagine 52 on the alpha subunit can bind to receptor and fail to activate adenylate cyclase (Butnev et al., 2002). However, there has been no formal proof that the transmembrane domains were involved. The present data suggest that the FSHR NAM stabilizes an inactive conformer of the FSH receptor. Opposite effects of a modulator on orthosteric ligand binding relative to function have also been described for an allosteric modulator (Org27569) of the CB1 receptor, another Family I GPCR (Price et al., 2005). While clearly being an allosteric antagonist of efficacy in recombinant and native tissue bioassays, this molecule behaved as an allosteric enhancer of agonist affinity as shown by an increase in [3H]CP 55940 binding. Those findings together with the one described here raise important questions regarding the classical models currently used (allosteric ternary complex and allosteric two state models, for review see (Keov et al., 2010)). In fact, an additional state to the ones already described (active and inactive receptors) needs to be postulated despite the fact that it is very difficult to envisage it thermodynamically. An interesting way of describing those kinds of modulators has been advanced by Keov and collaborators where they called them: “use-dependent antagonists”, a term usually used for modulators of voltage-gated ion channels (Keov et al., 2010).
Recently, it has been demonstrated that glycosylation variants of deglycosylated equine LH, which bind to FSH receptor exhibit biased agonism (Rajagopal et al., 2010), stimulating beta arrestin dependent signaling but not cAMP production through FSH receptor (Wehbi et al., 2010). Interestingly, the deglycosylated equine LH also triggered receptor internalization. The present study is complementary to that work because biased agonism anticipates biased antagonism. The present results demonstrate that biased antagonism at the FSH receptor is also possible. It is only now becoming appreciated that the receptor–effector complexes persist within the cell distinct from the plasma membrane (Calebiro et al., 2009). For example, internalized adrenergic receptor bound to the adaptor protein arrestin continues to signal via alternate pathways (Kovacs et al., 2009). Future studies will aim at elucidating the pathways that contribute specifically to estrogen production, because such an understanding may provide for therapies for estrogen dependent disease.
The FSHR NAM ADX61623, which was a very effective inhibitor of FSH action in vitro, exhibited no statistically significant inhibitory effect in vivo when administered subcutaneously. This may be due to its inability to suppress FSH induced estradiol production. Nevertheless, the effect of the FSHR NAM on estradiol production was clearly surprising and resembles a similar effect observed by Niswender et al. using MMPIP an mGluR7 NAM (Niswender et al., 2010). In that study, MMPIP effectively blocked L-AP4 mediated calcium mobilization while it was completely ineffective in blocking either L-AP4 induced cAMP accumulation or L-AP4-mediated depression of synaptic transmission at the Schaffer collateral-CA1 synapses. The different effect of ADX61623 on progesterone vs. estradiol production is to our knowledge the first example of a compound with the potential for context-dependant blockade or permissive antagonism (Kenakin, 2005) of Family I GPCRs responses by NAM.
The activation of G protein by occupied receptor is considered to be a two step process of coupling and then activation (Herrmann et al., 2004; Nanoff et al., 2006). The three dimensional structures of several GPCRs have become available in recent years and most notably, the newest structure of activated opsin, has provided some insight into how this GPCR activates G protein (Scheerer et al., 2008). That new finding shows that in the presence of activated ligand, transmembrane helix six moves outward creating a binding pocket for the Gαs. That suggests that the FSHR NAM is acting by stabilizing a conformer of FSHR which cannot respond to FSH binding with a conformational change. The increase in binding affinity in the presence of FSHR NAM further suggests that normally, upon binding to FSH the hormone receptor complex enters into a lower affinity metastable binding state.
The simultaneously occupied receptor (agonist + NAM) does not block FSH induced estradiol production suggesting pleiotropy of FSHR action and interaction. If indeed the FSHR NAM prevents a conformational change of FSHR that would allow G protein binding, then it would follow that there are other modes of binding to other G-proteins such as Gq (Escamilla-Hernandez et al., 2008), Gi (Arey et al., 2008) or Gh (Lin et al., 2006). In support of this notion, stimulation of undifferentiated granulosa cells with FSH induced P450 aromatase and LH receptors but forskolin which activates adenylate cyclase has no effect on these two parameters (Bebia et al., 2001). In addition, this pathway may require internalization of a high affinity hormone receptor complex such as the one stabilized by the FSHR NAM, that activates this arm of the FSHR signal transduction pathway. This scenario could involve cytoplasmic adapter proteins (Dias et al., 2005) which may enable transit and directionality of the FSHR signalosome potential effectors of this process. This possibility will be the focus of future efforts.
Acknowledgments
We thank Dr. George Bousfield for the pituitary extract GTN which was used to purify human follicle stimulating hormone and for consulting on the granulosa cell culture assays. We also thank Dr. Gordon Niswender for providing us with antisera which we used for assaying progesterone and estrogen in granulosa cell culture media. We would like to acknowledge the excellent participation of the Wadsworth Center Animal Facility, especially Frank S. Blaisdell, D.V.M., Antigone M. McKenna, D.V.M., Dierdre Torrisi and Jeannine Schneider. Supported by Addex Pharmaceuticals S.A. and by NIH Grant HD18407.
References
- Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- Arey BJ. Allosteric modulators of glycoprotein hormone receptors: discovery and therapeutic potential. Endocrine. 2008;34:1–10. doi: 10.1007/s12020-008-9098-2. [DOI] [PubMed] [Google Scholar]
- Arey BJ, Yanofsky SD, Claudia PM, Holmes CP, Wrobel J, Gopalsamy A, Stevis PE, Lopez FJ, Winneker RC. Differing pharmacological activities of thiazolidinone analogs at the FSH receptor. Biochem Biophys Res Commun. 2008;368:723–728. doi: 10.1016/j.bbrc.2008.01.119. [DOI] [PubMed] [Google Scholar]
- Bebia Z, Somers JP, Liu G, Ihrig L, Shenker A, Zeleznik AJ. Adenovirus-directed expression of functional luteinizing hormone (LH) receptors in undifferentiated rat granulosa cells: evidence for differential signaling through follicle-stimulating hormone and LH receptors. Endocrinology. 2001;142:2252–2259. doi: 10.1210/endo.142.6.8017. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B: Biol Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Brinsden P, Akagbosu F, Gibbons LM, Lancaster S, Gourdon D, Engrand P, Loumaye E. A comparison of the efficacy and tolerability of two recombinant human follicle-stimulating hormone preparations in patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2000;73:114–116. doi: 10.1016/s0015-0282(99)00450-1. [DOI] [PubMed] [Google Scholar]
- Butnev VY, Singh V, Nguyen VT, Bousfield GR. Truncated equine LH beta and asparagine(56)-deglycosylated equine LH alpha combine to produce a potent FSH antagonist. J Endocrinol. 2002;172:545–555. doi: 10.1677/joe.0.1720545. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de FT, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Menon KM. Regulation of ovarian steroidogenesis. The disparity between 125I-labelled choriogonadotropin binding cyclic adenosine 3′,5′-monophosphate formation and progesterone synthesis in the rat ovary. Biochim Biophys Acta. 1976;444:23–32. doi: 10.1016/0304-4165(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink HJ, Fauser BC, Out HJ. Recombinant follicle-stimulating hormone (FSH; Puregon) is more efficient than urinary FSH (Metrodin) in women with clomiphene citrate-resistant, normogonadotropic, chronic anovulation: a prospective, multicenter, assessor-blind, randomized, clinical trial. European Puregon Collaborative Anovulation Study Group. Fertil Steril. 1998;69:19–25. doi: 10.1016/s0015-0282(97)00423-8. [DOI] [PubMed] [Google Scholar]
- Cohen BD, Bariteau JT, Magenis LM, Dias JA. Regulation of follitropin receptor cell surface residency by the ubiquitin-proteasome pathway. Endocrinology. 2003;144:4393–4402. doi: 10.1210/en.2002-0063. [DOI] [PubMed] [Google Scholar]
- Conti M. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod. 2002;67:1653–1661. doi: 10.1095/biolreprod.102.004952. [DOI] [PubMed] [Google Scholar]
- Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. In: Litwack G, editor. Vitamins and Hormones. Academic Press; New York, NY: 2002. pp. 249–322. [DOI] [PubMed] [Google Scholar]
- Dias JA, Nechamen CA, Atari R. Identifying protein interactors in gonadotropin action. Endocrine. 2005;26:241–247. doi: 10.1385/ENDO:26:3:241. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ. Analysis of allosterism in functional assays. J Pharmacol Exp Ther. 2005 Nov;:740–754. doi: 10.1124/jpet.105.090886. [DOI] [PubMed] [Google Scholar]
- Escamilla-Hernandez R, Little-Ihrig L, Zeleznik AJ. Inhibition of rat granulosa cell differentiation by overexpression of Galphaq. Endocrine. 2008;33:21–31. doi: 10.1007/s12020-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389. doi: 10.1210/mend.15.3.0603. [DOI] [PubMed] [Google Scholar]
- Guo T, Adang AEP, Dolle RE, Dong GZ, Fitzpatrick D, Geng P, Ho KK, Kultgen SG, Liu RY, McDonald E, McGuinness BF, Saionz KW, Valenzano KJ, van Straten NCR, Xie D, Webb ML. Small molecule biaryl FSH receptor agonists. Part 1: lead discovery via encoded combinatorial synthesis. Bioorg Med Chem Lett. 2004a;14:1713–1716. doi: 10.1016/j.bmcl.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Guo T, Adang AEP, Dong G, Fitzpatrick D, Geng P, Ho KK, Jibilian CH, Kultgen SG, Liu RY, McDonald E, Saionz KW, Valenzano KJ, van Straten NCR, Xie D, Webb ML. Small molecule biaryl FSH receptor agonists. Part 2: lead optimization via parallel synthesis. Bioorg Med Chem Lett. 2004b;14:1717–1720. doi: 10.1016/j.bmcl.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Guo T, Dong GZ, Fitzpatrick D, Geng P, Ho KK, Jibilian CH, Kultgen SG, Liu RY, McDonald E, Saionz KW, Valenzano KJ, Xie D, Adang AEP, van Straten NCR, Webb ML. Discovery of potent biaryl diketopiperazine FSH receptor agonists: rapid lead optimization through parallel synthesis. Abstr Pap Am Chem Soc. 2004c;228:U911–U912. doi: 10.1016/j.bmcl.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heck M, Henklein P, Henklein P, Kleuss C, Hofmann KP, Ernst OP. Sequence of interactions in receptor-G protein coupling. J Biol Chem. 2004;279:24283–24290. doi: 10.1074/jbc.M311166200. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Lin YF, Tseng MJ, Hsu HL, Wu YW, Lee YH, Tsai YH. A novel follicle-stimulating hormone-induced G alpha h/phospholipase C-delta 1 signaling pathway mediating rat sertoli cell Ca2+-influx. Mol Endocrinol. 2006;20:2514–2527. doi: 10.1210/me.2005-0347. [DOI] [PubMed] [Google Scholar]
- Liu WK, Burleigh BD, Ward DN. Steroid and plasminogen activator production by cultured rat granulosa cells in response to hormone treatment. Mol Cell Endocrinol. 1981;21:63–73. doi: 10.1016/0303-7207(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Maclean D, Holden F, Davis AM, Scheuerman RA, Yanofsky S, Holmes CP, Fitch WL, Tsutsui K, Barrett RW, Gallop MA. Agonists of the follicle stimulating hormone receptor from an encoded thiazolidinone library. J Comb Chem. 2004;6:196–206. doi: 10.1021/cc0300154. [DOI] [PubMed] [Google Scholar]
- McDonald CA, Millena AC, Reddy S, Finlay S, Vizcarra J, Khan SA, Davis JS. Follicle-stimulating hormone-induced aromatase in immature rat sertoli cells requires an active phosphatidylinositol 3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway. Mol Endocrinol. 2006;20:608–618. doi: 10.1210/me.2005-0245. [DOI] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nanoff C, Koppensteiner R, Yang Q, Fuerst E, Ahorn H, Freissmuth M. The carboxyl terminus of the Galpha-subunit is the latch for triggered activation of heterotrimeric G proteins. Mol Pharmacol. 2006;69:397–405. doi: 10.1124/mol.105.016725. [DOI] [PubMed] [Google Scholar]
- Nechamen CA, Dias JA. Human follicle stimulating hormone receptor trafficking and hormone binding sites in the amino terminus. Mol Cell Endocrinol. 2000;166:101–110. doi: 10.1016/s0303-7207(00)00281-1. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Miller NR, Ayala JE, Luo Q, Williams R, Saleh S, Orton D, Weaver CD, Conn PJ. Context-dependent pharmacology exhibited by negative allosteric modulators of metabotropic glutamate receptor 7. Mol Pharmacol. 2010;77:459–468. doi: 10.1124/mol.109.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SS, McKenna S, Arkinstall S. Discovery of new molecules for future treatment of infertility. Reprod Biomed Online. 2005;10:45–54. doi: 10.1016/s1472-6483(11)60390-8. [DOI] [PubMed] [Google Scholar]
- Perez MM, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85:3365–3369. doi: 10.1210/jcem.85.9.6789. [DOI] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, Westwood P, Marrs J, Thomson F, Cowley P, Christopoulos A, Pertwee RG, Ross RA. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Schmidt A, MacColl R, Lindau-Shepard B, Buckler DR, Dias JA. Hormone-induced conformational change of the purified soluble hormone binding domain of follitropin receptor complexed with single chain follitropin. J Biol Chem. 2001;276:23373–23381. doi: 10.1074/jbc.M100057200. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Braun T, Nikolics K, Segaloff DL, Seeburg PH. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol. 1990;4:525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Hamel F, Viger RS. Protein kinase A-dependent cooperation between GATA and CCAAT/enhancer-binding protein transcription factors regulates steroidogenic acute regulatory protein promoter activity. Endocrinology. 2002;143:3935–3945. doi: 10.1210/en.2002-220413. [DOI] [PubMed] [Google Scholar]
- Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, Bourrier E, Ansanay H, Leroy C, Michaud A, Durroux T, Maurel D, Malhaire F, Goudet C, Pin JP, Naval M, Hernout O, Chretien F, Chapleur Y, Mathis G. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem. 2006;358:126–135. doi: 10.1016/j.ab.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Zarinan T, Pasapera AM, Casas-Gonzalez P, Dias JA. Multiple facets of follicle-stimulating hormone receptor function. Endocrine. 2007;32:251–263. doi: 10.1007/s12020-008-9041-6. [DOI] [PubMed] [Google Scholar]
- van Straten NCR, van Berkel THJ, Demont DR, Karstens WJF, Merkx R, Oosterom J, Schulz J, van Someren RG, Timmers CM, van Zandvoort PM. Identification of substituted 6-amino-4-phenyltetrahydroquinoline derivatives: potent antagonists for the follicle-stimulating hormone receptor. J Med Chem. 2005;48:1697–1700. doi: 10.1021/jm049676l. [DOI] [PubMed] [Google Scholar]
- Wehbi V, Tranchant T, Durand G, Musnier A, Decourtye J, Piketty V, Butnev VY, Bousfield GR, Crepieux P, Maurel MC, Reiter E. Partially deglycosylated equine LH preferentially activates beta-arrestin-dependent signaling at the follicle-stimulating hormone receptor. Mol Endocrinol. 2010;24:561–573. doi: 10.1210/me.2009-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Vensel T, Sistrom CL, Kipersztok S, Rhoton-Vlasak A, Drury K. Pregnancy rates in varying age groups after in vitro fertilization: a comparison of follitropin alfa (Gonal F) and follitropin beta (Follistim) Am J Obstet Gynecol. 2003;189:342–346. doi: 10.1067/s0002-9378(03)00728-2. [DOI] [PubMed] [Google Scholar]
- Yanofsky SD, Shen ES, Holden F, Whitehorn E, Aguilar B, Tate E, Holmes CP, Scheuerman R, Maclean D, Wu MM, Frail DE, Lopez FJ, Winneker R, Arey BJ, Barrett RW. Allosteric activation of the follicle-stimulating hormone (FSH) receptor by selective, nonpeptide agonists. J Biol Chem. 2006;281:13226–13233. doi: 10.1074/jbc.M600601200. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Saxena D, Little-Ihrig L. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 2003;144:3985–3994. doi: 10.1210/en.2003-0293. [DOI] [PubMed] [Google Scholar]