Abstract
Fully-automated radiosynthesizers are continuing to be developed to meet the growing need for the reliable production of positron emission tomography (PET) tracers made under current good manufacturing practice (cGMP) guidelines. There is a current trend towards supporting “kit-like” disposable cassettes that come preconfigured for particular tracers, thus eliminating the need for cleaning protocols between syntheses and enabling quick transitions to synthesizing other tracers. Though ideal for production, these systems are often limited for the development of novel tracers due to pressure, temperature, and chemical compatibility considerations. This study demonstrates the versatile use of the ELIXYS fully-automated radiosynthesizer to adapt and produce eight different 18F-labeled PET tracers of varying complexity.
Methods
Three reactor syntheses of D-[18F]FAC, L-[18F]FMAU, and D-[18F]FEAU along with the one reactor syntheses of D-[18F]FEAU, [18F]FDG, [18F]FLT, [18F]Fallypride, [18F]FHBG, and [18F]SFB were all produced using ELIXYS without the need for any hardware modifications or reconfiguration. Synthesis protocols were adapted, and slightly modified from literature, but not fully optimized. Furthermore, [18F]FLT, [18F]FDG, and [18F]Fallypride were produced sequentially on the same day and used for preclinical imaging of A431 tumor-bearing SCID mice and wild-type BALB/c mice, respectively. To assess future translation to the clinical setting, several batches of tracers were subjected to a full set of quality control tests.
Results
All tracers were produced with radiochemical yields comparable to those in literature. [18F]FLT, [18F]FDG, and [18F]Fallypride were successfully used to image the mice with results consistent with literature. All tracers subjected to clinical quality control tests passed.
Conclusion
The ELIXYS radiosynthesizer facilitates rapid tracer development and is capable of producing multiple 18F-labeled PET tracers suitable for clinical applications using the same hardware setup.
Keywords: Automated radiosynthesis, radiochemistry kits, [18F]FAC, [18F]FEAU, [18F]FMAU
INTRODUCTION
Positron emission tomography (PET) is a powerful tool for in vivo imaging, and the diversity of its research and clinical uses have significantly grown in the recent years (1). To support these PET studies, many fully-automated radiosynthesizers have been developed to aid radiochemists in routinely producing PET tracers (2). For [18F]fluoride radiochemistry (3,4), these systems are typically based on either a fixed-tubing design, that requires cleaning after every synthesis run, or a disposable cassette approach (5,6). Some fixed-tubing systems can be modified to produce different tracers, though it can be difficult for operators to reconfigure both the tubing layout and software for new tracers. Furthermore, once a fixed system is configured and optimized for a particular tracer, the radiochemist is often reluctant to modify any aspects of the synthesizer, or use the same system for another tracer, in order to maintain reproducible production conditions. Disposable cassette-based systems enable the radiochemist to purchase pre-configured cassettes and software programs to avoid the need for operator reconfiguration while enabling the production of several different tracers on the same system. The avoidance of cleaning simplifies operation and expedites compliance with current good manufacturing practice (cGMP) guidelines by eliminating the need for validated cleaning methodologies. However, cassette-based systems are often not amenable to custom modifications or novel tracer development, and thus tracer diversity is limited by the spectrum of kits available from the manufacturer making them less desirable in research environments.
Within each of the two classes of radiosynthesizers, the features of the system ultimately dictate their capacity for performing complex reactions. Examples of such features include the number of reaction vessels and intermediate purification capabilities, reaction conditions (e.g. temperature and pressure limitations), and reagent compatibility (e.g. volatility and corrosiveness). In some cases, significant modifications to the reaction conditions or even the synthesis pathway have been necessary to adapt optimized manual protocols to the capabilities of existing automated radiosynthesizers, increasing the barriers to synthesis automation.
To address these current challenges in automated radiosynthesis, we combined the benefits of both classes and have developed a fully-automated three-reactor radiosynthesizer (ELIXYS) that is based on a disposable-cassette design but is capable of handling high reaction temperatures and pressures (7). The unique reagent delivery and vial sealing mechanisms allow fluidic connections to be dynamically configured in software, rather than hardware, to accommodate diverse synthesis protocols without need for any custom plumbing modifications. The versatility of the radiosynthesizer is accomplished by mobilizing the reaction vessels, which removes the need for large numbers of valves, including those that isolate the reaction vessel during reaction steps (and that can lead to limitations in reaction temperatures). This allows for a single disposable cassette design to be used for developing and reliably producing a wide variety of PET tracers encompassing a broad range of reaction conditions and complexities. The system is, therefore, suitable for both novel tracer development and also supports routine production of multiple tracers on a single system (7).
Synthesis protocols are generated using the accompanying user-friendly software that allows the user to string together sequences of chemistry-oriented unit operations (8). The cassettes were designed to handle any combination of the unit operations (e.g. Add Reagent, React, Evaporate), therefore different tracers could be synthesized using the same cassette design. For more complex syntheses, up to three reactors can be linked with optional intermediate purification (i.e. trapping of products on solid phase extraction (SPE) cartridges and subsequently eluting them) between each reactor.
Here, we demonstrate the flexibility of the ELIXYS radiosynthesizer through reproducibly synthesizing various [18F]fluoride-labeled PET tracers with known chemistry: 2-deoxy-2-[18F]fluoro-β-D-arabinofuranosylcytosine (D-[18F]FAC), 2-deoxy-2-[18F]fluoro-5-methyl-β-L-arabinofuranosyluracil (L-[18F]FMAU), 2-deoxy-2-[18F]fluoro-5-ethyl-β-D-arabinofuranosyluracil (D-[18F]FEAU), 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG), 3-deoxy-3-[18F]fluoro-L-thymidine ([18F]FLT), (S)-N-((1-Allyl-2-pyrrrolidinyl)methyl)-5-(3-[18F]fluoropropyl)-2,3-dimethoxybenzamide ([18F]Fallypride), 9-(4-[18F]-fluoro-3-hydroxymethylbutyl)-guanine ([18F]FHBG), and N-succinimidyl-4-[18F]fluorobenzoate ([18F]SFB). Batches that were subject to quality control (QC) analysis passed all tests. We further show that the three reactors can be leveraged to sequentially synthesize multiple single-reactor tracers ([18F]FLT, [18F]FDG, and [18F]Fallypride) with very minimal manual intervention to enable preclinical imaging with multiple tracers in the same day from a single synthesizer.
MATERIALS AND METHODS
Materials
No-carrier-added [18F]fluoride was produced by the (p,n) reaction of [18O]H2O (84%, 98% isotopic purity, Medical Isotopes; Pelham, NH, USA) in a RDS-112 cyclotron (Siemens; Knoxville, TN, USA) using a 1 mL target (11 MeV) or in a PETtrace (GE Healthcare, Milwaukee, WI, USA) using a 2.5 mL target (16 MeV). Anhydrous grade acetonitrile (MeCN), dimethylsulfoxide (DMSO), ethyl acetate, toluene, 1,2-dichloroethane (DCE), dicholoromethane (DCM), methanol (MeOH), 2,3-dimethyl-2-butanol (thexyl alcohol), hexane, N,N,N′,N′-Tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate (TSTU), potassium carbonate (K2CO3), potassium bicarbonate (KHCO3), potassium phosphate monobasic (KH2PO4), ammonium phosphate monobasic (NH4H2PO4), ammonium acetate (NH4OAc), ammonium formate, 0.5 M sodium methoxide (NaOMe) in MeOH, 33% hydrobromic acid in acetic acid (HBr solution), 2 N sodium hydroxide (NaOH), trifluoroacetic acid (TFA), 1 M tetramethylammonium hydroxide in water (TMAOH), 4% tetrabutylammonium hydroxide, triethylamine (TEA), 37% hydrochloric acid (HCl, further diluted with water to form a 6 N solution), ammonium sulfate ((NH4)2SO4), hexamethyldisilazane (HMDS), trimethylsilyl trifluoromethanesulfonate (TMSTfO), and 5-ethyluracil were purchased from Sigma-Aldrich (Milwaukee, WI, USA). 1 N HCl was purchased from Fisher Scientific (Pittsburg, PA, USA). The tC18 (WAT036810) and silica cartridges (WAT020520 and WAT043400) were purchased from Waters (Milford, MA, USA). FDG purification cartridges (Chromabond® Set V, 730883.1129785) were purchased from MACHEREY-NAGEL GmbH & Co. KG (Düren, Germany). 0.45 μm regenerated cellulose (RC) membrane syringe filters (AF0-2103-12) were purchased from Phenomenex (Torrance, CA, USA). F18 Trap & Release Cartridges (MP1) for the one reactor D-[18F]FEAU synthesis were purchased from ORTG (Oakdale, TN, USA). 2-O-(trifluoromethylsulfonyl)-1,3,5-tri-O-benzoyl-alpha-D-ribofuranose (D-sugar), 2-O-(trifluoromethylsulfonyl)-1,3,5-tri-O-benzoyl-alpha-L-ribofuranose (L-sugar), bis(trimethylsilyl)cytosine (FAC precursor), 5-methyl-2,4-bis[(trimethylsilyl)oxy]pyrimidine (FMAU precursor), mannose triflate, FB-precursor, 3-N-Boc-5′-O-dimethoxytrityl-3′-O-nosyl-thymidine (FLT precursor), Tosyl-Fallypride, Tosyl-FHBG, 4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane (Kryptofix K222), tetrabutylammonium bicarbonate (TBAHCO3), preconditioned quarternary methylammonium (QMA) cartridges, sterile collection vials, and all cold standards were purchased from ABX (Advanced Biochemical Compounds; Radeberg, Germany). D-sugar and TBAHCO3 used for the one reactor D-[18F]FEAU was prepared as reported by Chin et al (9). 200-proof ethanol (EtOH) was purchased from the UCLA Chemistry Department (Los Angeles, CA, USA). Any water used was purified to 18 MΩ and 0.1 μm filtered for all cleaning, preparation, syntheses, and purification. Unless otherwise specified, all reagents were used as received.
Radiosynthesizer setup
QMA cartridges were used as received. MP1 cartridges were preconditioned with 1 mL of a 0.84 M KHCO3 solution, rinsed with 1 mL of water, and then dried under helium. FDG purification and tC18 cartridges were preconditioned with 5 mL of EtOH followed by 10 mL of water, and silica cartridges with 10 mL of anhydrous hexane. Unless otherwise specified, all conditioned cartridges were kept wet prior to use.
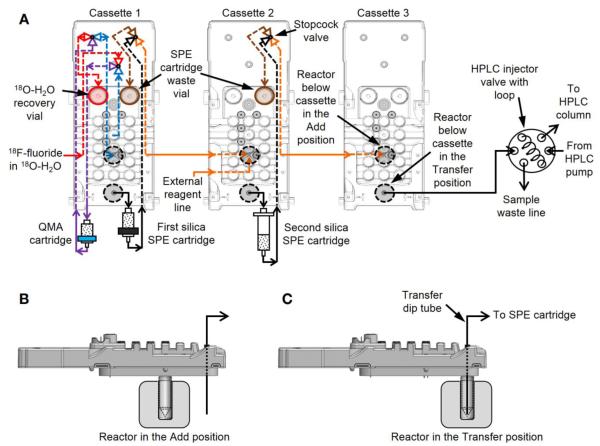
5mL glass V-vials (W986259NG, Wheaton; Millville, NJ, USA) with magnetic stir bars (14-513-65, Fisher Scientific) were installed to serve as the reaction vessel(s) for each synthesis. Reagent vials were prepared with the respective reagents and solvents and loaded into disposable cassettes (Supplemental Table 1), and the cassettes were subsequently installed into the system. For multi-reactor syntheses, the installation of intermediate purification cartridges and connections between cassettes were made via Luer-fittings pre-assembled on the cassettes (Figure 1). When high performance liquid chromatography (HPLC) purification was needed, the last cassette in the synthesis was connected to the HPLC injector valve, and samples were loaded remotely into the HPLC system.
FIGURE 1.
Disposable cassette fluidic paths used for the three-reactor syntheses. (A) Top-view with fluidic connections inside (dashed) and outside (solid) each cassette, including purification cartridges. Side view of the cassettes display the reactor in the Add position (B), where fluid is delivered into the reaction vessel, and the Transfer position (C), where fluid is transferred out of the reaction vessel.
Chromatography
Semi-preparative HPLC was performed with a WellChrom K-501 HPLC pump (Knauer; Berlin, Germany) or Dionex P680 quaternary gradient pump (Fisher Scientific), reversed-phase Gemini-NX column (5 μm, 10 × 250 mm, Phenomenex) or Luna (5 μm, 10 × 250 mm, Phenomenex) columns, ultraviolet (UV) detector (254 nm, WellChrom Spectro-Photometer K-2501 or K-2001, Knauer) and gamma-radiation detector and counter (B-FC-3300 and B-FC-1000; Bioscan Inc.; Washington, DC, USA). Analytical HPLC was performed on a Knauer Smartline HPLC system with a Phenomenex reverse-phase Luna column (5 μm, 4.6 × 250 mm) with inline Knauer UV (254 nm) and gamma-radiation coincidence detector and counter (B-FC-4100 and B-FC-1000; Bioscan, Inc.), or an Agilent 1200 Series (Agilent Technology; Santa Clara, CA, USA) with ChemStation software equipped with a quaternary pump, UV diode-array detector, and model 105S single-channel radiation detector using a Phenomenex Gemini C18 column (5 μm, 250 × 4.6 mm). Unless otherwise specified, chromatograms were collected by a GinaStar (raytest USA, Inc.; Wilmington, NC, USA) analog-to-digital converter and GinaStar software (raytest USA, Inc.). HPLC mobile phases, flow rates, and retention times are listed in Supplemental Table 2. Radio-thin-layer-chromatography (radio-TLC) was performed on a miniGita Star (raytest USA, Inc.) using precut silica plates (Baker-flex®, J.T.Baker; Phillipsburg, NJ, USA) developed in 95% MeCN in water (v/v).
Synthesis of tracers
For each of the eight tracers, a synthesis program was created and tested using the ELIXYS software (8). After setup and cassette installation, the program was run. Note that after all reaction and evaporation operations, the reactor was cooled to 35°C, with the exception of the [18F]fluoride and base solution drying where cooling was only applied after the last azeotropic drying step. All evaporation steps were performed by supplying both vacuum and inert gas to the reaction vessel while heating. Unless otherwise stated, inert gas supply during evaporation was set to 10 psig. All reaction steps were performed with stirring. Both the three-reactor and one-reactor D-[18F]FEAU syntheses were conducted at the Cyclotron and Radiochemistry Facility in the Stanford University Medical Center Department of Radiology, while all other tracers were synthesized at the Crump Radiochemistry and Cyclotron Technology Center at University of California, Los Angeles.
[18F]fluoride loading
Preparation of [18F]fluoride was performed in the same manner for all the tracers listed in this study. A vial of [18F]fluoride in [18O]H2O was connected via the dedicated transfer line to the first cassette on ELIXYS and transferred via pressure supplied by an inert gas line from the system. Starting radioactivity ranged from 0.3 – 37 GBq (8 mCi – 1 Ci) and averaged around 3.7 GBq (100 mCi).
[18F]fluoride was then trapped by passing the solution through a preconditioned strong anion-exchanging QMA or MP1 cartridge that allowed the [18O]H2O to be collected in a recovery vial installed on the cassette. Release of the [18F]fluoride was achieved by passing a base solution (Supplemental Table 1) to release the [18F]fluoride from the cartridge. The [18F]fluoride and base solution eluate was delivered to reaction vessel one and subsequently dried at 110°C until almost dry (2.5 min). Anhydrous MeCN (1 mL) was then added to reaction vessel one to azeotropically remove water from the solution at 110°C until complete dryness (1.5 min). This was repeated one additional time to ensure activation (i.e. sufficient removal of water).
Three reactor syntheses of D-[18F]FAC, L-[18F]FMAU, and D-[18F]FEAU
Syntheses of these nucleoside tracers were adapted from literature (7,10–17). Programmed unit operations were identical for the synthesis of each tracer and varied slightly from previously published work (7). Details are described in the Supplemental Method section.
One reactor synthesis of D-[18F]FEAU
One reactor synthesis of D-[18F]FEAU is based on previously reported methods (9,18). D-sugar solution (10 mg in 1 mL MeCN, 17 mM) was added to dried tetrabutylammonium [18F]fluoride in reaction vessel one and reacted at 160°C for 15 min. After 18F-labeling, the solvent was evaporated at 110°C (2.5 min). A fresh 5-ethyluracil solution was previously prepared by mixing 30 mg 5-ethyluracil, 4.5 mg (NH4)2SO4, 200 μL HMDS, and 200 μL MeCN and refluxing the mixture at 95°C under helium for 1 hr, then all liquid was removed by evaporation and the residue was reconstituted with 100 μL HMDS, 150 μL TMSTfO and 150 μL DCM. The 5-ethyluracil solution was added to the dried fluorinated sugar. Coupling reaction was then performed at 95°C for 30 min, and afterwards the solvent was evaporated at 85°C (5 min). 2.5 M NaOMe in MeOH (1.5 mL) was then added and reacted at 105°C for 5 min. 6 N HCl (1 mL) was then added to quench the reaction. The crude product was partially evaporated at 85°C for 1.5 min to remove MeOH. 1 mL water was added to the reaction vessel, and the mixture was passed through a filter (0.45 μm RC membrane, Phenomenex) before loading onto the semi-preparative HPLC.
[18F]FDG
Synthesis was adapted from multiple literature reports with slight modifications (19,20). Mannose triflate (20 mg in 0.7 mL MeCN, 59 mM) was added to the activated [18F]fluoride residue in reaction vessel one, and the solution reacted at 130°C for 3 min. After the [18F]fluorination reaction, water (2 mL) was added to reaction vessel one and the solution mixed for 15 s. The solution was then passed through a preconditioned tC18 cartridge to trap the fluorinated intermediate, and waste was collected in a vial on the cassette. The tC18 cartridge was subsequently washed with water (2 mL) and sent to waste. For deprotection of the intermediate, 2 N NaOH (2 mL) at room temperature was slowly (4 psig) passed through the tC18 cartridge, and then immediately to the FDG purification cartridge. Before entering a vented sterile collection vial, the product was passed through a 0.22 μm sterile filter. Five subsequent rinses with water (2 mL) were performed to remove product from the cartridges. Radiochemical purity was determined by radio-TLC.
[18F]FLT
Synthesis was adapted from multiple literature sources with slight modifications (21–26). FLT precursor (30 mg in 0.25 mL MeCN and 0.75 mL thexyl alcohol, 36 mM) was added to the activated [18F]fluoride residue in reaction vessel one, and the solution reacted at 120°C for 6 min. The crude fluorinated product solution was evaporated until almost dry at 100°C (3.5 min). 1 N HCl (1 mL) was then added to reaction vessel one and the solution reacted at 130°C for 5 min. 2 N NaOH (0.5 mL) was added to reaction vessel one to neutralize. HPLC mobile phase (1 mL, Supplemental Table 2) was added to reaction vessel one, and the mixture was passed through a filter (0.45 μm RC membrane, Phenomenex) before loading onto the semi-preparative HPLC.
[18F]Fallypride
Synthesis was adapted from literature with slight modifications (27). Tosyl-Fallypride (4 mg in 0.3 mL of MeCN and 0.7 mL thexyl alcohol, 8 mM) was added to the activated [18F]fluoride residue in reaction vessel one, and the solution reacted at 105°C for 7 min. The crude fluorinated product solution in reaction vessel one was evaporated to dryness at 100°C (3 min) and reconstituted in HPLC mobile phase (1 mL, Supplemental Table 2) prior to injection into semi-preparative HPLC for purification.
[18F]FHBG
Synthesis was adapted from multiple literature sources with slight modifications (28–30). Tosyl-FHBG (4 mg in 1 mL MeCN, 4 mM) was added to the activated [18F]fluoride residue in reaction vessel one, and the solution reacted at 135°C for 10 min. Solution was evaporated until almost dry at 110°C (2 min). 1 N HCl (1 mL) was then added to reaction vessel one and the solution reacted at 105°C for 5 min. 2 N NaOH (0.5 mL) was added to reaction vessel one to neutralize. HPLC mobile phase (1 mL, Supplemental Table 2) was added to reaction vessel three prior to injection into semi-preparative HPLC for purification.
[18F]SFB
Synthesis was adapted from multiple literature sources with slight modifications (31–37). FB-precursor (5 mg in 1 mL DMSO, 14mM) was added to the activated [18F]fluoride residue in reaction vessel one, and the solution reacted at 110°C for 15 min. 1 M TMAOH solution (0.025 mL in 1 mL MeCN) was then added to reaction vessel one, and the solution was evaporated at 110°C for 7 min with 5 psig inert gas supply. Immediately after evaporation, anhydrous MeCN (2 mL) was added to reaction vessel one, and the solution was evaporated at 110°C for 3 min with 5 psig inert gas supply to further remove traces of water. The TSTU solution (22.8 mg in 1 mL MeCN, 76 mM) was then added to reaction vessel one, and the mixture was reacted at 110°C for 5 min. HPLC mobile phase (1 mL, Supplemental Table 2) was added to reaction vessel three prior to injection into semi-preparative HPLC for purification.
Post-synthesis procedures and measurements
Starting activity was assayed prior to transfer into the first cassette, and start of synthesis was marked at the initiation of the automated synthesis program. All activity measurements were collected from a calibrated CRC 25PET dose calibrator (Capintec, Inc.; Ramsey, NJ, USA) and decay corrected to the start of synthesis. For all HPLC purified samples, the purified fraction from semi-preparative HPLC was collected through a selector valve into a collection tube, marking the end of synthesis. The collected purified fraction was assayed, and a sample taken to analytical HPLC for radio-purity analysis and verification of identity by co-injection with cold standard. Specific activity was determined via analytical HPLC and calculated using the activity of the sample at time of injection into HPLC. The sample’s mass was determined by a standard curve created for each tracer. For all tracers, radiochemical yield (RCY) was calculated as the percentage of starting activity that was collected as purified product. For [18F]FDG, radiochemical purity was determined by radio-TLC.
Sequential syntheses of three tracers with preclinical imaging
To demonstrate the high-throughput capabilities of the ELIXYS synthesizer, [18F]FLT, [18F]FDG, and [18F]Fallypride were synthesized sequentially in a single day on the same instrument with very minimal manual intervention. Each tracer was synthesized in one of the reactors using the above methods, with the exception that a formulation step was performed, and had its own reagents, cartridges, and starting [18F]fluoride solution. Preparation for all three syntheses was completed prior to introducing radioactivity into the system. Since ELIXYS currently has only one inert gas supply for transferring [18F]fluoride to the cassette, an inert gas stopcock manifold mounted outside of the hot cell was used to remotely control the pressurization of each vial containing [18F]fluoride solution. Also, since both [18F]FLT and [18F]Fallypride require HPLC purification, one quick manual swap of the lines to the HPLC injection valve was required during the [18F]FDG synthesis. These two tracers required the same semi-preparative HPLC column, therefore the HPLC system could be cleaned and reconditioned without exposure. All tracers were reformulated for preclinical imaging after purification. All QC testing was performed using the reformulated products. After purification, [18F]FLT and [18F]Fallypride were rotary evaporated, dissolved with pH 7.4 phosphate buffered saline (PBS), and transferred to a sterile vial for injection. For [18F]FDG, the purified product was delivered through a 0.22 μm sterile filter into a sterile vial to which 3 mL of PBS was previously added, thus making a 15 mL final solution.
For the preclinical imaging of [18F]FLT, six 6–8 week old female severe combined immunodeficient (SCID) mice were injected subcutaneously with 1.0×106 A431 cells in a 1:1 mixture of DMEM:Matrigel (BD Biosciences; San Jose, CA, USA). For [18F]FDG and [18F]Fallypride imaging, two and four non-tumor bearing 9-10 week old BALB/c mice were used, respectively. Micro-PET was performed as previously described (7). Briefly, conscious tumor-bearing SCID or non-tumor bearing BALB/c mice were injected with 20 μCi of [18F]FLT, [18F]FDG, or [18F]Fallypride followed by 60 min uptake. Prior to scanning, mice were anesthetized with 2% isoflurane and placed in a dedicated imaging chamber (Sofie Biosciences, Inc.; Culver City, CA, USA). Whole-body PET images were acquired using a GENISYS4(Sofie Biosciences, Inc.) and images were acquired for 10 min and analyzed using OsiriX Imaging software (Pixmeo; Geneva, Switzerland). All animal experiments were approved by the UCLA Animal Research Committee and were performed according to the guidelines of the Division of Laboratory Animal Medicine at UCLA.
Quality control analysis
Multiple batches of D-[18F]FAC and [18F]FDG were subjected to QC testing for clinical use. The D-[18F]FAC samples were reformulated by rotary evaporation of the HPLC purified fractions, dissolving with PBS, and transferring the product through a sterile filter to a sterile vial prior to testing. The [18F]FDG batches did not require further reformulation after purification. These tracers were chosen as models for multi-reactor HPLC purified and single-reactor SPE cartridge purified methods of production. The tests included optical clarity via visual inspection, filter integrity test, pH, radionuclide identity and purity via half-life and energy, radiochemical identity and purity via analytical HPLC, residual solvent analysis via gas chromatography, Kryptofix K222 spot test, pyrogenicity, and sterility. Detailed methods for these tests can be found in our previous work (17).
RESULTS
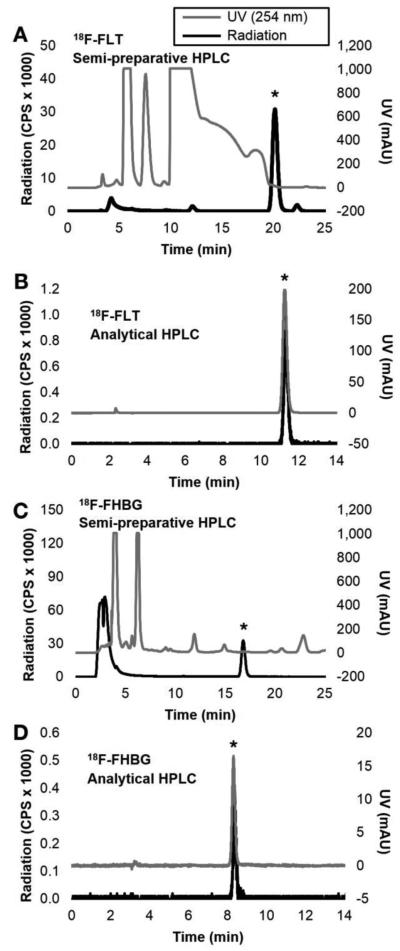
Summary of decay-corrected RCY, duration of synthesis (including purification, not including reformulation), and specific activity at time of injection into analytical HPLC is listed in Table 1 for the individual syntheses. All tracers had greater than 99% radiochemical purity. Examples of the semi-preparative and co-injected analytical HPLC chromatograms can be found in Figure 2.
TABLE 1.
Summary of synthesis data
| Tracer | Decay corrected Radiochemical yield (%) |
Synthesis duration (min)* |
Specific activity (GBq/μmol [Ci/μmol])† |
|---|---|---|---|
| D-[18F]FAC | 31 ± 4 (n=11) | 163 | 37 – 44 [1.0 – 1.2] |
|
| |||
| L-[18F]FMAU | 49 ± 7 (n=7) | 170 | 100 – 170 [2.7 – 4.6] |
|
| |||
| D-[18F]FEAU (three reactor) | 39 ± 4 (n=3) | 180 | 3.8 – 5.1 [0.10 – 0.14]‡ |
|
| |||
| D-[18F]FEAU (one reactor) | 28 ± 4 (n=3) | 140 | 7.4 – 20 [0.20 – 0.53]‡ |
|
| |||
| [18F]FDG | 70 ± 9 (n=3) | 38 | N/A |
|
| |||
| [18F]FLT | 69 ± 3 (n=5) | 65 | 67 – 481 [1.8 – 13] |
|
| |||
| [18F]Fallypride | 66 ± 8 (n=6) | 56 | 15 – 78 [0.4 – 2.1]‡ |
|
| |||
| [18F]FHBG | 11 ± 2 (n=3) | 87 | 92 – 189 [2.5 – 5.1] |
|
| |||
| [18F]SFB | 69 ± 8 (n=6) | 78 | 63 [1.7] |
Radiochemical yields are mean ± SD
Synthesis duration was defined from start of synthesis to end of synthesis, including purification but not including reformulation.
Range of specific activities measured at time of injection into the analytical HPLC.
Starting activity was lower than average and ranged from 1.3 – 1.9 GBq (35 – 50 mCi)
FIGURE 2.
Examples of HPLC chromatograms. [18F]FLT semi-preparative HPLC (A) and analytical HPLC of purified sample with co-injection of standard (B). [18F]FHBG semi-preparative HPLC (C) and analytical HPLC of purified sample with co-injection of standard (D). (*) Denotes the radioactive peak of interest. Chromatography conditions found in Supplemental Table 2.
[18F]FLT required an additional 20 min for reformulation (total synthesis duration of 85 min), and [18F]Fallypride reformulation required 10 min (total synthesis duration of 66 min). Analysis of the reformulated products showed no distinguishable variation in purity to their respective HPLC purified samples collected from the individual syntheses. Yields, synthesis durations, and specific activities of the three tracers likewise were equivalent to having been synthesized individually. Additionally, the pH (7–7.5) and the radiochemical purity were acceptable for both preclinical and clinical applications.
Sequentially synthesized [18F]FLT, [18F]FDG, and [18F]Fallypride were evaluated in vivo on the same day by microPET. High [18F]FLT tumor uptake in A431 tumor bearing mice was observed (Figure 3A), consistent with previous results (38). In non-tumor bearing mice, [18F]FDG demonstrated the anticipated uptake in brain and heart (Figure 3B) while the striatum could be clearly identified with [18F]Fallypride (Figure 3C) (39).
FIGURE 3.
Micro-PET imaging using same-day sequentially synthesized PET tracers on a single automated radiosynthesizer. Sample images for [18F]FLT (A), [18F]FDG (B), and [18F]Fallypride (C). Percent injected dose per gram (%ID/g) scale is listed for each image. BL: bladder; BM: bone marrow; GB: gallbladder; GI: gastrointestinal tract; HR: heart; KD: kidneys; LG: lacrimal glands; ST: striatum; TM: A431 implanted tumor.
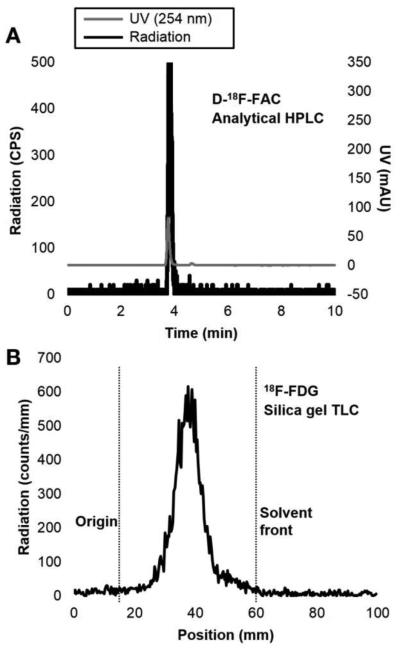
The reformulated batches of D-[18F]FAC and [18F]FDG passed full clinical QC testing, and their respective chromatograms can be found in Figure 4. The specific activity and pH of all the reformulated products were within acceptable range for both clinical and preclinical production.
FIGURE 4.
Chromatograms of purified and reformulated products. (A) D-[18F]FAC analytical HPLC with co-injection of standard. (B) [18F]FDG radio-TLC. Chromatography conditions found in Supplemental Table 2.
DISCUSSION
The various radiotracer syntheses were performed on the ELIXYS radiosynthesizer without hardware modifications regardless of complexity of the synthesis or reagents utilized. The only changes needed were in the programmed sequences, the reagents and purification cartridges installed in the cassettes, and the methods of HPLC purification. This contrasts other fully-automated cassette-based radiosynthesizers currently available (e.g. IBA Synthera®, GE FASTlab and TRACERlab MX, Eckert & Ziegler PharmTracer, TRASIS AllinOne, and Siemens Explora® One models), which tend to be limited in the number of reactors, reagents, purification capabilities, and/or reaction temperatures and pressures. In some reports, synthesizer limitations have been overcome by custom hardware modifications, but this often makes it difficult to accommodate the syntheses of various tracers, particularly novel tracer development, using only one of these systems. Universal cassettes and software-controlled fluid paths utilized by ELIXYS may obviate the need for such custom modifications.
Syntheses performed in this study were not extensively optimized as this was intended as a proof-of-concept demonstration. However, yields and synthesis durations were comparable to literature (7,9–37), and there may be room for improvement with further optimization. The tracers described here included some purified by cartridge and some by semi-preparative HPLC. For multiple sequential syntheses requiring HPLC, it was necessary to clean the HPLC system between syntheses. For several tracers of wide interest (e.g., [18F]FLT (40)), there have been efforts toward developing cartridge purification methods. These methods could be adapted to ELIXYS, thus simplifying the procedure for multi-tracer synthesis.
Three tracers were successfully produced on the same day and used for preclinical imaging using one system. Sample tracers also passed full clinical QC testing. These qualities combined with a wide range of synthesizable 18F-labeled tracers demonstrate the effective use of the ELIXYS radiosynthesizer to adapt previously developed synthesis protocols for immediate transition to production.
CONCLUSION
We have demonstrated the syntheses of eight well-known [18F]fluoride-labeled PET tracers on the ELIXYS radiosynthesizer without the need for any hardware reconfiguration. The software enabled automated synthesis programs to be quickly created from published protocols. All tracers were produced with radiochemical yields and synthesis durations comparable to literature. Several batches were reformulated and passed clinical level QC testing. In addition to supporting diverse synthesis protocols, the three reactors of ELIXYS could be leveraged to accomplish multiple single-reactor syntheses sequentially with very minimal manual intervention.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Department of Energy Office of Biological and Environmental Research (DE-SC0001249 and DE-FG02-06ER64249), the UCLA Foundation from a donation made by Ralph and Marjorie Crump for the Crump Institute for Molecular Imaging, and Sofie Biosciences, Inc. We greatly appreciate Dr. Saman Sadeghi and his staff for providing [18F]fluoride from the UCLA Biomedical Cyclotron Facility. We also thank Lisa Ta, Larry Pang, and Dr. Liu Wei for their assistance with PET imaging studies.
Footnotes
DISCLOSURE
Drs. Moore and van Dam are owners of Sofie Biosciences, Inc. Dr. van Dam is also a consultant for Sofie Biosciences, Inc., and Dr. Moore and Mr. Farhoud are employed by Sofie Biosciences, Inc. Mr. Collins and Mr. Maraglia work in part on collaborative research between UCLA and Sofie Biosciences, Inc. No other conflicts of interest were reported.
FOOT LINE
Diverse PET tracer synthesis on ELIXYS
REFERENCES
- 1.Keng PY, Esterby M, van Dam RM. Emerging Technologies for Decentralized Production of PET Tracers. In: Hsieh C-H, editor. Positron Emission Tomography - Current Clinical and Research Aspects. 2012. pp. 153–182. InTech. [Google Scholar]
- 2.Sachinidis JI, Poniger S, Tochon-Danguy HJ. Automation for Optimised Production of Fluorine-18-Labelled Radiopharmaceuticals. Curr Radiopharm. 2010;3:248–253. [Google Scholar]
- 3.Banister S, Roeda D, Dolle F, Kassiou M. Fluorine-18 Chemistry for PET: A Concise Introduction. Curr Radiopharm. 2010;3:68–80. [Google Scholar]
- 4.Cai L, Lu S, Pike VW. Chemistry with [18F]Fluoride Ion. Eur J Org Chem. 2008;2008:2853–2873. [Google Scholar]
- 5.Boschi S, Lodi F, Malizia C, Cicoria G, Marengo M. Automation synthesis modules review. Appl Radiat Isot. 2013;76:38–45. doi: 10.1016/j.apradiso.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Krasikova R. Synthesis Modules and Automation in F-18 Labeling. In: Schubiger PA, Lehmann L, Friebe M, editors. PET Chemistry. Vol. 62. Springer Berlin Heidelberg; 2007. pp. 289–316. [DOI] [PubMed] [Google Scholar]
- 7.Lazari M, Quinn KM, Claggett SB, et al. ELIXYS - a fully automated, three-reactor high-pressure radiosynthesizer for development and routine production of diverse PET tracers. EJNMMI Res. 2013;3:52. doi: 10.1186/2191-219X-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claggett SB, Quinn KM, Lazari M, Moore MD, van Dam RM. Simplified programming and control of automated radiosynthesizers through unit operations. EJNMMI Res. 2013;3:53. doi: 10.1186/2191-219X-3-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin FT, Namavari M, Levi J, et al. Semiautomated Radiosynthesis and Biological Evaluation of [18F]FEAU: A Novel PET Imaging Agent for HSV1-tk/sr39tk Reporter Gene Expression. Mol Imaging Biol. 2007;10:82–91. doi: 10.1007/s11307-007-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangner TJ, Klecker RW, Anderson L, Shields AF. Synthesis of 2′-deoxy-2′-[18F]fluoro-[beta]-D-arabinofuranosyl nucleosides, [18F]FAU, [18F]FMAU, [18F]FBAU and [18F]FIAU, as potential PET agents for imaging cellular proliferation: synthesis of [18F]labelled FAU, FMAU, FBAU, FIAU. Nucl Med Biol. 2003;30:215–224. doi: 10.1016/s0969-8051(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay U, Pal A, Gelovani JG, Bornmann W, Alauddin MM. Radiosynthesis of 2′-deoxy-2′-[18F]-fluoro-5-methyl-1-β-l-arabinofuranosyluracil ([18F]-l-FMAU) for PET. Appl Radiat Isot. 2007;65:941–946. doi: 10.1016/j.apradiso.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Turkman N, Gelovani JG, Alauddin MM. A novel method for stereospecific fluorination at the 2′-arabino-position of pyrimidine nucleoside: synthesis of [18F]-FMAU. J Label Compd Radiopharm. 2010;53:782–786. [Google Scholar]
- 13.Li Z, Cai H, Conti PS. Automated synthesis of 2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-d-arabinofuranosyluracil ([18F]-FMAU) using a one reactor radiosynthesis module. Nucl Med Biol. 2011;38:201–206. doi: 10.1016/j.nucmedbio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Soghomonyan S, Hajitou A, Rangel R, et al. Molecular PET imaging of HSV1-tk reporter gene expression using [18F]FEAU. Nat Protoc. 2007;2:416–423. doi: 10.1038/nprot.2007.49. [DOI] [PubMed] [Google Scholar]
- 15.Paolillo V, Riese S, Gelovani JG, Alauddin MM. A fully automated synthesis of [18F]-FEAU and [18F]-FMAU using a novel dual reactor radiosynthesis module. J Label Compd Radiopharm. 2009;52:553–558. [Google Scholar]
- 16.Radu CG, Shu CJ, Nair-Gill E, et al. Molecular imaging of lymphoid organs and immune activation using positron emission tomography with a new 18F-labeled 2′-deoxycytidine analog. Nat Med. 2008;14:783–788. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amarasekera B, Marchis PD, Bobinski KP, et al. High-pressure, compact, modular radiosynthesizer for production of positron emitting biomarkers. Appl Radiat Isot. 2013;78:88–101. doi: 10.1016/j.apradiso.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Li Z, Conti PS. The improved syntheses of 5-substituted 2′-[18F]fluoro-2′-deoxy-arabinofuranosyluracil derivatives ([18F]FAU, [18F]FEAU, [18F]FFAU, [18F]FCAU, [18F]FBAU and [18F]FIAU) using a multistep one-pot strategy. Nucl Med Biol. 2011;38:659–666. doi: 10.1016/j.nucmedbio.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Gomzina NA, Vasil′ev DA, Krasikova RN. Optimization of Automated Synthesis of 2-[18F]-Fluoro-2-deoxy-D-glucose Involving Base Hydrolysis. Radiochemistry. 2002;44:403–409. [Google Scholar]
- 20.Lemaire C, Damhaut P, Lauricella B, et al. Fast [18F]FDG synthesis by alkaline hydrolysis on a low polarity solid phase support. J Label Compd Radiopharm. 2002;45:435–447. [Google Scholar]
- 21.Lee SJ, Oh SJ, Chi DY, Lee BS, Ryu JS, Moon DH. Comparison of synthesis yields of 3′-deoxy-3′-[18F]fluorothymidine by nucleophilic fluorination in various alcohol solvents. J Label Compd Radiopharm. 2008;51:80–82. [Google Scholar]
- 22.Oh SJ, Mosdzianowski C, Chi DY, et al. Fully automated synthesis system of 3′-deoxy-3′-[18F]fluorothymidine. Nucl Med Biol. 2004;31:803–809. doi: 10.1016/j.nucmedbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Teng B, Wang S, Fu Z, Dang Y, Wu Z, Liu L. Semiautomatic synthesis of 3′-deoxy-3′-[18F]fluorothymidine using three precursors. Appl Radiat Isot. 2006;64:187–193. doi: 10.1016/j.apradiso.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Tang G, Tang X, Wen F, Wang M, Li B. A facile and rapid automated synthesis of 3′-deoxy-3′-[18F]fluorothymidine. Appl Radiat Isot. 2010;68:1734–1739. doi: 10.1016/j.apradiso.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Yun M, Oh SJ, Ha H-J, Ryu JS, Moon DH. High radiochemical yield synthesis of 3′-deoxy-3′-[18F]fluorothymidine using (5′-O-dimethoxytrityl-2′-deoxy-3′-O-nosyl-β-D-threo pentofuranosyl)thymine and its 3-N-BOC-protected analogue as a labeling precursor. Nucl Med Biol. 2003;30:151–157. doi: 10.1016/s0969-8051(02)00409-2. [DOI] [PubMed] [Google Scholar]
- 26.Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol. 2000;27:143–156. doi: 10.1016/s0969-8051(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 27.Seok Moon B, Hyung Park J, Jin Lee H, et al. Highly efficient production of [18F]fallypride using small amounts of base concentration. Appl Radiat Isot. 2010;68:2279–2284. doi: 10.1016/j.apradiso.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Chang CW, Lin M, Wu SY, et al. A high yield robotic synthesis of 9-(4-[18F]-fluoro-3-hydroxymethylbutyl)guanine ([18F]FHBG) and 9-[(3-[18F]fluoro-1-hydroxy-2-propoxy)methyl]guanine([18F] FHPG) for gene expression imaging. Appl Radiat Isot. 2007;65:57–63. doi: 10.1016/j.apradiso.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Hun Kang S, Jun Oh S, Ju Lee S, et al. Comparison of two full automatic synthesis methods of 9-(4-[18F]fluoro-3-hydroxymethylbutyl)guanine using different chemistry modules. Appl Radiat Isot. 2009;67:1758–1763. doi: 10.1016/j.apradiso.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Niedermoser S, Pape M, Gildehaus FJ, et al. Evaluation of an automated double-synthesis module: efficiency and reliability of subsequent radiosyntheses of FHBG and FLT. Nucl Med Biol. 2012;39:586–592. doi: 10.1016/j.nucmedbio.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Hou S, Phung DL, Lin W-Y, Wang M, Liu K, Shen CK-F. Microwave-assisted One-pot Synthesis of N-succinimidyl-4-[18F]fluorobenzoate ([18F]SFB) J Vis Exp. 2011;(52):e2755. doi: 10.3791/2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann U, Yeoh SD, Sachinidis JI, Poniger SS, Scott AM, Tochon-Danguy HJ. A simplified protocol for the automated production of succinimidyl 4-[18F]fluorobenzoate on an IBA Synthera module. J Label Compd Radiopharm. 2011;54:671–673. [Google Scholar]
- 33.Bejot R, Elizarov AM, Ball E, et al. Batch-mode microfluidic radiosynthesis of N-succinimidyl-4-[18F]fluorobenzoate for protein labelling. J Label Compd Radiopharm. 2011;54:117–122. [Google Scholar]
- 34.Tang G, Tang X, Wang X. A facile automated synthesis of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) for 18F-labeled cell-penetrating peptide as PET tracer. J Label Compd Radiopharm. 2010;53:543–547. [Google Scholar]
- 35.Johnström P, Clark JC, Pickard JD, Davenport AP. Automated synthesis of the generic peptide labelling agent N-succinimidyl 4-[18F]fluorobenzoate and application to 18F-label the vasoactive transmitter urotensin-II as a ligand for positron emission tomography. Nucl Med Biol. 2008;35:725–731. doi: 10.1016/j.nucmedbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Marik J, Sutcliffe JL. Fully automated preparation of n.c.a. 4-[18F]fluorobenzoic acid and N-succinimidyl 4-[18F]fluorobenzoate using a Siemens/CTI chemistry process control unit (CPCU) Appl Radiat Isot. 2007;65:199–203. doi: 10.1016/j.apradiso.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Mäding P, Füchtner F, Wüst F. Module-assisted synthesis of the bifunctional labelling agent N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) Appl Radiat Isot. 2005;63:329–332. doi: 10.1016/j.apradiso.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Waldherr C, Mellinghoff IK, Tran C, et al. Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3′-Deoxy-3′-18F-fluorothymidine PET. J Nucl Med. 2005;46:114–120. [PubMed] [Google Scholar]
- 39.Mukherjee J, Yang Z-Y, Brown T, et al. Preliminary assessment of extrastriatal dopamine d-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol. 1999;26:519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 40.Pascali C, Bogni A, Fugazza L, et al. Simple preparation and purification of ethanol-free solutions of 3′-deoxy-3′-[18F]fluorothymidine by means of disposable solid-phase extraction cartridges. Nucl Med Biol. 2012;39:540–550. doi: 10.1016/j.nucmedbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.