Abstract
For 20 years, monoclonal antibodies (mAbs) have been a standard component of cancer therapy, yet there is still much room for improvement. Efforts continue to build better cancer therapeutics based on mAbs. Anti-cancer mAbs function via a variety of mechanisms including directly targeting the malignant cells, modifying the host response to the malignant cells, delivering cytotoxic moieties to the malignant cells or retargeting cellular immunity towards the malignant cells. Characteristics of mAbs that affect their efficacy include antigen specificity, overall structure, affinity for the target antigen and how a mAb component is incorporated into a construct that can trigger target cell death. This article reviews the various approaches to using mAb-based therapeutics to treat cancer, the strategies used to take advantage of the unique potential of each approach, and provides examples of current mAb-based treatments.
More than two centuries have passed since we began to understand the remarkable characteristics and potential of antibodies1. Indeed, polyclonal antisera have been used in treatment of select infectious diseases for decades2.
Soon after the first description of monoclonal antibodies (mAbs) in 19753 (a discovery that led to a Nobel Prize ten years later4), mAbs were recognized as unique biological tools and quickly became invaluable in pathologic diagnosis and basic laboratory investigation. Their ability to bind to specific antigenic epitopes allowed for rapid assessment of the molecular make up of blood cells and subsequently other tissues. Molecules, identified by mAb binding, were given cluster of differentiation (CDs) numbers5 that are still used extensively today in diagnosis. MAb are now used extensively in immunohistochemistry, flow cytometry and related technologies. At the time mAb technology was first described, there was equal excitement about its therapeutic potential based on the ability to manufacture mAb of defined specificity and class in essentially unlimited amounts. Theoretically, this would allow for highly specific targeting of cancer cells based on their molecular makeup.
However, early clinical results exploring mAb-based therapeutics were disappointing6 and until just 20 years ago, some experts considered cancer treatment with antibody-based therapy a failed hypothesis. The first mAbs evaluated in the clinic as cancer treatments were murine mAbs. Although there were intriguing hints that mAb therapy could be successful7, problems associated with administering murine mAb to humans limited their clinical utility. These problems included development of an immune response against the therapeutic mAb itself, rapid clearance of the mAb, and suboptimal ability of the murine mAb to interact with the human immune in a manner that led to immune destruction of the cancer.
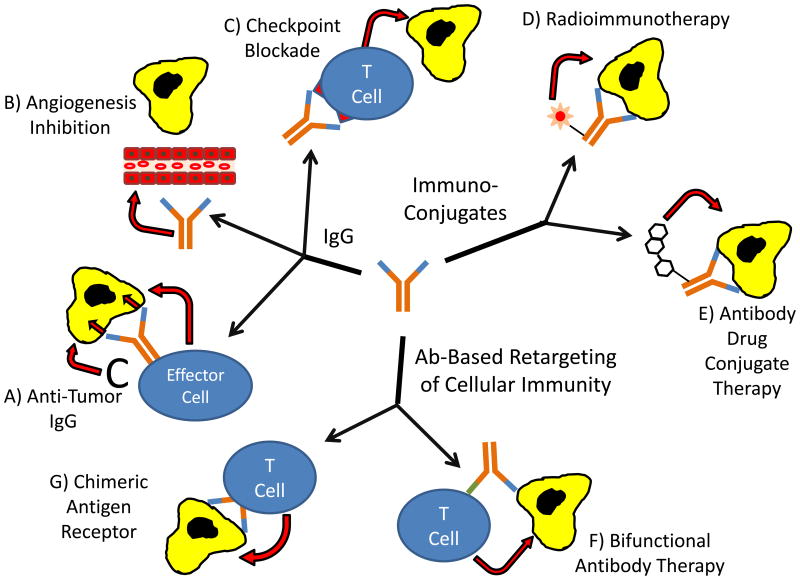
Fortunately, persistent investigators continued to explore how mAb could be used in cancer treatment. They evaluated various strategies including using IgG to target cancer directly, alter the host response to cancer, deliver cytotoxic substances to cancer, and retarget the cellular immune response towards cancer (Text Box).
Text Box: MAb-based therapy of cancer. One foundation - many approaches.
As a foundation for molecularly-based cancer therapeutics, mAbs have a number of major advantages. IgGs are proteins that bind to specific molecular epitopes, interact with effector arms of the immune system, have long half-lives and distribute in both the intravascular and extravascular compartments. MAb technology allows for production of essentially unlimited quantities of recombinant human IgG with predetermined properties. IgGs are naturally-occurring proteins and are well tolerated as therapeutic agents by the host. Given their long half-lives and effective biodistribution, clinically practical therapeutic schedules result in therapeutic systemic levels of mAb that last for weeks to months – long enough in many cases to mediate a prolonged anti-cancer response. Modification of mAbs to enhance aspects of their therapeutic effect can impact on a variety of characteristics of unmodified IgG. MAb can target and eliminate cancer cells by binding to tumor-associated antigens and altering signaling or targeting immune effector mechanisms towards the cancer cells. MAbs specific for molecules that impact on the host can block tumor angiogenesis thereby inhibiting tumor growth, or target inhibitory immunologic checkpoint signals thereby enhancing the anti-cancer cellular immune response. Decades of research and testing have illustrated the pros and cons of various mAb modifications, and have demonstrated that some modifications can be clinically beneficial. Immunoconjugates, including both antibody-drug conjugates and radioimmunoconjugates, can deliver a toxic payload to the cancer cell. Bifunctional antibodies and Chimeric Antigen Receptor T cells are able to use the specificity of mAb to retarget the cellular immune system towards cancer cells. Research is accelerating in each one of these areas, and leading to progress in both producing better mAb-based therapeutic agents and the ability to use them to help patients.
The current era of successful mAb therapy began with development of techniques that allowed for genetic modification of murine mAb to produce chimeric mouse – human, or humanized mAb that behave in most ways, like naturally occurring human IgG 8,9. Such mAb are less likely to be recognized by the host immune system as a foreign antigen, have half-lives similar to those of natural human IgG and interact well with the effector arm of the human immune system. They can be administered on a schedule that is practical for patients (in many cases weekly or monthly), and are present in the circulation of patients at therapeutic levels for months at a time. They distribute to both the intravascular and extravascular compartments and are present within the tumor mass for long periods of time where they interact with the malignant cells, stromal cells, benign lymphocytes, extracellular matrix and vasculature.
After many years of research, a variety of mAb-based approaches to cancer therapy being used with considerable success, and progress using mAb therapeutics based on a variety of different strategies (Figure 1) is continuing at a remarkable pace. Oncologists today see mAb-based cancer therapies as a vital component of state-of-the-art cancer care, and even better mAb-based treatments are on the horizon.
Figure 1. MAb-based cancer therapeutic strategies.
Successful mAb-based therapeutics have been based on a number of strategies. IgGs that bind to target cancer cells can (A) mediate ADCC by immune effector cells, induce CMC, or result in direct signaling induced death of cancer cells (e.g. herceptin and rituximab). MAb IgG can also be used to (B) inhibit angiogenesis (e.g. bevacizumab) or (C) block inhibitory signals thereby resulting in a stronger anti-tumor T cell response (e.g. ipilimumab and nivolumab). Radioimmunoconjugates (D) (e.g. I131 tositumomab and ibritumomab tiuxetan) deliver radioisotopes to the cancer cells while antibody-drug conjugates (E) (e.g. brentuximab vedotin and trastuzumab emtansine) deliver highly potent toxic drugs to the cancer cells. MAb variable regions are also used to retarget immune effector cells towards cancer cells through use of bispecific mAb that recognize the cancer cells with one arm and an activating antigen on immune effector cells with the other (F) (e.g. blinatumomab) or through a gene therapy approach where DNA for a mAb variable region fused to signaling peptides is transferred to T cells, thereby rendering those Chimeric Antigen Receptor T cells (G) specific for the tumor.
This article provides a review of recent progress in development and application of mAb-based approaches to cancer therapy, including a description of mAb and mAb-based constructs that directly target the cancer, alter the host response to the cancer, deliver cytotoxic moieties to the cancer or redirect T cells towards the cancer.
[H1]Targeting the cancer cell
MAb have been used to target a broad variety of antigens expressed on the surface of cancer cells 10. Characteristics that make antigens attractive as targets for mAb therapy include the density and consistency of expression of that target molecule by the malignant cells, limited expression of the target molecule on physiologically vital benign cells, lack of high levels of soluble target, and limited tendency of antigen-negative tumor variants to emerge. Desirable characteristics of target tumor antigens vary based on the mAb construct being considered, the nature of the malignancy (e.g. hematologic versus solid tumor) and the mechanism of action of such constructs.
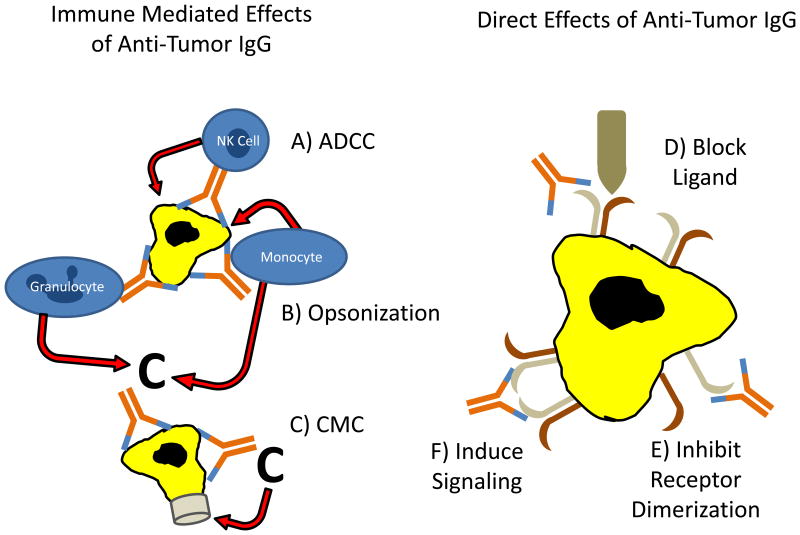
In vitro and animal model data indicate that some mAbs that target antigens on the surface of malignant cells are able to induce apoptosis by direct transmembrane signaling [G]11. There is also evidence that mAbs kill target cells by complement mediated cytotoxicity (CMC) [G]12 and inducing antibody dependent cellular cytotoxicity (ADCC) [G]13. Determining which of these various mechanisms (Figure 2) is most important for a given mAb in a given clinical scenario remains an ongoing challenge.
Figure 2. Mechanisms of action for mAb that target cancer cells.
MAb that bind directly to cancer cells can mediate their anti-tumor effects by a variety of mechanisms. These mechanisms are routinely identified in vitro, but their relative impact on clinical response to mAb therapy is difficult to determine. MAb can mediate ADCC (A) with NK cells, monocytes/macrophages or granulocytes playing a role as immune effector cells. Fixation of complement can opsonize the target cell and enhance lysis by monocytes and granulocytes (B). CMC can result directly in target cell death through development of a membrane attack complex (C). MAb can also have direct effects on target cells by blocking binding of an activating ligand responsible for the survival of the cancer cell (D), inhibiting dimerization of a receptor, thereby blocking an activation signal (E) or inducing an apoptotic signal by cross-linking a receptor (F).
[H2]MAb-Mediated Cell Signaling
Some mAbs can induce death of malignant cells in vitro, and presumably in vivo, in the absence of immune effector mechanisms. The strength of this effect varies considerably depending on the mAb, the target antigen and the target cell14,15. Assessment of whether a given mAb can mediate signaling-induced death in the target cell in vitro sometimes requires cross-linking with secondary antibodies such as anti-human IgG 16 Secondary cross linking results in enhanced aggregation of receptors that can lead to a more robust signal. This is not necessarily an artificial way of replicating cross-linking and signalling observed in vivo, where cross-linking of mAb bound to malignant cells likely occurs to a certain extent by binding of the constant region of IgG to Fc receptors (FcR) [G] expressed by a variety of cells in the tumor microenvironment. It is not clear whether artificial cross-linking of mAbs can speed up and strengthen the physiological effect of mAb signaling so it can be measured in a quick laboratory assay, or, by contrast, provides such a strong signal that non-physiologic changes are induced, which may not be relevant clinically.
In vitro measurement of signaling needs to be interpreted with caution as it varies greatly from the clinical environment17. In vitro studies usually utilize cells that have been selected to grow rapidly and consistently outside the body, i.e. independent of their normal environment. This can impact on sensitivity to a broad variety of signals. Study of primary cells obtained directly from patients avoids this problem. However, evaluation of such cells requires extensive manipulation including mincing, filtering and washing, before the cells can be evaluated. This obliterates the normal architecture of the microenvironment, alters cell growth properties and often leads to activation of apoptotic signaling pathways independent of additional therapy. In vitro signaling assays are usually done over minutes to hours while clinical response to mAb is measured over weeks to months. Despite these limitations, detection of signaling-induced apoptosis has become a common approach to in vitro analysis of the potential signalling effects of mAbs 18.
Resistance to small molecules that inhibit signaling pathways can develop when cells with alternative or compensatory signaling pathways emerge 19. Similar processes can result in the emergence of resistance to mAb that mediate their therapeutic effects by signaling 20. As with small molecules, use of combination therapy is one strategy to overcome this mechanism of resistance.
Even when a mAb is known to alter signaling properties and is effective clinically, it is difficult to use in vitro assays to determine whether the therapeutic effect of the mAb results from the mAb interrupting the interaction between an activating ligand and the receptor, mAb inhibiting dimerization of the receptor, or the mAb having a direct effect on receptor signaling21. This complexity has been studied most extensively with the ErbB family of receptors22, their various ligands, and mAbs such as Trastuzumab and Pertuzumab that recognize various receptor family members23. Individual receptors in this family can have multiple ligands, mAb can alter dimerization properties, and a mAb can have has different signaling properties depending on whether it is targeting a homodimer or a heterodimer receptor24. Understanding this complexity is not just an academic exercise, as it can have a major impact on development and clinical testing of novel therapeutics including mAb combinations25.
[H2] Complement
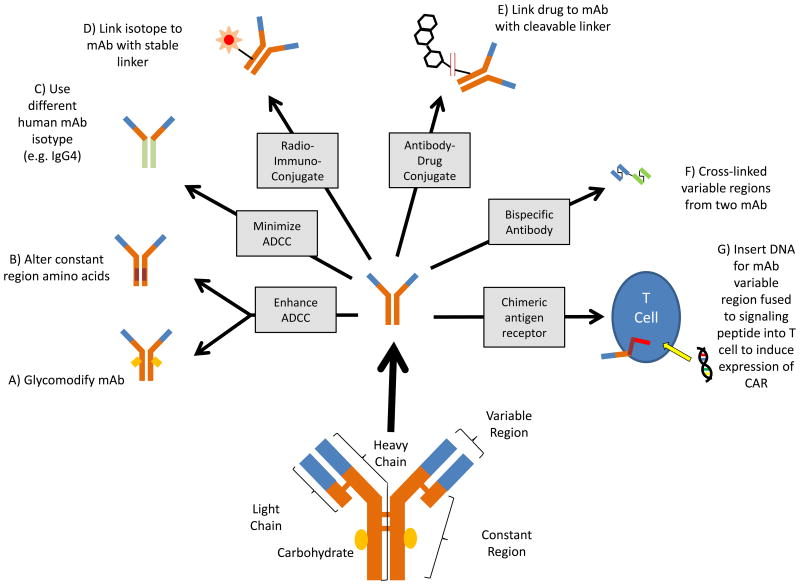
The first studies demonstrating the very potent ability of complement fixation [G] to enhance antibody-mediated cytolysis were performed over a century ago. They involved evaluation of the effect of cobra venom on lysis of red cells and bacteria26, and took place when our understanding of both antibodies and complement was obviously quite limited. We have since learned an amazing amount about complement in general, and how complement can impact on the efficacy of mAb in particular12. We now know that some, but not all, mAbs can mediate CMC27. The ability of a given mAb to fix complement and induce CMC is dependent in part on antigen concentration, orientation in the membrane, and whether the antigen occurs as a monomer or a polymer. CMC can also depend on the mAb isotype [G] and characteristics of the target cell including whether or not the cell expresses complement neutralizing molecules. Even mAb that are the same isotype and target the same antigen can vary in their ability to fix complement28. Some human IgG1 anti-CD20 (for example, rituximab) are classified as Type I anti-CD20 mAb. Type I anti-CD20 mAbs cross link between CD20 tetramers and fix complement. By contrast, type II IgG1 anti-CD20 mAbs, such as obinutuzumab, do not cross link between tetramers or fix complement well29 . Antibody engineering to alter either the constant region protein sequence or glycosylation [G] of the mAb allows for dialing up or down of a number of characteristics including its ability to fix complement30,31(Figure 3).
Figure 3. Modifying mAb structure.
MAb structure can be modified based on the desired mechanism of action. IgG1 is the most effective naturally occurring human IgG isotype at mediating ADCC. Glycomodified afucosylated mAb (A) such as obinutuzumab demonstrate enhanced binding to Fcg receptors and enhanced ADCC. Afucosylated mAb are produced using cell lines that lack enzymes responsible for fucosylation. Modifying the amino acid sequence of mAb Fc (B), as was done to produce ocaratuzumab, can also result in enhanced binding to Fcg receptors and enhanced ADCC. For some mechanisms of action where ADCC is not desirable, IgG4 is a more appropriate isotype since IgG4 mAb does not mediate ADCC to the same degree as IgG1 (C). Nivolumab, an IgG4 mAb that blocks PD-1 on T cells, is one such example. Producing radioimmunoconjugates involves linking the radioisotope to the mAb. A stable linker is most desirable (D) to limit leakage of free radioactive isotope. On the other hand, optimal ADCs utilize a cleavable linker (E). To avoid non-specific toxicity, it is desirable for drugs used in ADCs to be cytotoxic once inside the target cell, but non-toxic when bound to the mAb in the circulation. Linkers that are pH sensitive or enzymatically cleaved are now a standard component of ADCs. Bispecific antibodies require removal of a functional constant region so they do not non-specifically cross-link activating receptors and activate T cells (F). The lack of a constant region on such constructs results in a short half life, thus requiring continuous infusion to achieve the desired exposure. Chimeric antigen receptor T cells get their specificity from mAb variable regions, but are a form of genetic, not protein, therapeutics. They are produced by inserting DNA coding for the mAb variable region fused to signaling peptides into T cells (G).
Most studies exploring CMC use serum as a source of complement, which speaks to the importance of CMC in the circulation. Indeed, CMC appears to contribute most to the therapeutic effect of mAb in hematologic malignancies where target cells are exposed to complement in the circulation32. However, complement binding to target cells does not necessarily result in lysis of the target cell. When anti-CD20 binds to circulating chronic lymphocytic leukemia (CLL) cells and fixes complement, the entire antigen-mAb-complement complex may get sheared off the surface of the leukemic cell as it circulates through the liver and spleen in a process known as “shaving” or trogocytosis33. This results in circulating malignant cells that lack the target antigen at least temporarily, and are resistant to mAb specific for that antigen. The relative concentration of various complement components in the extravascular fluid is not well understood, and may be inadequate to mediate CMC34. It is generally accepted that CMC plays a limited role in the efficacy of mAb that recognize target antigens on malignancies outside the vascular compartment, i.e. solid tumors.
[H2] ADCC
MAbs can induce ADCC mediated by binding to FcR expressed by a variety of immune effector cells including natural killer (NK) cells, granulocytes and monocytes/macrophages35-38. Some of these effector cells express FcR with immunoreceptor tyrosine-based activation motifs (ITAMs). To trigger ADCC, mAb bound to the target cell interacts with FcR that signal through ITAMs and induce effector cell activation 13. Other cells express immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that can inhibit ADCC39. MAb-coated target cells can induce production and release of cytokines by immune effector cells that express FcRs40. These cytokines can then activate other immune effector cells in the tumor microenvironment41. Thus, immune cell activation via FcRs can contribute to direct ADCC as well as to the production of cytokines that contribute in other ways to control tumor growth.
[H2] Interacting mechanisms of mAb-induced cytotoxicity
There is growing evidence that there are extensive interactions – both synergistic and antagonistic – between various mechanisms of action that can affect the anti-tumor effects of a single mAb. A mAb may be developed with one mechanism in mind, yet other mechanisms may be important as well42. Complement fixation has very complex effects43. On the one hand, the anti-CD20 mAbs rituximab and ofatumumab, and the anti-CD52 antibody alemtuzumab, kill target cells rapidly in vitro via CMC. On the other hand, complement fixation can block interaction between mAb and activating FcR on NK cells, and reduce ADCC44. Thus, in some circumstances, complement fixation may induce CMC and enhance response to mAb, while in others it could inhibit ADCC and the subsequent development of an active anti-cancer immune response, therefore blocking the response to mAb. Signaling that results when a mAb binds to a receptor on a cancer cell can alter the sensitivity of that target cell to ADCC11. MAb-induced cancer cell lysis can lead to enhanced uptake of the targeted antigen and subsequent cross-presentation by cells such as dendritic cells, thereby leading to an enhanced T cell response, at least in preclinical models45. Malignant B cells that express FcR without acting motifs may actually contribute positively to the anti-tumor effect of a mAb. These FcR can allow for autocrine cross linking of anti-B cell mAb that bind to target antigen via the variable region and the FcR expressed by the same or neighbouring malignant cells46.
An antigen that can be effectively targeted by a cancer-specific mAb needs to be found on the surface of the cancer cell in high enough concentration to trigger one or more of the effector mechanisms outlined above once the mAb has bound to the surface. However, the antigen should not be expressed to a similar degree by a vital population of benign cells47 or found in high concentrations as a soluble antigen in the circulation48. There continue to be efforts to identify mAb that recognize novel tumor-associated antigens although it could be argued that most of the highly promising antigens that can be effectively targeted by unmodified IgG have already been identified. Ongoing efforts to enhance the efficacy of IgGs that directly target cancer cells involve identifying mAb with unique signaling properties such as HER2 mAbs that vary in their ability to interfere with receptor heterodimerization 49, or modifying the IgG constant region to enhance its ability to interact with the human immune system (Figure 3). Approaches to enhancing this interaction include changing the amino acid sequence 50 or the glycosylation pattern of the IgG in a way that enhances interaction with FcR on effector cells 51. MAb with such modifications can enhance the in vitro efficacy of mAb, and appear to be safe in clinical trials 52. One glycomodified mAb, obinutuzumab, was recently approved by the FDA for the treatment of CLL 53. Obinutuzumab is unique in other ways such as the manner by which it cross links its target antigen CD20, and the actual therapeutic contribution of changing IgG glycosylation remains to be determined. There have also been studies exploring mAb based on other antibody isotypes such as IgA 54. These agents have some intriguing properties but are not currently in wide clinical development.
T-Cell Receptor (TCR)-like mAbs
Many tumor associated antigens are not expressed on the surface of the cancer cell. This has led to generation of mAbs that bind to peptides expressed by HLA molecules55. These mAbs recognize peptides derived from intracellular oncoproteins such as PR1 and WT1 56,57. Such mAb are HLA restricted (i.e. only bind to antigen expressed by cells from patients with a given HLA genotype) and are early in development, but represent a novel approach to broadening the potential targets for mAb-based therapy.
[H1]Altering the Host Response
[H2]Inhibiting angiogenesis
In the early 1970s, Dr. Judah Folkman and others hypothesized that tumors require new blood vessels to grow, and that growth of such vessels is stimulated by proangiogenic factors produced by the cancer58. A number of substances were subsequently identified, including vascular endothelial growth factor (VEGF), that stimulate intratumoural blood vessel growth. MAbs, including bevacizumab, were produced to inhibit angiogenesis by interfering with the ability of VEGF to stimulate new intratumoural blood vessel growth. This, in turn, has an anti-tumor effect by depriving a growing tumor of nutrients and oxygen provided by the new blood vessels59. Since such angiogenesis inhibitors do not directly target the cancer, and are generally assumed to inhibit cancer growth rather than induce cancer cell death, they are most often used in combination with agents that are cytotoxic60. A significant advantage of this approach is that it is not dependent on expression by the tumor cell of a specific target antigen, but instead is based on the role VEGF plays in neoangiogenesis that is vital for growth of tumors in general. Bevacizumab has been shown to be effective, at least in some clinical trials and scenarios, in a variety of cancers including colorectal, lung, breast, renal, brain and ovarian cancer61.
The unique mechanism of action of angiogenesis inhibition creates a unique challenge in evaluating efficacy62. A standard approach to assessing response for most anti-cancer agents involves radiologic determination of tumor shrinkage. It has been proposed that angiogenesis inhibitors may temporarily shrink the size of a cancer by impacting on vascularity, without having a significant impact on the growth of the malignant cells. Thus, while it remains controversial, some experts claim that evaluation of efficacy for angiogenesis inhibitors should be based on overall survival rather than radiographic determination of tumor shrinkage63. Trials with survival as an endpoint are more challenging, and robust debate continues over the clinical value of angiogenesis inhibition in various cancer types.
[H2] T cell checkpoint blockade [G]
Years of basic immunology exploring the exquisite control of T-cell immunity have demonstrated that co-regulatory ligand-receptor pairs play a central role in the balance between activation and inhibition of antigen-specific (and tumor-specific) T cells64. Such precise control is central to the ability of the immune system to respond to infection, yet avoid the autoimmunity that could result from an uncontrolled T-cell response. Over the past decade, mAbs have been developed that interfere with the inhibitory signals responsible for limiting T-cell activation. These mAb, called checkpoint blockade mAb, can maintain the T-cell activation phenotype and enhance T-cell-mediated lysis. In the case of cancer, such mAbs can induce a more robust and sustained anti-tumor T-cell response64,65.
Cytotoxic T-lymphocyte protein 4 (CTLA4), also known as CD152, is a receptor expressed by activated T cells that results in downregulation of the T-cell response. Physiologically, CTLA4, and other negative regulators of T cell activation, play a role in blunting the T cell response. Such blunting of the T cell response is important for avoiding autoimmunity, but is undesirable when it comes to generation and maintenance of an effective anti-tumor T cell response66. Blocking this negative signal would be expected to enhance and maintain such a T cell response. This has proven to be the case. Ipilimumab, a mAb that blocks CTLA4, has been approved by the FDA, and has had a major impact on treatment of patients with melanoma67. Clinical responses to ipilimumab can be profound and long lasting. Ongoing studies in a variety of cancers are exploring various regimens that incorporate ipilimumab both alone and in combination with other agents, particularly when there is reason to think such therapy could be inducing a T cell response such as with vaccines or agents that induce release of antigen68. The toxicity of checkpoint blockade mAb are also unique, and as would be expected, include autoimmunity69.
A number of mAb that interfere with a different T-cell regulatory pathway, the PD-1/PDL-1 axis, are also generating considerable excitement. Indeed, the FDA has recently approved two anti-PD1 mAbs (pembrolizumab and nivolumab) for treatment of melanoma. A PD-L1 has been granted breakthrough based on its promise in early phase clinical trials 70.CTLA-4 regulates de novo immune responses, while the PD-1 axis has a greater influence on ongoing T-cell immune responses. Thus the concept of checkpoint blockade is similar but the efficacy and toxicity of mAb that target these two pathways are likely to be different. Anti-PD-1 mAb has been found to be active in melanoma patients refractory to anti-CTLA-4 therapy71. Combination trials exploring inhibition of both CTLA-4 and PD-1 are promising72 although blocking both pathways does raise concerns about enhanced autoimmunity.
MAb targeting the PD-1 axis include mAb that bind to the ligand and others that bind to the receptor70,73. Both approaches are very promising in early clinical trials, and we are sure to learn much more about relative value of these approaches in the years ahead. The PD-1 axis appears to be particularly important in classic Hodgkin's Lymphoma where Reed- Sternberg cells have recently been shown to express PD-L1 and PD-L2 which allows them to turn off T cells. This helps explain the hallmark of Hodgkin's that has intrigued hematologists for decades – how malignant Reed-Sternberg cells are able to survive despite being surrounded by benign immune cell infiltrates. An early phase clinical trial of single agent PD-1 blockade in relapsed or refractory Hodgkin's lymphoma demonstrated very positive results74.
[H1]Delivering cytotoxic moieties
Military analogies have been part of immunology since the days of Paul Ehrlich and the description of antibodies as “magic bullets”. In more recent years, the phrase “smart bombs” has been used to describe mAb that deliver cytotoxic moieties to cancer cells. The types of payloads that have been shown to be successfully delivered by mAb-based “smart bombs” are quite varied and include radioactive molecules, cytotoxic small molecules, and cellular components of the immune system (Figure 3).
[H2]Radioimmunoconjugates [G]
131I treatment for thyroid cancer was the first, highly effective targeted cancer treatment, and was successful because of the specificity of elemental iodine for the thyroid. 131I is a beta emitter that also emits a modest amount of gamma radiation and so can be used to both treat and image. This success led investigators to explore approaches to delivering radioisotopes directly to target molecules expressed by other cancers using various carrier molecules including antibodies. Attempts to use such radioimmunoconjugates to treat cancer began with polyclonal antibodies before mAb technology was available. Order and colleagues produced polyclonal antisera from various species including rabbits, pigs, monkeys and cows immunized with human ferritin. Anti-sera from these animals was labeled with 131I and administered to patients with tumors known to express high levels of ferritin receptors75. The results, particularly the images demonstrating evidence for some targeting of the agent to the tumor, were intriguing, but use of polyclonal antiserum from various species demonstrated little clinical efficacy and posed many problems. These included significant consistency and quality control issues, and development of host immune responses against the foreign proteins (similar to classic serum sickness)76.
MAb technology solved some of these problems, and resulted in a renewed interest in radioimmunoconjugates and radioimmunotherapy. However, challenges have persisted and limited the clinical utility of radioimmunotherapy. Radioisotopes decay continually and cause non-specific radiation damage to normal tissue when the agent is in the circulation or is taken up non-specifically by normal tissues77. Bone marrow is particularly radiosensitive and is affected by circulating radioimmunoconjugate. Kidney and liver receive high doses of radiation because of their role in clearing the radioimmunoconjugate and free radioisotope. Only a small fraction of the injected radiation dose ends up in the tumor, even for the most specific agents77. Optimizing the very narrow therapeutic window of radioimmunotherapy requires careful choice of the mAb, the radioisotope, the chemistry that links the two together. Experience over the past 25 years has taught us that radioimmunoconjugates directed towards solid tumors can be useful as diagnostic agents, but have limited impact therapeutically due to radioresistance of the tumors78. A radioimmunoconjugate consisting of a radioiodinated mAb that targets to necrotic tumor cells has been approved for clinical use in China79. Ongoing studies are evaluating radioimmunotherapy combined with chemotherapy.
Lymphoma is uniquely suited for radioimmunotherapy because of the availability of highly specific target antigens and its relative radiosensitivity. Two radioimmunoconjugates, ibritumomab tiuxetan80 and tositumomab81, have been approved by the FDA as therapeutic agents for treatment of lymphoma. They are both based on anti-CD20 mAbs but use different isotopes (90Y and 131I respectively). The logistics of administration, need for dosimetry, and pharmacokinetics of these two agents are distinct, but their clinical efficacy and toxicity are similar. Despite the clear clinical efficacy and limited toxicity of lymphoma radioimmunotherapy, clinical use of this modality has been surprisingly limited. This appears to be due to the complex logistics of providing radioimmunotherapy (that requires experienced nuclear medicine physicians), and the emergence of a number of other new therapies for lymphoma that are less complex to deliver. Recent and ongoing studies are exploring additional clinical questions related to lymphoma radioimmunotherapy, including the timing of therapy (at diagnosis at relapse), which lymphoma subtypes should be treated (most effective for follicular lymphoma) and how they should be combined with other treatments including bone marrow transplantation82 and novel agents83.
Ongoing studies are also exploring radioimmunoconjugates that contain novel radioisotopes, including alpha-emitters84. The advantage of alpha emitters is that the ionizing radiation is delivered very locally, within just a few cell diameters, thus limiting radiation exposure to cells that expresses the target antigen and sparing neighboring benign cells. This highly local delivery of radiation is allowing treatment of malignancies such as leukemia that are not geographically isolated but are highly radiosensitive. The radiochemistry of working with these isotopes is challenging, in part because of their very short half-life, and production of isotopes with promising properties such as astatine-211 requires a cyclotron 85. However, not all alpha-particle emitting isotopes are short-lived. Preliminary preclinical and clinical results utilizing alpha particles are intriguing86 and studies are ongoing.
[H2]Antibody Drug Conjugates [G]
In the 1970s and 1980s, a number of investigators began developing and testing immunotoxins composed of mAb linked to a variety of very potent protein toxins such as ricin, pseudomonas exotoxin or diphtheria toxin. For an immunotoxin to be effective, it needs to bind to a surface antigen on a cancer cell, enter the cell by endocytosis, and traffic within the cell to the site where the toxin can kill the cell. The toxins have to be modified by removing the moieties responsible for non-specific binding to normal cells so that cellular targeting and uptake is controlled by the mAb specificity. Initial studies of immunotoxins were done using protein conjugation techniques such as heterobifunctional reagents that linked the mAb to the toxin. Subsequent efforts involved development of recombinant immunotoxins. These agents were found to be highly potent, but the immunogenicity and non-specific toxicity of protein toxins posed major problems and limited further development87.
Many of the important lessons learned from the study of immunotoxins were applied to development and testing of Antibody Drug Conjugates (ADCs) - a field that is showing great promise88. ADCs combine the cytotoxic potential of drugs with the specificity of mAbs, and theoretically can overcome the limitations of both nonspecific cytotoxic drugs and specific but often ineffective mAbs. The choice of the target antigen and selection of the mAb for ideal ADCs have some characteristics that are different from those used for unlabeled mAbs89. Tumor antigens for ADCs can be expressed at lower concentrations when compared to unlabeled mAbs because delivery of a highly toxic drug by a small number of ADC molecules can result in death of the target cancer cell. Depending on the drug used, just a few molecules delivered intracellularly may be capable of killing a target cell. Internalization properties are also important. For unlabeled mAb, it is helpful for the mAb-antigen complex to remain on the surface of the cell so it can be recognized by the host immune system. For ADCs, internalization needs to take place so the drug can be released inside the cancer cell and mediate its toxic effect.
ADCs utilize small molecules instead of protein toxins, thereby reducing immunogenicity. The drugs used as components of ADCs include potent drugs including calicheamicin that binds to the minor groove in DNA and causes strand scission90, auristatin that blocks polymerization of tubulin91, maytansine that also inhibits assembly of microtubules92, and most recently pyrrolobenzodiazepines that cross-links DNA 93. These are extremely potent drugs, indeed some were evaluated as stand-alone chemotherapy agents and rejected because of their toxicity at very low doses94,90. The linker that connects the mAb to the drug is vital. It needs to attach the drug to the mAb in a manner that does not alter the specificity of the mAb, renders the drug non-toxic while bound to the mAb, remains stable in the circulation, and releases the drug in the appropriate intracellular compartment when the ADC is internalized so the drug can kill the target cell95.
As with radioimmunoconjugates, ADCs seem to be particularly effective in lymphoma, but ADCs are showing promise in solid tumors as well. Brentuximab Vedotin (for lymphoma) 96,97 and ado-trastuzumab emtansine (for breast cancer)98 were the first ADCs approved by the FDA. The field is rapidly expanding with over 30 ADCs currently in development and in clinical trials 99, many with encouraging preliminary results 100,101,102,103. The ADC field is currently in its infancy, and major advances based on the design of new ADCs, and a better understanding of how to use them, are sure to come.
An approach that combines targeting of the cancer cell directly by a mAb with the ability to alter the tumor microenvironment is use of immunocytokines that can mediate their anti-tumor effect via both the direct effect of the mAb and their ability to deliver a cytokine, such as IL2 or GMCSF, to the tumor. Most notable in this area of research have been immunocytokines based on anti-GD2 with clinical development based mostly on treatment of neuroblastoma104,105 . These agents have shown remarkable efficacy in this rare childhood malignancy. A modified version of such immunocytokine, designed to mediate reduced complement fixation that is thought to mediate some of its neurotoxicity, has shown promise in an early phase clinical trial as well 106.
[H1]Retargeting T cells
Tumor immunology has taught us that T cell immunity is key to immune rejection of many cancers. The initial step of cell-mediated cytolysis involves the formation of conjugates between T cells and target cells. The appropriate engagement of receptors and co-receptors on T cells is followed by the triggering of cytotoxic responses that result in death of the cancer cell. Two broad approaches to combining the specificity of mAb with the power of the T cell immune response have been explored with hopes of enhancing immune rejection of cancer (Figure 3). Both of these approaches are designed to retarget large numbers of T cells towards the tumor by bypassing the need for the T cell receptor to recognize target antigen as processed and expressed by target cell MHC.
[H2]Bispecific antibodies [G]
The first approach involves creation of bispecific antibody-like molecules that have one arm that binds to the target cell, and the other that binds to activating receptors on cytotoxic cells such as T cells or NK cells. Most bispecific antibodies involve constructs that bind to an antigen on a cancer cell with one arm and CD3 on T cells with the other. This, as with many of the strategies centered around using mAb to treat cancer, is not a new concept, but has benefited from a steady improvement in technology, and lessons learned from earlier preclinical and clinical studies107. For example, early studies demonstrated bispecific antibodies with intact Fc activate T cells non-specifically and result in unacceptable toxicity. Smaller bispecific molecules lacking Fc have short half-lives, and need to be given by continuous infusion, but result in less non-specific immune activation than do intact bifunctional mAb. Recent positive clinical trials with the bifunctional mAb blinatumomab led to approval by the FDA. Additional bispecific antibodies, including some with different structures that have longer half-lives and may not require continuous infusion, are under development108,109.
[H2]Chimeric Antigen Receptor T cells [G]
Another example of how persistence has paid off in mAb-based cancer immunotherapy is demonstrated by the decades of research that preceded the current high level of excitement surrounding Chimeric Antigen Receptor (CAR) T cells 110. CAR T cells are genetically modified to respond to target cells expressing a given antigen. CAR T cells consist of a mAb variable region linked to a T cell activating motif. A number of investigators worked for many years refining various antigen-specific constructs and approaches to transferring those constructs into primary T cells 111. Recent clinical trials based on some of the newer constructs show CAR T cells can lead to robust therapeutic responses 112,113. They also result in significant, but increasingly manageable toxicity due to cytokine storm that occurs as a consequence of massive activation and proliferation of CAR T cells induced when they come in contact with a large tumor burden. CAR T cells are unique among treatment strategies based on mAb in that the specificity provided by the mAb expands with the cellular immune response and is passed on to daughter cells.
Indeed, long-lived CAR T memory cells have been observed in a number of patients114. This memory response represents both an advantage as well as a challenge. On the one hand, it likely contributes to the very gratifying long-term clinical responses seen in some patients treated with CAR T cells. On the other hand, such long term immune memory can result in long term, perhaps even permanent, depletion of any cells that express the target antigen. In the case of B cell malignancies, long term loss of benign B cells that expressed CD19 may be an acceptable toxicity. However, most tumor antigens are not as specific as CD19, and long term toxicity to vital normal tissues can result if such tissues express even low levels of the target antigen recognized by CAR T cells. This may limit the number and types of cancers that can be treated with this approach.
Bispecific antibodies and CAR T cells each has advantages and disadvantages. Bispecific antibodies are off the shelf reagents and can be stopped if autoimmune toxic effects are observed, but are challenging to administer. CAR T cells need to produced individually for each patient, but expand in response to tumor and can result in long term anti-tumor immune responses. Both approaches can result in rapid activation of large numbers of T cells interacting with large numbers of cells expressing the target antigen 115. The resulting massive release of cytokines can result in a clinical cytokine storm with potentially devastating physiologic effects including cardiovascular collapse. Cytokine storm can be limited by stopping infusion for bispecific antibodies, or using mAb against the select cytokines such as anti-IL6 for CAR-T cells. Growing experience with CAR-T cells is leading to development of protocols to prevent or treat this challenging complication 116. Additional studies are needed to determine the relative efficacy, toxicity and value of these two exciting new strategies for using mAb to treat cancer.
[H1]Conclusion
MAb therapy of cancer has come a long way since the days when unmodified murine mAb were first explored as anti-cancer agents. A broad variety of mAb-based strategies have proven to be useful in treating patients with cancer. These include use of unlabelled IgG that binds directly to cancer cells, mAb that alter the active host response to the cancer, immunoconjugates that delivery cytotoxic moieties to the cancer, and constructs that use the specificity of mAb to retarget cellular immunity towards the cancer cell. The demonstration of clinical efficacy for each of these approaches was made possible by the dedication and persistence of investigators who would not give up on a good idea.
Indeed, investment in mAb-based therapy of cancer by both the public and private sector, and the speed of progress, has never been greater. Particularly exciting are recent advances and positive clinical results in checkpoint blockade, ADCs and retargeting T cells via CAR T cells and bispecific antibodies. We are in a period of unprecedented progress in mAb-based therapy with rapid development of new therapeutic agents and constructs, enhanced understanding of their biologic effects, and growing clinical experience based on both clinical trials and community use of FDA approved drugs. With dedicated attention to basic, translational and clinical research geared towards additional progress, we will certainly be able to build even better anti-cancer mAbs in the years ahead. Equally important will be studies evaluating how best to use these agents, alone and in combination, to better serve our patients.
Table 1. MAb-based therapeutics.
| MAb-based therapeutic | Structure | Characteristics of target antigen | Example of major ongoing research questions |
|---|---|---|---|
| Anti-tumor mAb | Unmodified IgG or IgG modified to mediate enhanced ADCC | Tumor-associated surface antigen | Are IgGs with enhanced affinity for Fc receptors more effective clinically than unaltered IgG? |
| Angiogenesis inhibition | Unmodified IgG | Host molecules that control angiogenesis | What is the best way to evaluate clinical response in patients treated with angiogenesis inhibitors? |
| T cell checkpoint blockade | IgG1 (blocks checkpoint and mediates ADCC) or IgG4 (blocks checkpoint without mediating extensive ADCC) | Molecules that limit the anti-cancer T cell response | How should we combine checkpoint blockade mAbs with each other, other immunotherapeutics, and other anti-cancer agents? |
| Radioimmuno-therapy | Unmodified IgG or mAb fragment | Tumor-associated antigen that is not shed or present in circulation | How can the logistics of administering successful radioimmunotherapeutic agents be simplified to enhance their clinical utility? |
| Antibody-drug conjugate | IgG modified with cleavable linker and drug | Highly specific tumor-associated antigen that can internalize when bound by mAb | What is the best combination of linkers and drugs with each mAb and target antigen? |
| Bifunctional antibody | Variable regions from cancer-specific mAb linked to variable region specific for activating receptor on T cell | Tumor-associated antigen that is not commonly absent in antigen-loss resistant variants | Can bispecific constructs be developed that are effective and have modified kinetics thereby avoiding the logistic complexities of continuous infusion? |
| Chimeric antigen receptor T cell | Gene therapy approach to modifying T cells by inserting DNA coding for the mAb variable region fused to signaling peptides | Highly tumor-specific antigen that is not commonly absent in antigen-loss resistant variants | Can very promising preliminary results be extended to solid tumors or will toxicity be associated with expression of low levels of target antigen by benign cells? |
Key points.
Monoclonal antibody (mAb)-based therapeutics are now standard in the treatment of cancer, and the number and variety of clinically applicable mAb-based approaches continues to grow.
Effective mAb-based treatments of cancer include those that directly target the cancer directly, alter the host response to the cancer, deliver cytotoxic moieties to the cancer, or retarget T cells towards the cancer.
MAb-based treatments that target the cancer directly mediate their effect through direct signaling, antibody dependent cellular cytotoxicity, and complement mediated lysis. Differentiating which of these mechanisms is most important for a given mAb can be difficult, but is important when working to identify better mAb-based treatments.
MAb-based treatments that alter the host response can alter tumor angiogenesis or alter the T cell response through T cell checkpoint blockade. Checkpoint blockade mAb are showing particular promise.
MAb-based treatments that deliver cytotoxic agents to the cancer include radioimmunotherapy and antibody-drug conjugates. Antibody-drug conjugates are complex because of the need to match target cancer to the right mAb, linker and drug, but early results are promising and many new antibody-drug conjugates are in development.
MAb-based treatments that retarget T cells towards cancer include bispecific antibodies and chimeric antigen receptor T cells. Both approaches are challenging logistically, but have also demonstrated exciting early results, particularly in B cell malignancies.
Each of these approaches has advantages and disadvantages that need to be considered in the development and evaluation.
Rapid progress is taking place in the development of new agents, and testing of new approaches both alone and in combination in each of these areas.
Acknowledgments
The author would like to acknowledge support from the US National Institutes of Health grants P30 CA086862 and P50 CA97274.
Glossary
- Transmembrane Signaling
The process by which an extracellular signal mediated by a natural ligand or a alternative agent such as a monoclonal antibody, binds to a membrane receptor and generates an intracellular signal that can impact on a broad range of cellular functions including cell growth, differentiation or death.
- Complement mediated cytotoxicity (CMC)
Activation of the complement cascade by a mAb that leads to formation of a membrane attack complex on the surface of the cell thereby resulting in the death of the cell. Also referred to as complement dependent cytotoxicity (CDC).
- Antibody dependent cellular cytotoxicity (ADCC)
Lysis of a target cell by an immune effector cell such as an NK cell, monocyte, macrophage or granulocyte, induced by an antibody bound to the surface of the target cell.
- Fc receptors
A family of protein receptors specific for an epitope on the constant region of an antibody. When Fc receptors on immune effector cells come in contact with an antibody-coated target cell they can result in immune effector cell activation (those Fc receptors with ITAM or immunoreceptor tyrosine-based activation motifs) or inhibition (those Fc receptors with ITIM or immunoreceptor tyrosine-based inhibition motifs).
- Complement fixation
Initiation of the complement cascade by an antibody bound to an antigen. Complement fixation can lead to CMC of the target cell as well as other complex effects mediated by the activation of various complement components.
- mAb isotype
The subtype of a mAb based on the amino acid sequence of the constant region. Various mAb subtypes (IgG1, IgG2, IgG3, IgG4) vary in their ability to mediate ADCC and CMC.
- Glycosylation
The Fc region of IgG includes carbohydrate moieties. Altering the enzymes responsible for glycosylation in a cell line that produces a mAb can alter the glycosylation of the mAb produced by that cell line thereby altering the ability of that mAb to activate immune effector cells.
- Checkpoint blockade
Immune checkpoints are inhibitory pathways expressed by T cells that serve to limit T cell activation in order to maintain self-tolerance and prevent autoimmunity. Checkpoint blockade inhibits these negative signals thereby allowing for more a more robust T cell response.
- Radioimmunoconjugate
Antibodies linked to radioactive isotopes allowing the antibody to deliver the radiation directly to the surface of the malignant cell. Depending on the energy of the radioactive isotope radioimmunoconjugates can also result in radiation exposure to bystander cells that are near the cell expressing the target antigen.
- Antibody drug conjugate
Antibodies linked to cytotoxic drugs allowing the antibody to deliver the cytotoxic drug directly to the malignant cell. For antibody-drug conjugates to be effective the drug needs to be released from the antibody and get into the target cell compartment where it has its therapeutic effect.
- Bispecific antibody
An antibody with two arms that have different specificities. Most often one arm is specific for an antigen on the cancer cell, and the other arm for an activating antigen on an immune effector cell, thereby allowing the bispecific antibody to retarget the immune effector cell towards the cancer cell.
- Chimeric antigen receptor
Engineered receptors which graft an alternative specificity onto an immune effector cell most often a T cell. Most current constructs for engineered receptors include a single chain mAb variable region linked to activating transmembrane domains that activate the T cell when it comes in contact with a target cell recognized by the mAb variable region.
Footnotes
Competing interests statement: The author declares no competing interests.
References
- 1.Ehrlich P. Collected studies on immunity. 1. J. Wiley & sons; 1906. [Google Scholar]
- 2.Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clinical microbiology reviews. 2000;13:602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495. doi: 10.1038/256495a0. Landmark publication outlining both how to produce monoclonal antibodies as well as their therapeutic potential. [DOI] [PubMed] [Google Scholar]
- 4.Milstein C. From the structure of antibodies to the diversification of the immune response. Nobel lecture, 8 December 1984. Bioscience reports. 1985;5:275–297. doi: 10.1007/BF01116899. [DOI] [PubMed] [Google Scholar]
- 5.Bernard A, Boumsell L. The clusters of differentiation (CD) defined by the First International Workshop on Human Leucocyte Differentiation Antigens. Hum Immunol. 1984;11:1–10. doi: 10.1016/0198-8859(84)90051-x. [DOI] [PubMed] [Google Scholar]
- 6.Vaickus L, Foon KA. Overview of monoclonal antibodies in the diagnosis and therapy of cancer. Cancer investigation. 1991;9:195–209. doi: 10.3109/07357909109044230. [DOI] [PubMed] [Google Scholar]
- 7.Meeker TC, et al. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985;65:1349–1363. An early clinical trial that demonstrated both the potential efficacy of mAb-based therapy, and many of the challenges associated with such therapy. [PubMed] [Google Scholar]
- 8.LoBuglio AF, et al. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:4220–4224. doi: 10.1073/pnas.86.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maloney DG, et al. Idec-C2b8 (Rituximab) Anti-CD20 Monoclonal Antibody Therapy Patients With Relapsed Low-Grade Non-Hodgkins Lymphoma. Blood. 1997;90:2188–2195. The first clinical trial demonstrating the clear clinical efficacy of a chimeric human/murine monoclonal antibody. [PubMed] [Google Scholar]
- 10.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature reviews. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 11.Tutt AL, et al. Monoclonal antibody therapy of B cell lymphoma: signaling activity on tumor cells appears more important than recruitment of effectors. J Immunol. 1998;161:3176–3185. [PubMed] [Google Scholar]
- 12.Taylor RP. Of mice and mechanisms: identifying the role of complement in monoclonal antibody-based immunotherapy. Haematologica. 2006;91:146a. [PubMed] [Google Scholar]
- 13.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beers SA, et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan D, Ledbetter JA, Press OW. Apoptosis Of Malignant Human B Cells By Ligation Of Cd20 Monoclonal Antibodies. Blood. 1998;91:1644–1652. [PubMed] [Google Scholar]
- 16.Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood. 2002;99:1314–1319. doi: 10.1182/blood.v99.4.1314. [DOI] [PubMed] [Google Scholar]
- 17.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan D, Ledbetter JA, Press OW. Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother. 2000;48:673–683. doi: 10.1007/s002620050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the Treatment of Breast Cancer. Annual review of medicine. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 20.Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med. 2014;12:132. doi: 10.1186/s12916-014-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews. Molecular cell biology. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 22.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nature reviews. Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 23.Franklin MC, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 24.Le XF, et al. Differential signaling by an anti-p185(HER2) antibody and heregulin. Cancer research. 2000;60:3522–3531. [PubMed] [Google Scholar]
- 25.Swain SM, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. The lancet oncology. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flexner S, Noguchi H. Snake Venom in Relation to Haemolysis, Bacteriolysis, and Toxicity. J Exp Med. 1902;6:277–301. doi: 10.1084/jem.6.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SY, Weiner G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert opinion on biological therapy. 2008;8:759–768. doi: 10.1517/14712598.8.6.759. [DOI] [PubMed] [Google Scholar]
- 28.Teeling JL, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 29.Alduaij W, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117:4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natsume A, Shimizu-Yokoyama Y, Satoh M, Shitara K, Niwa R. Engineered anti-CD20 antibodies with enhanced complement-activating capacity mediate potent anti-lymphoma activity. Cancer science. 2009;100:2411–2418. doi: 10.1111/j.1349-7006.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mossner E, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawluczkowycz AW, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–758. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 33.Beum PV, et al. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. J Immunol. 2011;187:3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 34.Wang SY, et al. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dall'Ozzo S, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer research. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 36.Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother. 2006;29:388–397. doi: 10.1097/01.cji.0000203081.43235.d7. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Ilizaliturri FJ, et al. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin's lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–5873. [PubMed] [Google Scholar]
- 38.Beers SA, Glennie MJ. Neutrophils: “neu players” in antibody therapy? Blood. 2013;122:3093–3094. doi: 10.1182/blood-2013-09-525451. [DOI] [PubMed] [Google Scholar]
- 39.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. An important publication illustrating the importance of both activation and inhibitory Fc receptors in mediating the anti-tumor effects of monoclonal antibodies. [DOI] [PubMed] [Google Scholar]
- 40.Bowles JA, Weiner GJ. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. Journal of immunological methods. 2005;304:88–99. doi: 10.1016/j.jim.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmeyer F, Witte K, Schmidt RE. The high-affinity Fc gamma RI on PMN: regulation of expression and signal transduction. Immunology. 1997;92:544–552. doi: 10.1046/j.1365-2567.1997.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Molecular immunology. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 43.Rogers LM, Veeramani S, Weiner GJ. Complement in monoclonal antibody therapy of cancer. Immunol Res. 2014;59:203–210. doi: 10.1007/s12026-014-8542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harbers SO, et al. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaughan AT, et al. Inhibitory FcgammaRIIb (CD32b) becomes activated by therapeutic mAb in both cis and trans and drives internalization according to antibody specificity. Blood. 2014;123:669–677. doi: 10.1182/blood-2013-04-490821. [DOI] [PubMed] [Google Scholar]
- 47.Feldman AM, Lorell BH, Reis SE. Trastuzumab in the treatment of metastatic breast cancer : anticancer therapy versus cardiotoxicity. Circulation. 2000;102:272–274. doi: 10.1161/01.cir.102.3.272. [DOI] [PubMed] [Google Scholar]
- 48.Nadler LM, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer research. 1980;40:3147–3154. [PubMed] [Google Scholar]
- 49.de Goeij BE, et al. HER2 monoclonal antibodies that do not interfere with receptor heterodimerization-mediated signaling induce effective internalization and represent valuable components for rational antibody-drug conjugate design. MAbs. 2014;6:392–402. doi: 10.4161/mabs.27705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowles JA, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006 doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalle S, et al. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther. 2011;10:178–185. doi: 10.1158/1535-7163.MCT-10-0385. [DOI] [PubMed] [Google Scholar]
- 52.Salles G, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119:5126–5132. doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 53.Cartron G, et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124:2196–2202. doi: 10.1182/blood-2014-07-586610. [DOI] [PubMed] [Google Scholar]
- 54.Lohse S, et al. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol. 2011;186:3770–3778. doi: 10.4049/jimmunol.1003082. [DOI] [PubMed] [Google Scholar]
- 55.Dao T, Liu C, Scheinberg DA. Approaching untargetable tumor-associated antigens with antibodies. Oncoimmunology. 2013;2:e24678. doi: 10.4161/onci.24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubrovsky L, et al. A TCR-mimic antibody to WT1 bypasses tyrosine kinase inhibitor resistance in human BCR-ABL+ leukemias. Blood. 2014;123:3296–3304. doi: 10.1182/blood-2014-01-549022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sergeeva A, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011;117:4262–4272. doi: 10.1182/blood-2010-07-299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. Description of the potential therapeutic impact of altering angiogenesis. [DOI] [PubMed] [Google Scholar]
- 59.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature reviews. Drug discovery. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 60.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nature reviews. Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lima AB, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen PY, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 63.Cristofanilli M, Charnsangavej C, Hortobagyi GN. Angiogenesis modulation in cancer research: novel clinical approaches. Nature reviews. Drug discovery. 2002;1:415–426. doi: 10.1038/nrd819. [DOI] [PubMed] [Google Scholar]
- 64.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annual review of medicine. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 66.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Current opinion in immunology. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. Successful clinical trial of checkpoint blockade in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 69.Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nature reviews. Drug discovery. 2011;10:411–412. doi: 10.1038/nrd3463. [DOI] [PubMed] [Google Scholar]
- 70.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England Journal of Medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 72.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ansell SM, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1411087. An early phase clinical trial that demonstrates how an enhanced understanding of the immune response can lead to a development of a new therapeutic approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Order SE. The history and progress of serologic immunotherapy and radiodiagnosis. Radiology. 1976;118:219–223. doi: 10.1148/118.1.219. [DOI] [PubMed] [Google Scholar]
- 76.Order SE, et al. Radiolabeled antibody in the treatment of primary and metastatic liver malignancies. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 1986;100:307–314. doi: 10.1007/978-3-642-82635-1_38. [DOI] [PubMed] [Google Scholar]
- 77.Pouget JP, et al. Clinical radioimmunotherapy--the role of radiobiology. Nature reviews. Clinical oncology. 2011;8:720–734. doi: 10.1038/nrclinonc.2011.160. [DOI] [PubMed] [Google Scholar]
- 78.Jain M, Venkatraman G, Batra SK. Optimization of radioimmunotherapy of solid tumors: biological impediments and their modulation. Clin Cancer Res. 2007;13:1374–1382. doi: 10.1158/1078-0432.CCR-06-2436. [DOI] [PubMed] [Google Scholar]
- 79.Chen S, et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23:1538–1547. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 80.Witzig TE, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 81.Kaminski MS, et al. Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. The New England journal of medicine. 1993;329:459–465. doi: 10.1056/NEJM199308123290703. An early clinical trial demonstrating the efficacy of radioimmunotherapy. [DOI] [PubMed] [Google Scholar]
- 82.Kolstad A, et al. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood. 2014;123:2953–2959. doi: 10.1182/blood-2013-12-541953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Witzig TE, et al. A phase I trial of immunostimulatory CpG 7909 oligodeoxynucleotide and yttrium ibritumomab tiuxetan radioimmunotherapy for relapsed B-cell non-hodgkin lymphoma. Am J Hematol. 2012;53:211–217. doi: 10.1002/ajh.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allen BJ. Can alpha-radioimmunotherapy increase efficacy for the systemic control of cancer? Immunotherapy. 2011;3:455–458. doi: 10.2217/imt.11.13. [DOI] [PubMed] [Google Scholar]
- 85.Zalutsky MR, Pruszynski M. Astatine-211: production and availability. Current radiopharmaceuticals. 2011;4:177–185. doi: 10.2174/1874471011104030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheinberg DA, McDevitt MR. Actinium-225 in targeted alpha-particle therapeutic applications. Current radiopharmaceuticals. 2011;4:306–320. doi: 10.2174/1874471011104040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghetie V, Vitetta E. Immunotoxins in the therapy of cancer: From bench to clinic. Pharmacol Ther. 1994;63:209–234. doi: 10.1016/0163-7258(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 88.Zolot RS, Basu S, Million RP. Antibody-drug conjugates. Nature reviews. Drug discovery. 2013;12:259–260. doi: 10.1038/nrd3980. [DOI] [PubMed] [Google Scholar]
- 89.Mathur R, Weiner GJ. Picking the optimal target for antibody-drug conjugates. American Society of Clinical Oncology educational book / ASCO. American Society of Clinical Oncology Meeting. 2013:103–107. doi: 10.14694/EdBook_AM.2013.33.e103. [DOI] [PubMed] [Google Scholar]
- 90.Hinman LM, et al. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer research. 1993;53:3336–3342. [PubMed] [Google Scholar]
- 91.Pettit GR, et al. Antineoplastic agents 337. Synthesis of dolastatin 10 structural modifications. Anti-cancer drug design. 1995;10:529–544. [PubMed] [Google Scholar]
- 92.Kupchan SM, et al. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. Journal of the American Chemical Society. 1972;94:1354–1356. doi: 10.1021/ja00759a054. [DOI] [PubMed] [Google Scholar]
- 93.Cooper N, et al. Synthesis of novel C2-aryl pyrrolobenzodiazepines (PBDs) as potential antitumour agents. Chemical communications. 2002:1764–1765. doi: 10.1039/b205136b. [DOI] [PubMed] [Google Scholar]
- 94.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annual review of medicine. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 95.Shen BQ, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 96.Younes A, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gopal AK, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. The New England journal of medicine. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. Clinical trial demonstring the potential efficacy of a drug-antibody conjugate in a solid tumor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nature reviews. Drug discovery. 2013;12:329–332. doi: 10.1038/nrd4009. [DOI] [PubMed] [Google Scholar]
- 100.Bendell J, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2014;32:3619–3625. doi: 10.1200/JCO.2013.52.5683. [DOI] [PubMed] [Google Scholar]
- 101.Ott PA, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J Clin Oncol. 2014;32:3659–3666. doi: 10.1200/JCO.2013.54.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tannir NM, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Investigational new drugs. 2014;32:1246–1257. doi: 10.1007/s10637-014-0151-0. [DOI] [PubMed] [Google Scholar]
- 103.Ribrag V, et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2014;20:213–220. doi: 10.1158/1078-0432.CCR-13-0580. [DOI] [PubMed] [Google Scholar]
- 104.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. The New England Journal of Medicine. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shusterman S, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navid F, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32:1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Segal DM, Weiner GJ, Weiner LM. Bispecific antibodies in cancer therapy. Current opinion in immunology. 1999;11:558–562. doi: 10.1016/s0952-7915(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 108.Nagorsen D, et al. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leukemia & lymphoma. 2009;50:886–891. doi: 10.1080/10428190902943077. [DOI] [PubMed] [Google Scholar]
- 109.Topp MS, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. Clinical trial demonstrating potential efficacy of bispecific antibody therapy. [DOI] [PubMed] [Google Scholar]
- 110.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annual review of medicine. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.June C, Rosenberg SA, Sadelain M, Weber JS. T-cell therapy at the threshold. Nat Biotechnol. 2012;30:611–614. doi: 10.1038/nbt.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. Early demonstration of the potential of CAR T cells including the potential to develop CAR T memory cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer journal. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DeFrancesco L. CAR-T cell therapy seeks strategies to harness cytokine storm. Nat Biotechnol. 2014;32:604. doi: 10.1038/nbt0714-604. [DOI] [PubMed] [Google Scholar]