Abstract
Myogenesis is facilitated by four myogenic regulatory factors and is significantly inhibited by myostatin. The objective of the current study was to examine embryonic gene regulation of myostatin/myogenic regulatory factors, and subsequent manipulations of protein synthesis, in broiler embryos under induced hyperammonemia. Broiler eggs were injected with ammonium acetate solution four times over 48 hours beginning on either embryonic day (ED) 15 or 17. Serum ammonia concentration was significantly higher (P < 0.05) in ammonium acetate injected embryos for both ED17 and ED19 collected samples when compared to sham-injected controls. Expression of mRNA, extracted from pectoralis major of experimental and control embryos, was measured using real-time quantitative PCR for myostatin, myogenic regulatory factors myogenic factor 5, myogenic determination factor 1, myogenin, myogenic regulatory factor 4, and paired box 7. A significantly lower (P < 0.01) myostatin expression was accompanied by a higher serum ammonia concentration in both ED17 and ED19 collected samples. Myogenic factor 5 expression was higher (P < 0.05) in ED17 collected samples administered ammonium acetate. In both ED17 and ED19 collected samples, myogenic regulatory factor 4 was lower (P ≤ 0.05) in ammonium acetate injected embryos. No significant difference was seen in myogenic determination factor 1, myogenin, or paired box 7 expression between treatment groups for either age of sample collection. Additionally, there was no significant difference in BrdU staining of histological samples taken from treated and control embryos. Myostatin protein levels were evaluated by Western blot analysis, and also showed lower myostatin expression (P < 0.05). Overall, it appears possible to inhibit myostatin expression through hyperammonemia, which is expected to have a positive effect on embryonic myogenesis and postnatal muscle growth.
Keywords: broiler, hyperammonemia, myogenesis, myogenic regulatory factor, myostatin
Introduction
It is widely accepted in both agricultural and human research that embryonic and immediate post-natal environment plays an important role in muscle development. In poultry, it is well established that muscle fiber number is determined during embryonic development and that post hatch growth is dependent on hypertrophy of the existing fibers (Remignon et al., 1995; Mozdziak et al., 1997). Embryonic muscle hyperplasia, or increase in myoblast proliferation, is controlled by a group of four basic helix-loop-helix transcription factors called myogenic regulatory factors (MRFs).
Two of the four MRFs, myogenic determination factor 1 (MyoD), and myogenic factor 5 (MyF5), are regulators of myogenic progenitor specification. These transcription factors are evident early in embryonic development supporting that they play a crucial role in the determination of embryonic stem cells to become committed myogenic cells. Research in MyoD and MyF5 knockout mice has demonstrated the importance of these regulatory factors on myoblast determination. Mice that lack MyoD or MyF5 develop normally, suggesting that there is redundancy in the role of these genes, while mutant mice for both MyoD and MyF5 completely lack skeletal muscle and do not survive (Rudnicki et al., 1992 and 1993). Myogenin (MYOG), and myogenic regulatory factor 4 (MRF4), also known as MyF6 or herculin, are expressed later in embryonic development. MYOG is the major determinant of myoblast differentiation, while MRF4 is expressed in mature myocytes (Nabeshima et al., 1993). Paired box 7 (PAX7) is a transcription factor that is expressed in proliferating myoblasts, but is downregulated during myoblast differentiation (Seale et al. 2000). PAX7 is also expressed in quiescent and proliferating satellite cells in mature muscle (Zammit et al. 2004).
Myostatin (MSTN), a transforming growth factor-β (TGF-β) family member, is the most powerful negative regulator of myogenesis, but is also expressed in adult muscles, suggesting it also inhibits postnatal muscle growth (McPherron et al., 1997; Lee and McPherron, 2001; Amthor et al., 2004). MSTN knockout mice display an extreme hyperplasic and hypertrophic phenotype termed double muscling (McPherron and Lee, 1997). Mutations in the MSTN gene were deemed responsible for the same phenotype observed, as a result of genetic selection for muscle growth, in Belgian Blue and Piedmontese cattle proving that the role of the MSTN gene is highly conserved across species (McPherron and Lee, 1997). MSTN has been shown to inhibit myogenesis by downregulating expression of the crucial growth factors MyoD, MyF5, and MYOG (Langley et al., 2002; Amthor et al., 2004; Dasarathy et al., 2004).
In agricultural research, the importance of MSTN and MRF expression in early stages of development is well understood to impact meat quality and ultimate meat yield. Though MSTN mutant livestock have significantly increased muscle yield, MSTN mutations also presents disadvantages. Increased calving difficulty and reduced reproductive performance are associated with MSTN mutant hypermuscular animals preventing the selection for this phenotype in commercial practice (Wiener et al., 2009). There is evidence that performing selective breeding for livestock that carry only one mutant MSTN allele may be a more realistic opportunity to benefit from increased hyperplasia and hypertrophy observed with MSTN mutations (Wiener et al., 2009; Hope et al., 2013). It is therefore of particular interest for agricultural advancement to metabolically adjust the expression of MSTN, and subsequently MRFs, without completely eliminating its role in normal regulation of muscle growth.
Increased MSTN expression has been noted in the investigation of diseases, such as cancer, heart and kidney failure, and cirrhosis, where muscle wasting is frequently a secondary, but serious, side effect (Dasarathy et al., 2004; Han and Mitch, 2011; Qiu et al., 2013). Regulation of MSTN expression is targeted as a potential therapy for sarcopenia and cachexia (Dasarathy et al., 2004, Han and Mitch, 2011). In a recent study, Qiu et al. (2013), observed increased MSTN expression under induced hyperammonemia using rats as a model for cirrhosis. Additionally, hyperammonemia has been observed in other diseases including chronic obstructive lung disease and heart failure (Andrews et al., 1997; Calvert et al., 2010). These clinical and mechanistic observations suggest that hyperammonemia has an adverse effect on muscle structure and function in a broad range of disorders.
Ammonia is generated in the developing embryos when amino acids are catabolized to generate energy (Terjesen et al., 2002; He et al., 2007). Ewe and murine embryos are adversely affected by hyperammonemia, while bovine embryos have been reported resistant to increased ammonia concentrations in vitro (Bishonga et al., 1996; McEvoy et al., 1997; Hammon et al., 2000). Similar results of the adverse effects of ammonia have been reported in amphibians and echinoderms (Webb and Charbonneua, 1987). Further, previous studies have reported that ammonia inhibits transcription of ribosomal RNA in Xenopus embryos, therefore inhibiting protein synthesis (Shiokawa et al., 1986; Shiokawa et al., 2010). Thus, the adverse effects of ammonia on the growing embryo are inconsistent in contrast to the consistent negative effects in adults. There is no data on the role of ammonia toxicity in the developing embryo of broilers. The objective of the present study was to investigate chick embryonic gene manipulation of MSTN and MRFs under induced hyperammonemia, and the effect of the gene manipulation on protein synthesis and growth.
Materials and methods
Injection of eggs
Fertilized broiler eggs (Ross 708 x Ross 344) were incubated at 37°C to embryonic day (ED) 15 or 17. In late stage broiler embryos, secondary muscle fiber formation is occurring and a population of adult myoblasts, or satellite cells, is being established, providing a great opportunity for growth enhancement between ED15 and ED19 (Stockdale et al., 1981; Maier, 1993). Ammonium Acetate (Sigma Life Science, St. Louis, MO) was diluted in Hanks’ Balanced Salt Solution and blue food coloring (FD&C Blue 1; Food Lion, LLC, Salisbury, NC) was added to the solution (2%). In preparation for injections, the shell surface was disinfected using ethyl alcohol. The amnion was identified by candling, and ammonium acetate injections were delivered using a 25-gauge needle. A total of 200 mg (50 mg per dose) of ammonium acetate was delivered into the amniotic sac, via injection of 0.2 mL (250 mg/mL) every 12 hours, over 2 days. The ammonium acetate dosage and frequency of administration was empirically derived, using non-toxic intraparitoneal dosages from murine models (2.5 mmol/kg) and intraparitoneal LD50 toxicity observed in 4-week-old chicks as guidelines for an initial low (2 mmol/kg) and high (10 mmol/kg) amount of ammonium acetate to be administered (Wilson et al., 1968; Yonden et al., 2010). The average body weight (12g) of ten ED15 embryos was used to calculate dosages for all injections. Empirically, it was determined that a dose of 50 mmol/kg every twelve hours imparts a significant increase in serum ammonia concentration without any adverse effects on the embryo. Control eggs were injected with a solution consisting of Hanks’ Balanced Salt Solution and food coloring only. After each injection, entry holes in the shell were sealed using cellophane tape and eggs were immediately returned to the incubator. Blood and tissue samples were collected 12 hours after the last injection (i.e. for groups beginning injections in ED15, samples were harvested on ED17, and those beginning on ED17, harvesting was performed on ED19). There was no observed toxicity, or marked difference in health, in experimental embryos, as compared to controls, at the time of sampling. Additionally, upon sampling from the ED19 group, internal pipping was noted in both the experimental and control groups, indicating that chicks in each group were preparing for hatch.
Collection of samples
Blood samples were drawn directly from blood vessels using beveled glass capillary tubes and placed in 0.5 mM EDTA treated tubes. Samples were centrifuged at 12,000 rpm for 10 min, the serum was separated, and placed in fresh tubes for storage at −80°C. The embryos were removed from the shell, killed via decapitation and total body weight was recorded. Pectoralis major tissue was dissected and stored in RNAlater (Ambion Inc., Grand Island, NY) at −20°C for total RNA extraction, or snap frozen in liquid nitrogen and stored at −80°C for total protein extraction.
Serum ammonia analysis
Serum samples were analyzed in duplicate for ammonia concentration using an ammonia assay kit (AA0100; Sigma Aldrich, St. Louis, MO) using methods previously reported by Qiu et al., 2013. In brief, serum samples were diluted 1:3 in ultra pure water. The enzyme, L-Glutamate dehydrogenase, was diluted 1:4 with 0.1 M Phosphate buffer (KH2PO4; pH 7.5) as needed prior to each assay. The working volume of reagent blank, test, and standard reactions were reduced by a factor of 5 to accommodate the working volume of a 96-well plate.
RNA extraction, reverse transcription, and quantitative real time-PCR
Total RNA was isolated using the RNeasy Mini Kit protocol (Qiagen, Venlo, Limburg). Approximately 30 g of RNAlater (Ambion Inc., Grand Island, NY) preserved pectoralis muscle was placed in 600 μL of the provided buffer for homogenization using a Mini-Beadbeater-1 (BioSpec Products, Bartlesville, OK). Total RNA concentration was determined by measuring absorbance at 260 nm. RNA quality was assessed using agarose gel electrophoresis. Reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Grand Island, NY) using the reverse transcriptase from the murine leukemia virus and random hexamers.
Five samples for each treatment (ED17 AA, ED17 C, ED19 AA, ED19 C) were prepared for qPCR analysis of MSTN, MyoD, MYOG, MyF5, MRF4, and PAX7 genes. These genes were chosen because they are known to play a critical regulatory role in muscle development and differentiation. β-actin was used as an internal control for normalization of each sample because our preliminary data showed no change in expression under our experimental conditions (data not shown). Primer sequences are shown in Supplementary Table S1. Real-time PCR for quantification of mRNA was performed on a fluorescent thermocycler (Applied Biosystems Inc., Grand Island, NY) using a SYBR protocol on the fluorescence temperature cycler. Standard curves for amplification efficiency were produced for each set of primers by performing serial dilutions of pooled cDNA (1:5, 1:25, 1:125, 1:625). At each extension step, fluorescence was measured and the cycle threshold (Ct) was calculated by the StepOne software (version 2.1, Applied Biosystems Inc., Grand Island, NY). All experiments were run in triplicate. Fold changes were calculated by method described by Pfaffl (2001). qPCR products for each gene were visualized by agarose gel electrophoresis and DNA sequence verified to ensure amplification specificity (Eton Bioscience, Research Triangle Park, NC).
Protein extraction, SDS-PAGE, and Western blot analysis
Total protein was extracted using a modification of methods previously described (Dasarathy et al., 2004). In brief, previously weighed muscle samples (25 mg) were homogenized in 250 μL cold lysis buffer (20 mM Tris-HCL pH 7.5, 10 mM NaCl, 10 mM KCl, 3 mM MgCl2) using a bead beater and immediately placed on ice. The homogentate was centrifuged at 12,000 rpm for 4 min, returned to ice for 4 min, and centrifuged again for an additional 4 min to remove undissolved tissue debris. Protein concentration in the supernatant was measured using the Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories Inc., Richmond, CA).
Samples (20 μg protein) were boiled 1:1 in Laemmli buffer (Bio-Rad Laboratories Inc., Richmond, CA) and separated by SDS-PAGE using a 10% Mini-PROTEAN TGX precast gel (Bio-Rad Laboratories Inc., Richmond, CA) with a 10–245 kDa Prism Ultra Protein Ladder (Abcam, Cambridge, MA). The protein was transferred to a polyvinylidene difluoride membrade (PVDF) via wet transfer, using a 0.02 M Tris/0.15 M Glycine buffer with 18% Methanol, at 400 mA for 1 hour. The membrane was Ponceau S stained to observe loading and transfer accuracy, then destained using ultra pure water.
The membrane was blocked with 5% goat serum (Life Technologies Corporation, Carlsbad, CA) in TBS-T (50mM Tris HCL, 200 mM NaCl, pH 7.5, 0.5% Tween-20) for 1.5 hrs on an orbital shaker at room temperature. The primary antibody for MSTN (Abcam, Cambridge, MA) is a rabbit polyclonal to MSTN C-terminal peptide. The MSTN primary antibody was diluted 1:250 in 5% goat serum and incubated at 4°C overnight. Three washes with TBS-T, 10 min each, followed primary antibody incubation. The secondary antibody used to detect the MSTN primary antibody is a goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP; Abcam, Cambridge, MA). The secondary antibody was diluted 1:500 in 5% goat serum and incubated at room temperature on an orbital shaker for 1.5 hrs. The membrane was washed three times with TBS-T, 10 minutes each wash. HRP activity was detected using chemiluminescent reagent (Bio-Rad Laboratories Inc., Richmond, CA). The membrane was stripped of the MSTN antibodies using an acidic glycine buffer, as outlined in the Abcam mild stripping protocol (Abcam, Cambridge, MA). Chemiluminescent reagent was used to confirm the antibodies had been removed from the membrane.
After the PVDF membrane was stripped of the antibodies detecting myostatin, the membrane was re-blocked with 5% goat serum, washed, and re-probed with β-actin for a loading control. The primary antibody for β-actin (Thermo Scientific, Rockford, IL) is a mouse monoclonal to β-actin N-terminal peptide. The β-actin antibody was diluted 1:1000 in 5% goat serum and incubated overnight at 4°C. Following primary antibody incubation, the membrane was washed three times with TBS-T, then probed with goat anti-mouse IgG secondary antibody conjugated with HRP (SouthernBiotech, Brimingham, AL), diluted 1:10,000 in 5% goat serum, for 1.5 hours on an orbital shaker at room temperature. Three washes with TBS-T were performed and HRP was detected using Chemiluminescent reagent. Semi-quantitative analysis of protein was determined by band intensity using Kodak 1D Scientific Imaging Systems (v.3.6.2, New Haven, CT). MSTN intensity of each band was normalized to β-actin prior to comparing experimental and control samples.
Statistical Analysis
Statistical analysis was performed using JMP Pro (v.10.0.0, SAS Institute Inc., Cary, NC) and SAS (v. 9.4, SAS Institute Inc., Cary, NC). All experiments were performed in triplicate and data is presented as mean ± SE. Significance (P < 0.05) was determined by oneway ANOVA. PCR results of mRNA content are expressed as mean fold-changes relative to the internal control, β-actin, ± SE.
Results
Serum ammonia concentration
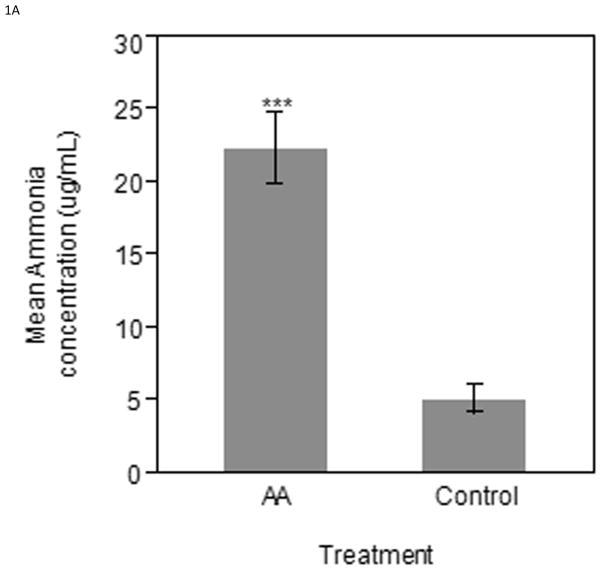
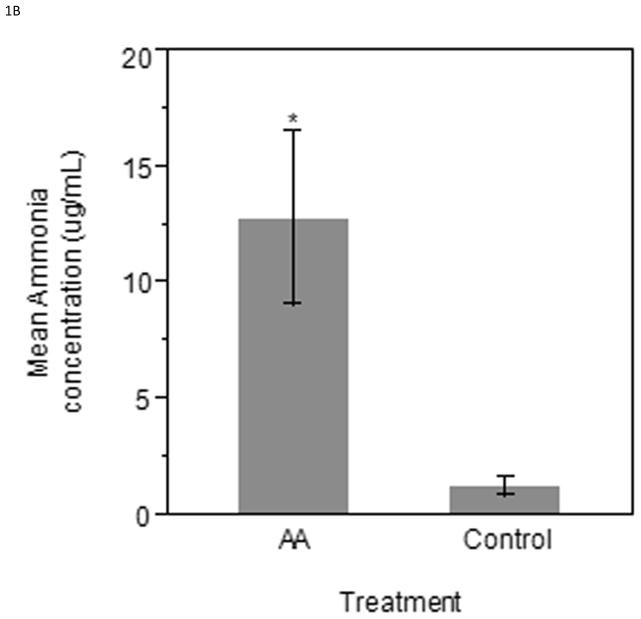
Eggs injected with ammonium acetate had a higher serum ammonia level in both the ED17 and ED19 groups when compared to the controls. The ED17 collected samples (n=6 per treatment) were found to have significantly higher (P < 0.0001) serum ammonia concentration in ammonium acetate administered embryos compared to controls (Figure 1A). Similarly, the ED19 group (n=5 per treatment) had higher serum ammonia concentrations (P < 0.05) in ammonium acetate administered embryos as compared to control injected embryos (Figure 1B).
Figure 1.
Serum ammonia concentrations taken from ammonium acetate and control injected embryos 12 hours after the last injection on A) ED17 or B) ED19. Bars represent mean ammonia concentration (μg/mL) ± SE. *** Indicates significance of (P < 0.0001); * Indicates significance of (P < 0.05).
mRNA expression
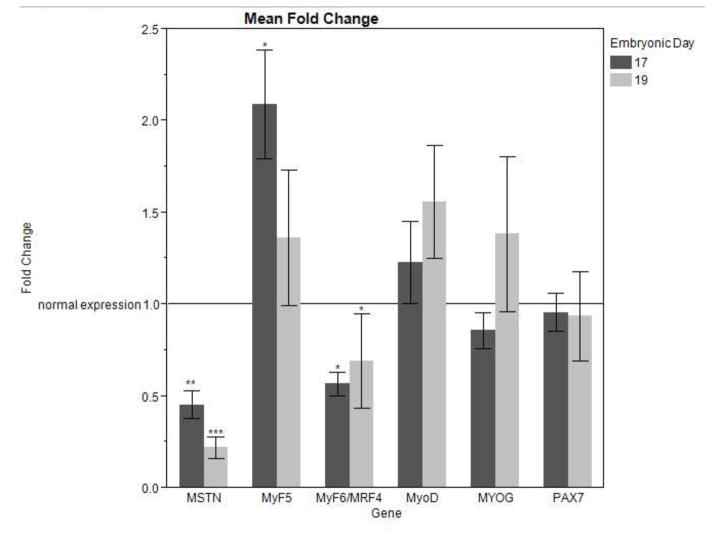
In both ED17 and ED 19 collected groups, the increase in serum ammonia concentration was accompanied by a highly significant reduction in MSTN expression (ED17 P < 0.01; E19 P < 0.0001) (Figure 2). ED17 collected samples showed a significant increase in MyF5 expression (P < 0.05); while there was no significant difference in MyF5 expression in ED19 collected samples (Figure 2). In both groups, MRF4 expression was decreased with increased serum ammonia concentration (ED17 P < 0.05; ED19 P = 0.05) (Figure 2). No significant difference was found in MyoD, MYOG, or PAX7 expression in either sample population.
Figure 2.
mRNA expression of MSTN and MRFs in ED17 and ED19 collected pectoralis major samples after 48 hours of induced hyperammonemia (Four injections of [50 mmol/kg] ammonium acetate) in experimental samples. mRNA expression was measured by quantitative real-time PCR and each sample was normalized using β-actin as an internal control. Bars represent the mean fold change ± SE. *** indicates significance of (P < 0.001); ** indicates significance of (P < 0.01); * indicates significance of (P < 0.05).
Protein expression
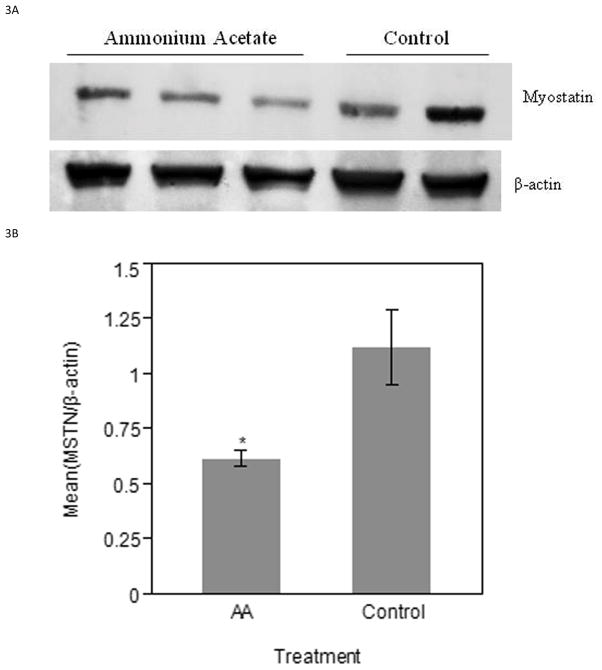
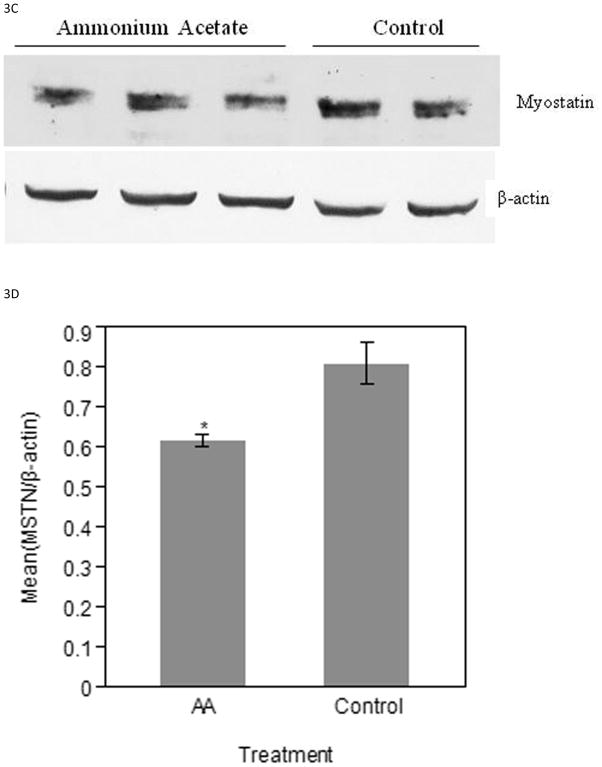
MSTN protein expression was detected by Western blot and normalized to β-actin for relative quantification and statistical significance. The MSTN band assessed is approximately 43 kDa. ED17 ammonium acetate injected samples had a lower amount of MSTN expression when compared to the control injected samples (Figure 3A). Relative intensity measurements confirm the visual observation of decreased MSTN protein (P < 0.05) in experimental samples (Figure 3B). Similarly, the ED19 experimental samples showed a lower amount of MSTN expression, when the β-actin loading control showed consistent results between the two treatment groups (Figure 3C). The relative mean intensity of experimental samples was decreased (P < 0.05) as compared to controls (Figure 3D).
Figure 3.
Western blot analysis of MSTN protein in ED17 and ED19 collected samples. A) PVDF membrane images of ED17 MSTN and β-actin bands. B) Relative intensity measurements of ED 17 PVDF membrane images. C) PVDF membrane images of ED19 MSTN and β-actin bands. D) Relative intensity measurements of ED19 PVDF membrane images. Bars represent the mean relative intensity, normalized to β-actin, ± SE. * indicates a significance of (P < 0.05).
Discussion
The aim of this study was to investigate changes in chick embryonic myogenesis under conditions of increased serum ammonia concentration, as seen in cirrhotic patients. Based upon previous studies reporting increases in plasma ammonia concentration negatively effecting satellite cell proliferation, via increased MSTN expression and reduced expression of proliferation factors, the present study aimed to understand the effects of hyperammonemia in late stage broiler embryos (Dasarathy et al., 2004; Qiu et al., 2012). During late chick embryonic development a transition from fetal myoblasts to adult myoblasts occurs. By ED18, adult myoblasts, also called satellite cells, become the predominant type of myoblasts, and are virtually exclusive at hatch (Hartley et al., 1992). Altering the proliferative activity of satellite cells, which provide essential nuclei to growing post-hatch myofibers, affects the reservoir of myogenic cells available for myonuclear donation and can have an impact post-hatch growth.
Previous studies, using portacaval anastamosis (PCA) rats as a model, investigating the mechanisms of muscle wasting secondary to cirrhosis have demonstrated a strong correlation between hyperammonemia and increased MSTN expression (Dasarathy et al., 2004; Qiu et. al., 2013). Therefore, it was expected that increasing serum ammonia in broiler embryos would upregulate MSTN expression and have a negative effect on muscle growth. The ammonium acetate administration protocol was successful in increasing serum ammonia concentration that is consistent with previous reports of inducing hyperammonemia in murine models (Kosenko et al., 2004; Qiu et al., 2013). These authors induced hyperammonemia in adult animals and reported adverse effects. However, the current results show a significant downregulation of MSTN mRNA expression in both ED17 and ED19 collected samples under induced hyperammonemia. Western blot analysis on MSTN protein content confirmed the downregulation of mRNA observed by qPCR in pectoralis samples obtained from embryos with increased serum ammonia concentration.
Potential reasons for suppression of MSTN by hyperammonemia include the relatively short term exposure to hyperammonemia compared to the previously reported PCA rat model with a long term hyperammonemic condition or the murine model with induced hyperammonemia (Qiu et al. 2012). Also, the primary mechanism of excess ammonia excretion in mammals is producing urea to be eliminated as a urinary waste product. Avian species do not have a developed urea cycle and the principal mode of ammonia disposal is through uric acid generation (Wiggins et al., 1982). Administration of low levels of ammonium acetate to the embryonic environment may have provided substrate, acetic acid, for acetyl coenzyme A synthesis, which is oxidized via the citric acid cycle for energy production and increased nitrogen availability for protein synthesis. Additionally, expression of glutamine synthetase is high in avian tissue and the glutamine synthesized is used for purine and uric acid biosynthesis (Campbell and Vorhaben, 1976). Glutamine synthesis is driven by the reaction between ammonia and α-ketoglutarate from the tricarboxylic acid cycle to generate glutamate that combines with another molecule of ammonia to generate glutamine (Hod et al., 1982). Glutamine synthesis is potentially a major mechanism of skeletal muscle ammonium detoxification (Thompson and Wu, 1991; He et al., 2010). Glutamine also plays an important role in the metabolic activity of muscle and has been reported to inhibit MSTN expression (Hickson et al., 1995; Salehian et al., 2006; Bonetto et al., 2011). Thus increased skeletal muscle glutamine synthesis in response to hyperammonemia may be responsible for reduced MSTN expression in the broiler embryos. Furthermore, glutamine has been shown to upregulate gluconeogenesis via increased expression of phoshoenolpyruvate carboxykinase (PEPCK), a rate limiting enzyme of the gluconeogenesis pathway, providing energy in the form of glucose to the developing embryo (Lavoinne et al., 1996). This model needs to be evaluated further since it provides a potential mechanism of MSTN suppression and increased muscle yield in the avian system.
In addition to downregulating MSTN expression, a significant increase in MyF5 was observed in ED17 collected samples. Increased expression of MyF5 during myogenesis supports myoblast proliferation, which is determined during embryonic development. The downregulation of MRF4, which is expressed in mature myocytes, further suggests that ammonium acetate administration creates a proliferative embryonic environment. Increasing myoblast activity, and ultimately increasing myofiber number, during development not only provides a greater potential for postnatal muscle growth, but may be a more efficient manner to increase ultimate meat yield in poultry. Given these interesting results, it would be necessary to examine the functional impact of these transcriptional changes induced by hyperammonemia on skeletal muscle mass, protein content, translational efficiency, rates of protein synthesis and breakdown in the embryos.
There were no significant changes in satellite cell activity between ammonium acetate treated and control embryos (BrdU immunostaining data not shown). Additionally, no significant difference in PAX7 expression was observed in the ammonium acetate treated embryos as compared to the controls, where increased PAX7expression would suggest increased satellite cell specification and proliferation. However, based upon in vitro studies, which indicate that significant differences in myoblast proliferation are not apparent until 5 days after initiation, the embryos were not maintained for a sufficient time to reveal impacts on the myoblast proliferative compartment (McCroskery et al., 2003). Further supporting this notion, the rat model that precedes the current model of hyperammonemia was under hyperammonemic conditions for 4 weeks, which resulted in significant changes in muscle mass (Dasarathy et al., 2004). Therefore, it was not expected that the data collected in this study would show a significant changes in the downstream targets of these alterations in gene expression.
In summary, the results of the current study suggest that increased serum ammonia concentration in developing broiler embryos leads to a reduction of MSTN. Muscle mass is positively affected by reductions in MSTN expression. Therefore, it is possible that a transient reduction in MSTN expression in the embryo can increase post hatch muscle mass. An investigation of the downstream effects on growth, an understanding of the biochemical pathway that leads to a transient downregulation of MSTN expression, and the amount of time required for this gene regulation to have a effect on embryonic myogenesis are needed to optimally employ these unanticipated findings as a strategy for improving muscle growth and ultimate meat yield in poultry.
Supplementary Material
Implications.
This study was designed to observe the effect of induced hyperammonemia on myostatin and myogenic regulatory factor expression in developing broiler embryos. The findings are important because they have the potential to positively impact growth. The results from this research may lead to a novel strategy of improving muscle growth and ultimate meat yield in avian species.
Acknowledgments
The authors express their appreciation to Shelly Nolin (North Carolina State University, Raleigh, NC), Rizwana Ali (North Carolina State University, Raleigh, NC), and Dee Hodgson (North Carolina State University, Raleigh, NC) for technical support. In addition, thanks to Jason Osborne (North Carolina State University, Raleigh, NC) for assistance with statistical analysis. Research funding and support was provided, in part, by NIH project number 5R01DK083414-04.
References
- Andrews R, Walsh JT, Evans A, Curtis S, Cowley AJ. Abnormalities of skeletal muscle metabolism in patients with chronic heart failure: evidence that they are present at rest. Heart. 1997;77:159–163. doi: 10.1136/hrt.77.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Developmental Biology. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Bishonga C, Robinson JJ, McEvoy TG, Findlay P, Aitken RP, Robertson I. Excess dietary urea intake in ewes and its effect on ovulation rate and embryo development. The Japanese Journal of Veterinary Research. 1996;44:139–151. [PubMed] [Google Scholar]
- Bonetto A, Penna F, Minero VG, Reffo P, Costamagna D, Bonelli G, Baccino FM, Costelli P. Glutamine prevents myostatin hyperexpression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-α. Amino Acids. 2011;40:585–594. doi: 10.1007/s00726-010-0683-3. [DOI] [PubMed] [Google Scholar]
- Calvert LD, Steiner MC, Morgan MD, Singh SJ. Plasma ammonia response to incremental cycling and walking tests in COPD. Respiratory Medicine. 2010;104:675–681. doi: 10.1016/j.rmed.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Campbell JW, Vorhaben JE. Avian mitochondrial glutamine metabolism. The Journal of Biological Chemistry. 1976;251:781–786. [PubMed] [Google Scholar]
- Dasarathy S, Dodig M, Muc SM, Kalhan SC, McCullough AJ. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;287:1124–1130. doi: 10.1152/ajpgi.00202.2004. [DOI] [PubMed] [Google Scholar]
- Hammon DS, Wang S, Holyoak GR. Ammonia concentration in bovine follicular fluid and its effect during in vitro maturation on subsequent embryo development. Animal Reproduction Science. 2000;58:1–8. doi: 10.1016/s0378-4320(99)00069-x. [DOI] [PubMed] [Google Scholar]
- Han HQ, Mitch WE. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Current Opinion in Supportive and Palliative Care. 2011;5:334–341. doi: 10.1097/SPC.0b013e32834bddf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. Skeletal muscle satellite cells appear during late chicken embryogenesis. Developmental Biology. 1992;153:206–216. doi: 10.1016/0012-1606(92)90106-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hakvoort TBM, Vermeulen JLM, Lamers WH, Van Roon MA. Glutamine synthetase is essential in early mouse embryogenesis. Developmental Dynamics: an official publication of the American Association of Anatomists. 2007;236:1865–1875. doi: 10.1002/dvdy.21185. [DOI] [PubMed] [Google Scholar]
- He Y, Hakvoort TBM, Köhler SE, Vermeulen JLM, de Waart DR, de Theije C, ten Have GAM, van Eijk HMH, Kunne C, Labruyere WT, Houten SM, Sokolovic M, Ruijter JM, Deutz NEP, Lamers WH. Glutamine synthetase in muscle is required for glutamine production during fasting and extrahepatic ammonia detoxification. The Journal of Biological Chemistry. 2010;285:9516–9524. doi: 10.1074/jbc.M109.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson RC, Czerwinski SM, Wegrzyn LE. Glutamine prevents downregualtion of myosin heavy chain synthesis and muscle atrophy from glucocorticoids. American Journal of Physiology. Endocrinology and Metabolism. 1995;268:730–734. doi: 10.1152/ajpendo.1995.268.4.E730. [DOI] [PubMed] [Google Scholar]
- Hod G, Chaouat M, Haskel Y, Lernau OZ, Nissan S, Mayer M. Ammonia uptake by skeletal muscle in the hyperammonaemic rat. European Journal of Clinical Investigation. 1982;12:445–450. doi: 10.1111/j.1365-2362.1982.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Hope M, Haynes F, Oddy H, Koohmaraie M, Al-Owaimer A, Geesink G. The effects of the myostatin g+6723G>A mutation on carcass and meat quality of lamb. Meat Science. 2013;95:118–122. doi: 10.1016/j.meatsci.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Montoliu C, Giordano G, Kaminsky Y, Venediktova N, Buryanov Y, Felipo V. Acute ammonia intoxication indices an NMDA receptor-mediated increase in poly(ADP-ribose) polymerase level and NAD+ metabolism in nuclei of rat brain cells. Journal of Neurochemistry. 2004;89:1101–1110. doi: 10.1111/j.1471-4159.2004.02426.x. [DOI] [PubMed] [Google Scholar]
- Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. The Journal of Biological Chemistry. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- Lavoinne A, Husson A, Quillard M, Chédeville A, Fairand A. Glutamine inhibits the lowering effect of glucose on the level of phosphoenolpyruvate carboxykinase mRNA in isolated rat hepatocytes. European Journal of Biochemistry. 1996;242:537–543. doi: 10.1111/j.1432-1033.1996.0537r.x. [DOI] [PubMed] [Google Scholar]
- Lee S-J, McPherron AC. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A. Development of chicken intrafusal muscle fibers. Cell and Tissue Research. 1993;274:383–391. doi: 10.1007/BF00318757. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. The Journal of Cell Biology. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy TG, Robinson JJ, Aitken RP, Findlay PA, Robertson IS. Dietary excesses of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Animal Reproduction Science. 1997;47:71–90. doi: 10.1016/s0378-4320(96)01627-2. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee S-J. Double muscling in cattle due to mutations in the myostatin gene. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdziak PE, Schultz E, Cassens RG. Myonuclear accretion is a major determinant of avian skeletal muscle growth. American Journal of Physiology. Cell Physiology. 1997;272:565–571. doi: 10.1152/ajpcell.1997.272.2.C565. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y-i. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, McDonald C, Almasan A, Hazen SL, Naga Prasad SV, Dasarathy S. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. American Journal of Physiology. Endocrinology and Metabolism. 2012;303:983–993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, Stark GR, Welle S, Naga Prasad SV, Dasarathy S. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remignon H, Gardahaut M-F, Marche G, Ricard F-H. Selection for rapid growth increases the number and the size of muscle fibers without changing their typing in chickens. Journal of Muscle Research and Cell Motility. 1995;16:95–102. doi: 10.1007/BF00122527. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up- regulation of the myogenic HLH gene Myf-5 and results in apparently normal development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold H-H, Jaenisch R. MyoD or Myf- 5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Salehian B, Mahabadi V, Bilas J, Taylor WE, Ma K. The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism Clinical and Experimental. 2006;55:1239–1247. doi: 10.1016/j.metabol.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shiokawa K, Kawazoe Y, Nomura H, Miura T, Nakakura N, Horiuchi T, Yamana K. Ammonium ion as a possible regulator of the commencement of rRNA synthesis in Xenopus laevis embryogenesis. Developmental Biology. 1986;115:380–391. doi: 10.1016/0012-1606(86)90257-5. [DOI] [PubMed] [Google Scholar]
- Shiokawa K, Aso M, Kondo T, Takai J-I, Yoshida J, Mishina T, Fuchimukai K, Ogasawara T, Kariya T, Tashiro K, Igarashi K. Effects of S-adenosylmethionine decarboxylase, polyamines, amino acids, and weak bases (amines and ammonia) on development and ribosomal RNA synthesis in Xenopus embryos. Amino Acids. 2010;38:439–449. doi: 10.1007/s00726-009-0403-z. [DOI] [PubMed] [Google Scholar]
- Stockdale FE, Raman N, Baden H. Myosin light chains and the developmental origin of fast muscle. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:931–935. doi: 10.1073/pnas.78.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terjesen BF, Finn RN, Norberg B, Ronnestad I. Kinetics and fates of ammonia, urea, and uric acid during oocyte maturation and ontogeny of the atlantic halibut (Hippoglossus hippoglossus L.) Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology. 2002;131:433–455. doi: 10.1016/s1095-6433(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Wu G. The effect of ketone bodies on nitrogen metabolism in skeletal muscle. Comparative Biochemistry and Physiology Part B: Molecular biology and Biochemistry. 1991;100:209–216. doi: 10.1016/0305-0491(91)90363-i. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Charbonneau M. Weak bases inhibit cleavage and embryogenesis in amphibians and echinoderms. Cell Differentiation. 1987;20:33–44. doi: 10.1016/0045-6039(87)90463-5. [DOI] [PubMed] [Google Scholar]
- Wiener P, Woolliams JA, Frank-Lawale A, Ryan M, Richardson RI, Nute GR, Wood JD, Homer D, Williams JL. The effects of a mutation in the myostatin gene on meat and carcass quality. Meat Science. 2009;83:127–134. doi: 10.1016/j.meatsci.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Wiggins D, Lund P, Krebs HA. Adaptation of urate synthesis in chicken liver. Comparative Biochemistry and Physiology Part B: Molecular biology and Biochemistry. 1982;72:565–568. doi: 10.1016/0305-0491(82)90507-7. [DOI] [PubMed] [Google Scholar]
- Wilson RP, Muhrer ME, Bloomfield RA. Comparative ammonia toxicity. Comparative Biochemistry and Physiology. 1968;25:295–301. doi: 10.1016/0010-406x(68)90936-5. [DOI] [PubMed] [Google Scholar]
- Yonden Z, Aydin M, Kilbas A, Demrin H, Sutcu R, Delibas N. Effects of ammonia and allopurinol on rat hippocampal NMDA receptors. Cell Biochemistry and Function. 2010;28:159–163. doi: 10.1002/cbf.1636. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? The Journal of Cell Biology. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.