Summary
Motivational interviewing (MI) is a client-centred method of intervention focused on enhancing intrinsic motivation and behaviour change. A previous review of the literature and meta-analyses support the effectiveness of MI for weight loss. None of these studies, however, focused on the bourgeoning literature examining MI for weight loss among adults within primary care settings, which confers unique barriers to providing weight loss treatment. Further, the current review includes 19 studies not included in previous reviews or meta-analyses. We conducted a comprehensive review of PubMed, MI review papers, and citations from relevant papers. A total of 24 adult randomized controlled trials were identified. MI interventions typically were provided individually by a range of clinicians and compared with usual care. Few studies provided adequate information regarding MI treatment fidelity. Nine studies (37.5%) reported significant weight loss at post-treatment assessment for the MI condition compared with control groups. Thirteen studies (54.2%) reported MI patients achieving at least 5% loss of initial body weight. There is potential for MI to help primary care patients lose weight. Conclusions, however, must be drawn cautiously as more than half of the reviewed studies showed no significant weight loss compared with usual care and few reported MI treatment fidelity.
Keywords: Motivational interviewing, obesity, primary care, review
The global prevalence of people who are obese has risen dramatically, with a projected global rate of 1.12 billion meeting criteria for obesity by 2030 (1). The consequences of excess weight are dire and include increased risk of cardiovascular disease, hypertension, stroke and metabolic syndrome (2–5). With such life-threatening consequences, there is an urgent need for effective and easy-to-disseminate weight loss interventions that address (i) scalability and (ii) adherence (6).
Consistent with these two areas of concentration is MI. MI is a client-centred, time-limited, method of intervention focused on enhancing intrinsic motivation and behaviour change by discussing and addressing ambivalence (7). MI clinicians use accurate reflections and open-ended questioning to help patients discuss reasons for change. The focus on change talk, which includes reasons, optimism, and intent for change, combined with clinician empathy and avoidance of confrontation, are thought to underlie the basis for behaviour change (7). A review of the literature (8) and meta-analyses (9–11) support the effectiveness of MI for weight loss and weight-related behaviour change.
To reach the millions of people who struggle with excess weight, an important place to address weight loss and scalability may be within primary care centres. While we can learn from specialty clinics incorporating MI for weight loss (8), recruiting and treating patients from primary care centres may differ from community referrals (12). There are a number of barriers unique to primary care centres that may hinder incorporating empirically supported MI weight loss treatments. Primary care providers often are busy and potentially overburdened, necessitating briefer interventions than are typical of specialty weight loss clinics. Further, primary care providers may not have prior experience or training in weight loss interventions and may be without access or financial means for trained weight loss clinicians. As such, weight loss treatment provided by primary care centres often is limited (13–18). Patients do want their providers to discuss weight loss, but feel their providers’ ability to provide resources is insufficient (16), and providers are unlikely to provide weight loss counselling at appointments (13,19–21). Fortunately, MI is a time-limited approach to weight loss and general medical practitioners, without prior therapeutic training, can be trained to provide MI, increasing the opportunity for widespread treatment dissemination (22–24).
Consequently, medical offices have begun to incorporate relatively low- to moderate-intensity MI treatments for weight loss (25). Because of the potential for MI to help patients successfully lose weight and the aforementioned unique challenges to implementing weight loss treatment into primary care centres, it is important to examine this emerging literature. Moreover, 19 additional randomized controlled trials examining MI for weight loss in primary care have been published since and/or were not included in the most recent review and meta-analysis (8,9). These two papers also focused primarily on weight loss outcomes; the current review will also examine weight-related outcomes, with specific focus on trials in primary care. To our knowledge, this is the first paper to review randomized controlled trials of MI for weight loss in primary care centres.
Method
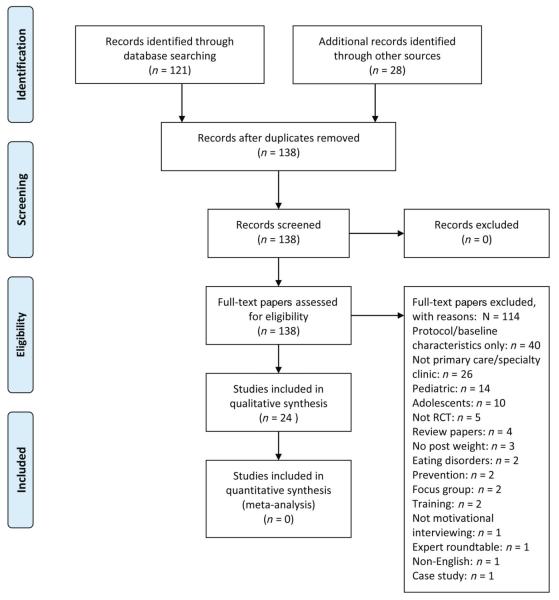
A comprehensive review was conducted by searching PubMed, MI review papers and citations from relevant papers. Search terms included, but were not limited to, ‘randomized controlled trial’ or ‘RCT’ or ‘trial’, and ‘weight’ or ‘weight loss’ or ‘overweight’ or ‘obese’ or ‘obesity’, and ‘motivational interviewing’ or ‘motivational counseling’, yielding 121 papers from PubMed. Date last searched was 1 June 2014. Inclusion criteria consisted of randomized controlled trials of MI in primary care settings with weight as an outcome; however, weight as a secondary outcome was also included. Each paper was reviewed to search for the aforementioned terms. Common exclusion criteria included baseline data only and no post-treatment assessment, specialty care as opposed to primary care clinics, and paediatric or adolescent samples. The authors met to discuss studies deemed unclear based on our inclusion criteria (i.e. whether or not a study was primary care or a specialty clinic). In addition, authors met to review findings of the final selected papers. For instance, if a study was unclear on fidelity ratings, the authors met to discuss and emailed the corresponding author of the study if necessary. Figure 1 depicts the process of identifying papers for the present review.
Figure 1.
PRISMA flow diagram. RCT, randomized controlled trial. From Moher et al. (66). For more information, visit http://www.prisma-statement.org.
Results
A total of 24 RCTs (25–48) examining MI weight loss treatments for adults in PC were identified. In addition to the publications of the main studies, we also included three publications of corresponding follow-up assessment data (Table 1; 49–51). Five of 24 RCTs controlled for some form of cluster effects in their statistical approach.
Table 1.
Randomized controlled trials of motivational interviewing for weight loss in primary care among adults
| Study | Baseline sample characteristics |
Treatment length, Min of treatment, F/U‡‡ |
Training, Fidelity |
Provider | Groups: treatment retention |
MI kg change per hour, MI 5% WL |
Summary of significant relevant findings between MI and comparison |
|---|---|---|---|---|---|---|---|
| Armit et al. 2009 (25) |
n = 136 Age: 58 BMI: NI Women: 60% Men: 40% % Minority: NI |
3 months 60–75 min F/U: 3 months |
Training: No Fidelity: No |
Exercise specialists | 1. UC+ Pedometer+ MI: 100% 2. UC+ Pedometer: 100% 3. UC: 100% |
0 kg h−1 5% WL: NI |
Weight: NS Physical activity: NS* Diet: NI Other: Blood pressure: NS* Post-exercise HR: NS* |
| Barnes et al. 2014 (26) |
n = 89 Age: 47.9 BMI: 35.3 Women: 76.4% Men: 23.6% % Minority: 34.8% |
3 months 140 min F/U: 3 months |
Training: Yes Fidelity: Yes |
Medical assistants | 1. MI: 93.3% 2. Nutrition: 90% 3. UC: 96.7% |
−0.64 kg h−1 5% WL: 25% |
Weight: NS* Physical activity: NI Diet: NI Other: Blood pressure: NS* Triglycerides: NS* HbA1C: NS Eating disorder: NS* |
| Bennett et al. 2010 (27) |
n = 101 Age: 54.4 BMI: 34.6 Women: 47.5% Men: 52.5% % Minority: 50.5% |
3 months 80 min F/U: No |
Training: No Fidelity: No |
Registered dietician | 1. MI: 98.0% 2. UC: 94% |
−1.71 kg h−1 5% WL: 25.6% |
Weight: Sig BMI: Sig Waist circumference: NS Physical activity: NI Diet: NI Other: Blood pressure: NS* |
| Bennett et al. 2012 (28) |
n = 365 Age: 54.5 BMI: 36.99–37.03 Women: 68.5% Men: 31.5% % Minority: 96.8% |
24 months 270–360 min F/U: No |
Training: No Fidelity: No |
Community health educators |
1. MI: 82.2%, 70.6% of telephone counselling calls 2. UC: 89.7% |
−0.26 kg h−1 5% WL: 20% |
Weight: Sig Physical activity: NI Diet: NI Other: Blood pressure: NS* |
| Christian et al. 2008 (29) |
n = 310 Age: 53.0–53.4 BMI: 34.8–35.4 Women: 65–68% Men: 32–35% % Minority: Hispanic/Latino 50% |
12 months 100 min F/U: No |
Training: Yes Fidelity: No |
Physicians | 1. MI: 91.0% 2. UC: 85.2% |
−0.05 kg h−1 5% WL: 21% |
Weight: NS 5% WL at 12 months: Sig Waist circumference: NS* Physical activity: Sig Moderate level: NS* Diet: NS* Other: Blood pressure: NS* Total cholesterol: Sig LDL: Sig HDL: NS* HbA1C: NS* |
| Cochrane et al. **2012 (30) |
n = 601 Age: 63.3–63.9 BMI: 27.5–28.7 Women: 9.9–13.6% Men: 86.4–90.1% % Minority: 3–4.2% |
12 months 360 min F/U: No |
Training: No Fidelity: No |
Lifestyle coach | 1. MI + NHS Health check: 84.7% 2. UC + NHS Health check: 83.8% |
−0.12 kg h−1 5% WL: NI |
Weight: NS BMI: NS Physical Activity: NS* Diet: NI Other: Blood pressure: NS Cholesterol: NS CVD risk: NS* |
| Drevenhorn et al. 2012 (31) |
n = 213 Age: NI BMI: 30.9–31.4 Women: NI Men: NI % Minority: NI |
24 months Min: NI F/U: No |
Training: Yes Fidelity: Yes |
Nurses | 1. MI: 96.1% 2. UC: 94.8% |
Kg h−1: NI 5% WL: NI |
Weight: NS* BMI: NS* Waist circumference: NS* Waist–hip ratio: NS* Physical Activity: Sig Diet: NI Other: Blood pressure: NS* Heart rate: Sig Total cholesterol: NS* LDL: NS* HDL: Sig |
| Ely et al. 2008 (32) |
n = 107 Age: 49–50 BMI: 36–37 Women: 77% Men: 23% % Minority: 13% |
6 months 8 calls, Min: NI F/U: No |
Training: No Fidelity: No |
Master’s counsellors | 1. MI: NI 2. UC: NI Overall: 50% |
Kg h−1: NI 5% WL: NI Overall: 17.8% |
Weight: Sig Physical activity: NS Diet: NS Other: NI |
| Greaves et al. 2008 (33) |
n = 141 Age: 53.3–54.5 BMI: NI Women: 64% Men: 36% % Minority: NI |
6 months 374 min avg F/U: No |
Training: Yes Fidelity: Yes |
Ex-NHS staff health visitor, rehabilitation nurse, and postgraduates students in sports and health science |
1. MI: 94.4% 2. Standardized information pack: 100% |
−0.05 kg h−1 5% WL: 24% |
Weight: Sig %5 WL: Sig Physical activity: NS* Diet: NI Other: NI |
| Groeneveld et al. 2010 (34) |
n = 595 PA/Diet group: Age: 47.4 BMI 28.8 Women: 0% Men: 100% % Minority: NI |
6 months 195–300 min F/U: 6 months |
Training: Yes Fidelity: Yes |
Occupational physician or occupational nurse |
1. MI: 66.4% 2. UC: 100% |
−0.14 kg h−1 5% WL: 20.4% |
Weight: Sig BMI: Sig Physical activity: NI Diet: NI Other: Blood pressure: NS* HDL cholesterol: NS* Cholesterol ratio: NS* HbA1c: NS* |
| Hardcastle et al. 2008 (35) and 2013 (49) |
n = 334 Age: 50.22 BMI: 33.67–34.28 Women: 67%* Men: 33%* % Minority: NI |
6 months 20–150 min F/U: 12 months |
Training: Yes Fidelity: Yes |
Registered dietician and physical activity specialist (graduate in exercise science, specializing in exercise psychology) |
1. MI: NI 2. Standard exercise and nutrition information: NI Overall F/U: 65% |
−0.25 kg h−1 5% WL: NI |
Weight: NS by 18 months Physical activity: Sig Walking: Sig % inactive: Sig Vigorous and moderate: NS Diet: fruit/vegetable: NS* Fat: NS Other: Diastolic blood pressure: Sig Cholesterol: NS* LDL: NS HDL: NS Triglycerides: NS CHD risk: NS |
| Harris et al., **††2013 (37) |
n = 804 Age: overall NI 67% ≥ 60 BMI: NI, 75% overweight/obese Women: 49.3% Men: 50.7% % Minority: 0.5% aboriginal/Torres Strait Islander, 4.4% non-English |
6 months Min: NI F/U: NI |
Training: Yes Fidelity: No |
Community health nurses |
1. MI: NI 2. UC: NI |
kg h−1: NI 5% WL: NI |
Weight: NS Physical activity: NS Diet: NS Other: NI |
| Harris et al. **2012 (36) |
n = 814 Age: 40–64, Mean NI BMI: NI Women: 49.3% Men: 50.7% % Minority: NI |
12 months 540 min F/U: No |
Training: No Fidelity: No |
Physician, practice nurse |
1. MI: 95.1% 2. UC: 97.5% |
−0.01 kg h−1 5% WL: NI |
Weight: NS Physical activity: Sig Diet: Sig only at 6 months Other: Blood pressure: NS Lipids: NS CVD risk: NS |
| Heinrich et al. 2010 (38) |
n = 618 Age: 59 BMI: NI Women: 44.9% Men: 55.1% % Minority: NI |
24 months 160 min F/U: No |
Training: Yes Fidelity: Yes |
Nurses | 1. MI: NI 2. UC: NI Overall F/U: 72% |
Kg h−1: NI 5% WL: NI |
Weight: NS Physical activity: NS Diet: fruit/vegetable: NS Fat intake: NS Other: Blood pressure: NS Total cholesterol: NS LDL: NS HDL: NS Triglycerides: NS HbA1c: NS |
| Martin et al. **2006 (50) and 2008 (39) |
n = 137 Age: 40.7–43.0 Women: 100% Men: 0% % Minority: 100% |
6 months 90 min F/U: 3, 6, and 12 months |
Training: Yes Fidelity: No |
Physician | 1. MI: 71% 2. UC: 88% |
−0.96 kg h−1 5% WL: 12.5% |
Weight: Sig 5% WL: NS Physical activity: NI Diet: NI Other: NI |
| McDoniel et al. 2010a (40) |
n = 74 Age: 46.6 BMI: 36.4 Women: 58% Men: 42% % Minority: 19–22% |
6 months 120 min F/U: No |
Training: No Fidelity: No |
Master’s-level exercise physiologist |
1. MI + energy Plan based on REE†: NI 2. MI + Standard Diet: NI Overall: 75.7% |
kg h−1: 2.15 5% WL: ≈6% |
*Both groups MI Weight: NS* Physical activity: NI Diet: eating behaviour: NS* Dietary restraint: NS* Uncontrolled eating: NS* Other: Metabolic: NS |
| McDoniel, et al. 2010b (41) |
n = 111 Age: 45.5 BMI: 37.0 Women: 62% Men: 38% % Minority: 19.9–23.2% |
3 months Min: NI F/U: No |
Training: No Fidelity: No |
Master’s level exercise physiologist |
1. MI + Nutrition Program based on RMR‡: 70.9% 2. MI + Standard Nutrition Plan: 73.2% |
kg h−1: NI 5% WL: ≈9% |
*Both groups MI Weight: NS* Physical activity: NI Diet: NI Other: Blood pressure: NS* |
| Nanchahal et al. 2012 (42) |
n = 381 Age: 48.8 BMI: 33.5 Women: 72.2% Men: 27.8% % Minority: 27.4% |
12 months 420 min F/U: No |
Training: Yes Fidelity: No |
Trained non-specialists |
1. MI: 96.3% 2. UC: NI |
−0.39 kg h−1 5% WL: 32.7% |
Weight: NS* BMI: NS* Waist circumference: NS* % Body fat: NS* 5% WL: Sig Physical activity: NI Diet: NI Other: Blood pressure: NS* Heart rate: Sig |
| Penn et al. 2009 (43) |
n = 102 Age: 57 BMI: 34 Women: 58.8% Men: 41.2% % Minority: NI |
60 months 540 min F/U: No |
Training: No Fidelity: No |
Dietician and physiotherapist |
1. MI: 41.2% 2. UC: 41.2% at 60 months |
−0.46 kg h−1 at 12 months 5% WL: NI |
Weight: Sig at 12 months only Physical activity: NS Diet: NS Other: Cumulative diabetes incidence: NS* |
| Vermunt et al. **2011 (44) and 2012 (51) |
n = 925 Age: 57.9–59.5 BMI: 28.5–29 Women: 55.9–61.2% Men: 38.8–44.1% % Minority: NI |
30 months 580 min F/U: No |
Training: Yes Fidelity: No |
Nurse and physician |
1. MI: 85.4% 2. UC: 86.8% |
−0.09 kg h−1 5% WL: NI |
Weight: NS* BMI: NS* Waist circumference: NS Physical activity: NS Diet: energy intake: NS* Total saturated fat: NS Total dietary fibre: Sig Other: Fasting plasma glucose: NS* 2-h plasma glucose: NS Diabetes incidence: NS |
| Whittemore et al. **2009 (45) |
n = 58 Age: 48.2–43.2 BMI: 37.4–40.0 Women: 92% Men: 8% % Minority: 55% |
6 months 240 min F/U: No |
Training: Yes Fidelity: No |
Nurse practitioners | 1. MI: 77.4% 2. UC: 100% |
−0.11 kg h−1§ 5% WL: 25% |
Weight: % WL: NS* Physical activity: NS* Diet: NS* Other: HDL: NS* LDL: NS Glucose: NS |
| Williams et al. 2014 (46) |
n = 54 Age: 47.3 BMI: 25.1 Women: 100% Men: 0% % Minority: NI |
12 months 300 min F/U: No |
Training: No Fidelity: Yes |
Dietician and exercise physiologists |
1. MI: 78.6% 2. Self-directed intervention: 69.2% |
−0.50 kg h−1 (healthy weight pts.) −0.7 kg h−1 (overweight pts.) 5% WL: NI (reported 7% by weight category) |
Weight: Sig Overweight group: NS* Percentage of body fat: NS Percentage of lean muscle: NS Waist circumference: Sig Physical activity: NS Diet: NS Other: Diastolic blood pressure: Sig Total cholesterol: NS LDL and HDL: NS Glucose: NS |
| Woollard et al. ¶1995 (47) |
n = 166 Age: 58–59 BMI: NI Women: 44.2–50% Men: 50–55.8% % Minority: NI |
18 weeks 270 min (MI-High) 75 min (MI-Low) F/U: No |
Training: No Fidelity: No |
Nurse counsellors | 1. MI-High: NI 2. MI-Low: NI 3. UC: NI |
−0.38 kg h−1 (MI-High) −0.80 kg h−1 (MI-Low) 5% WL: NI |
Weight: Sig (MI-High) Physical activity: NI Diet: Salt: Sig (MI-Low) Other: Blood pressure: Sig (MI-High) |
| Woollard et al. ¶2003 (48) |
n = 212 Age: 59.5–61.0 BMI: Women: 50.5% Men: 49.1% % Minority: NI |
12 months 720 min (MI-High) 180 min (MI-Low) F/U: 6 months |
Training: Yes Fidelity: Yes |
Nurses | 1. MI-High: 64.9% 2. MI-Low: 71.0% 3. UC: 76.8% |
0 kg h−1 (MI-High) 0 kg h−1 (MI-Low) 5% WL: NI |
Weight: NS BMI: NS Waist–hip ratio: NS Physical activity: NI Diet: total energy intake: NS*(MI-High at 18 months) Fat: NS* Sodium: NS* Potassium and fibre: NS Other: Total cholesterol: NS* (MI-High at 18 months) LDL: NS* HDL: NS* (MI-Low at 18 months) Triglycerides: NS* Ratio of plasma n3 to n6 fatty acids: NS* |
Note: For baseline characteristics, ranges were used when means were reported by group, and not for the overall sample.
Percentages based on those accepting invitation to participate (n = 358), not those randomized (n = 334).
Resting energy expenditure.
Resting metabolic rate measured by a validated hand-held device.
Percentage of weight loss was used for this calculation as weight loss was unavailable.
MI-High, high intervention was longer in time spent with clinician compared with low intervention; summary results reported for Woollard et al. 1995 and Woollard et al. 2003 are for treatment groups compared with usual care.
Indicates study controlled for cluster effects in RCT.
Indicates unclear whether study controlled for cluster effects.
F/U refers to a follow-up assessment following a period of treatment cessation.
BMI, body mass index; CVD, cardiovascular disease; F/U, follow-up; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, motivational interviewing; NI, not indicated/unclear; NS, not statistically different; NS*, MI group improved but did not statistically differ from comparison group; Pts, participants; Sig, significant change in MI intervention compared to UC/Control; UC, usual care; WL, weight loss.
MI training and treatment adherence
The individuals recruited to provide MI and the training they received varied greatly among the studies reviewed. Many RCTs (n = 7, 29.2%) utilized mixed intervention teams to provide the MI intervention (33–35,37,43,44,46,49,51), with clinicians of varying backgrounds, including professionals such as dieticians, nurses/nurse practitioners, medical doctors, or sports and health science specialists. Other RCTs (n = 6, 25.0%) incorporated one type of interventionist such as nurses/nurse practitioners (31,36,38,45,47,48), physicians (n = 2, 8.3%) (29,39,50), exercise specialists (n = 3, 12.5%) (25,40,41), dieticians (n = 1, 4.2%) (27), ‘Masters-level counsellors’ (n = 1, 4.2%) (32), medical assistants (n = 1, 4.2%) (26) and the remaining did not specify educational background (e.g. ‘trained non-specialists’) (n = 3, 12.5%) (28,30,42).
Many studies (n = 12, 50.0%) provided no or minimal (e.g. ‘trained’) details about how MI clinicians were trained (25,27,28,30,32,34,37,40,41,43,46,47), although Groeneveld et al. (34) included this information in a corresponding publication (52). Of the 12 (50%) studies reporting information about MI training (26,29,31,33,35,36,38,39,42,44,45,48–51), most (n = 8) did not specify who provided the training (29,31,36, 39,42,44,45,48,50,51); training provided in the remaining four studies was from MI ‘accredited’ or ‘certified’ trainers (n = 2) (33,38), the study investigators (n = 1) (35,49), and a Motivational Interviewing Network of Trainers-certified trainer (n = 1) (26). Of the 12 studies reporting information about MI training length (26,29,31,33,35,36,38, 39,42,44,45,48–51), it is difficult to determine exactly how much training was received as almost half of these studies (n = 5) reported training in terms of ‘days’ or ‘evenings’ instead of hours (31,33,36,42,44,51). Of those that did report hours (n = 7, 29.2%) (26,29,35,38,39,45,48–50), training length ranged from 3 (29) to 170 hours (48). Most studies did not describe any ongoing MI supervision for clinicians (n = 17, 70.8%). Of those that reported specific information regarding supervision (n = 7, 29.2%) (26,28,35,36,38,44,45,49,51), the supervision provided ranged from weekly (28) to every 6 months (44,51), most typically once every 3–4 weeks (26,35,45,49).
Assessing treatment fidelity is an important part of ensuring that the training MI clinicians receive translates to the treatment provided. Despite its important, most RCTs did not include descriptions of treatment fidelity assessment (n = 17, 70.8%) (25,27–30,32,34,36,37,39–45,47,50,51), although Groeneveld et al. (34) included this information in a corresponding publication (52). Methods of providing feedback to the MI clinicians were based on actual (n = 1, 4.2%) (38) and mock (n = 2, 8.3%) (31,48) recorded sessions, or fidelity ratings of mock sessions with a standardized rating system (n = 1, 4.2%) (33). Only three studies (12.5%) described using standardized fidelity rating systems of real patient sessions (26,35,46,49), although information for Groeneveld et al. (34) was included in a corresponding publication (52). Of the three studies using real patient sessions with standardized MI fidelity ratings, Hardcastle et al. (35,49) did not report the results, Williams et al. (46) reported MI treatment scores below proficiency, and Barnes et al. (26) reported satisfactory MI treatment fidelity.
MI treatment comparison conditions
Most commonly, the MI-based intervention was compared with a standardized dietary advice or usual care (UC), with no control for attention/time (n = 17, 70.8%) (25,27–35,37–39,42–44,46,49–51). One study (4.2%) compared the MI intervention to an ‘enhanced’ standard care (45), one study (4.2%) compared UC to MI with and without a pedometer (25), two studies (8.3%) compared two interventions (e.g. standard dietary advice vs. metabolic diet) with both groups receiving MI (40,41), and two (8.3%) compared high- and low-intensity MI interventions to UC (47,48). Only one (4.2%) intervention included a non-MI attentional control, nutrition psychoeducation (26).
MI treatment format and other treatments
Most trials (n = 18, 75.0%) tested individual therapy (25–27,29,31–35,38–42,45–50), while others (n = 6, 25.0%) tested a mixed model of individual and group therapy (28,30,36,37,43,44,51), and none examined group only. Most MI-based interventions also incorporated behavioural weight loss (n = 21, 87.5%) (25–32,34,35,37–47,49–51) or cognitive behavioural techniques (n = 2, 8.3%) (33,48), such as self-monitoring. The majority (n = 18, 75.0%) also implemented the MI intervention above and beyond typical primary care appointments (25–28,30,32–35,40–49,51); in the remaining studies (n = 6, 25.0%), practitioners implemented MI into their regularly scheduled primary care appointments (29,31,36–39,50).
Treatment length, follow-up assessments, and retention
Intervention length ranged from 3 (25,26) to 60 months (43). Most commonly, interventions were 6 months or longer (n = 18, 75.0%) (28–35,37–40,42–46,48–51) or 3 months (n = 4, 16.7%) (25–27,41). In terms of exposure to treatment, most studies (n = 20, 83.3%) reported the typical length of sessions or treatment (25–30,33–35,37–40,42–51), the lowest MI exposure was 60–75 min over 3 months (25) and the highest was 720 min over 12 months (48). The majority of studies relied on post assessments immediately after treatment cessation, only four studies (16.7%) clearly indicated including follow-up assessments after a period of treatment cessation in the original publication (25,26,34,48), and two published follow-up data in subsequent papers (35,39,49,50). Treatment retention rates tended to be high for intervention conditions, with most studies reporting rates in the 80–89% (n = 5, 20.8%; 28,30,39,44,45,50,51) and 90–100% (n = 8, 33.3%; 25–27,29,31,33,37,42) ranges; the lowest MI intervention retention rates ranges were 50–69% (32,34,48).
Supplemental materials and technology
Because of time constraints, PC interventions may also incorporate supplemental weight loss resources. Many studies (n = 11, 45.8%) (25,30,33,34,36–38,40, 44,45,47,51) did not report incorporating any such materials. The traditional supplemental materials included a pedometer and weight loss resources (e.g. encouraging physical activity; n = 3, 12.5%) (28,32,42), booklet/newsletter/intake logs with psychoeducational weight loss information (n = 9, 37.5%) (29,31,32,35,41–43,46,48,49), menus/recipes (n = 1, 4.2%) (39,50), and an empirically supported self-help behavioural weight loss manual (n = 1, 4.2%) (26).
All but two studies (47,48) reviewed were published since 2006; however, only 7 of these 22 incorporated computer, internet or email technology (26–28,38,40–42). The use of such technology varied from study to study, but often included a place for patients to track their food intake and exercise via a website, typically designed specifically for the study, or computer program and/or emails including topics such as goal setting.
Patient characteristics
Patients’ average age ranged from 40s to 60s. The sex breakdown ranged from 8% men (45) to 55% men (38); although two studies limited recruitment to either women (39,50) or men (34) only. Inclusion of minority patients ranged from 3% (30) to 97% (27), with two studies (8.3%) recruiting African–American or Hispanic/Latino participants only (29,39,50). Almost 50% of studies did not include information regarding patients’ racial/ethnic background (n = 11, 45.8%) (25,31,33–35,37,38,43,44,47–49,51). Over one-third of studies also limited recruitment to individuals already diagnosed with hypertension or hyperlipidaemia (n = 1, 4.2%) (37), hypertension (n = 5, 20.8%) (27,28,31,47,48), and at risk for (n = 2, 8.3%) (44,45,51) or diagnosed with type two diabetes (n = 2, 8.3%) (29,38). The majority of studies (n = 10, 41.7%) required patients to be at least overweight (26,29,31–33,35,39,42,43,45,49,50) or obese (n = 5, 20.8%) (27,28,34,40,41). The remaining studies were restricted to normal or overweight (n = 1, 4.2%) (46) or (n = 8, 33.3%) incorporated body mass index into either broader requirements or was not a criterion (25,30,36–38,44,47,48,51).
Weight loss
To maintain consistency among studies, we present weight data based on changes from baseline to post-treatment assessment. A number of studies (n = 10, 41.7%) reported less than 1 kg (25,29,30,33,35–38,44,45,48,49,51) of weight loss for the treatment condition or weight gain with these interventions ranging from 12 weeks (25) to 2 years (38). Half of the studies (n = 12) reported average weight losses of 1.0–4.9 kg for MI interventions (26–28,31,32,34,39,41–43,46,47,50), ranging from 12 weeks (26,27,41) to 5 years (43). Only one study reported an average weight loss over 5 kg (i.e. 5.8 kg; 40). When the information was available, we calculated weight loss per hour of treatment, and it ranged from 0 (25) to 2.15 kg (40; Table 1).
In addition to overall weight loss for intervention participants, an important outcome is weight loss relative to UC or control groups. When examining all 24 studies, 12 studies (50.0%) reported no significant weight loss compared with UC (25,29–31,36–38,42–45,48,51), 9 studies (37.5%) reported significant weight loss compared with UC or control groups (27,28,32–35,39,46,47,49,50), 1 (4.2%) reported a trend towards significance ( (26), P = 0.053) and 2 (8.3%) provided MI to both conditions (40,41). When comparing the studies that implemented the MI intervention in addition to typical primary care appointments to those that incorporated MI into regularly scheduled appointments, 9 (27,28,32–35,46,47,49) of 17 studies (52.9%) versus 2 (39,50) of 7 (28.6%) studies reported the MI group experienced significant weight loss compared with control groups, respectively.
Losing 5% of initial body weight is associated with ameliorating weight-related health consequences. Many studies (n = 11, 45.8%) did not include this measurement (25,30,31,35–38,43,44,47–49,51). Studies that reported and achieved a 5% loss of initial weight (n = 13, 54.2%) (26–29,32–34,39–42,45,46,50) by post-treatment included ranges from 12.5% (39,50) to 35.7% (46) of participants reaching this goal. When examining the 13 studies reporting data on 5% weight loss, a few patterns emerged. Almost all were individual treatment (n = 12) (26,27,29,32–34,39–42,45,46,50), incorporated telephone sessions (n = 4) (32–34,45), technology via computer, internet, or emails (n = 3) (40–42), or a combination of both (n = 3) (26–28) to support weight loss. The treatment duration most typically was 6 months (n = 5) (33,34,39,40,45,50); while 4 (26,27,32,41) were 12 weeks and 4 (28,29,42,46) were longer than 6 months. Most of these studies included some information regarding MI training or fidelity (n = 8) (26,29,33,34,39,42,45,46,50). The majority (n = 11) (26–28,32–34,40–42,45,46) implemented the MI intervention as additional treatment provided above and beyond implementing MI within regular primary care visits.
When examining the 11 studies (45.8%) that did not report data on 5% weight loss (25,30,31,35–38,43,44,47–49,51), approximately half were individual treatment (n = 6) (25,31,35,38,47–49) and few incorporated telephone sessions (n = 3) (25,47,48) and/or technology via computer, internet or emails (n = 1) (38). The treatment duration most typically was longer than 6 months (n = 7) (30,31,37,38,43,44,48,51), 2 (25,47) were less than 6 months and 1 (35,49) was exactly 6 months. Half of these studies included some information regarding MI training or fidelity (n = 5) (31,35,38,44,48,49,51). Most (n = 8) (25,30,35,37,43,44,47–49,51) implemented the MI intervention as additional treatment provided in addition to implementing MI into regular primary care visits.
Other treatment outcomes
In addition to weight, other weight-related variables were measured; most often, these outcomes included physical activity, food intake, metabolic and physiological outcomes. Of those that measured physical activity (n = 14, 58.3%) (25,29–33,35–38,43–46,49,51), only 4 reported significant improvements in physical activity compared with UC/control groups (29,31,35,37,49) and 4 reported significant increases for both MI and UC/control groups (25,29,33,36,45).
More than half of the studies (n = 13, 54.2%) also examined changes in food-related behaviours (29,32,35–38,40,43–49,51) and of them, 10 (29,32,35,36,38,43–46,48,49,51) found no treatment-related improvements compared with UC/control conditions and one (40) did not have a non-MI control group. MI treatment resulted in decreased salt intake in the low-MI intervention group (less time in treatment relative to the high-MI group) (47), and increased fruit and vegetable consumption (37), but these changes were not maintained at follow-up.
The most common metabolic and physiological measures were blood pressure (BP, n = 15, 62.5%; 25–31, 34,35,37,38,41,42,46,47,49), lipid panel (n = 11; 45.8%) (26,29–31,34,35,37,38,45,46,48,49), and/or glucose/HbA1c (n = 8, 33.3%) (26,29,34,38,43–46,51). Of the 15 studies measuring BP, 3 reported significant BP decreases compared with UC/control (35,46,47,49). In terms of cholesterol levels, one study reported significant increases in high-density lipoproteins only (31) and one reported significant decreases in total cholesterol and low-density lipoprotein (29). No studies reported significant changes in glucose/HbA1c overtime when compared with UC/controls.
Disordered eating
Only two studies (8.3%) examined variables related to disordered eating behaviours (26,40); one included a clinician-led interview (i.e. Eating Disorder Examination) to diagnose binge eating disorder (BED) and monitor changes in disordered eating (26); and one used a self-report measure, the Three-Factor Eating Questionnaire (40). In the first study, Barnes and colleagues (26) identified and diagnosed patients meeting DSM-5 criteria for BED and stratified treatment randomization by BED status. BED was unrelated to treatment outcomes. Overall eating disorder symptoms decreased overtime, regardless of treatment condition. In the second study, McDoniel et al. (40) showed that all participants (MI provided in each condition) reported significantly increased dietary restraint and decreased uncontrolled eating.
Discussion
To our knowledge, this is the first review of the literature to examine MI for weight loss among adults, specifically within primary care. Over one-third of the studies examined showed that participants treated with MI lost significantly more weight than UC controls, and approximately half reported participants losing 5% of initial weight. Approximately one-third of studies examining weight-related outcomes, such as physical activity, food intake and metabolic measurements, reported improvements compared with controls. No studies, however, reported improvements in glucose/HbA1c. Many studies failed to report details related to training MI clinicians, supervision for MI clinicians, and treatment fidelity. Treatment participants tended to be White women in their 50s with obesity.
Participants receiving MI for weight loss in primary care achieved no to modest weight loss on average when compared with UC controls, with just over one-third of the studies reporting significant weight changes for MI participants compared with controls. The benefits of MI weight loss interventions in primary care may be overlooked when examining only the average weight loss, which ranges from +1 kg gained to −5.7 kg lost (40,48). A key benefit of incorporating these treatments into primary care is the increased dissemination of treatments when compared with specialty weight loss clinics. Approximately half of the studies showed that approximately 6% (40) to 35.7% (46) of patients lost at least 5% of their initial body weight, a parameter associated with important health-related improvements (53). If primary care offices worldwide were able to help this percentage of their overweight and obese patients lose enough weight to experience health benefits, perhaps such interventions are worth the investment, despite the modest average weight losses. While other weight loss treatment options, such as weight loss surgery (54) or intensive lifestyle interventions (55), may result in greater weight loss than those presented here, there has been a call for scalable and easily accessible obesity treatments (6). This recent shift is to broaden the impact of weight loss interventions and to help the significant percentage of individuals with overweight and obesity who may not have access to more intensive options. MI, therefore, may offer benefits to patients without access to specialty clinics who seek weight loss through primary care.
The following information is based on studies that reported data on 5% weight loss. We cannot assume that studies not reporting this information did not have participants achieve this goal. Interesting patterns, however, emerged when examining studies that did versus did not achieve or examine this outcome. Studies reporting patients meeting this goal were more likely to provide individual treatment as opposed to mixed (individual and group), were twice as likely to incorporate phone sessions and/or technology via computer, internet, and/or emails, and treatment tended to be 6 months or less versus longer than 6 months in duration. They were also slightly more likely to include information about MI training or fidelity and to incorporate the MI intervention in addition to regularly scheduled appointments instead of within regularly scheduled primary care appointments.
In addition to weight loss, other outcomes included changes in physical activity, food intake, metabolic/physiological measurements and disordered eating. Of those examining improvements in physical activity and food-related behaviours, most reported no significant improvements compared with UC. One-third of the studies examining BP reported significant improvements, few reported improvements in cholesterol, and no studies reported significant improvements for glucose/HbA1c relative to UC control groups. Even fewer studies examined disordered eating generally or BED, the latter of which may be common among treatment-seeking individuals with overweight or obesity (26). Based on the limited literature, conclusions cannot be drawn about the impact of MI on disordered eating within the context of weight loss treatments in primary care.
Part of the recent increase of incorporating MI into primary care is that previous literature suggests nonspecialists can be trained in MI (23). The breakdown of chosen clinicians somewhat follows guidelines of the Centers for Medicare and Medicaid, which currently only provide coverage for lifestyle interventions provided by primary care physicians, physician assistants, and nurse practitioners. It is important to note, however, that many studies utilized providers (e.g. exercise specialists) who may not be readily available in most primary care centres.
It is quite surprising that most studies did not discuss MI treatment fidelity, as the use of treatment fidelity measures has been linked to improved weight loss outcomes when using MI for weight loss (9). Only two studies (8.3%) reported the results of standardized fidelity ratings of real patient sessions (26,46). One reported inadequate delivery and one reported adequate delivery of MI. It is truly difficult, therefore, to make conclusions regarding the quality of the MI provided in most of these studies. Ideally, MI clinicians receive ongoing training and supervision that incorporates rating-based feedback (56,57). These rating systems typically require that MI clinicians demonstrate MI adherence and competence prior to providing MI in a treatment trial (56,57), and are also used by independent raters at the end of the trial to assess treatment fidelity. However, such implementation may be difficult to reconcile within busy primary care offices.
Guidelines for treating obesity include treatment duration of at least 6 months (58). This recommendation was supported by a meta-analysis reporting improved weight loss outcomes following MI treatments of 6 months or longer (9). Similarly, current studies reporting better weight loss outcomes tended to be 6 months in duration. While preliminary, reviewed interventions longer than 6 months did not appear to confer additional weight loss benefits.
Importantly, few studies statistically controlled for cluster effects; failure to do so is a significant limitation as it considerably reduces the ability to accurately interpret findings (59). For example, studies relying on group-randomized trial design (e.g. medical centres randomly assigned to treatment arms) must use appropriate statistical methods to best clarify outcomes (59). Results from studies failing to do so must be interpreted with caution. Controlling for cluster effects, when appropriate based on study design, should be a significant consideration in future research assessing MI in primary care centres.
Limitations of the current study include publication bias or ‘file–drawer problem’ such that non-significant results tend not to be published. Despite our extensive searches, both electronically and through the reference lists of relevant publications, it is possible that we missed relevant publications. The current review also did not include a meta-analysis; however, this allowed for examination of a broader range of published literature. As only one study (26) provided detailed information on MI training, standardized MI fidelity measurement and satisfactory MI adherence outcomes, comparing the weight loss outcomes among studies that did and did not include this information was not possible. Lastly, the terms used throughout the literature to describe MI interventions range (e.g. ‘structured by MI principles’, ‘motivational coaching’, ‘MI approach’). We chose to include all studies that referenced MI in some manner. While this may cast a broad net for study inclusion, without fidelity data, it is not possible to determine based on the MI terms used alone which studies may or may not have implemented a valid MI intervention.
There are a number of future research areas that will benefit the field. First, these treatment designs often are chosen as a cost-effective option when compared with traditional therapy. Only one study, however, included cost-effectiveness analyses. While such analyses may be out of the scope of publishing an RCT, providing more details regarding treatment (including minutes of treatment received) will allow for future cost-effectiveness analyses and also treatment replications. Second, as stated previously, the average intervention patient is a White woman in her 50s with obesity, and approximately half of the studies neglected to report patients’ race and ethnicities. Future studies must incorporate specific outreach to increase inclusion of minorities, men, and younger patients. Because of the high rates of overweight and obesity within minority populations (60), it is very important for future studies to report the ethnic and racial breakdown of their patients, and to include specific outreach to recruit more diverse samples. Similarly, average ages of participants ranged from mid-40s–60s. Considering that most individuals continue to gain weight each year (61), perhaps at even more significant rates for those with BED (62), it is important for primary care to engage young adults. Third, the internet may have extensive public health potential for widespread dissemination of interventions, particularly given the time and resource restraints within primary care centres. A meta-analysis of internet treatments for weight loss concluded that the internet is a viable means for providing treatment for overweight and obesity when used as an adjunct to clinician interaction (63). Despite this, only a handful of studies incorporated such technology, and none reported examining the benefits of smart-phone technology. Such technology appears to be related to improved weight loss outcomes, but more research is needed (64). Fourth, weight-related variables such as metabolic syndrome and BED were overlooked. Both are related to weight, medically costly, and common within primary care (65). Fifth, weight loss medications combined with clinician support may result in improved weight loss outcomes; however, none of the reviewed studies examined a combination of medications and MI. Sixth, an important step in improving how we determine the impact of MI on weight loss in primary care is the inclusion of attention–control conditions (9). Only one study compared the MI intervention to UC and an attention–control condition (26), and in fact, the attention–control outperformed MI when compared with UC.
In summary, primary care offices may be a unique opportunity to address a recent mandate to focus on scalability and retention (6). This review suggests there is potential for MI to help primary care adult patients lose weight and improve weight-related variables, such as decreasing BP. Conclusions, however, must be drawn cautiously as more than half of the reviewed studies showed no significant weight loss compared with UC/control groups and there was little existing evidence regarding MI treatment fidelity.
Acknowledgement
We would like to thank Jessica Barber, Ph.D. for providing feedback on an earlier draft of this paper.
Funding: This study was supported by a career development award from the National Institutes of Health, K23-DK092279 for RDB.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation. Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Ervin RB. Prevalence of Metabolic Syndrome among Adults 20 Years of Age and Over, by Sex, Age, Race and Ethnicity, and Body Mass Index: United States, 2003–2006. National Center for Health Statistics; Hyattsville, MD: 2009. National Health Statistics Reports; No 13. [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Melchionda N, Apolone N, et al. The metabolic syndrome in treatment-seeking obese persons. Metabolism. 2004;53:435–540. doi: 10.1016/j.metabol.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Pagota SL, Appelhans BM. A call for an end to the diet debates. JAMA. 2013;310:687–688. doi: 10.1001/jama.2013.8601. [DOI] [PubMed] [Google Scholar]
- 7.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 3rd edn Guilford Press; New York: 2013. [Google Scholar]
- 8.DiLillo V, Smith West D. Motivational interviewing for weight loss. Psychiatr Clin N Am. 2011;34:861–869. doi: 10.1016/j.psc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 10.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 11.Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Soc Work Pract. 2010;20:137–160. [Google Scholar]
- 12.Margitić S, Sevick MA, Miller M, et al. Challenges faced in recruiting patients from primary care practices into a physical activity intervention trial. Activity counseling trial research group. Prev Med. 1999;29:277–286. doi: 10.1006/pmed.1999.0543. [DOI] [PubMed] [Google Scholar]
- 13.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns. 2011;82:123–129. doi: 10.1016/j.pec.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis NJ, Emerenini A, Wylie-Rosett J. Obesity management: physician practice patterns and patient preference. Diabetes Educ. 2006;32:557–561. doi: 10.1177/0145721706290437. [DOI] [PubMed] [Google Scholar]
- 15.Galuska DA, Will JC, Serdula MK, Ford ES. Are health care professional advising obese patients to lose weight? JAMA. 1999;282:1576–1578. doi: 10.1001/jama.282.16.1576. [DOI] [PubMed] [Google Scholar]
- 16.Malterud K, Ulriksen K. Obesity in general practice: a focus group study on patient experiences. Scand J Prim Health Care. 2010;28:205–210. doi: 10.3109/02813432.2010.526773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JG, Cohen D, DiCicco-Bloom B, et al. Speaking of weight: how patients and primary care clinicians initiate weight loss counseling. Prev Med. 2004;38:819–827. doi: 10.1016/j.ypmed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Tsai AG, Wadden TA. Treatment of obesity in primary care practices in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson SE, Wardle J, Johnson F, Finer N, Beeken RJ. The impact of a health professional recommendation on weight loss attempts in overweight and obese British adults: a cross-sectional analysis. BMJ Open. 2013;3:e003693. doi: 10.1136/bmjopen-2013-003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraschnewski JL, Sciamanna CN, Pollak KI, Stuckey HL, Sherwood NE. The epidemiology of weight counseling for adults in the United States: a case of positive deviance. Int J Obes. 2013;37:6751–6753. doi: 10.1038/ijo.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care. 2013;51:186–192. doi: 10.1097/MLR.0b013e3182726c33. [DOI] [PubMed] [Google Scholar]
- 22.Haeseler F, Fortin AH, Pfeiffer C, Walters C, Martino S. Assessment of motivational interviewing curriculum for year 3 medical students using a standardized patient case. Patient Educ Couns. 2011;84:27–30. doi: 10.1016/j.pec.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns. 2011;84:16–26. doi: 10.1016/j.pec.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36:101–109. doi: 10.1016/j.jsat.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Armit CM, Brown WJ, Marshall AL, et al. Randomized trial of three strategies to promote physical activity in general practice. Prev Med. 2009;48:156–163. doi: 10.1016/j.ypmed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Barnes RD, White MA, Martino S, Grilo C. A randomized controlled trial comparing scalable weight loss treatments in primary care. Obesity. 2014;22:2508–2516. doi: 10.1002/oby.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: a randomized controlled trial. Obesity. 2010;18:308–313. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian JG, Bessesen DH, Byers TE, Christian KK, Goldstein MG, Bock BC. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168:141–146. doi: 10.1001/archinternmed.2007.13. [DOI] [PubMed] [Google Scholar]
- 30.Cochrane T, Davey R, Iqbal Z, et al. NHS health checks through general practice: randomized trial of population cardiovascular risk reduction. BMC Public Health. 2012;12:944–954. doi: 10.1186/1471-2458-12-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drevenhorn E, Bengtson A, Nilsson PM, Nyberg P, Kjellgren KI. Consultation training of nurses for cardiovascular prevention: a randomized study of 2 years duration. Blood Press. 2012;21:293–299. doi: 10.3109/08037051.2012.680734. [DOI] [PubMed] [Google Scholar]
- 32.Ely AC, Banitt A, Befort C, et al. Kansas primary care weighs in: a pilot randomized trial of a chronic care model program for obesity in 3 rural Kansas primary care practices. J Rural Health. 2008;24:125–132. doi: 10.1111/j.1748-0361.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 33.Greaves CJ, Middlebrooke A, O’Loughlin L, et al. Motivational interviewing for modifying diabetes risk: a randomized controlled trial. Br J Gen Pract. 2008;58:535–540. doi: 10.3399/bjgp08X319648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groeneveld IF, Proper KI, van der Beck AJ, van Mechelen W. Sustained body weight reduction by an individual-based lifestyle intervention for workers in the construction industry at risk for cardiovascular disease: results of a randomized controlled trial. Prev Med. 2010;51:240–246. doi: 10.1016/j.ypmed.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Hardcastle S, Taylor AH, Bailey MP, Castle R. A randomized controlled trial on the effectiveness of a primary health care based counseling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008;70:31–39. doi: 10.1016/j.pec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Harris MF, Chan BC, Laws RA, et al. The impact of a brief lifestyle intervention delivered by generalist community nurses (CN SNAP trial) BMC Public Health. 2013;13:375–385. doi: 10.1186/1471-2458-13-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris MF, Fanaian M, Jayasinghe UW, et al. A cluster randomized controlled trial of vascular risk factor management in general practice. Med J Aust. 2012;197:387–393. doi: 10.5694/mja12.10313. [DOI] [PubMed] [Google Scholar]
- 38.Heinrich E, Candel MJJM, Schaper NC, de Vries NK. Effect evaluation of a motivational interviewing based counseling strategy in diabetes care. Diabetes Res Clin Pract. 2010;90:270–278. doi: 10.1016/j.diabres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity. 2008;16:2462–2467. doi: 10.1038/oby.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDoniel SO, Hammond RS. A 24-week randomized controlled trial comparing usual care and metabolic-based diet plans in obese adults. Int J Clin Pract. 2010a;64:1503–1511. doi: 10.1111/j.1742-1241.2010.02464.x. [DOI] [PubMed] [Google Scholar]
- 41.McDoniel SO, Wolskee P, Shen J. Treating obesity with a novel hand-held device, computer software program, and Internet technology in primary care: the SMART motivational trial. Patient Educ Couns. 2010b;79:185–191. doi: 10.1016/j.pec.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 42.Nanchahal K, Power T, Holdsworth E, et al. A pragmatic randomized controlled trial in primary care of the Camden Weight Loss programme. BMJ Open. 2012;2:e000793. doi: 10.1136/bmjopen-2011-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penn L, White M, Oldroyd J, Walker M, Alberti GMM, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9:342–355. doi: 10.1186/1471-2458-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermunt PWA, Milder IEJ, Wielaard F, de Vries JHM, van Oers HAM, Westert GP. Lifestyle counseling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabetes Care. 2011;34:1919–1925. doi: 10.2337/dc10-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the diabetes prevention program to primary care: a pilot study. Nurs Res. 2009;58:2–12. doi: 10.1097/NNR.0b013e31818fcef3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams LT, Hollis JL, Collins CE, Morgan PJ. Can a relatively low-intensity intervention by health professionals prevent weight gain in mid-age women? 12-month outcomes of the 40-something randomized controlled trial. Nutr Diabetes. 2014;4:e116–e123. doi: 10.1038/nutd.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woollard J, Beilin L, Lord T, Puddey I, MacAdam D, Rouse I. A controlled trial of nurse counseling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466–468. doi: 10.1111/j.1440-1681.1995.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 48.Woollard J, Burke V, Beilin LJ, Verheijden M, Bulsara MK. Effects of a general practice-based intervention on diet, body mass index, and blood lipids in patients at cardiovascular risk. J Cardiovasc Risk. 2003;10:31–40. doi: 10.1097/01.hjr.0000050718.61003.30. [DOI] [PubMed] [Google Scholar]
- 49.Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomized controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act. 2013;10:40. doi: 10.1186/1479-5868-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin PD, Rhode PC, Dutton GR, Redmann SM, Ryan DH, Brantley PJ. A primary care weight management intervention for low-income African–American women. Obesity. 2006;14:1412–1420. doi: 10.1038/oby.2006.160. [DOI] [PubMed] [Google Scholar]
- 51.Vermunt PWA, Milder IEJ, Wielaard F, et al. A lifestyle intervention to reduce Type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med. 2012;29:e223–e231. doi: 10.1111/j.1464-5491.2012.03648.x. [DOI] [PubMed] [Google Scholar]
- 52.Groeneveld IF, Proper KI, van der Beek AJ, van Duivenbooden C, van Mechelen W. Design of a RCT evaluating the (cost-) effectiveness of a lifestyle intervention for male construction workers at risk for cardiovascular disease: the Health under Construction Study. BMC Public Health. 2008;8 doi: 10.1186/1471-2458-8-1. doi:10.1186/1471-2458-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3(Suppl. 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 54.Sjostrom L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 55.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ball SA, Martino S, Corvino J, Morganstern J, Carroll KM. Independent tape rater guide. 2005. Unpublished psychotherapy tape rating.
- 57.Martino S, Ball SA, Nich C, Frankforter TL, Carroll LM. Community program therapist adherence and competence in motivational enhancement therapy. Drug Alcohol Depend. 2008;96:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;24(25 Suppl. 2):129, S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Public Health. 2004;94:423–432. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Beydoun MA. The obesity epidemic in the United States – gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 61.Williamson DF. Weight change in middle-aged Americans. Am J Prev Med. 2004;27:81–82. doi: 10.1016/j.amepre.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 62.Ivezaj V, Kalebjian R, Grilo CM, Barnes RD. Comparing weight gain in the year prior to treatment for overweight and obese patients with and without binge eating disorder in primary care. J Psychosom Res. 2014;77:151–154. doi: 10.1016/j.jpsychores.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kodama S, Saito K, Tanaka S, et al. Effect of web-based lifestyle modifications on weight control: a meta-analysis. Int J Obes (Lond) 2012;36:675–685. doi: 10.1038/ijo.2011.121. [DOI] [PubMed] [Google Scholar]
- 64.Hartmann-Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P, Behavioural Weight Management Review Group Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obes Rev. 2014;15:920–932. doi: 10.1111/obr.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnes RD, Boeka AG, McKenzie KC, et al. Metabolic syndrome in obese patients with binge-eating disorder in primary care clinics: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:e1–e7. doi: 10.4088/PCC.10m01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systemic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]