Abstract
Exon skipping is currently one of the most promising molecular therapies for Duchenne muscular dystrophy (DMD). We have recently developed multiple exon skipping targeting exons 6 and 8 in dystrophin mRNA of canine X-linked muscular dystrophy (CXMD), an animal model of DMD, which exhibits severe dystrophic phenotype in skeletal muscles and cardiac muscle. We have induced efficient exon skipping both in vitro and in vivo by using cocktail antisense 2’ O-methyl oligonucleotides (2’OMePS) and cocktail phosphorodiamidate morpholino oligomers (morpholinos, or PMOs) and ameliorated phenotype of dystrophic dogs by systemic injections. The multiple exon skipping (double exon skipping) shown here provides the prospect of choosing deletions that optimize the functionality of the truncated dystrophin protein for DMD patients by using a common cocktail that could be validated as a single drug and also potentially applicable for more than 90% of DMD patients.
Keywords: Multiple exon skipping, Morpholinos (phosphorodiamidate morpholino oligomers), 2′ O-methylated antisense oligomers (phosphorothioate), Dystrophic dogs (canine X-linked muscular dystrophy), Duchenne/Becker muscular dystrophies
1. Introduction
Duchenne muscular dystrophy (DMD), a progressive and fatal X-linked myopathy, and its milder form, Becker muscular dystrophy (BMD), are caused by mutations in the DMD gene (1). Exon skipping using antisense oligonucleotides (AOs) is currently one of the most promising molecular therapies for DMD (2–4). Synthetic derivatives of nucleic acids have been designed and synthesized, where the backbone of RNA and DNA is replaced with other chemistries. One uses a morpholino backbone phosphorodiamidate morpholino oligomers (morpholinos, or PMOs) developed by AVI BioPharma, Portland, Oregon. Recently, we have successfully induced dystrophin expression by using morpholino-mediated systemic multiple exon skipping and ameliorated dystrophic pathology in dogs (5). Another antisense chemistry 2’O-methylated phosphorothioate (2’OMePS) has been also shown to effectively induce dystrophin expression systemically in mice in vivo (6).
The canine X-linked muscular dystrophy (CXMD) model contains a point mutation within the acceptor splice site of exon 7. This leads to exclusion of exon 7 from the mRNA transcript (7, 8). To restore the open reading frame, at least two further exons (exons 6 and 8) must be skipped (multiple exon skipping, or multiexon-skipping). Therefore, it is more challenging to rescue dystrophic dogs with exon-skipping strategy. Here, we summarize the method and protocol of antisense-mediated exon skipping in vitro and in vivo in dystrophic CXMD dogs.
2. Materials
2.1. Design of Antisense Oligos
Web sites for exonic splicing enhancer ESE targeting. ESE finder [http://rulai.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi?process=home] and Rescue ESE [http://genes.mit.edu/burgelab/rescue-ese/].
2.2. Transfection of Antisense 2’OMePS into Dog Myoblasts
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Bethesda, MD, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, Ogden, UT, USA).
0.25% Trypsin and 1 mM ethylenediamine tetraacetic acid (EDTA) (Gibco).
Teflon cell scrapers (Fisher, Waltham, MA, USA).
Ham’s F-10 nutrient mixture with HEPES (Gibco) (9).
Fetal calf serum (FCS) (Gibco).
Human recombinant basic fibroblast growth factor (bFGF) (Sigma-Aldrich, Natick, MA, USA).
Penicillin (200 U/mL) and streptomycin (200 µg/mL) (Sigma-Aldrich).
AOs (2’OMePS) (Eurogentec, Liège, Belgium) against exons 6 and 8 of the canine dystrophin gene. Ex6A (GUU GAUUGUCGGACCCAGCUCAGG), Ex6B (ACCUAUGA CUGUGGAUGAGAGCGUU), and Ex8A (CUUCCUGG AUGGCUUCAAUGCUCAC).
Lipofectin (Invitrogen, Carlsbad, CA, USA).
2% Horse serum (Gibco).
Six-well plates (IWAKI, Funabashi, Japan).
Opti-MEM (Gibco).
Culture dish (10, 15 cm noncoat and 10, 15 cm collagen coat) (IWAKI).
Phosphate buffer saline (PBS).
Human recombinant insulin (10 mg/mL) (Sigma-Aldrich).
Proliferation medium: Nutrient Mixture F-10 Ham (Ham’s F-10; developed by Ham et al. for mammalian cell proliferation (9)) supplemented with 200 U/mL penicillin, 200 µg/mL streptomycin, 2.5 ng/mL bFGF, and 20% FBS.
Differentiation medium: DMEM supplemented with 200 U/mL penicillin, 200 µg/mL streptomycin, and 10 µg/mL insulin.
2.3. Intramuscular Injections of Antisense Oligos in Dogs
CXMD dogs and wild type littermates.
Antisense morpholinos (Gene-tools, Philomath, OR, USA) against exons 6 and 8 of the dog dystrophin gene. Ex6A (GTTGATTGTCGGACCCAGCTCAGG), Ex6B (ACCTAT GACTGTGGATGAGAGCGTT), and Ex8A (CTTCCTGG ATGGCTTCAATGCTCAC) (see Note 1).
Saline (Ohtsuka-Pharmaceutical, Tokyo, Japan).
27G Needles (TERUMO, Tokyo, Japan).
Thiopental sodium (Mitsubishi Tanabe Pharma, Osaka, Japan).
Isoflurane (Abbott laboratories, Chicago, IL, USA).
Butorphanol tartrate (Bedford Laboratories, Bedford, OH, USA).
Gauze (Johnson and Johnson, New Brunswick, NJ, USA).
Pledget (Johnson and Johnson).
Veterinary surgical instruments: forceps, scalpels, scissors, suture needles, threads, and needle holders (Mizuho, Narashino, Japan).
Povidone iodine (Meiji Seika, Tokyo, Japan).
Heparin sodium (Fuji Pharmaceutical, Tokyo, Japan).
Surgical glove (Ansell, Red bank, NJ, USA).
Surgical drape (Nagai Leben, Tokyo, Japan).
Sepham antibiotics (Cefamezine or Syncl) (Astellas, Tokyo, Japan, or Asahi-kasei, Tokyo, Japan).
2.4. Systemic Injections of Antisense Morpholinos
CXMD dogs and wild-type littermates.
Syringe infusion pump (Muromachi, Tokyo, Japan).
22G Indwelling needles (TERUMO).
50 mL syringe (TERUMO).
Antisense morpholinos (Gene-tools)against exons 6 and 8 of the dog dystrophin gene. Ex6A (GTTGATTGTCGGA CCCAGCTCAGG), Ex6B (ACCTATGACTGTGGATGA GAGCGTT), and Ex8A (CTTCCTGGATGGCTTCAATG CTCAC).
2.5. RNA Extraction
Eppendorf tubes (Eppendorf, Hamburg, Germany).
Trizol (Invitrogen).
Chloroform (Sigma-Aldrich).
Isopropanol (Sigma-Aldrich).
75% Ethanol (Sigma-Aldrich).
2.6. RT-PCR
One-Step RT-PCR kit (Qiagen, Venlo, The Netherlands).
Forward primer in exon 5: CTGACTCTTGGTTTGA TTTGGA (Invitrogen).
Reverse primer in exon 10: TGCTTCGGTCTCTGTCAATG (Invitrogen).
RNAsin (Promega, Madison, WI, USA).
2.7. cDNA Sequencing
Gel extraction kit (Qiagen).
BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA).
ExoSap-IT® (USB, Santa Clara, CA, USA).
MicroAmp® Reaction Plates (Applied Biosystems).
Qiagen gel extraction kit (Qiagen).
Hidi-formamide (Applied Biosystems).
ABI 3130 Genetic Analyzer (Applied Biosystems)
2.8. Muscle Sampling from Necropsy of Dogs
Tragacanth gum (Sigma-Aldrich).
Isopentane (Sigma-Aldrich).
Liquid nitrogen.
Cork disks (Iwai-kagaku, Tokyo, Japan).
Dry ice.
2.9. Immunostaining for Dog Muscles
Poly-l-lysine–coated slides (Fisher, Hampton, NH, USA).
Cover glasses (Fisher).
Cryostat Microsystem cm1900 (Leica, Wetzlar, Germany).
Dystrophin antibodies including DYS1 and DYS2 (Novocastra, Newcastle, UK).
Alexa 594 goat antimouse IgG1, Alexa 594 goat antimouse IgG2, highly cross-absorbed (Invitrogen).
DAPI containing mounting agent (Invitrogen).
Goat serum (Invitrogen).
Moisture chamber (Scientific Devise Laboratory, Des Plaines, IL, USA).
Chamber slide (Lab-tek, Naperville, IL).
4% Paraformaldehyde (PFA).
2.10. Western Blotting from Dog Muscles
Lysis buffer: 75 mM Tris–HCl (pH 6.8), 10% SDS, 10 mM EDTA, and 5% 2-mercaptoethanol.
Bradford reagent (Bio-Rad, Hercules, CA, USA).
Bovine serum albumin (BSA) (Sigma-Aldrich).
2× Laemmli SDS-loading buffer: 0.1 M Tris–HCl (pH 6.6), 2% (w/v) SDS, 2% (0.28 M) beta-mercaptoethanol, 20% glycerol, 0.01% bromophenol blue.
Ready-made 5% resolving SDS gels (Bio-Rad).
PVDF membrane (GE, Fairfield, CT, USA).
Transfer buffer (10×): 250 mM of Tris-Base, 1,920 mM of Glycine.
Transfer buffer (1×): 10% 10× buffer, 20% methanol.
Dystrophin antibodies including DYS1 and DYS2 (Novocastra) and desmin antibody (Abcam, Cambridge, MA, USA).
ECL plus kit (GE).
ECL and autography film (GE).
ImageJ software (NIH, Bethesda, MD, USA).
2.11. Clinical Grading of Dogs
Video camera.
Stop watch.
3. Methods
3.1. Design of Antisense Oligos
Identify ESE sites in exons using Rescue ESE and ESEfinder.
Design antisense sequences to target ESEs of exon 6 (Ex6A) and exon 8 (Ex8A), or exon/intron boundary between exon 6 and intron 6 (Ex6B), or between exon 8 and intron 8 (Ex8B) (see Note 2).
Select antisense oligonucleotide chemistries. 2’OMePS is preferred for myoblast experiment (see Note 3). PMOs are used for in vivo studies (see Note 1).
3.2. Transfection of Antisense 2’OMePS into Dog Myoblasts
Use standard preplating method to obtain primary myoblast cells from neonatal CXMD dogs (10).
Culture WT or CXMD myoblasts (1.5 × 105 cells) in growth medium containing F-10, FCS (20%), bFGF (2.5 ng/mL), penicillin (200 U/mL), and streptomycin (200 µg/mL) for 72 h, on six-well plates.
Dilute lipofectin to a total of 100 mL in opti-MEM media at a ratio of 2:1 for lipofectin: RNA (Use 10 mL lipofectin for 5 mg RNA).
Allow to stand still at RT for 30–45 min, then dilute AOs to a final volume of 100 µL in opti-MEM media.
Combine diluted lipofectin and AOs and mix gently.
Incubate at RT for 10–15 min.
Remove serum-containing medium from cells and wash them with opti-MEM reduced serum media.
Add 0.8 mL opti-MEM media to the tube containing the lipofectin DNA complexes.
Mix gently and overlay the complex onto the cells.
Return cells to the incubator and after 3 h replace opti-MEM media with differentiation medium and wait 3–10 days until they differentiate into myotubes.
3.3. Intramuscular Injections of Antisense Oligos in Dogs
Induce general anesthesia by 20 mg/kg of thiopental sodium injections and maintain by isoflurane inhalation (2.0–3.0%).
Cut skin above tibialis anterior (TA) muscle with scalpel.
Stitch the fascia of TA muscles at two different points at 2 cm intervals as markers; i.e., inner side distal/outer side proximal.
Bend needles (10°) to inject PMOs horizontally using 27 G needle, inject PMO solutions slowly into muscles and wait 1 min before removing the needle to prevent leakage.
Inject butorphanol tartrate (0.2 mg/kg) before and after procedure.
Administer sepham antibiotics (Cefamezine or Syncl) for three days after surgical procedures.
3.4. Systemic Injections of Antisense Morpholinos
Dissolve 120–200 mg/kg of morpholinos Ex6A, Ex6B, and Ex8A at 32 mg/mL in saline.
Inject them into saphenous vein of a dog using 22 G indwelling needles for each injection using infusion pumps to inject at 50 mL/20 min.
Inject morpholinos for 5–11 times at weekly or biweekly intervals.
3.5. RNA Extraction from Myotubes
Remove medium.
Put 1 mL Trizol for each well of six-well plates.
Wait 10 min.
Add 200 µL of chloroform (for RNA)
Shake well.
Wait 2 min.
You can see three layers including the RNA layer (top), DNA layer (middle), and protein layer (bottom).
Centrifuge at 12,000 × g for 15 min at 4°C.
Take 400 mL carefully from top layer. Remove supernatant from the top layer, and put in another tube.
Add 500 mL isopropanol.
Keep in −80°C for O/N.
Centrifuge at 12,000 × g, 10 min, 4°C.
Decant fluid. You can see a pellet of RNA in bottom.
Wash with 75% EtOH.
Centrifuge at 8,000 × g, 5 min, 4°C.
Dry up, keep upside down for 15 min or O/N.
Add 15–30 µL water, then quantify RNA concentration.
3.6. RT-PCR
Make Reaction mix containing 1.5 µL 10 mM forward primer, 1.5 µL 10 mM reverse primer, 1 µL dNTP, 5 µL one-step PCR kit buffer, 0.7 µL RNAsin, 1 µL enzyme mixture from one-step PCR kit, and 200 ng RNA and add water to the total of 25 µL.
Perform RT-PCR in the thermocycler with 1 cycle of 50°C 30 min, 1 cycle of 95°C 15 min, 35 cycles of 94°C 1 min, 60°C 1 min and 72°C 1 min. Finally add 1 cycle of 72°C 10 min and then store PCR product in 4°C.
3.7. cDNA Sequencing
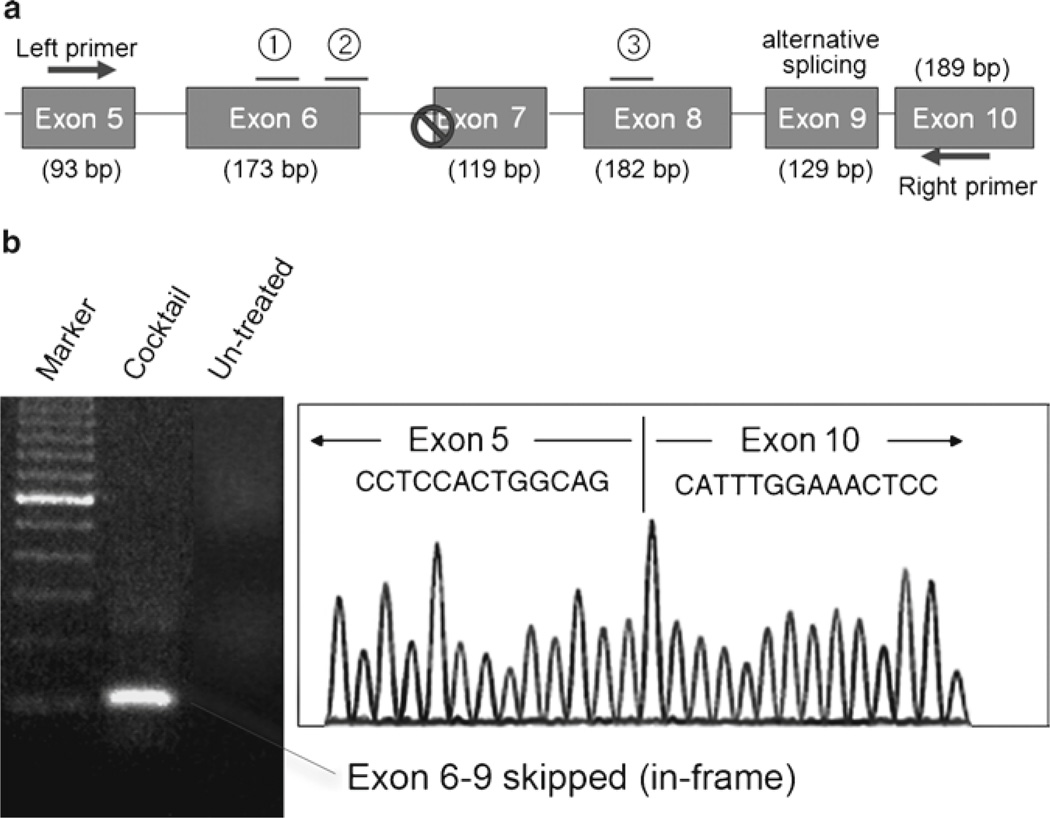
Use Qiagen gel extraction kit to excise the band of interest for subsequent cDNA sequencing according to manufacturer’s instructions. Exon 6–9 skipped band (101 bp) is identified by electrophoresis using 2% agarose gel (Fig. 1).
Use BigDye® Terminator v3.1 cycle sequencing kit for cDNA sequencing with the same primers following manufacturer’s instructions (Fig. 1).
Fig. 1.
Multiple exon skipping in dystrophic dogs. (a) Schematic outline of the protocol. A splice site mutation in intron 6 leads to deletion of exon 7 at mRNA level in dystrophic dogs. To restore the reading frame, two additional exons (exon 6 and exon 8) need to be skipped (removed) by three oligo cocktail of antisense. Exon 9 is known as alternative splice site. (b) RT-PCR and cDNA sequencing after exon skipping in dystrophic dogs. Left panel; RT-PCR reveals exon 6–9 skipped in-frame products (101 bp) in dystrophic dogs after the treatment of cocktail oligos. Alternative splice site Exon 9 is also mostly removed from the resulting mRNA. Right panel; Exon-skipping patterns are further confirmed by cDNA sequencing.
3.8. Muscle Sampling from Necropsy of Dogs
Inject with thiopental sodium for induction of general anesthesia, then maintain anesthetic status by isoflurane.
Euthanize dogs by bleeding from the carotid artery.
Collect following muscles by necropsy of dogs 2 weeks after final injection of oligos. These muscles include TA, extensor digitorum longus (EDL), Gastrocnemius, soleus, biceps femoris, rectus femoris, biceps brachii, triceps brachii, deltoid, extensor carpi ulnaris (ECU), extensor carpi radialis (ECR), flexor carpi ulnaris (FCU), flexor carpi radialis (FCR), gracilis, intercostal, abdominal muscles, diaphragm, lateral dorsi, esophagus, sternocleidomastoid, and the heart.
Dissect muscles into small portion to stand on cork disks (1.2 cm diameter) labeled with the ID of the animal and muscle name on the back side.
Mix a portion of tragacanth gum (10–20 mL) well with equal amount of water until it becomes soft and sticky. Put them into 10 mL or 25 mL syringes. Unused gum in the syringe can be stored in freezer.
Put tragacanth gum to fix the muscle specimen on cork disks.
Put liquid nitrogen in a metal container and isopentane in a smaller metal container.
Lower the isopentane with the container into the liquid nitrogen. Wait for a couple of minutes until it becomes slushy and ready for freezing.
Put a portion of gum on the cork.
Dissect out muscles and put it on the cork at RT. Place it on the cork longitudinally and put some gum blob around the bottom of them so that the longitudinal axis of the muscle is perpendicular to the cork and stable.
Place the muscle on cork into the cooled isopentane and shake vigorously for 1 min.
Place it on dry ice (Fig. 2).
Put samples in a glass vials, and store at −80°C.
Fig. 2.
Frozen muscle samples from dystrophic dogs.
3.9. Immunostaining for Dog Muscles
Set up cryostat for sectioning. The working temperature should be −25°C. Set the section thickness at 8 µm for immunohistochemistry and 12 µm for HE staining. Put in a blade.
Place muscle blocks on dry ice for transportation.
Label slide glasses in pencil with animal IDs, cut date, and muscle name.
Mount cork with muscle sample block and fix in place with water. Attach the chuck with tissue specimen onto the holder.
Start slicing the muscle until approximately one fourth of the way in the muscle.
Touch and transfer individual sections onto RT slide glass and leave at RT to dry.
Place every sixth section on the same slide (sections 1, 6, 11 on slide #1; sections 2, 7, 12 on slide #2) and cut them at interval of every 200 mm until you have five sections collected per slide. Keep sections clustered as closely as possible to reduce the amount of antibody solutions required
When finished, allow slides to dry at RT for at least an additional 90 min. Slides can be stored at −80°C.
For immunohistochemistry, put slides in moisture chamber (and dry them for 30 min if they were stored in a freezer).
Blocking; 2 h in PBS with 15% goat serum at RT.
Incubate with a primary antibody; antidystrophin rod (DYS-1) or C-terminal monoclonal antibody (DYS-2) for dog dystrophin staining (1:150 dilutions) for overnight at 4°C.
Wash with PBS 5 min × 3 times.
Incubate with a secondary antibody, Alexa 594 goat antibody against mouse IgG1 or IgG2 (highly cross-absorbed) (1:2,500) for 30 min at RT.
Wash with PBS 5 min × 5 times.
Wipe off excess liquid and mount with DAPI-containing mounting agent for nuclear staining and then put cover glasses.
Count the number of positive fibers for DYS1 under fluorescent microscope and compare in sections where their biggest number of the positive fibers were as previously described (see Note 4) (11).
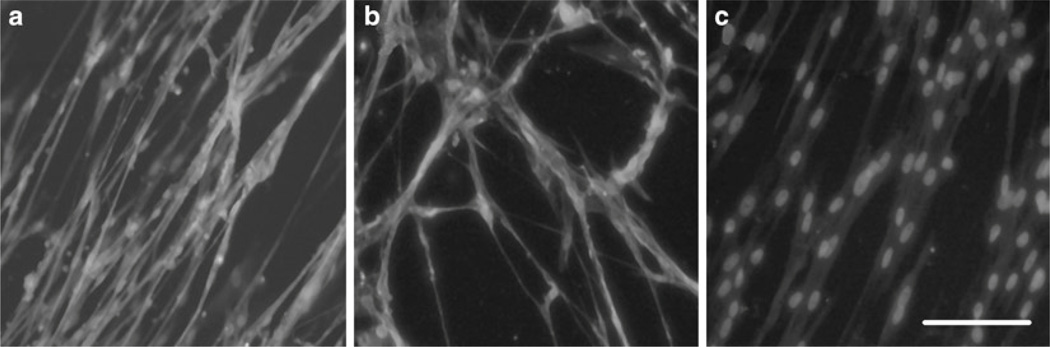
Immunohistochemistry is also applicable for myotubes. Use slide glasses with chambers to culture them and fix them by 4% PFA for 10 min (Fig. 3).
Fig. 3.
Recovery of dystrophin expression after cocktail antisense transfection in dystrophic dog myoblast. Dystrophin expression and DAPI nuclear double staining of wild-type myotubes (a), cocktail of Ex6A, Ex6B, and Ex8A 2’OMePS transfected myotubes (b), and nontreated CXMD myotubes (c). Dystrophin C-terminal antibody DYS-2 is used. Bar: 50 µm.
3.10. Western Blotting from Dog Muscles
Collect the 30–40 of cryo-sections of 15 µm in 1.5 mL tube on dry ice.
Add 150 mL of sample buffer and homogenize on ice.
Boil them for 3–5 min and centrifuge for 15 min at 16,500 × g.
Collect supernatant and keep the aliquot at –70°C.
Dilute an aliquot of protein 100-fold with distilled water to reduce final SDS concentration less than 0.1%. Measure protein concentration of the diluted protein sample with Bradford protein assay. Specifically, record the absorbance at 570 nm using a photospectrometer and calculate the concentration from standard curve.
For SDS-PAGE, set the glass plates for readymade minigel (5%).
Mix the samples with 2× Laemmli SDS-loading buffer.
Boil samples for 3 min, then load 20 mg of samples in each lane.
Run the gel at 150 V for approximately 3 h.
After running the gel, incubate the gel for 20 min in transfer buffer + 0.1 % SDS (optional for transferring high molecular weight proteins).
Wet four pieces of sponge and Whatman paper with ddH2O, then soak them in the transfer buffer, and soak PVDF membrane using methanol for 1 min to prewet it, and then pouroff methanol and add H2O, make sure that the membrane does not float. Leave it in water for 3 min.
Set the gel and membrane as shown in the manual.
Run 40–50 V o/n in cold room.
For blotting, prepare 2,000 mL of 0.05% PBS/Tween 20 (PBST). Wash the membrane briefly with 20 mL PBS.
Prepare 100 mL of PBST/5% milk powder, and incubate in 50 mL PBST/5% milk powder for 2 h.
Incubate the blot with primary antibody in the appropriate dilution with PBS/5% milk powder (1:100 dilution for Dys1 dystrophin antibody) for 1 h or O/N.
Wash the blot for 15 min each with 3× 100 mL PBST, then incubate the blot with the HRP conjugated secondary antibody.
Wash the blot for 20 min each with 3× 200 mL PBST.
Use ECL plus kit for detection. Mix two solutions at 40:1, and incubate with membrane for 1 min. Then use film and developer for the detection.
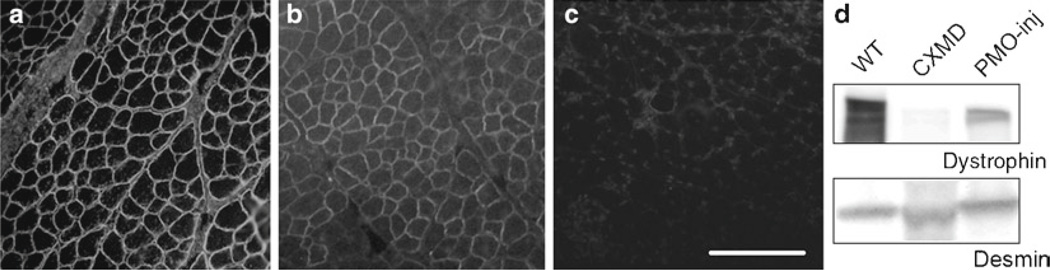
To preserve for the spare blot, rinse the blot with PBST, and store it in PBST at 4°C for a few weeks. Desmin antibody is used to normalize intersample loading amount. Signals are analyzed and quantified using Adobe Photoshop and ImageJ software (Fig. 4).
Fig. 4.
Recovery of dystrophin expression after 7 × 200 mg/kg intravenous cocktail morpholino injections. Dystrophin expression of wild-type dog muscle (a), cocktail of Ex6A, Ex6B, and Ex8A PMOs injected dog muscle (b), nontreated CXMD dogs (c), Western blotting analysis with dystrophin antibody (d). Bar: 100 µm.
3.11. Clinical Grading of Dogs
Let a dog walk and evaluate gait disturbance: grade 1 = none, grade 2 = sitting with hind legs extended, grade 3 = bunny hops with hind legs, grade 4 = shuffling walk, and grade 5 = unable to walk (12).
Evaluate mobility disturbance: grade 1 = none, grade 2 = lying down more than normal, grade 3 = cannot jump on hind legs; grade 4 = increasing difficulty moving around, and grade 5 = unable to get up and move around.
Palpate limb or temporal muscle atrophy: grade 1 = none, grade 2 = suspect hardness, grade 3 = can feel hardness or apparently thin, grade 4 = between grades 3 and 5, and grade 5 = extremely thin or hard.
Evaluate drooling: grade 1 = none, grade 2 = occasionally dribbles saliva when sitting, grade 3 = some drool when eating and drinking, grade 4 = strings of drool when eating or drinking, and grade 5 = continuous drool.
Evaluate macroglossia: grade 1 = none, grade 2 = slightly enlarged, grade 3 = extended outside dentition, grade 4 = enlarged and slightly thickened, and grade 5 = enlarged and thickened.
Evaluate dysphagia: grade 1 = none; grade 2 = takes time and effort in taking food, grade 3 = difficulty in taking food from plate, grade 4 = difficulty in chewing, swallowing, or drinking, and grade 5 = unable to eat.
Add up the total score.
For running test, encourage each dog to run one time for 15 m, and record elapsed time.
Acknowledgments
Authors thank Drs. Terence Partridge, Stephanie Duguez (Children’s National Medical Center, Washington DC), Masanori Kobayashi, Yoshitsugu Aoki, Takashi Saito, Katsutoshi Yuasa, Naoko Yugeta, Sachiko Ohshima, Jin-Hong Shin, Michiko Wada, Kazuhiro Fukushima, Satoru Masuda, Kazue Kinoshita, Hideki Kita, Shin-ichi Ichikawa, Yumiko Yahata, Takayuki Nakayama, Akinori Nakamura (National Institute of Neuroscience, Tokyo, Japan), Adam Rabinowitz, and Jonathan Beauchamp (Imperial College, London, UK), Qi-long Lu (Carolinas Medical Center) for discussions and technical assistance. This work was supported by the Foundation to Eradicate Duchenne, the Department of Defense CDMRP program, the Jain Foundation, the Crystal Ball of Virginia Beach (Muscular Dystrophy Association USA), the National Center for Medical Rehabilitation Research, the NIH Wellstone Muscular Dystrophy Research Centers, and the Ministry of Health, Labor, and Welfare of Japan (Research on Nervous and Mental Disorders, 16B-2, 19A-7; Health and Labor Sciences, Research Grants for Translation Research, H19-translational research-003, Health Sciences Research Grants Research on Psychiatry and Neurological Disease and Mental Health, H18-kokoro-019).
Footnotes
Alternatively, one can also use 2’O-MePs (Eurogentec) against exons 6 and 8 of the dog dystrophin gene. These include Ex6A (GUUGAUUGUCGGACCCAGCUCAGG), Ex6B (ACCUAUGACUGUGGAUGAGAGCGUU), and Ex8A (CUUCCUGGAUGGCUUCAAUGCUCAC).
The efficacy of antisense oligos is highly unpredictable, and hence several antisense oligos should be designed for each target exon. A preferred antisense sequence contains 40–60% of GC, does not have more than three consecutive guanine, and does not lead to self dimers or hetero dimers when injected as a cocktail. We have designed more than ten antisense sequences against exon 6 and exon 8 of dogs, and optimized the most efficient combination of cocktail antisense oligos both in vitro and in vivo (Saito et al., Unpublished).
For 2′OMePS, U (uracil) is used instead of T (thymidine).
Occasionally dystrophin-positive revertant fibers can be detected in dystrophic dog muscles (5, 12). Revertant fibers cannot be distinguished from antisense-mediated dystrophin expression by immunohistochemistry unless an epitope-specific antibody is used. Therefore, the expression level should be carefully compared with untreated controls.
References
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Yokota T, Duddy W, Partridge T. Optimizing exon skipping therapies for DMD. Acta Myol. 2007;26:179–184. [PMC free article] [PubMed] [Google Scholar]
- 3.Yokota T, Takeda S, Lu QL, Partridge TA, Nakamura A, Hoffman EP. A renaissance for antisense oligonucleotide drugs in neurology: exon skipping breaks new ground. Arch Neurol. 2009;66:32–38. doi: 10.1001/archneurol.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokota T, Pistilli E, Duddy W, Nagaraju K. Potential of oligonucleotide-mediated exon-skipping therapy for Duchenne muscular dystrophy. Expert Opin Biol Ther. 2007;7:831–842. doi: 10.1517/14712598.7.6.831. [DOI] [PubMed] [Google Scholar]
- 5.Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, Hoffman E. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, Jadoon A, Bou-Gharios G, Partridge T. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, Tomohiro M, Kornegay JN, Nonaka I, Takeda S. Canine X-linked muscular dystrophy in Japan (CXMDJ) Exp Anim. 2003;52:93–97. doi: 10.1538/expanim.52.93. [DOI] [PubMed] [Google Scholar]
- 8.Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S, Hung WY, Constantinou CD, Dykstra MJ, Roses AD, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- 9.Ham RG. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 11.Yokota T, Lu QL, Morgan JE, Davies KE, Fisher R, Takeda S, Partridge TA. Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J Cell Sci. 2006;119:2679–2687. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- 12.Shimatsu Y, Yoshimura M, Yuasa K, Urasawa N, Tomohiro M, Nakura M, Tanigawa M, Nakamura A, Takeda S. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy in Japan, CXMDJ. Acta Myol. 2005;24:145–154. [PubMed] [Google Scholar]